Abstract
Background
Patients with chronic kidney disease (CKD) and secondary hyperparathyroidism (SHPT) who require dialysis are at increased risk for cardiovascular events and bone fractures. To assist in economic evaluations, this study aimed to estimate the disutility of these events beyond the impact of CKD and SHPT.
Methods
A basic one-year health state was developed describing CKD and SHPT requiring dialysis. Further health states added acute events (cardiovascular events, fractures, and surgical procedures) or chronic post-event effects. Acute health states described a year including an event, and chronic health states described a year subsequent to an event. General population participants in Canada completed time trade-off interviews from which utilities were derived. Pairwise comparisons were made between the basic state and event, and between comparable health states.
Results
A total of 199 participants (54.8% female; mean age = 46.3 years) completed interviews. Each health state had ≥130 valuations. The mean (SD) utility of the basic health state was 0.60 (0.34). For acute events, mean utility differences versus the basic state were: myocardial infarction, −0.06; unstable angina, −0.05; peripheral vascular disease (PVD) with amputation, −0.33; PVD without amputation, −0.11; heart failure, −0.14; stroke, −0.30; hip fracture, −0.14; arm fracture, −0.04; parathyroidectomy, +0.02; kidney transplant, +0.06. Disutilities for chronic health states were: stable angina, −0.09; stroke, −0.27; PVD with amputation, −0.30; PVD without amputation, −0.12; heart failure, −0.14.
Conclusions
Cardiovascular events and fractures were associated with lower utility scores, suggesting a perceived decrease in quality of life beyond the impact of CKD and SHPT.
Electronic supplementary material
The online version of this article (doi:10.1186/s12955-015-0266-9) contains supplementary material, which is available to authorized users.
Keywords: Utility, Chronic kidney disease, Secondary hyperparathyroidism, End-stage renal disease, Standard gamble
Background
Chronic kidney disease (CKD) involves a gradual and usually permanent loss of kidney function. The final stage, end-stage renal disease (ESRD; Stage 5), is characterized by kidney failure and the requirement for dialysis [1–3]. Most stage 5 CKD patients have abnormal blood mineral levels and elevated levels of parathyroid hormone (PTH), a condition known as secondary hyperparathyroidism (SHPT) [4–6]. Patients with CKD and SHPT typically experience a wide range of symptoms such as bone and joint pain, as well as psychological symptoms that are associated with lower health-related quality of life [7–11]. In patients with CKD, SHPT has been found to be associated with increased resource utilization [12], treatment costs [13], and mortality [14, 15].
CKD and SHPT are also associated with increased risk of serious cardiovascular and bone-related complications. Cardiovascular disease is the leading cause of death and disability in this population [16–21]. Elevated PTH, calcium, and phosphorus levels are associated with increased risk of cardiovascular events and cardiovascular-related death in patients with CKD [18]. Sustained control of these biomarkers is associated with increased survival [22]. Furthermore, abnormal levels of PTH and other mineral metabolism indicators are associated with increased risk of bone fractures [23, 24]. Pharmacological treatments for PTH and mineral imbalances in CKD include vitamin D sterols, phosphate binders, and calcimimetic agents [2, 25–28]. When pharmacotherapy and other minimally invasive treatment options are ineffective, surgical interventions (e.g., parathyroidectomy) may be necessary [28, 29].
As new treatments are developed for patients with CKD and SHPT, it is important to evaluate their cost-effectiveness to demonstrate their value to clinicians and third-party payers. Many of the cost-effectiveness analyses of CKD treatments are cost-utility models, which incorporate the preferences of individuals for various health states and treatment-related outcomes [5, 30–33]. In these models, treatment outcome is quantified in terms of quality-adjusted life years (QALYs), which combine duration of life and quality of life into a single metric. The quality of life component is based on utilities, which are values representing the strength of preferences for health states, anchored on a scale with 1 representing full health and 0 representing dead [34, 35].
Although utility values for CKD have been published [36, 37], little is known about the utility or disutility (i.e., utility decrease) associated with cardiovascular events and bone fractures in patients with CKD and SHPT. Consequently, modelers have often had to use utility data derived from other populations such as patients with osteoporosis or cardiovascular disease without CKD [30, 32, 33, 38], leading to considerable uncertainty in the resulting cost-utility estimates. Therefore, the objective of the current study was to obtain utility values associated with cardiovascular events and fractures in the context of SHPT and CKD requiring dialysis. In addition, utilities were obtained for parathyroidectomy and kidney transplant, the two surgical procedures that are common in this population.
Methods
Health state development
Health state descriptions were drafted based on patient interviews, interviews with healthcare providers, and literature review. Qualitative research was conducted with 54 patients diagnosed with CKD and SHPT who were receiving hemodialysis. These interviews elicited descriptions of patients’ experience with CKD and SHPT, including symptoms and impact. Information obtained from the patient interviews was used when drafting the structured interview guides for subsequent clinician interviews.
Telephone interviews were conducted with three Canadian-based physicians (co-authors DM, PM, and SS) who specialize in treatment of patients with CKD and SHPT as well as related cardiovascular events and fractures. Interviews were first conducted to inform health state development, with questions focusing on symptoms and impact of CKD and SHPT on a patient’s life, as well as the impact of dialysis treatment. Physicians also answered questions about cardiovascular events and fractures within this patient population, as well as differences or similarities with these events in non-CKD patients. Health states were subsequently drafted and then reviewed by the physicians for clarity and accuracy.
Literature was reviewed to inform the clinician interview questions and ensure that the health state descriptions were consistent with published research. Literature searches focused on CKD and SHPT [4, 8, 39]; cardiovascular events in the context of CKD and SHPT [40–47]; fractures in the context of CKD and SHPT [4, 5]; and surgical interventions for patients with CKD and SHPT [5, 39, 48].
Health states were tested in a pilot study conducted with 19 general population participants (13 female; mean age = 31.6 years; age range = 18 to 48) recruited through an online advertisement. Each participant valued the health states using both time-trade off (TTO) and standard gamble (SG) methods [49] in order to determine which of the methods was most appropriate for the time horizon of the task (one year). The order in which participants completed the two tasks was randomized (11 completed SG first; eight completed TTO first). Both TTO and SG methods yielded logical data (i.e., utilities in a reasonable range, with logical discrimination among health states). After completing the TTO and SG tasks to rate the health states, participants answered a series of qualitative questions designed to identify any issues with the rating tasks and health states. Although the TTO and SG methods elicited similar results, participants reported that the TTO was easier to understand and complete. Subsequently, the TTO method with a one-year time horizon was used in the main study. Following feedback from pilot study participants, minor edits to health states were made to improve clarity and ease of comprehension.
Final health states administered in time trade-off interviews
The final set of health state descriptions included a “basic health state” (health state A), which was designed to represent a patient with CKD and SHPT on dialysis. This health state included statements in four categories: a description of CKD with SHPT, symptoms, impact, and dialysis. The full text of this health state and all other health states is presented in Additional file 1. An Additional 15 “event health states” (health states B–P) included this basic health state, plus the addition of statements describing a cardiovascular event, a fracture, or a surgical procedure. Every health state was presented to participants as lasting for a single hypothetical year.
Eleven of the additional health states represented cardiovascular events (B–G and L–P). Because some of the cardiovascular events are likely to have long-term effects that are different from the acute experience of the event, both acute and chronic health states were developed. The acute health states described a year in which the event occurred, beginning with the event itself followed by ongoing consequences for the remainder of the year. The chronic health states described the ongoing functioning of a patient who had experienced one of these events in a previous year. These chronic health states can be characterized as post-event health states that describe the chronic ongoing impact of an event that occurred in the past, instead of the acute impact of the event itself.
Six acute health states (B–G) described acute cardiovascular events: myocardial infarction, unstable angina, heart failure exacerbation, peripheral vascular disease (PVD) with amputation, PVD without amputation, and stroke. Five chronic health states (L–P) were designed to represent ongoing long-term impact of previous cardiovascular events: stable angina (which is a possible chronic condition that may follow MI or unstable angina), heart failure, PVD with amputation, PVD without amputation, and stroke. In each of these chronic health states, the timing of the event was clearly explained. For example, the chronic stroke health state (N) included the following explanation: “Prior to this year, you experienced a stroke…The continuing effects on your life include the following symptoms and impact”.
Two health states described a fracture of the hip (H) and a fracture of the arm (I). Consistent with clinicians’ input during the interviews, the hip fracture included statements with more serious functional impairment than the arm fracture. These two fracture locations were chosen for inclusion in the health states in order to represent a broad range of the potential impact of a fracture. Two surgical procedure health states describing parathyroidectomy (J) and kidney transplant (K) were also included to represent the potential surgical treatment options for this patient group. These surgical procedures were selected based on review of the literature and input from clinicians indicating that they are common treatment options for patients with CKD and SHPT on dialysis [5, 39, 48, 50–52]. Parathyroidectomy was considered important for the current set of health states because it is directly related to the mineral imbalances associated with SHPT.
Participants
All participants were required to be (1) at least 18 years old; (2) able to understand the assessment procedures; (3) able and willing to give written informed consent; and (4) residing in Canada. Inclusion criteria did not specify particular clinical characteristics because interviews were intended to yield utilities that may be used in cost-utility analyses for submission to reimbursement authorities, most of whom prefer utilities that represent general population values [53–56]. Participants were recruited via local newspaper and online advertisements in Toronto, Canada.
Time trade-off utility interview procedures and scoring
Utilities were derived by eliciting values for the health state descriptions in a TTO utility interview with a one-year time horizon conducted by a single interviewer. The one-year time horizon was used so that the interviews could capture the impact of relatively brief health-related events. Each participant rated the basic health state (A) describing CKD and SHPT with dialysis, followed by 10 of the 15 additional health states (B–P). The 10 event health states to be rated by each participant were determined by block randomization. To introduce participants to the health state descriptions, a ranking exercise was conducted prior to TTO utility elicitation. Health states were presented in random order on individual cards, and each participant ranked the health states in order of preference.
After completing the introductory ranking task, health state utilities were obtained using the TTO method. For each health state, participants were offered a choice between spending a one-year period in this health state versus spending shorter amounts of time (in one-month increments) in the full health state. Choices varied by one-year increments and were presented in the following pattern: 12 months in full health, 0, 11, 1, 10, 2, 9, 3, 8, 4, 7, 5, 6. Choices were presented visually with a booklet illustrating one choice per page. Each health state rated as better than dead received a utility value on a scale with the anchors of dead (0) and full health (1). The assigned value was calculated based on the choice in which the respondent is indifferent between y months in the health state being evaluated and x months in full health (followed by y – x months dead). The resulting utility estimate (u) is calculated as u = x/y.
If participants indicated that a health state was worse than dead, the interviewer altered the task and offered a choice between immediate death (alternative 1) and a one-year life span (alternative 2) beginning with varying amounts of time in the health state being rated, followed by full health for the remainder of the one-year timeframe. For these health states, the current study used a bounded scoring approach, which is commonly used to avoid highly skewed distributions for negative utilities [49]. This scoring approach limits the utility range of health states worse than dead to values between 0 and −1. To compute these negative values, the current study used the Dolan method [57] as described by Rowen & Brazier [35]: u = −x/t, where x is the number of months in full health, and t is the total life span of alternative 2 in the TTO choice. In the current study, t was 12 months, which is the number of months in the health state being rated plus subsequent months in full health.
Data collection and statistical analysis procedures
Each participant attended one interview conducted in Toronto, Canada in May 2013. Participants provided written informed consent, completed a brief demographic and clinical form, and then participated in a utility interview. Participants received remuneration of C$50 for their time. All procedures and materials were approved by an independent Institutional Review Board (Schulman Associates IRB; Protocol number 20130125). Statistical analyses were completed using SAS version 9.2 (SAS Institute, Cary, NC).
Continuous variables are summarized as means and standard deviations, and categorical variables are summarized as frequencies and percentages. The difference in utility, which may be either a disutility (i.e., utility decrease) or added utility (i.e., utility increase), associated with each health-related event or treatment modality was calculated by subtracting the utility of each event health state (B–P) from the utility of health state A. This utility difference quantifies the impact of each event on preferences for one-year health states in the context of CKD, SHPT, and dialysis. A series of independent t-tests were conducted to compare utility scores among various demographic subgroups (age, gender, religiosity).
Pairwise comparisons between health states, using t-tests, were conducted to examine utility differences between health states. T-tests were considered exploratory, and therefore no adjustments were made for multiple comparisons. The basic health state (A) was compared to each of the other health states that included a description of a cardiovascular, fracture, or surgical event (B–P). T-tests were also performed to compare between selected pairs of event health states that were conceptually comparable to each other: acute vs. chronic stroke; arm vs. hip fracture; myocardial infarction vs. unstable angina; and PVD with amputation vs. PVD without amputation. These analyses were performed to examine whether there were differences in valuations between specific pairs of comparable health states.
Results
Sample description
A total of 530 individuals responded to the advertisements, and 264 of these were reached for screening. Of the 264 screened participants, 260 were eligible, 226 were scheduled for interviews, and 202 attended interviews. Three of the 202 participants were unable to complete the TTO interview procedures. Thus, a total of 199 valid interviews were completed (Table 1). The sample had a mean age of 46.3 years (SD = 13.8) and was 54.8 % female. Approximately half of the sample (49.7 %) reported no health conditions, 22.1 % reported one condition, 14.6 % reported two, and 13.6 % reported greater than two. The most common health conditions were depression (17.1 %), arthritis (15.1 %), anxiety (13.6 %), hypertension (13.1 %), and diabetes (9.0 %). Cardiovascular conditions were reported less frequently: heart attack or heart disease (2.5 %) and stroke (0.5 %). No respondents reported having kidney disease or other renal conditions.
Table 1.
Demographic characteristics
| Demographic characteristics | Statistics |
|---|---|
| (N = 199) | |
| Age (mean, SD) | 46.3, 13.8 |
| Gender (n, %) | |
| Female | 109 (54.8 %) |
| Male | 90 (45.2 %) |
| Ethnicity* (n, %) | |
| White | 107 (53.8 %) |
| Black | 24 (12.1 %) |
| Asian | 52 (26.1 %) |
| Latin American | 6 (3.0 %) |
| Other/Multiple | 10 (5.0 %) |
| Marital status† (n, %) | |
| Single | 109 (54.8 %) |
| Married | 51 (25.6 %) |
| Other | 39 (19.6 %) |
| Employment status‡, (n, %) | |
| Full-time work | 65 (32.7 %) |
| Part-time work | 57 (28.6 %) |
| Student | 21 (10.6 %) |
| Retired | 23 (11.6 %) |
| Unemployed | 16 (8.0 %) |
| Other | 17 (8.5 %) |
| Education Level (n, %) | |
| Completed University Degree | 125 (62.8 %) |
| Did not Complete University Degree | 74 (37.2 %) |
*Other includes participants who reported multiple categories (n = 9) and participants who reported “other” (n = 1)
†Other includes divorced (n = 22), separated (n = 7), widowed (n = 8), and “other” (n = 2)
‡Other includes homemaker (n = 4), disabled (n = 7), and “other” (n = 6)
Health state utilities
Results of the introductory health state ranking task are presented in Table 2, and TTO utilities are presented in Fig. 1. Health state A was valued by all 199 participants who completed the interview, while every other health state was valued by approximately two-thirds of the sample (n = 130 to 135). The basic health state (A) describing CKD and SHPT on dialysis without additional clinical events had a mean utility of 0.60. Compared with health state A, the health states describing surgical interventions had higher mean utilities of 0.61 (J, parathyroidectomy) and 0.65 (K, kidney transplant).
Table 2.
Health state ranking
| Health state | N | Mean ranking* | SD | Range |
|---|---|---|---|---|
| A. Basic HS: CKD and SHPT | 199 | 2.0 | 0.6 | 1.0–3.0 |
| B. Acute HS: Myocardial Infarction | 130 | 5.7 | 1.9 | 2.0–11.0 |
| C. Acute HS: Unstable Angina | 133 | 4.7 | 1.6 | 2.0–9.0 |
| D. Acute HS: PVD without amputation | 135 | 6.1 | 1.8 | 2.0–11.0 |
| E. Acute HS: PVD with amputation | 130 | 9.6 | 1.6 | 3.0–11.0 |
| F. Acute HS: Heart Failure | 133 | 7.5 | 2.0 | 4.0–11.0 |
| G. Acute HS: Stroke | 135 | 9.5 | 1.6 | 2.0–11.0 |
| H. Acute HS: Hip Fracture | 130 | 6.3 | 2.0 | 3.0–11.0 |
| I. Acute HS: Arm Fracture | 133 | 3.8 | 1.6 | 2.0–9.0 |
| J. Acute HS: Parathyroidectomy | 135 | 2.3 | 1.8 | 1.0–11.0 |
| K. Acute HS: Kidney Transplant | 130 | 1.6 | 1.7 | 1.0–11.0 |
| L. Chronic HS: PVD without amputation | 133 | 6.6 | 2.0 | 2.0–10.0 |
| M. Chronic HS: PVD with amputation | 135 | 9.6 | 1.6 | 4.0–11.0 |
| N. Chronic HS: Stroke | 130 | 9.1 | 1.7 | 3.0–11.0 |
| O. Chronic HS: Stable Angina | 133 | 6.3 | 1.9 | 2.0–11.0 |
| P. Chronic HS: Heart Failure | 135 | 7.3 | 2.1 | 3.0–11.0 |
*A lower mean ranking indicates that a health state was more preferable, while a higher mean ranking indicates that a health state was less preferable
HS = health state; PVD = peripheral vascular disease; CKD = chronic kidney disease; SHPT = secondary hyperparathyroidism
Fig. 1.
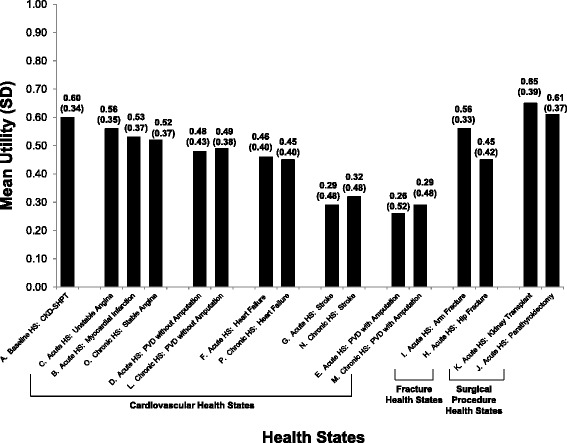
Time Trade-Off* Utility Scores.
*TTO utility scores are on a scale anchored with 0 representing dead and 1 representing full health. HS = health state; TTO = time trade-off; PVD = peripheral vascular disease; CKD = chronic kidney disease; SHPT = secondary hyperparathyroidism. All 199 participants rated health state A. Mean utilities for other health states are based on evaluations from subsets of 130 to 135 participants. The precise number of participants who rated each health state is presented in Table 2. In this figure, acute cardiovascular health states are presented (grouped with their corresponding chronic states) in descending order from highest utility to lowest utility. The fracture and surgical procedure health states are also ordered from highest utility to lowest utility
All other health states had lower mean utilities than health state A. Utilities for health states including acute cardiovascular events (B–G) ranged from 0.26 (E, PVD with amputation) to 0.56 (C, unstable angina). Utilities for chronic health states following a cardiovascular event (L–P) ranged from 0.29 (M, PVD with amputation) to 0.52 (O, stable angina). Health state H (hip fracture) had a utility of 0.45, while health state I (arm fracture) had a utility of 0.56.
There were no statistically significant differences in health state utilities between younger (n = 96) and older (n = 103) participants categorized based on a median split (median age = 48); male (n = 90) and female (n = 109) participants; or between participants who considered themselves religious (n = 103) and those who did not (n = 96).
Disutilities associated with major clinical events
The disutility associated with the addition of each clinical event to health state A was computed by subtracting the utility of each event health state from the utility of health state A (Table 3). The event health states were identical to health state A other than the addition of the event. Therefore, any difference in utility score between health state A and the other health states represents the impact of the event on respondent preference. The two surgical events were associated with utilities higher than the utility for health state A, with mean differences of 0.02 for parathyroidectomy (J) and 0.06 for kidney transplant (K), indicating that the addition of these two events resulted in better perceived health-related quality of life.
Table 3.
Differences in utility scores associated with major clinical events
| Health State | N | Mean difference score* | SD | T value | P value |
|---|---|---|---|---|---|
| B-A. Acute HS: Myocardial Infarction | 130 | −0.06 | 0.13 | −5.75 | <0.0001 |
| C-A. Acute HS: Unstable Angina | 133 | −0.05 | 0.10 | −5.62 | <0.0001 |
| D-A. Acute HS: PVD without amputation | 135 | −0.11 | 0.22 | −5.84 | <0.0001 |
| E-A. Acute HS: PVD with amputation | 130 | −0.33 | 0.39 | −9.76 | <0.0001 |
| F-A. Acute HS: Heart Failure | 133 | −0.14 | 0.25 | −6.51 | <0.0001 |
| G-A. Acute HS: Stroke | 135 | −0.30 | 0.35 | −9.96 | <0.0001 |
| H-A. Acute HS: Hip Fracture | 130 | −0.14 | 0.22 | −7.18 | <0.0001 |
| I-A. Acute HS: Arm Fracture | 133 | −0.04 | 0.09 | −5.44 | <0.0001 |
| J-A. Acute HS: Parathyroidectomy | 135 | 0.02 | 0.15 | 1.27 | 0.21 |
| K-A. Acute HS: Kidney Transplant | 130 | 0.06 | 0.25 | 2.73 | 0.0072 |
| L-A. Chronic HS: PVD without amputation | 133 | −0.12 | 0.22 | −6.25 | <0.0001 |
| M-A. Chronic HS: PVD with amputation | 135 | −0.30 | 0.32 | −10.72 | <0.0001 |
| N-A. Chronic HS: Stroke | 130 | −0.27 | 0.35 | −8.69 | <0.0001 |
| O-A. Chronic HS: Stable Angina | 133 | −0.09 | 0.17 | −5.96 | <0.0001 |
| P-A. Chronic HS: Heart Failure | 135 | −0.14 | 0.20 | −7.86 | <0.0001 |
*This difference score was computed for each participant by subtracting the value of health state A from the value of the other health state
HS = health state; PVD = peripheral vascular disease
All other events were associated with disutilities (i.e., lower utility) ranging from a mean of −0.04 to −0.33. Mean disutilities for acute cardiovascular events (B–G) ranged from −0.05 (C, unstable angina) to −0.33 (E, PVD with amputation), while mean disutilities for chronic health states following a cardiovascular event (L–P) ranged from −0.09 (O, stable angina) to −0.30 (M, PVD with amputation). Of the two fracture health states, hip fracture (H) was associated with a larger mean disutility (−0.14) than arm fracture (I; −0.04). Differences between the utility of health state A and utilities of all cardiovascular and fracture health states (B–I, L–P) were statistically significant (p < 0.0001) (Table 3).
Among selected event health states that were conceptually comparable to each other, the following pairwise comparisons were statistically significant (Table 3): hip fracture (H) and arm fracture (I) (utility difference = 0.08); PVD with and without amputation, acute (D vs. E; utility difference = 0.19) and chronic health states (L vs. M; utility difference = 0.16); myocardial infarction and unstable angina (B vs. C; utility difference = 0.03).
Discussion
In the current study cardiovascular events and fractures were associated with substantial disutility in the context of SHPT and CKD on dialysis. It has been suggested that differences among health state utilities of at least 0.05 can be considered clinically important [34]. The disutilities of all cardiovascular events and hip fracture met or exceeded this threshold, indicating that cardiovascular events and major fractures have an important impact on utility in the context of CKD and SHPT on dialysis. In addition, the utilities of all cardiovascular and fracture health states were significantly lower than the utility of CKD and SHPT without these added complications. In contrast, the two health states describing surgical interventions (J and K) were associated with higher utility values than the basic CKD and SHPT health state (A) due to the perceived improvements that could result from these surgeries. While the difference associated with parathyroidectomy was relatively small (+0.02), kidney transplant was associated with a more substantial utility increase (+0.06) over the basic health state. In light of these results, it is recommended that researchers conducting cost-utility models of treatment for CKD and SHPT on dialysis consider incorporating the utility differences associated with these important clinical events.
A unique contribution of the current study is the identification of disutilities associated with cardiovascular events and fractures in the context of CKD and SHPT. Previous cost-utility analyses of treatments for CKD and SHPT have used utilities for cardiovascular events and fractures from outside of the context of CKD due to the lack of utility data available for these events in this population [30–33]. However, the literature for cardiovascular events is highly variable [58], making it difficult to select the appropriate values for use in a model focusing on treatment of CKD. In addition, the majority of utilities for fractures have come from utility estimates in osteoporosis, which may not be relevant to patients with CKD [30–33]. Current health states were drafted based on clinicians’ descriptions of cardiovascular and fracture events specifically in the CKD context, and the impact of these events on utility were rated in this context. Therefore, current utility values may be uniquely appropriate for use in models focused on treatment for CKD and SHPT with dialysis.
While the current cardiovascular and fracture utilities are new in the CKD context, previous studies have reported utility values for CKD itself, as well as for patients who have received kidney transplants. The utility score of 0.60 for the health state representing CKD and SHPT on dialysis (A) is within the range of utilities for this type of patient obtained via a range of methods in previous studies. For example, a review found that TTO utilities for patients on hemodialysis across multiple studies ranged from 0.42 to 0.73 with a mean of 0.61, and utilities derived from the EQ-5D ranged from 0.44 to 0.62 with a mean of 0.56 [36]. Similar to results of the current study, this review reported that kidney transplant was associated with higher TTO (mean = 0.78) and EQ-5D (mean = 0.81) utilities. Another review reported average TTO utility means of 0.70 across studies for patients on dialysis, 0.82 for patients with kidney transplant, and an EQ-5D range from 0.44 to 0.71 for patients on dialysis [37]. The utility values for kidney transplant were higher in both of these reviews than in the current study, which is not unexpected since the utilities are representing different health concepts. These previous studies reported utilities of patients who had previously undergone a transplant and were living in a post-transplant state at the time of utility assessment. In contrast, the kidney transplant health state valued in the current study represented a year that included both the surgical procedure and the subsequent quality of life improvements. While the benefits of the surgery would lead to a greater utility value, the procedure itself was likely viewed as a negative event by most respondents, which would attenuate the utility gain associated with this health state. In sum, the previously published values represent a post-transplant health state, while the current utility of health state K represents a year including the transplant as well as subsequent improvements.
The TTO interviews in the current study were conducted with a one-year time horizon, and this methodology has yielded utility values with logical differences among health states (differences between health states were considered logical if the difference was in the expected direction, such as PVD with amputation having a lower utility than PVD without amputation). In TTO procedures, the duration of time spent in the health state being rated (i.e., the time horizon) is an important component of the task, and this time horizon varies across studies. Longer time horizons, most commonly 10 years, are used more frequently, but time horizons as short as one or two years have been shown to be useful in several previous TTO studies [59–68]. In the current study, the one-year time horizon was selected because a primary goal was to quantify the utility impact of acute events. Whereas this acute impact could be obscured if presented as a brief event within a 10-year time horizon, respondents clearly viewed the acute events as important when considered within the context of a single year. Therefore, the relatively brief time horizon seems to be an effective approach for quantifying the utility impact of acute events.
The time horizon does have implications for the eventual use of the utility scores. The health states each described a single hypothetical year of life, often involving a path or sequence of events such as a fracture followed by a gradual recovery. Consistent with the one-year paths described in the health states, they were rated in a TTO task with a one-year time horizon. Therefore, the utilities derived from this study represent valuations for hypothetical paths through one year of life, and can therefore be used in a cost utility model as representing one year. For example, the disutility of −0.06 associated with a myocardial infarction represents utility decrement across a single year. This disutility value would likely be different if it were representing the impact of myocardial infarction on a longer or shorter period of time. Consequently, when using these values in a cost-utility model, researchers need to be mindful of the timeframe, and the current disutilities should be applied to one-year time periods.
Modelers who intend to use the current values should also be aware of the conceptual difference between the acute and chronic health states. The acute health states represent a year that includes the acute event, and the corresponding disutility represents the utility decrement across the full year in which the event occurs. The chronic health states represent a post-event year, which is a year following an event, but not including the event itself. As with the acute health states, it is recommended that disutilities associated with the chronic health states be applied to a full year when used in a cost-utility model. This one-year time horizon allows results to be conceptualized in terms of the impact of cardiovascular and fracture events on a QALY. For example, the difference between health states A and G represents the QALY decrement associated with an acute stroke in the context of CKD and SHPT. The utilities reported here can also be used by modelers to represent trajectories of health-related quality of life in the context of CKD with SHPT. For instance, a patient might be in health state A for one year (utility = 0.60), suffer a stroke in year 2 (acute health state G; utility = 0.29), and then transition to chronic health state N representing the chronic impact of stroke (utility = 0.32) in year 3. The total (undiscounted) number of QALYs for the three-year period would be 1.21, compared with 1.8 for a patient without the stroke (i.e., 0.60*3).
The utility scores estimated in this study were derived from members of the general population, rather than patients with the relevant medical conditions, in order to approximate the societal viewpoint. This general population approach is consistent with guidance provided by health technology assessment agencies in many countries [53–56]. An advantage of a general population sample is that, because of the widespread use of utilities derived from general population valuations, scores derived via this approach are comparable to general population valuations of other health states. There are, of course, tradeoffs between scores obtained from the general population and scores obtained from patients who may have experienced some of the relevant health states. Patients may have greater insight into the content of health state descriptions, and therefore, patient-based utility valuations may be grounded in a better understanding of the health states. In this study, a concerted effort was made to draft health state descriptions that accurately represented the experience of patients with SHPT and CKD requiring dialysis, which may attenuate some of this concern. Nonetheless, it would be useful in future studies to compare general population and patient scores for these health states. In the meantime, the scores presented here may be used by modelers to estimate QALYs for major clinical events in the context of SHPT and CKD requiring dialysis.
Although this study yielded logical utilities (i.e., with relationships among health states in the expected directions), several aspects of the study design suggest that the results should be interpreted with appropriate caution. For example, utility assessment involving hypothetical health states is limited by the accuracy and level of detail in the health state descriptions. Therefore, utility scores derived via these methods may differ from patients’ ratings of their own health. However, identifying utilities associated with major clinical events directly from patients rating their own health would be impractical for two reasons. First, it would be difficult to identify patients and obtain utility values at the time the cardiovascular events or fractures occur. Second, even if a patient provides a utility at the time of the event, it may not be feasible to isolate the impact of a cardiovascular event or fracture on utility, separate from the impact of CKD and SHPT. In contrast, the hypothetical health state vignette approach used in the current study (which could be completed by either patients or general population respondents) is well-suited for isolating the impact on utility of specific medical conditions, health-related events, or treatments.
Another limitation of the current study is that the health states specified the names of the disease and medical events that were described. Some research has suggested that including the disease label in a health state can affect respondents’ valuations, while other studies have reported situations when the label did not affect valuations [69–71]. Although some researchers recommend omitting the disease label from health states, others prefer to label the medical condition as it might minimize misunderstanding of the health state. Furthermore, inclusion of the label more closely mirrors the patient experience, as patients clearly know the name of their condition. The current study included a complex set of health states representing a diverse range of medical concepts, including kidney disease, SHPT, dialysis, cardiovascular disease, bone-related complications, two types of surgery, and acute versus chronic effects. Therefore, each medical issue was explicitly named to make it easier for respondents to comprehend and differentiate between the health states. Additionally, some of the cardiovascular states were similar to each other (e.g., myocardial infarction and unstable angina), and the labels were necessary to ensure that participants did not become confused during the TTO task. However, it should be acknowledged that inclusion of the labels could have influenced utility scores.
An additional limitation relates to negative utility values. The health states in this study describe severe medical conditions, and all health states were rated by at least one respondent as worse than dead. With TTO methods, health states worse than dead are rated in a slightly different procedure than states with positive scores, meaning that worse than dead valuations are not measured on the same scale as states with positive scores [49]. While this limitation is relevant to all utility assessment, it is a more important issue when assessing severe health states that are likely to elicit more negative values.
Sample selection may be considered another limitation. Although efforts were made to ensure that the sample was balanced with regard to key demographic variables such as age, gender, ethnic/racial background, and employment status, the sample was not recruited to be nationally representative. Furthermore, participants reported relatively high rates of some clinical conditions (e.g., depression, arthritis, anxiety). Therefore, generalizability to the Canadian population or the population of other countries is not known, although there is no reason to believe that current values would be systematically different from a nationally representative sample.
Despite limitations, the current study is a step toward more thorough and accurate modeling of treatment for patients with CKD and SHPT. The current utility differences associated with cardiovascular events and fractures may be used to represent these debilitating events in cost-utility analyses specifically focusing on patients with CKD and SHPT on dialysis. Future research may examine whether current values derived from health state descriptions are consistent with utilities derived from actual patients who are experiencing these important clinical events.
Acknowledgments
Funding for this study was provided by Amgen, Inc., Thousand Oaks, CA, USA. Two of the authors (GW, VB) are employees of Amgen, but their input into the conceptualization and interpretation of this study represented their own opinions rather than those of the company. Three of the authors (ED, LM, JK) are employees of Evidera, a company that received funding from Amgen for this research. The other authors (DF, SS, DM, PM) received funding from Amgen for time spent on this research.
The authors would like to thank Karen Malley for statistical programming; Loni Ajagbe, David Hengerer, Jessica Jordan, Erin Klepeis, Amanda Landrian, Mary Kay Margolis, Kelly McDaniel, Ann Peyser, and Anna Tate for assistance with data collection; Katie D. Stewart for protocol review, literature searching, and manuscript review; Ingela Wiklund and Lorraine Boyle for consultation; and Amara Tiebout for production assistance.
Additional file
Full text of all 16 health states.
Footnotes
Competing interests
Financial support for this study was provided by Amgen Inc. The following authors are employed by the sponsor: Gavin Worth and Vasily Belozeroff. Parts of this research were discussed in a poster presentation at the ISPOR 16th Annual European Congress held November 2–6, 2013 in Dublin, Ireland.
Authors’ contributions
ED and LM were the primary writers of this manuscript, and they co-directed the study including the study design, data collection, data analysis, and data interpretation. ED directed health state development. GW, DF, and VB made substantial contributions to the study design, data interpretation, and resulting manuscript. JK co-directed the data collection and made substantial contributions to the manuscript. SS, DM, and PM made substantial contributions to health state development and data interpretation. All authors provided input on multiple drafts of the manuscript and approval of the final draft.
Contributor Information
Evan W. Davies, Email: evan.davies@evidera.com
Louis S. Matza, Email: Louis.matza@evidera.com
Gavin Worth, Email: gworth@amgen.com.
David H. Feeny, Email: dafeeny@telus.net
Jacqueline Kostelec, Email: Jacqueline.kostelec@evidera.com.
Steven Soroka, Email: steven.soroka@cdha.nshealth.ca.
David Mendelssohn, Email: dmendy@gmail.com.
Philip McFarlane, Email: phil.mcfarlane@utoronto.ca.
Vasily Belozeroff, Email: vasilyb@amgen.com.
References
- 1.Eckardt KU, Coresh J, Devuyst O, Johnson RJ, Kottgen A, Levey AS, et al. Evolving importance of kidney disease: from subspecialty to global health burden. Lancet. 2013;382:158–169. doi: 10.1016/S0140-6736(13)60439-0. [DOI] [PubMed] [Google Scholar]
- 2.Disease K. Improving global outcomes CKDMBDWG: KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl. 2009;S1–130. [DOI] [PubMed]
- 3.Levin A, Hemmelgarn B, Culleton B, Tobe S, McFarlane P, Ruzicka M, et al. Guidelines for the management of chronic kidney disease. CMAJ. 2008;179:1154–1162. doi: 10.1503/cmaj.080351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fraser WD. Hyperparathyroidism. Lancet. 2009;374:145–158. doi: 10.1016/S0140-6736(09)60507-9. [DOI] [PubMed] [Google Scholar]
- 5.Garside R, Pitt M, Anderson R, Mealing S, Roome C, Snaith A, et al. The effectiveness and cost-effectiveness of cinacalcet for secondary hyperparathyroidism in end-stage renal disease patients on dialysis: a systematic review and economic evaluation. Health Technol Assess. 2007;11(iii):1–167. doi: 10.3310/hta11180. [DOI] [PubMed] [Google Scholar]
- 6.Michels TC, Kelly KM. Parathyroid disorders. Am Fam Physician. 2013;88:249–257. [PubMed] [Google Scholar]
- 7.Cengiz K, Ozkan A. Depression and secondary hyperparathyroidism in chronic renal failure. Nephron. 1998;79:508–509. doi: 10.1159/000045115. [DOI] [PubMed] [Google Scholar]
- 8.Chertow GM, Lu ZJ, Xu X, Knight TG, Goodman WG, Bushinsky DA, et al. Self-reported symptoms in patients on hemodialysis with moderate to severe secondary hyperparathyroidism receiving combined therapy with cinacalcet and low-dose vitamin D sterols. Hemodial Int. 2012;16:188–197. doi: 10.1111/j.1542-4758.2011.00642.x. [DOI] [PubMed] [Google Scholar]
- 9.Cunningham J, Danese M, Olson K, Klassen P, Chertow GM. Effects of the calcimimetic cinacalcet HCl on cardiovascular disease, fracture, and health-related quality of life in secondary hyperparathyroidism. Kidney Int. 2005;68:1793–1800. doi: 10.1111/j.1523-1755.2005.00596.x. [DOI] [PubMed] [Google Scholar]
- 10.Cunningham J, Floege J, London G, Rodriguez M, Shanahan CM. Clinical outcomes in secondary hyperparathyroidism and the potential role of calcimimetics. NDT Plus. 2008;1:i29–i35. doi: 10.1093/ndtplus/sfm042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curtin RB, Bultman DC, Thomas-Hawkins C, Walters BA, Schatell D. Hemodialysis patients' symptom experiences: effects on physical and mental functioning. Nephrol Nurs J. 2002;29(562):567–574. [PubMed] [Google Scholar]
- 12.Schumock GT, Andress DL, Marx SE, Sterz R, Joyce AT, Kalantar-Zadeh K. Association of secondary hyperparathyroidism with CKD progression, health care costs and survival in diabetic predialysis CKD patients. Nephron Clin Pract. 2009;113:c54–c61. doi: 10.1159/000228076. [DOI] [PubMed] [Google Scholar]
- 13.Joy MS, Karagiannis PC, Peyerl FW. Outcomes of secondary hyperparathyroidism in chronic kidney disease and the direct costs of treatment. J Manag Care Pharm. 2007;13:397–411. doi: 10.18553/jmcp.2007.13.5.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 15.Kovesdy CP, Ahmadzadeh S, Anderson JE, Kalantar-Zadeh K. Secondary hyperparathyroidism is associated with higher mortality in men with moderate to severe chronic kidney disease. Kidney Int. 2008;73:1296–1302. doi: 10.1038/ki.2008.64. [DOI] [PubMed] [Google Scholar]
- 16.Bhuriya R, Li S, Chen SC, McCullough PA, Bakris GL. Plasma parathyroid hormone level and prevalent cardiovascular disease in CKD stages 3 and 4: an analysis from the Kidney Early Evaluation Program (KEEP) Am J Kidney Dis. 2009;53:S3–S10. doi: 10.1053/j.ajkd.2008.11.029. [DOI] [PubMed] [Google Scholar]
- 17.Block GA, Raggi P, Bellasi A, Kooienga L, Spiegel DM. Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int. 2007;71:438–441. doi: 10.1038/sj.ki.5002059. [DOI] [PubMed] [Google Scholar]
- 18.Covic A, Kothawala P, Bernal M, Robbins S, Chalian A, Goldsmith D. Systematic review of the evidence underlying the association between mineral metabolism disturbances and risk of all-cause mortality, cardiovascular mortality and cardiovascular events in chronic kidney disease. Nephrol Dial Transplant. 2009;24:1506–1523. doi: 10.1093/ndt/gfn613. [DOI] [PubMed] [Google Scholar]
- 19.Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJ, Mann JF, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382:339–352. doi: 10.1016/S0140-6736(13)60595-4. [DOI] [PubMed] [Google Scholar]
- 20.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 21.Shantouf RS, Budoff MJ, Ahmadi N, Ghaffari A, Flores F, Gopal A, et al. Total and individual coronary artery calcium scores as independent predictors of mortality in hemodialysis patients. Am J Nephrol. 2010;31:419–425. doi: 10.1159/000294405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Danese MD, Belozeroff V, Smirnakis K, Rothman KJ. Consistent control of mineral and bone disorder in incident hemodialysis patients. Clin J Am Soc Nephrol. 2008;3:1423–1429. doi: 10.2215/CJN.01060308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blayney MJ, Tentori F. Trends and consequences of mineral bone disorder in haemodialysis patients: lessons from The Dialysis Outcomes and Practice Patterns Study (DOPPS) J Ren Care. 2009;35(Suppl 1):7–13. doi: 10.1111/j.1755-6686.2009.00048.x. [DOI] [PubMed] [Google Scholar]
- 24.Jadoul M, Albert JM, Akiba T, Akizawa T, Arab L, Bragg-Gresham JL, et al. Incidence and risk factors for hip or other bone fractures among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2006;70:1358–1366. doi: 10.1038/sj.ki.5001754. [DOI] [PubMed] [Google Scholar]
- 25.Block GA, Martin KJ, de Francisco AL, Turner SA, Avram MM, Suranyi MG, et al. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med. 2004;350:1516–1525. doi: 10.1056/NEJMoa031633. [DOI] [PubMed] [Google Scholar]
- 26.Goodman WG. The consequences of uncontrolled secondary hyperparathyroidism and its treatment in chronic kidney disease. Semin Dial. 2004;17:209–216. doi: 10.1111/j.0894-0959.2004.17308.x. [DOI] [PubMed] [Google Scholar]
- 27.Michael M, Garcia D. Secondary hyperparathyroidism in chronic kidney disease: clinical consequences and challenges. Nephrol Nurs J. 2004;31:185–194. [PubMed] [Google Scholar]
- 28.National Kidney Foundation: K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003, 42:S1-201. [PubMed]
- 29.Pasieka JL, Parsons LL. A prospective surgical outcome study assessing the impact of parathyroidectomy on symptoms in patients with secondary and tertiary hyperparathyroidism. Surgery. 2000;128:531–539. doi: 10.1067/msy.2000.108117. [DOI] [PubMed] [Google Scholar]
- 30.Boer R, Lalla AM, Belozeroff V. Cost-effectiveness of cinacalcet in secondary hyperparathyroidism in the United States. J Med Econ. 2012;15:509–520. doi: 10.3111/13696998.2012.664799. [DOI] [PubMed] [Google Scholar]
- 31.Garside R, Pitt M, Anderson R, Mealing S, D'Souza R, Stein K. The cost-utility of cinacalcet in addition to standard care compared to standard care alone for secondary hyperparathyroidism in end-stage renal disease: a UK perspective. Nephrol Dial Transplant. 2007;22:1428–1436. doi: 10.1093/ndt/gfl774. [DOI] [PubMed] [Google Scholar]
- 32.Iannazzo S, Carsi M, Chiroli S. A cost-utility analysis of cinacalcet in secondary hyperparathyroidism in five European countries. Appl Health Econ Health Policy. 2012;10:127–138. doi: 10.2165/11597980-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 33.Komaba H, Moriwaki K, Goto S, Yamada S, Taniguchi M, Kakuta T, et al. Cost-effectiveness of cinacalcet hydrochloride for hemodialysis patients with severe secondary hyperparathyroidism in Japan. Am J Kidney Dis. 2012;60:262–271. doi: 10.1053/j.ajkd.2011.12.034. [DOI] [PubMed] [Google Scholar]
- 34.Feeny D. Preference-based measures: utility and quality-adjusted life years. In: Fayers P, Hays R, editors. Assessing quality of life in clinical trials 2nd edition. New York: Oxford University Press; 2005. pp. 405–431. [Google Scholar]
- 35.Rowen D, Brazier J. Health Utility Measurement. In: Glied S, Smith P, editors. The Oxford Handbook of Health Economics. New York: Oxford University Press; 2011. pp. 788–813. [Google Scholar]
- 36.Liem YS, Bosch JL, Hunink MG. Preference-based quality of life of patients on renal replacement therapy: a systematic review and meta-analysis. Value Health. 2008;11:733–741. doi: 10.1111/j.1524-4733.2007.00308.x. [DOI] [PubMed] [Google Scholar]
- 37.Wyld M, Morton RL, Hayen A, Howard K, Webster AC. A systematic review and meta-analysis of utility-based quality of life in chronic kidney disease treatments. PLoS Med. 2012;9 doi: 10.1371/journal.pmed.1001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eandi M, Pradelli L, Iannazzo S, Chiroli S, Pontoriero G. Economic evaluation of cinacalcet in the treatment of secondary hyperparathyroidism in Italy. Pharmacoeconomics. 2010;28:1041–1054. doi: 10.2165/11538600-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 39.National Kidney Foundation [https://www.kidney.org/atoz/atozTopic_Transplantation.cfm]
- 40.American Heart Association (AHA). Cardiovascular conditions. [http://www.heart.org/HEARTORG/Conditions/Conditions_UCM_001087_SubHomePage.jsp] Accessed January 16, 2014.
- 41.Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined–a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36:959–969. doi: 10.1016/S0735-1097(00)00804-4. [DOI] [PubMed] [Google Scholar]
- 42.American Stroke Association. Effects of stroke. [http://www.strokeassociation.org/STROKEORG/AboutStroke/EffectsofStroke/Effects-of-Stroke_UCM_308534_SubHomePage.jsp] Accessed January 16, 2014.
- 43.European Stroke Organization. Tendera M, Aboyans V, Bartelink ML, Baumgartner I, Clement D, et al. ESC Guidelines on the diagnosis and treatment of peripheral artery diseases: Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: the Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology (ESC) Eur Heart J. 2011;32:2851–2906. doi: 10.1093/eurheartj/ehr211. [DOI] [PubMed] [Google Scholar]
- 44.Hamm CW, Bassand JP, Agewall S, Bax J, Boersma E, Bueno H, et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2011;32:2999–3054. doi: 10.1093/eurheartj/ehr236. [DOI] [PubMed] [Google Scholar]
- 45.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803–869. doi: 10.1093/eurjhf/hfs033. [DOI] [PubMed] [Google Scholar]
- 46.NICE . Lower limb peripheral arterial disease: diagnosis and management (NICE clinical guideline 147) 30. London: National Institute of Clinical Excellence; 2012. p. pp. 30. [Google Scholar]
- 47.NICE . Management of stable angina (NICE clinical guideline 126) London: National Institute of Clinical Excellence; 2011. p. p. 37. [PubMed] [Google Scholar]
- 48.NICE: Technology Appraisal Guidance 85: Immunosuppressive therapy for renal transplantation in adults. Reviewed August 2007 edition. pp. 45. London: National Institute for Clinical Excellence; September 2004:45.
- 49.Brazier JR, Ratcliffe J, Salomon JA, Tsuchiya A. Measuring and Valuing Health benefits for Economic Evaluation. In. New York: Oxford University Press; 2007. [Google Scholar]
- 50.Belozeroff V, Cooper K, Hess G, Chang CL. Healthcare use and costs before and after parathyroidectomy in patients on dialysis. BMC Health Serv Res. 2013;13:248. doi: 10.1186/1472-6963-13-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lafrance JP, Cardinal H, Leblanc M, Madore F, Pichette V, Roy L, et al. Effect of cinacalcet availability and formulary listing on parathyroidectomy rate trends. BMC Nephrol. 2013;14:100. doi: 10.1186/1471-2369-14-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li S, Chen YW, Peng Y, Foley RN, St Peter WL. Trends in parathyroidectomy rates in US hemodialysis patients from 1992 to 2007. Am J Kidney Dis. 2011;57:602–611. doi: 10.1053/j.ajkd.2010.10.041. [DOI] [PubMed] [Google Scholar]
- 53.Brazier J. Valuing health States for use in cost-effectiveness analysis. Pharmacoeconomics. 2008;26:769–779. doi: 10.2165/00019053-200826090-00007. [DOI] [PubMed] [Google Scholar]
- 54.CADTH (Canadian Agency for Drugs and Technologies in Health) Guidelines for the economic evaluation of health technologies. Canada: Ottawa: Canadian Agency for Drugs and Technologies in Health; 2006. [Google Scholar]
- 55.NICE (National Institute for Health and Clinical Excellence) Process and methods guides. London, UK: National Institute for Health and Clinical Excellence; 2013. [Google Scholar]
- 56.PBAC (Pharmaceutical Benefits Advisory Committee): Guidelines for preparing submissions to PBAC, Version 4.3.2. PBAC; 2008.
- 57.Dolan P, Gudex C, Kind P, Williams A. The time trade-off method: results from a general population study. Health Econ. 1996;5:141–154. doi: 10.1002/(SICI)1099-1050(199603)5:2<141::AID-HEC189>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 58.Smith DW, Davies EW, Wissinger E, Huelin R, Matza LS, Chung K. A systematic literature review of cardiovascular event utilities. Expert Rev Pharmacoecon Outcomes Res. 2013;13:767–790. doi: 10.1586/14737167.2013.841545. [DOI] [PubMed] [Google Scholar]
- 59.Arnesen T, Trommald M. Are QALYs based on time trade-off comparable?–a systematic review of TTO methodologies. Health Econ. 2005;14:39–53. doi: 10.1002/hec.895. [DOI] [PubMed] [Google Scholar]
- 60.Hamel MB, Phillips RS, Davis RB, Desbiens N, Connors AF, Jr, Teno JM, et al. Outcomes and cost-effectiveness of initiating dialysis and continuing aggressive care in seriously ill hospitalized adults. SUPPORT investigators. Study to understand prognoses and preferences for outcomes and risks of treatments. Ann Intern Med. 1997;127:195–202. doi: 10.7326/0003-4819-127-3-199708010-00003. [DOI] [PubMed] [Google Scholar]
- 61.Hurny C, van Wegberg B, Bacchi M, Bernhard J, Thurlimann B, Real O, et al. Subjective health estimations (SHE) in patients with advanced breast cancer: an adapted utility concept for clinical trials. Br J Cancer. 1998;77:985–991. doi: 10.1038/bjc.1998.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matza LS, Chung K, Van Brunt K, Brazier JE, Braun A, Currie B, et al. Health state utilities for skeletal-related events secondary to bone metastases. Eur J Health Econ. 2014;15:7–18. doi: 10.1007/s10198-012-0443-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Matza LS, Cong Z, Chung K, Stopeck A, Tonkin K, Brown J, et al. Utilities associated with subcutaneous injections and intravenous infusions for treatment of patients with bone metastases. Patient Prefer Adherence. 2013;7:855–865. doi: 10.2147/PPA.S44947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.O'Leary JF, Fairclough DL, Jankowski MK, Weeks JC. Comparison of time-tradeoff utilities and rating scale values of cancer patients and their relatives: evidence for a possible plateau relationship. Med Decis Making. 1995;15:132–137. doi: 10.1177/0272989X9501500205. [DOI] [PubMed] [Google Scholar]
- 65.Schulz MW, Chen J, Woo HH, Keech M, Watson ME, Davey PJ. A comparison of techniques for eliciting patient preferences in patients with benign prostatic hyperplasia. J Urol. 2002;168:155–159. doi: 10.1016/S0022-5347(05)64851-3. [DOI] [PubMed] [Google Scholar]
- 66.Stevenson LW, Hellkamp AS, Leier CV, Sopko G, Koelling T, Warnica JW, et al. Changing preferences for survival after hospitalization with advanced heart failure. J Am Coll Cardiol. 2008;52:1702–1708. doi: 10.1016/j.jacc.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tsevat J, Cook EF, Green ML, Matchar DB, Dawson NV, Broste SK, et al. Health values of the seriously ill support investigators. Ann Intern Med. 1995;122:514–520. doi: 10.7326/0003-4819-122-7-199504010-00007. [DOI] [PubMed] [Google Scholar]
- 68.Tsevat J, Dawson NV, Wu AW, Lynn J, Soukup JR, Cook EF, et al. Health values of hospitalized patients 80 years or older HELP investigators hospitalized elderly longitudinal project. JAMA. 1998;279:371–375. doi: 10.1001/jama.279.5.371. [DOI] [PubMed] [Google Scholar]
- 69.Gerard K, Dobson M, Hall J. Framing and labelling effects in health descriptions: quality adjusted life years for treatment of breast cancer. J Clin Epidemiol. 1993;46:77–84. doi: 10.1016/0895-4356(93)90011-O. [DOI] [PubMed] [Google Scholar]
- 70.Rowen D, Brazier J, Tsuchiya A, Young T, Ibbotson R. It's all in the name, or is it? The impact of labeling on health state values. Med Decis Making. 2012;32:31–40. doi: 10.1177/0272989X11408435. [DOI] [PubMed] [Google Scholar]
- 71.Sackett DL, Torrance GW. The utility of different health states as perceived by the general public. J Chronic Dis. 1978;31:697–704. doi: 10.1016/0021-9681(78)90072-3. [DOI] [PubMed] [Google Scholar]


