Abstract
Artemisinin, a powerful antimalarial medicine, is extracted from the Chinese herb, Artemisia annua L., and has the ability to inhibit the proliferation of cancer cells. Dihydroartemisinin (DHA), the major active metabolite of artemisinin, is able to inhibit the growth of a variety of types of human cancer. However, the effect of DHA on human glioma cells remains unclear. The aim of the present study was to investigate the effect of DHA on the proliferation of glioma cells, and whether DHA was able to enhance temozolomide (TMZ) sensitivity in vitro and in vivo. In total, 10 human glioma cell lines were used to analyze the growth inhibition ability of DHA by MTT assay. The typical autophagic vacuoles were monitored by the application of the autofluorescent agent, monodansylcadaverine. Western blotting was used to detect markers of apoptosis and autophagy, namely Caspase-3, Beclin-1 and LC3-B. The combination efficiency of DHA and TMZ was assessed in vitro and in vivo. The half maximal inhibitory concentration (IC50) of DHA differed among the ten human glioma cell lines. The number of autophagic vacuoles was higher in DHA-treated SKMG-4 cells; this was highest of all cell lines analyzed. The expression of autophagy molecular markers, Beclin-1 and LC3-B, was increased following DHA treatment, while no significant alteration was detected in the expression of apoptotic marker Caspase-3. When combined with DHA, the IC50 of TMZ decreased significantly in the four glioma cell lines analyzed. Furthermore, DHA enhanced the tumor inhibition ability of TMZ in tumor-burdened mice. The results of the present study demonstrated that DHA inhibited the proliferation of glioma cells and enhanced the tumor inhibition efficacy of TMZ in vitro and in vivo through the induction of autophagy.
Keywords: dihydroartemisinin, temozolomide, glioma, autophagy
Introduction
Glioma is the most common type of primary brain tumor, accounting for ~80% of all brain and central nervous system tumors. Glioma is divided into four grades (grade I-IV) according to criterion established by the World Health Organization. The survival rate of low-grade glioma (grade II) is higher than that of high-grade glioma (anaplastic astrocytomas and glioblastoma multiforme), at ~47% (1). High-grade gliomas are the most malignant tumors and are characterized by high cellularity, mitotic activity, vascular proliferation, central necrosis and aggressive invasion into normal brain tissue (2). Despite the combination of maximal tumor resection, concurrent radiotherapy, adjuvant chemotherapy and molecular targeted therapy for the treatment of high grade glioma, the life expectancy of patients remains poor, at just 12–14 months (3–6). Therefore, the identification of novel, effective therapies for the treatment of gliomas is required.
Artemisinin is derived from a traditional Chinese herb, which is used for the treatment of fever and malaria (7). Previous studies have revealed that artemisinin is able to inhibit the progression of chronic myelogenous leukemia, pancreatic adenocarcinoma, colon cancer, prostate cancer, non-small cell lung cancer and breast malignancies at low concentrations (8–10). Artemisinin and its derivatives have also been used in the screening of anticancer drugs by the National Cancer Institute (NCI) (11).
Dihydroartemisinin (DHA), the major active metabolite of artemisinin, has been found to inhibit the growth of cancer cells in a variety of cancers (12–15). Xie et al (16) reported that the concentration of DHA was two-fold higher in the brain than in the plasma, which indicates that DHA is able to penetrate the brain-blood barrier. DHA triggers the production of reactive oxygen species (ROS) and inhibits the activity of glutathione-S-transferase, thereby promoting the radiosensitivity of glioma cells (17). Furthermore, DHA has been found to potentiate the cytotoxic effect of temozolomide (TMZ) in rat C6 glioma cells (18). However, whether DHA is able to enhance the efficiency of TMZ in human glioma cells has remained to be elucidated. The present study aimed to assess the effect of DHA on glioma cell lines as well as whether DHA could enhance the cell response to TMZ.
Materials and methods
Cell lines and reagents
In total, 10 human glioma cell lines, namely MGR1, MGR3, SF295, SKMG-1, SKMG-4, T98G, U251, U251/CP2, U373 and U87, were used in the present study. All cells were maintained in Dulbecco's modified Eagle's medium /F-12 (Gibco Life Technologies, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (GE Healthcare Life Sciences, Chalfont, UK) and 1.0% penicillin/streptomycin (Gibco Life Technologies) in a humidified incubator at 37°C with 5% CO2. DHA (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich; 20 µg/ml) and stored at −20°C in the dark. TMZ (Sigma-Aldrich) was dissolved in DMSO, (stock concentration, 0.2 M) and stored at −20°C.
Cytotoxicity assay
The glioma cells were seeded into 96-well plates at a density of 5×103 cells/well and incubated overnight at 37°C. Next, the cells were treated with DHA at serial concentrations (0, 0.3125, 0.625, 1.25, 2.5, 5.0, 10.0 and 20.0 µg/ml) for 72 h. Cell viability was analyzed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. In order to detect the effect of DHA on TMZ efficiency, the cells were treated with 2 µM DHA in combination with various concentrations of TMZ (ranging between 31.25 and 2,000 µM). Following 72 h of incubation, the MTT assay was performed and the half maximal inhibitory concentration (IC50) was calculated. Briefly, MTT (Sigma-Aldrich) was added to cells following treatment and incubated for 4 h at 37°C. DMSO was subsequently added and absorbance was measured by microplate reader (Multiskan MK3; Thermo Fisher Scientific, Waltham, MA, USA) after shaking for ~30 sec.
Western blot analysis
The cell lysates were collected using radioimmunoprecipitation assay lysis buffer (1% NP-40, 1% sodium deoxycholate, 0.1% SDS, 150 mM NaCl and 10 mM Na2HPO4; pH 7.2; Beyotime Institute of Biotechnology, Japan), supplemented with a protease inhibitor cocktail (Roche Diagnostics GmbH, Mannheim, Germany). Immunoblotting was performed as previously described (19). Rabbit anti-human antibodies against LC3-B (polyclonal; catalog no. ab48394; dilution, 1:1,000; Abcam, Cambridge, MA, USA), Beclin-1 (monoclonal; catalog no. ab51031; dilution, 1:750; Abcam) and Caspase-3 (polyclonal; catalog no. ab90437; dilution, 1:100; Abcam) were added and incubated overnight at 4°C. A mouse anti-human β-actin antibody (catalog no. AA128; dilution, 1:1,000; Beyotime Institute of Biotechnology) was loaded as the internal control. The membranes were then washed three times with phosphate-buffered saline (PBS). Horseradish peroxidase-conjugated goat anti-mouse (dilution, 1:1,000; catalog no. A0216; Beyotime Institute of Biotechnology) and goat anti-rabbit (dilution, 1:1,000; catalog no. 7074; Cell Signaling Technology Inc., Danvers, MA, USA) secondary antibodies were applied for 30 min at room temperature. The membranes were then washed three times with PBS and incubated with enhanced chemiluminescence reagent (Millipore, Billerica, MA, USA) according to the manufacturer's instructions. Next, the membranes were exposed to ECL sensitive films (Hyperfilm ECL; GE Healthcare Bio-Sciences, Pittsburgh, PA, USA), and developed using an OPTIMAX X-Ray film processor (Protec GmbH & Co. KG, Oberstenfeld, Germany). All protein bands were normalized to β-actin, which was used as the internal loading control.
Monodansylcadaverine (MDC) fluorescence staining
The glioma SKMG-4 cells, which are sensitive to DHA, were seeded into a 30-mm culture dish and treated with 2 µg/ml DHA for 24 h. MDC (Sigma-Aldrich), at a concentration of 0.05 mM, was then added for 10 min. Next, the cells were fixed in 4% paraformaldehyde (Sigma-Aldrich) for 15 min and washed twice with phosphate-buffered saline. The cytoplasmic autophagic vacuoles were then observed under a fluorescence microscope (Olympus BX61; Olympus Corp., Tokyo, Japan).
In vivo glioma formation assay
The U251 cells were maintained in standard culture medium, adjusted to 5×106 cells/ml. Next, 300 µl cell suspension was injected into the axillary region of the nude mice (age, 4–5 weeks; mean weight, 22.6 g). Mice were maintained in specific-pathogen-free grade room, with 6 mice per cage, at 18–29°C and with light from 7:30 am to 19:30 pm. Two weeks later, the tumor masses were resected, cut into sections measuring 2 mm3 and transplanted subcutaneously into the backs of a second set of experimental nude mice. These mice were randomly separated into four groups (n=10) and treated for 5 continuous days with either DMSO (control), 50 mg/kg DHA only, 50 mg/kg TMZ only, or 50 mg/kg DHA plus 50 mg/kg TMZ. The tumor sizes were measured using a vernier caliper every three days during the following month. The tumor volumes (TV) were calculated according to the following formula: TV = 1/2 × length × width2 (20). The present study was approved by the ethics committee of Sun Yat-sen University Cancer Center (Guangzhou, China) according to Institutional Animal Care and Use Committee protocols. Chloral hydrate was applied for anesthesia (10%; 80 µl per mouse), and mice were sacrificed by cervical dislocation.
Statistical analysis
The data is expressed as the mean ± standard error of the mean. Student's t-test was used to determine any statistically significant differences. P-values are two sided, with P<0.05 considered to indicate a statistically significant difference. Statistical analysis was performed using SPSS 16.0 (SPSS, Inc., Chicago, IL, USA).
Results
DHA is cytotoxic to glioma cells in vitro
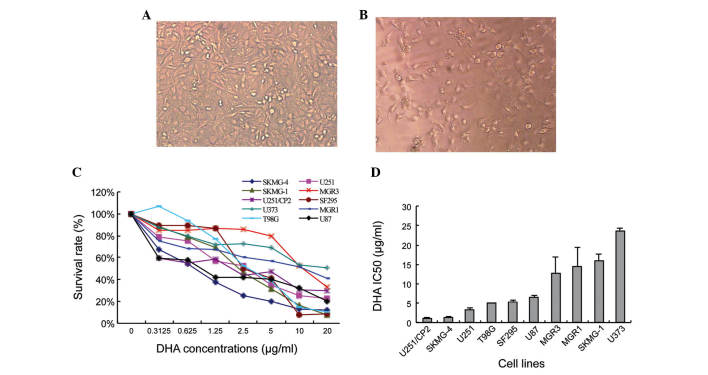
The IC50 of ten glioma cell lines treated with serial concentrations DHA was calculated. Following treatment, the SKMG-4 cells appeared to be low in density and exhibit abnormal morphologies compared with the non-treated cells (Fig. 1A and B). The extent of DHA toxicity was mild and differed amongst the glioma cell lines (Fig. 1C). The IC50 also varied between the ten human glioma cell lines, from 1.17±0.08 µg/ml in U251/CP2 cells to 23.57±0.80 µg/ml in U373 cells (Fig. 1D).
Figure 1.
DHA exhibits cytotoxicity in glioma cell lines in vitro. The density and cellular morphology of SKMG-4 cells cultured (A) without and (B) with DHA (magnification, ×200). The cells appeared lower in density and shrunken following treatment with DHA. (C) Survival curve of the 10 human glioma cell lines following treatment with serial concentrations of DHA for 72 h. (D) IC50 of the 10 glioma cell lines. Values are expressed as the mean ± standard deviation. DHA, dihydroartemisinin; IC50, half maximal inhibitory concentration.
DHA activates autophagy, rather than apoptosis, in SKMG-4 cells
The cell lysates were collected from SKMG-4 cells following treatment with DHA for 72 h. Markers of apoptosis and autophagy were then detected by western blotting. There were no significant alterations to the expression of Caspase-3 following DHA treatment. However, the expression of the autophagy markers, Beclin-1 and LC3-B, increased upon DHA treatment in a dose-dependent manner (Fig. 2A). The autofluorescent reagent MDC, a specific autophagic vacuole marker, was also applied in order to monitor the process of autophagy. Following culture with DHA and MDC, the SKMG-4 cells were fixed and observed under a fluorescent microscope. It was revealed that MDC accumulated in DHA-treated cells, but not in untreated control cells (Fig. 2B and C). The data therefore indicated that autophagy was induced by DHA in glioma cell lines.
Figure 2.
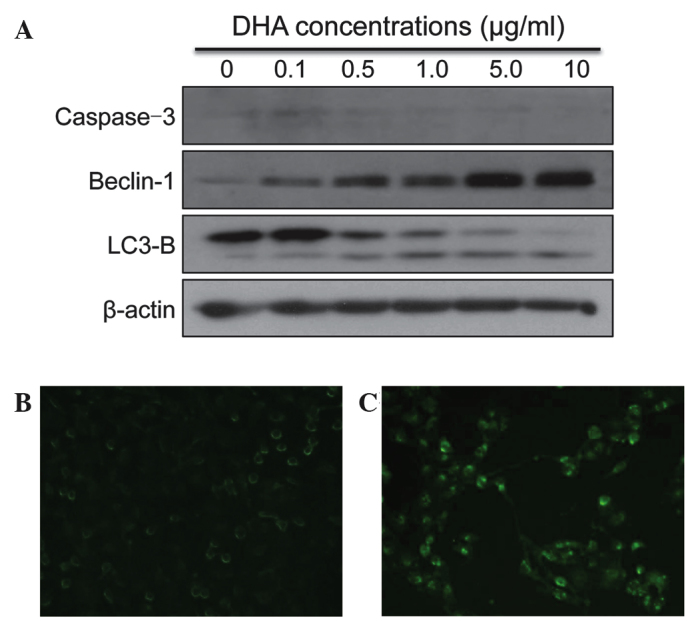
DHA activates autophagy in glioma cells. (A) The expression of the apoptosis molecular marker, Caspase-3, did not change following DHA treatment. However, the expression of autophagy markers Beclin-1 and LC3-B was markedly increased. (B) Monodansylcadaverine staining revealing that a 24-h treatment with 2 µg/ml DHA increased the formation of autophagic vacuoles in SKMG-4 cells compared with (C) the non-treated control cells (magnification, ×200). DHA, dihydroartemisinin.
DHA promotes the cytotoxicity of TMZ in glioma cells
The present study aimed to investigate whether combined treatment with DHA and TMZ was able to increase cell sensitivity to TMZ. In total, four glioma cell lines, namely SKMG-4, U251, U373 and T98G, were used for the combination treatment due to the differences in their sensitivity to DHA and their genetic background. The 10% inhibition concentration of TMZ (IC10) was calculated according to the results of the cytotoxicity assay (Fig. 1C). The concentration of DHA was fixed to the IC10 of each cell line and then combined with serial concentrations of TMZ. The IC50 of TMZ was calculated following co-treatment for 72 h. It was revealed that the application of DHA reduced the IC50 of TMZ in all four cell lines; from 417.36±17.92 to 143.90±28.74 µM in SKMG-4 cells (P<0.01), 455.06±64.39 to 246.84±26.14 µM in U251 cells (P<0.01), 759.03±76.96 to 537.15±11.88 µM in U373 cells (P<0.01) and 1274.72±159.70 to 863.20±73.26 µM in T98G cells (P<0.05) (Fig. 3). These data indicated that DHA sensitized glioma cell lines to TMZ.
Figure 3.
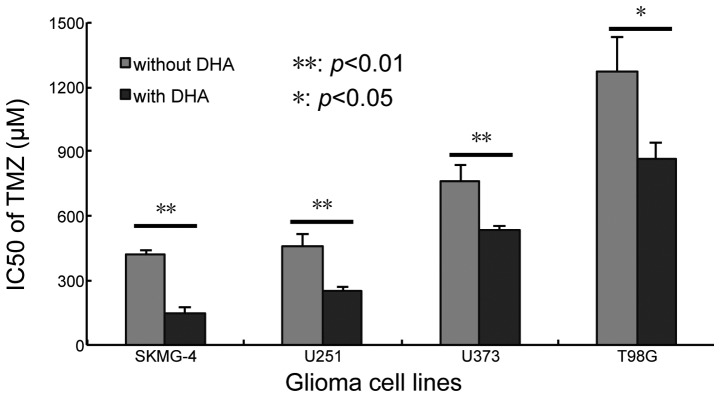
DHA increased the cytotoxic effect of TMZ in four glioma cell lines in vitro. The IC50 of TMZ markedly decreased when administered in combination with a low concentration of DHA (the 10% inhibition rate concentration) for 72 h in SKMG-4, U251, U373 and T98 G cells. Values are expressed as the mean ± standard deviation. **P<0.01, *P<0.05. DHA, dihydroartemisinin; TMZ, temozolomide; IC50, half maximal inhibitory concentration.
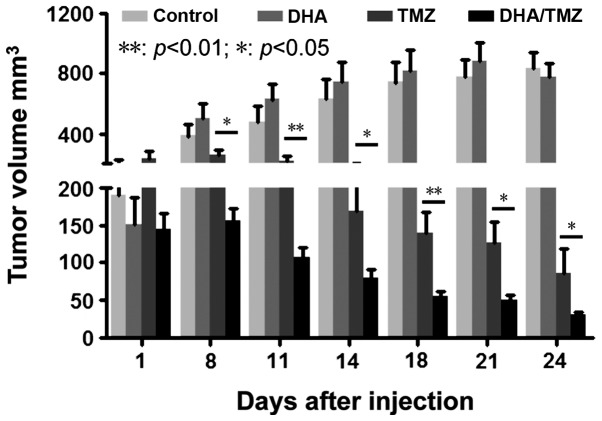
DHA strengthens the tumor inhibitory effect of TMZ
The glioma U251 cell line was used for the in vivo tumor formation and inhibition assay, due to its ability to form tumors in xenografts in vivo and its sensitivity to DHA (low IC50) in vitro. The tumor size was monitored every 3 days, following continuous treatment for 5 days with DMSO, DHA, TMZ and DHA+TMZ. It was revealed that 50 mg/kg DHA alone had no effect on tumor growth in vivo compared with the DMSO-treated control cells. By contrast, TMZ significantly suppressed tumor growth compared with that of the DMSO-treated control cells. Co-treatment with DHA and TMZ significantly reduced the tumor size compared with the effects of TMZ alone (Fig. 4; P<0.01, P<0.05). This indicated that DHA was able to strengthen the tumor inhibitory effect of TMZ in vivo. Taken together, the data demonstrated that DHA inhibited tumor cell proliferation and sensitized glioma cells to TMZ in vitro and in vivo, potentially through the activation of autophagy.
Figure 4.
DHA enhances the tumor inhibition effect of TMZ in vivo. Treatment with 50 mg/kg TMZ reduced tumor formation compared with the control (dimethyl sulfoxide) group. Although 50 mg/kg DHA alone had little effect on the tumor size of nude mice, it enhanced the action of TMZ in decreasing tumor burden, compared with the TMZ alone-treated group. Values are expressed as the mean ± standard deviation. **P<0.01, *P<0.05. DHA, dihydroartemisinin; TMZ, temozolomide.
Discussion
Glioma is the most common primary malignant brain tumor amongst adults. The median survival time of patients with high-grade glioma is generally <12 months, even with surgical resection, radiotherapy and chemotherapy (21–23). Therefore, the identification of novel, effective treatment strategies is necessary. Adjuvant chemotherapeutic reagents, as well as various combination therapies, have been extensively studied. Drugs used for combination therapies usually have clear pharmacokinetics, which leads to improved therapeutic efficiencies and tolerance, and reduces drug resistance and side effects (19,24,25).
Due to their efficiency and few side effects, traditional Chinese medicines have been extensively studied in anti-cancer research. Artemisinin is a Chinese herb derived from the plant, Artemisia annua L., and has been used in the treatment of fevers for >1,000 years (26). Rasheed et al (27) reported that artesunate, a derivative of artemisinin, was able to inhibit the proliferation and metastasis of non-small cell lung carcinomas in a chicken embryo model. A further study, which included 55 cancer cell lines, found that artesunate demonstrated anti-tumor activities in several types of tumor cell, including leukemia, as well as breast, ovarian, cervical, stomach, liver, pancreatic, colon and lung cancer (28). Artemisinin was therefore enrolled by the NCI in their drug-screening program. Previous data has suggested that artemisinin may be involved in multiple pathways. Screening approaches have established that artemisinin is involved in the inhibition of proliferation, the promotion of apoptosis and the inhibition of angiogenesis (28). Further studies have revealed that artemisinin contributes to the generation of ROS, which leads to heme-mediated endoperoxide cleavage, DNA damage and apoptosis (29). In addition, it has been found that artemisinin enhances the sensitivity of glioma cells to β, γ-rays (17).
As the major active metabolite of artemisinin, DHA has advantages over the original compound. The anti-cancer effects of DHA have been previously reported in several types of cancer, but not glioma. The present study identified that DHA mildly inhibited the growth of glioma cells. It was hypothesized that the inhibition of proliferation exerted by DHA may be cell-type specific, since its efficiency in the 10 human glioma cell lines evaluated was varied.
The findings of the present study indicated that the mechanisms underlying DHA-induced proliferation arrest may involve autophagy activation, since the expression of autophagic molecular markers increased following DHA treatment. Autophagy is a self-digestive process that degrades intracellular structures in response to oxidative stresses, ultimately leading to cell survival (30). However, cells die following prolonged autophagic activity. It has been established that cancer cells produce higher levels of ROS than those of normal cells, as a result of metabolic stress and proliferative requirements. A previous study reported that DHA induced autophagy in the mitochondria of leukemia K562 cells (31). A further study found that the sensitivity of cancer cells to artemisinin was attenuated following the overexpression of oxidative stress associated enzymes (32). The detailed mechanisms underlying the role of DHA in glioma cell autophagy will be investigated in future studies.
TMZ is an alkylating agent, whose therapeutic benefit depends upon its ability to alkylate DNA at the N7 or O6 positions of guanine residues. The low cytotoxicity, high lipid solubility and small molecular weight of TMZ facilitates its passage through the blood-brain barrier. These properties established TMZ as an effective, first-line chemotherapeutic drug for glioma patients. Kanzawa et al (33) identified that TMZ cytotoxicity resulted from the induction of autophagy, rather than apoptosis, in malignant glioma cells as the blockage of autophagy significantly decreased the anti-tumor effect of TMZ. The results of the present study revealed that DHA interacted with TMZ to enhance the anti-cancer effect in vitro and in vivo.
In conclusion, DHA may inhibit the growth of glioma cells through the induction of autophagy. Furthermore, cell response to TMZ may be enhanced significantly by co-treatment of DHA, both in vitro and in vivo. However, the mechanisms underlying the interaction between DHA and TMZ in autophagy and other signaling pathways require further investigation.
Acknowledgements
The present study was supported by grants from the National Natural Science Foundation of China (no. 81372685), the National High Technology Research and Development Program 863 (no 2012AA02A508), the Department of Science and Technology of Guangdong Province (no. 2011B031800178), the Natural Science Foundation of Guangdong Province (no. 10151040701000035), the Hong Kong Scholars Program (no. XJ2012059), the China Postdoctoral Science Foundation (no. 2013M540678) and the Fundamental Research Funds for Central Universities (no. 12ykpy49).
References
- 1.Smoll NR, Gautschi OP, Schatlo B, Schaller K, Weber DC. Relative survival of patients with supratentorial low-grade gliomas. Neuro Oncol. 2012;14:1062–1069. doi: 10.1093/neuonc/nos144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakazato Y. The 4th edition of WHO classification of tumours of the central nervous system published in 2007. No Shinkei Geka. 2008;36:473–491. (In Japanese) [PubMed] [Google Scholar]
- 3.Stupp R, Mason WP, van den Bent MJ, et al. National Cancer Institute of Canada Clinical Trials Group: Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 4.Davis FG, McCarthy BJ, Freels S, Kupelian V, Bondy ML. The conditional probability of survival of patients with primary malignant brain tumors: Surveillance, epidemiology and end results (SEER) data. Cancer. 1999;85:485–491. doi: 10.1002/(SICI)1097-0142(19990115)85:2<485::AID-CNCR29>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 5.Giese A, Bjerkvig R, Berens ME, Westphal M. Cost of migration: Invasion of malignant gliomas and implications for treatment. J Clin Oncol. 2003;21:1624–1636. doi: 10.1200/JCO.2003.05.063. [DOI] [PubMed] [Google Scholar]
- 6.Stupp R, Hegi ME, Mason WP, et al. European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group: Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 7.Klayman DL. Qinghaosu (artemisinin): An antimalarial drug from China. Science. 1985;228:1049–1055. doi: 10.1126/science.3887571. [DOI] [PubMed] [Google Scholar]
- 8.Qaderi A, Dadgar N, Mansouri H, Alavi SE, Esfahani MK, Akbarzadeh A. Modeling and prediction of cytotoxicity of artemisinin for treatment of the breast cancer by using artificial neural networks. Springerplus. 2013;2:340. doi: 10.1186/2193-1801-2-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim SH, Chun SY, Kim TS. Interferon-alpha enhances artemisinin-induced differentiation of HL-60 leukemia cells via a PKC alpha/ERK pathway. Eur J Pharmacol. 2008;587:65–72. doi: 10.1016/j.ejphar.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 10.Sadava D, Phillips T, Lin C, Kane SE. Transferrin overcomes drug resistance to artemisinin in human small-cell lung carcinoma cells. Cancer Lett. 2002;179:151–156. doi: 10.1016/S0304-3835(02)00005-8. [DOI] [PubMed] [Google Scholar]
- 11.Schmuck G, Haynes RK. Establishment of an in vitro screening model for neurodegeneration induced by antimalarial drugs of the artemisinin-type. Neurotox Res. 2000;2:37–49. doi: 10.1007/BF03033326. [DOI] [PubMed] [Google Scholar]
- 12.Ontikatze T, Rudner J, Handrick R, Belka C, Jendrossek V. Dihydroartemisinin is a hypoxia-active anti-cancer drug in colorectal carcinoma cells. Front Oncol. 2014;4:116. doi: 10.3389/fonc.2014.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li YJ, Zhou JH, Du XX, et al. Dihydroartemisinin accentuates the anti-tumor effects of photodynamic therapy via inactivation of NF-κB in Eca109 and Ec9706 esophageal cancer cells. Cell Physiol Biochem. 2014;33:1527–1536. doi: 10.1159/000358716. [DOI] [PubMed] [Google Scholar]
- 14.Park J, Lai HC, Singh M, Sasaki T, Singh NP. Development of a dihydroartemisinin-resistant Molt-4 leukemia cell line. Anticancer Res. 2014;34:2807–2810. [PubMed] [Google Scholar]
- 15.Gu HM, Warhurst DC, Peters W. Uptake of [3H] dihydroartemisinine by erythrocytes infected with Plasmodium falciparum in vitro. Trans R Soc Trop Med Hyg. 1984;78:265–270. doi: 10.1016/0035-9203(84)90296-7. [DOI] [PubMed] [Google Scholar]
- 16.Xie LH, Li Q, Zhang J, Weina PJ. Pharmacokinetics, tissue distribution and mass balance of radiolabeled dihydroartemisinin in male rats. Malar J. 2009;8:112. doi: 10.1186/1475-2875-8-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim SJ, Kim MS, Lee JW, et al. Dihydroartemisinin enhances radiosensitivity of human glioma cells in vitro. J Cancer Res Clin Oncol. 2006;132:129–135. doi: 10.1007/s00432-005-0052-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang XJ, Li CT, Zhang WP, Lu YB, Fang SH, Wei EQ. Dihydroartemisinin potentiates the cytotoxic effect of temozolomide in rat C6 glioma cells. Pharmacology. 2008;82:1–9. doi: 10.1159/000125673. [DOI] [PubMed] [Google Scholar]
- 19.Sun YC, Wang J, Guo CC, et al. MiR-181b sensitizes glioma cells to teniposide by targeting MDM2. BMC Cancer. 2014;14:611. doi: 10.1186/1471-2407-14-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naito S, von Eschenbach AC, Giavazzi R, Fidler IJ. Growth and metastasis of tumor cells isolated from a human renal cell carcinoma implanted into different organs of nude mice. Cancer Res. 1986;46:4109–4115. [PubMed] [Google Scholar]
- 21.Buckner JC. Factors influencing survival in high-grade gliomas. Semin Oncol. 2003;30(Suppl 19):S10–S14. doi: 10.1053/j.seminoncol.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 22.Curran WJ, Jr, Scott CB, Horton J, et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst. 1993;85:704–710. doi: 10.1093/jnci/85.9.704. [DOI] [PubMed] [Google Scholar]
- 23.De Angelis LM. Brain tumors. N Engl J Med. 2001;344:114–123. doi: 10.1056/NEJM200101113440207. [DOI] [PubMed] [Google Scholar]
- 24.Sai K, Li WY, Chen YS, et al. Triptolide synergistically enhances temozolomide-induced apoptosis and potentiates inhibition of NF-κB signaling in glioma initiating cells. Am J Chin Med. 2014;42:485–503. doi: 10.1142/S0192415X14500323. [DOI] [PubMed] [Google Scholar]
- 25.Fu J, Yang QY, Sai K, et al. TGM2 inhibition attenuates ID1 expression in CD44-high glioma-initiating cells. Neuro Oncol. 2013;15:1353–1365. doi: 10.1093/neuonc/not079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, Wu YL. An over four millennium story behind qinghaosu (artemisinin) - a fantastic antimalarial drug from a traditional Chinese herb. Curr Med Chem. 2003;10:2197–2230. doi: 10.2174/0929867033456710. [DOI] [PubMed] [Google Scholar]
- 27.Rasheed SA, Efferth T, Asangani IA, Allgayer H. First evidence that the antimalarial drug artesunate inhibits invasion and in vivo metastasis in lung cancer by targeting essential extracellular proteases. Int J Cancer. 2010;127:1475–1485. doi: 10.1002/ijc.25315. [DOI] [PubMed] [Google Scholar]
- 28.Efferth T, Sauerbrey A, Olbrich A, et al. Molecular modes of action of artesunate in tumor cell lines. Mol Pharmacol. 2003;64:382–394. doi: 10.1124/mol.64.2.382. [DOI] [PubMed] [Google Scholar]
- 29.Stockwin LH, Han B, Yu SX, et al. Artemisinin dimer anticancer activity correlates with heme-catalyzed reactive oxygen species generation and endoplasmic reticulum stress induction. Int J Cancer. 2009;125:1266–1275. doi: 10.1002/ijc.24496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scherz-Shouval R, Elazar Z. ROS, mitochondria and the regulation of autophagy. Trends Cell Biol. 2007;17:422–427. doi: 10.1016/j.tcb.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 31.Wang Z, Hu W, Zhang JL, Wu XH, Zhou HJ. Dihydroartemisinin induces autophagy and inhibits the growth of iron-loaded human myeloid leukemia K562 cells via ROS toxicity. FEBS Open Bio. 2012;2:103–112. doi: 10.1016/j.fob.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Efferth T, Briehl MM, Tome ME. Role of antioxidant genes for the activity of artesunate against tumor cells. Int J Oncol. 2003;23:1231–1235. [PubMed] [Google Scholar]
- 33.Kanzawa T, Germano IM, Komata T, Ito H, Kondo Y, Kondo S. Role of autophagy in temozolomide-induced cytotoxicity for malignant glioma cells. Cell Death Differ. 2004;11:448–457. doi: 10.1038/sj.cdd.4401359. [DOI] [PubMed] [Google Scholar]