Abstract
Genome-wide association studies implicate a variant in the neuronal nitric oxide synthase adaptor protein (CAPON) in electrocardiographic QT variation and sudden cardiac death. Interestingly, nitric oxide generated by neuronal NO synthase-1 reduces norepinephrine release; however, this pathway is downregulated in animal models of cardiovascular disease. Because sympathetic hyperactivity can trigger arrhythmia, is this neural phenotype linked to CAPON dysregulation? We hypothesized that CAPON resides in cardiac sympathetic neurons and is a part of the prediseased neuronal phenotype that modulates calcium handling and neurotransmission in dysautonomia. CAPON expression was significantly reduced in the stellate ganglia of spontaneously hypertensive rats before the development of hypertension compared with age-matched Wistar–Kyoto rats. The neuronal calcium current (ICa; n=8) and intracellular calcium transient ([Ca2+]i; n=16) were significantly larger in the spontaneously hypertensive rat than in Wistar–Kyoto rat (P<0.05). A novel noradrenergic specific vector (Ad.PRSx8-mCherry/CAPON) significantly upregulated CAPON expression, NO synthase-1 activity, and cGMP in spontaneously hypertensive rat neurons without altering NO synthase-1 levels. Neuronal ICa and [Ca2+]i were significantly reduced after CAPON transduction compared with the empty vector. In addition, Ad.PRSx8-mCherry/CAPON also reduced 3H-norepinephrine release from spontaneously hypertensive rat atria (n=7). NO synthase-1 inhibition (AAAN, 10 μmol/L; n=6) reversed these effects compared with the empty virus alone. In conclusion, targeted upregulation of CAPON decreases cardiac sympathetic hyperactivity. Moreover, dysregulation of this adaptor protein in sympathetic neurons might further amplify the negative cardiac electrophysiological properties seen with CAPON mutations.
Keywords: calcium, CAPON, hypertension, primary dysautonomias, sympathetic nervous system, synaptic transmission
Large genome-wide association studies have implicated the neuronal nitric oxide synthase adaptor protein (NOS1-AP or CAPON) as a potential molecular marker of both corrected QT interval on the ECG and increased risk of sudden cardiac death.1–5 Single-nucleotide polymorphisms in the CAPON gene have also been shown to be an important risk modifier in patients with inherited long-QT syndrome6–8 where sympathetic drive further exacerbates the electrophysiological phenotype.9
In ventricular myocytes, CAPON is colocalized with neuronal nitric oxide synthase (nNOS/NOS1).10,11 Increasing CAPON expression using adenoviral gene transfer accelerates cardiac repolarization (shortening action potential duration) by suppressing the L-type calcium current ICa,L and enhancing the delayed rectifier potassium current IKr.10 Furthermore, CAPON facilitates nNOS translocation to caveolae post-myocardial infarction, suggesting the interaction with CAPON is required for nNOS redistribution in injured myocardium.12
CAPON was first identified in neuronal tissue, where it interacts with the N-terminal PDZ domain of nNOS via C-terminal competition with PSD9513. CAPON escorts nNOS to specific target proteins, such as synapsin14 and the small monomeric G protein, Dexras115, and thus may play an important role in calcium-dependent exocytosis. Taken together with the observation that nNOS-generated NO acts via cGMP and phosphodiesterase 216 to reduce cAMP-protein kinase A–dependent neuronal calcium-handling and cardiac norepinephrine release,17,18 it is therefore conceivable that an impaired neuronal CAPON-nNOS interaction might augment cardiac sympathetic neurotransmission.
We hypothesized that CAPON is present, but it is reduced in cardiac sympathetic neurons from an animal model of dysautonomia.19 As a consequence, this contributes to a predisease neuronal phenotype that enhances calcium handling and neurotransmission. By developing an adenoviral vector with a noradrenergic neuron-specific promoter to increase CAPON expression, we also tested the hypothesis that CAPON reduces the neuronal calcium current, intracellular calcium transient, and cardiac norepinephrine release through an nNOS-cGMP–dependent pathway.
Methods
Age- and weight-matched prehypertensive young male spontaneously hypertensive rats (SHRs; n=51) and normotensive Wistar–Kyoto (WKY; n=45) rats were purchased from Harlan (Bicester, United Kingdom) and housed under standard laboratory conditions. This investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (Publication No. 85-23, revised 1996) and the Animals Scientific Procedures Act 1986 (United Kingdom). Procedures were performed under British Home Office license requirements (PPL 30/2360). Further methodological detail is available in the online-only Data Supplement.
Sympathetic Stellate Neuron Isolation
Sympathetic neurons were isolated and cultured using a previously published method,20 and the media used for isolation were based on modification of those previously described.21 Briefly, cardiac stellate ganglia were dissected, desheathed, and enzymatically digested. After a sequential mechanical trituration, cell suspension containing stellate neurons was plated onto poly-d/lysine/laminin-coated coverslips.
Adenovirus Vector Transduction
A novel adenoviral vector expressing CAPON fused in frame at the C-terminal end to red fluorescent protein mCherry with either a CMV promoter (Ad.CMV-mCherry/CAPON) or a noradrenergic cell-specific promoter (Ad.PRSx8-mCherry/CAPON) were transferred to isolated cardiac sympathetic neurons or whole stellate ganglia tissue. An adenoviral vector expressing only mCherry (Ad.CMV-mCherry or Ad.PRSx8-mCherry) served as a control. 2×109 pfu of adenoviral vector was used to infect neurons or ganglia in a 4-well pate (1.9 cm2/well; Nunc, Denmark). The virus-containing medium was left in the well for a maximum of 12 hours before replacing with fresh plating medium. The experiments were performed after 3 days post gene transfer for calcium transient measurements, and after 12 hours for measuring the calcium current because of the necessity to minimize space clamp error caused by dendritic growth.
For the local evoked norepinephrine release experiment, targeted percutaneous gene transfer to the right atrium was performed under isoflurane (Isocare; Animalcare Ltd) anesthesia (4% for induction and 2%–3% for maintenance in 100% O2), using a technique similar to that described previously.22 Animals received an injection of 1.6×1010 pfu of Ad.PRSx8-mCherry/CAPON or Ad.PRSx8-mCherry in 300 μL of PBS. Molecular and physiological phenotyping were investigated 5 days after gene transfer.
Immunofluorescence
Cultured primary neurons were fixed in cold acetone/methanol for 10 minutes. After permeabilization and blocking with 0.1% Triton X100 in PBS containing 1% BSA for 1 hour, the neurons were then incubated with primary antibody against CAPON (rabbit pAb, 1:200; Abcam) and tyrosine hydroxylase (mouse mAb, 1:200; Sigma) in 1% BSA overnight at 4°C. Signals were visualized with antirabbit antibody conjugated to Alexa-488 (1:1000; Molecular Probes) and antimouse antibody conjugated to Alexa-594 (1:1000; Molecular Probes). Nuclear staining was performed with 4′,6-diamidino-2-phenylindole (DAPI, 1:1000; Sigma). Imaging was performed on a Nikon Ti-U fluorescent microscope.
Western Blotting
Protein extraction and Western blotting were performed as previously described (details are available in the online-only Data Supplement).20
Patch-Clamp Recordings
Cells were patched on freshly isolated neurons for nongene transferred experiments. Calcium current was recorded using conventional whole cell techniques. Pipette resistance varied from 1.5 to 2 2MΩ when filled with the internal solution containing (in mmol/L) 140 CsCl, 10 HEPES, 0.1 CaCl2, 4 MgATP, 1 MgCl2, and 1 EGTA, adjusted to pH 7.4 with CsOH. The isolated neurons were superfused in a 36±0.5°C bath with external solution containing (in mmol/L) 145 TEACl, 10 HEPES, 4.5 KCl, 1 MgCl2 and 11 glucose, 1 NaHCO3, 2 BaCl2, and 0.001 TTX, adjusted to pH 7.4 with Sigma base 7 to 9. The bath was grounded by a Ag/AgCl electrode connected via a 3M KCl/agar salt bridge. Calcium currents were acquired using Clampex software via an Axopatch 200B amplifier. Series resistance was compensated between 75% and 90%. Current–voltage (I–V) relationships were elicited from a holding potential of −90 mV using 50-ms steps (5 s between steps) to test potentials over the range of −50 to +50 mV in 10-mV increments.
Measurement of Intracellular Calcium Concentration
Intracellular free calcium concentration [Ca2+]i of individual cultured stellate neurons was determined using Fura-2 acetoxymethyl ester (Fura-2/AM) fluorescence ratio imaging as previously described.19 The specific nNOS inhibitor, N-[(4S)-4-Amino-5-[(2-aminoetyl) amino]pentyl]-N′-nitroguanidine (AAAN; 10 μmol/L) was introduced separately after the first high K+ (50 mmol/L) stimulation (S1) to depolarize cell as previously described.20 After 10 minutes of incubation, neurons were stimulated again in the presence of the drug (S2).
Measurement of Tissue NOS Activity
The activity of NOS was assessed by measuring the conversion of [14C]l-arginine to [14C]l-citrulline as described previously.23,24 Stellates from 2 animals were pooled to provide protein for each NOS activity measure.
Measurement of Tissue cGMP Levels
Stellate ganglia tissue were isolated and transduced in 4-well plates, which contained 2×109 pfu of adenoviral vector in 1 mL plating medium that was kept at 37°C in 5% CO2. The virus containing medium was left in the well for a maximum of 12 hours before replacing with fresh plating medium. After 5 days of gene transfer, stellate ganglia were rapidly frozen in liquid nitrogen. Measurement of cGMP levels was performed using cGMP Direct Immunoassay Kit (Abcam) according to the manufacturer’s instructions.
Measurement of Atrial [3H]-Norepinephrine Release
Spontaneously beating atria were isolated and transferred to a preheated (37±0.2°C), constantly oxygenated (carbogen: 95% oxygen, 5% CO2), water-jacketed organ bath containing 3-mL Tyrode solution where they were pinned flat on a silver stimulating electrode. The method for determining the local release of 3H- norepinephrine to field stimulation (5 Hz, 20 V. 1-ms pulse width, for 1 minute) was identical to that which we have previously described.18
Data Analysis
Data are expressed as mean±SEM. All data passed a normality test (Shapiro–Wilk). Comparison within groups was performed using the paired Student t test and between groups using the unpaired Student t test, or ANOVA with Newman–Keuls post hoc analysis for multiple comparisons. A value of P<0.05 were considered statistically significant.
Results
Identification of Endogenous CAPON Protein in Cardiac Sympathetic Neurons
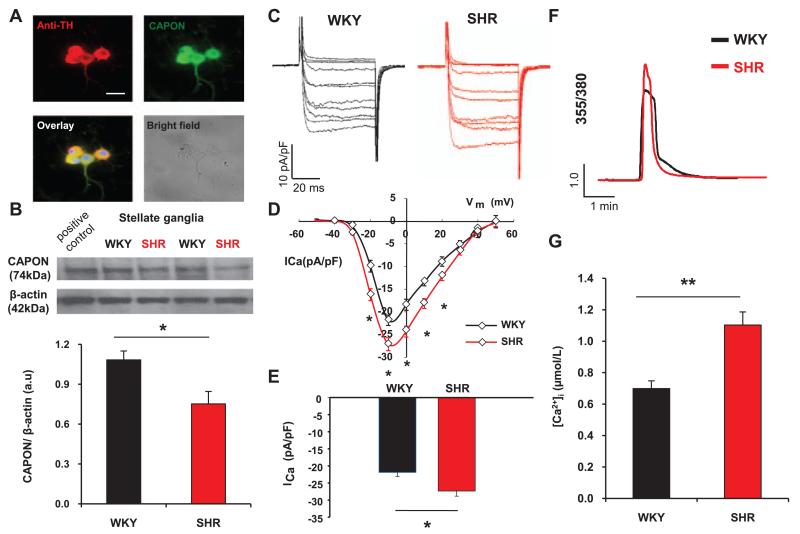
Immunostaining demonstrated endogenous CAPON protein colocalized in tyrosine hydroxylase positive neurons (Figure 1A). The endogenous expression of CAPON determined by Western blotting was significantly lower in the prehypertensive SHR (P<0.05; n=6) than in WKY controls (n=6) when normalized to β-actin (Figure 1B).
Figure 1.
A, Coimmunostaining of stellate neurons with anti–tyrosine hydroxylase (TH, red) and anti–neuronal nitric oxide synthase adaptor protein (CAPON, green) antibodies showed CAPON expression in sympathetic neurons. Nuclear staining with DAPI (4′,6-diamidino-2-phenylindole) is in blue. Scale bar, 25 μm. B, Representative Western blot and group mean data showing a significant reduction in CAPON protein expression relative to β-actin in stellate ganglia from 4-week-old spontaneously hypertensive rats (SHR; n=6) compared with age-matched Wistar–Kyoto controls (WKY; n=6; * P<0.05, unpaired t test). C, Representative traces elicited by depolarizing voltage steps (50 ms) from −50 to +50 mV in 10-mV increments from a holding potential of −90 mV in stellate neurons from 4-week-old SHR and WKY rats. D, Current density–voltage relationship curve of the neuronal calcium current (ICa) demonstrating significantly larger ICa at multiple voltages in stellate neurons from SHRs (n=8) when compared to WKY controls (n=8). E, The peak calcium current density in SHR stellate neurons (n=8) was larger than in WKY controls (n=8; *P<0.05, unpaired t test). F, Representative calcium fluorescence traces from 4-week-old SHR and WKY cardiac stellate neurons loaded with Fura2-AM and depolarized to evoke voltage-gated Ca2+ entry. G, Group data showing the difference peak evoked [Ca2+]i transients in 4-week-old SHR and WKY stellate neurons (n=16 versus n=13; **P<0.01, unpaired t test).
Neuronal Calcium Current and Calcium Transient Are Enhanced in Sympathetic Stellate Neurons From Young Prehypertensive Spontaneous Rat
The neuronal calcium current was recorded using the whole cell configuration of the patch-clamp technique. Currents were evoked by test pulses from a holding potential of −90 mV. The peak calcium current density was significantly increased by 25.04±0.01% in the cardiac stellate neurons of the SHR (−27.41±1.41 pA/pF; n=8) when compared with age-matched WKY (−21.92±1.13 pA/pF; n=8; P<0.05; Figure 1C–1E). Averaged peak current density–voltage relationships showed significant enhancement of ICa density from −20 to +20 mV (P<0.05) in the SHR (Figure 1D). As we have observed previously,25 the depolarization-induced [Ca2+]i transient was also significantly larger in SHR (n=16) than in WKY (n=13) stellate neurons (Figure 1F and 1G).
Effect of CAPON Gene Transfer
Western Blotting and cGMP Measurement
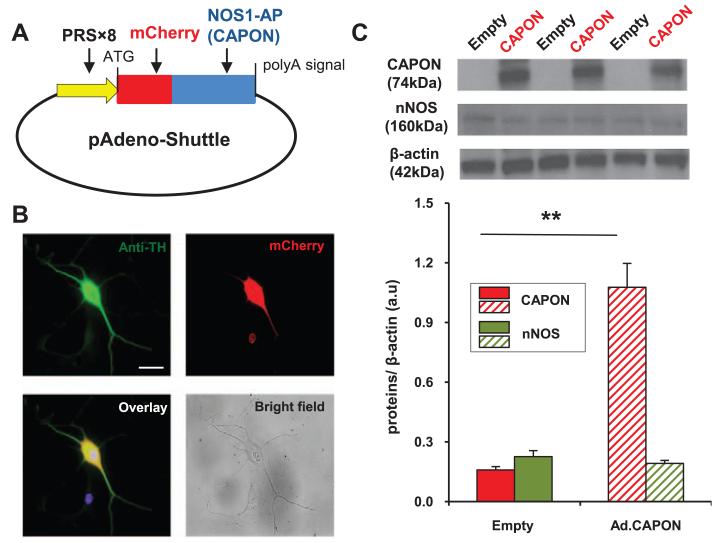
Western blotting revealed that Ad.PRSx8-mCherry/CAPON increased the expression of CAPON in SHR stellate ganglia in vitro compared with Ad.PRSx8-mCherry; however, it did not affect the level of nNOS protein (Figure 2C). Fluorescence microscopy demonstrated that Ad.PRSx8-mCherry/CAPON localized in tyrosine hydroxylase positive neurons from the stellate ganglia throughout the cytosol (Figure 2B).
Figure 2.
A, Map of adenoviral vector construct containing a noradrenergic neuron-specific promoter, (PRSx8), NOS1-AP (neuronal nitric oxide synthase adaptor protein [CAPON]) gene, and red mCherry fluorescent protein (Ad.PRSx8-mCherry/CAPON). As a control, the same construct without CAPON gene insert was used (Ad.PRSx8-mCherry). B, Coimmunostaining of stellate neurons with anti–tyrosine hydroxylase (TH, green) and mCherry (red) tagged viral construct showed viral transduction in sympathetic neurons. Nuclear staining with DAPI (4′,6-diamidino-2-phenylindole) is in blue. Scale bar, 25 μm. C, Representative Western blot and group mean data showing CAPON expression (74 kDa) and nNOS expression in stellate ganglia from 4-week-old SHR 3 days after transduction with Ad.PRSx8-mCherry/CAPON (Ad.CAPON, n=6) and Ad.PRSx8- mCherry (empty, n=6; **P<0.01, unpaired t test).
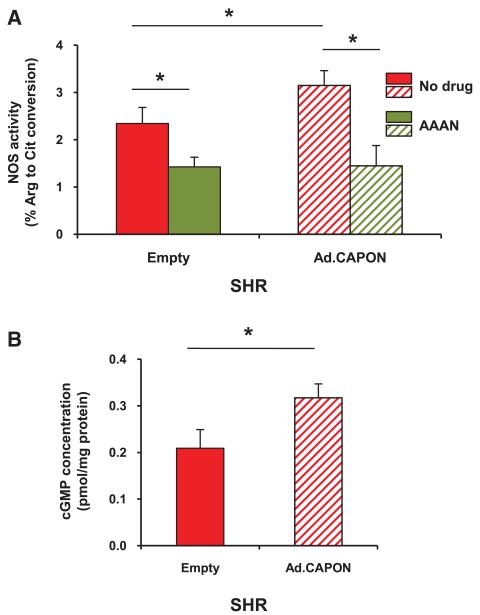
Although the level of nNOS expression in SHR stellate ganglia was unchanged after Ad.PRSx8-mCherry/CAPON transduction, NOS activity was significantly enhanced (n=3 measures, with each measure using pooled stellate protein from 2 animals) compared with Ad.PRSx8-mCherry (n=3; Figure 3A). The specific nNOS inhibitor AAAN normalized the difference in NOS activity after transduction with Ad.PRSx8-mCherry/CAPON (n=3) or Ad.PRSx8-mCherry (n=3). Furthermore, the level of cGMP in stellate ganglia from the SHRs was increased by ≈52% post CAPON adenoviral gene transfer compared with the empty vector (empty, 0.209±0.040 pmol/mg protein; n=10 versus Ad.CAPON:0.317±0.030 pmol/mg protein; n=10; P<0.05; Figure 3B).
Figure 3.
A, Gene transfer of neuronal nitric oxide synthase adaptor protein (CAPON; Ad.PRSx8-mCherry/CAPON) significantly increased NOS activity in stellate ganglia from spontaneously hypertensive rat (SHR) when compared with the empty vector (Ad.PRSx8-mCherry), control (n=3 measures, with each measure from tissue pooled from 2 animals). The specific nNOS inhibitor, S2, N-[(4S)-4-Amino-5-[(2-aminoetyl) amino] pentyl]-N′-nitroguanidine (AAAN, 10 μmol/L) normalized the difference in NOS activity after transduction with CAPON (n=3 measures) or the empty vector control (n=3 measures; *P<0.05, ANOVA). B, cGMP concentration in SHR stellate ganglia tissue was significantly enhanced by Ad.PRSx8-mCherry/CAPON transduction (n=10) when compared with empty controls (Ad.PRSx8-mCherry; n=10; *P<0.05, unpaired t test).
Calcium Current and Intracellular Free Calcium Transients in Cardiac Sympathetic Neurons
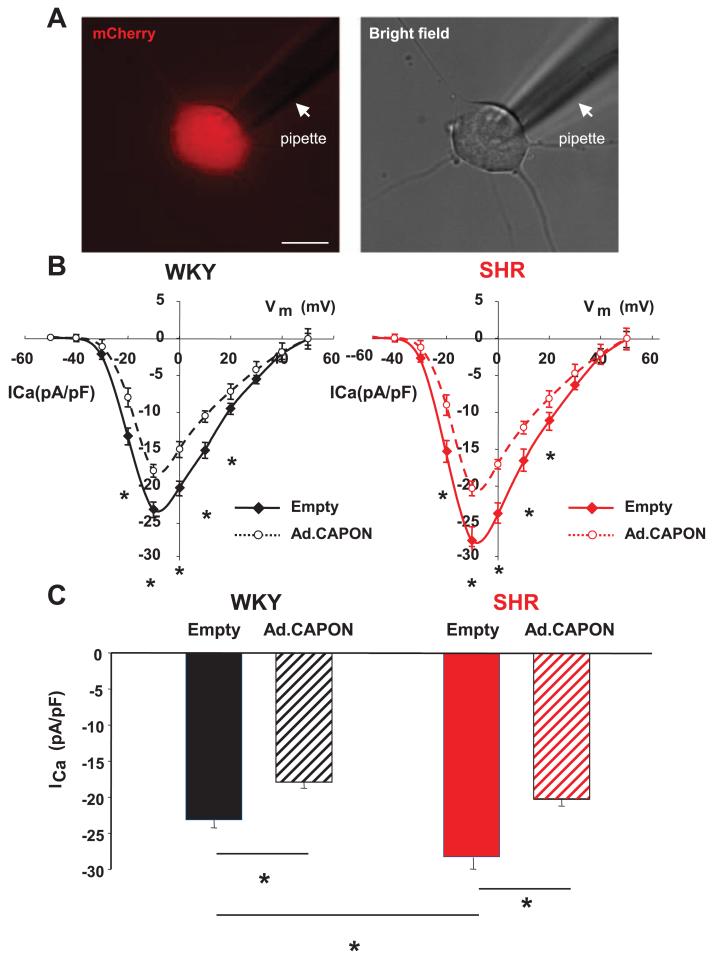
To explore whether CAPON overexpression also modulates the neuronal calcium current (ICa), this was recorded using the whole cell configuration of the patch-clamp technique on neurons 12 hours post viral transduction (Figure 4A). In CAPON gene transfer neurons from the SHR, the peak calcium current (ICa) density was significantly reduced by 19.48±1.57% (Ad.CMV-mCherry/CAPON, −20.29±0.78 pA/pF; n=6 and Ad.CMV-mCherry, −25.20±1.59 pA/pF; n=8; P<0.05) and by 22.69±0.09% in WKY (Ad.CMV-mCherry/CAPON, −17.92±0.84 pA/pF; n=7 and Ad.CMV-mCherry, −23.18±1.06 pA/pF; n=7; P<0.05; Figure 4C). In both strains, the averaged peak current density–voltage relationships showed significant attenuation of ICa from −20 mV to +20 mV (P<0.05) in neurons overexpressing CAPON when compared with empty vector controls (Figure 4B). The magnitude of the baseline current in cells transduced with the empty virus was similar to that measured in freshly isolated SHR or WKY neurons.
Figure 4.
A, Representative fluorescence (mCherry) and bright field images of single stellate neuron transduced with Ad.CMV-mCherry/neuronal nitric oxide synthase adaptor protein (CAPON; Ad.CAPON) with a patch pipette (pointed by a white arrow). Scale bar, 20 μm. B, Current density–voltage relationship curve of the neuronal calcium current (ICa) demonstrating attenuation of ICa at multiple voltages in CAPON-overexpressing stellate neurons from both 4-week-old spontaneously hypertensive rat (SHR) and age-matched Wistar–Kyoto (WKY) controls when compared with cells transduced with empty virus (Ad.CMV-mCherry). C, The peak ICa density in CAPON-overexpressing stellate neurons from both SHR (n=6) and WKY (n=7) was significantly less than in empty controls (SHR, n=8; WKY, n=7; *P<0.05, unpaired t test).
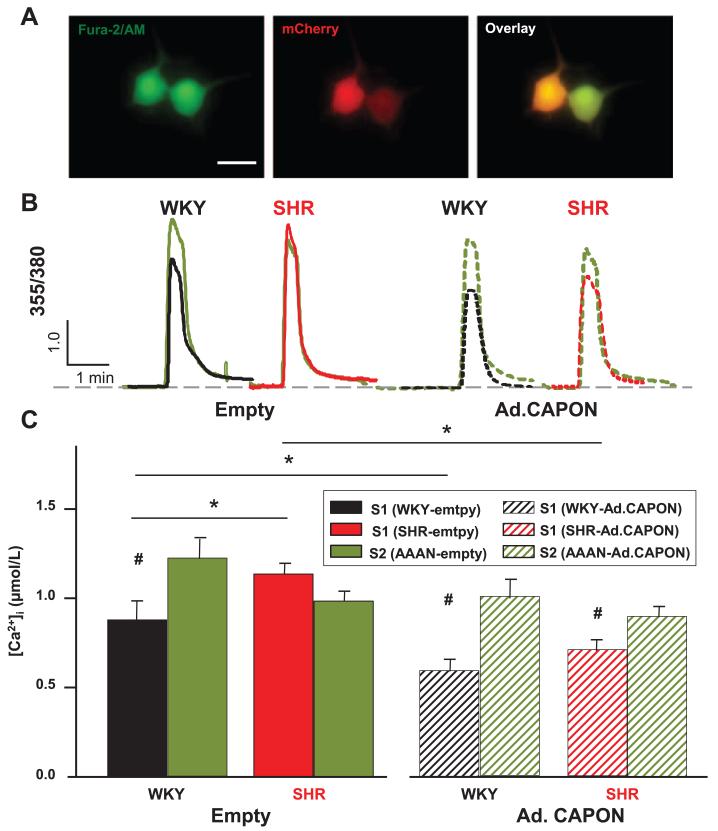
To determine whether the reduction in calcium current from CAPON gene transfer leads to a reduced intracellular calcium ([Ca2+]i) transient, fluorescence microscopy was used in cells that displayed both mCherry expression and Fura-2 AM loading, after transduction with Ad.PRSx8-mCherry/CAPON (Figure 5A) or its control vector (Ad.PRSx8-mCherry). In CAPON-overexpressing sympathetic neurons, the depolarization-induced [Ca2+]i transient was significantly reduced by 33.0±1.34% in the WKY rat (Ad.PRSx8-mCherry/CAPON, 0.62±0.06 μmol/L, n=11 and Ad.PRSx8-mCherry, 0.92±0.11 μmol/L; n=14; P<0.05) and 37.8±2.62%.in the SHRs (Ad. PRSx8-mCherry/CAPON, 0.74±0.07 μmol/L; n=13 and Ad.PRSx8-mCherry, 1.19±0.06 μmol/L; n=11; P<0.05) when compared with neurons transduced with empty viral vector (Figure 5B and 5C). The specific nNOS inhibitor AAAN reversed the effect of CAPON gene transfer on the depolarization induced [Ca2+]i transient and also normalized the differences between SHR and WKY neurons (Figure 5B and 5C).
Figure 5.
A, Stellate neurons demonstrating loading of Fura-2 AM by green fluorescence and viral transduction with mCherry (red). Scale bar, 25 μm. Representative ratio data trace (B) and group mean data (C) showing that the depolarization-induced intracellular calcium ([Ca2+]i) transient was significantly less in stellate neurons transduced with Ad.PRSx8-mCherry from 4-week-old Wistar–Kyoto (WKY; n=14) rats compared with age-matched with SHR n=11, *P<0.05, unpaired t test; #P<0.05, paired t test. Gene transfer with neuronal nitric oxide synthase adaptor protein (CAPON; Ad.PRSx8-mCherry/CAPON) significantly reduced the calcium transient in both WKY (n=11) and SHR (n=13) neurons to a similar levels. The effect of CAPON gene transfer in both strains can be reversed with a specific nNOS inhibitor (S2, N-[(4S)-4-Amino-5-[(2-aminoetyl) amino] pentyl]-N′-nitroguanidine, AAAN, 10 μmol/L).
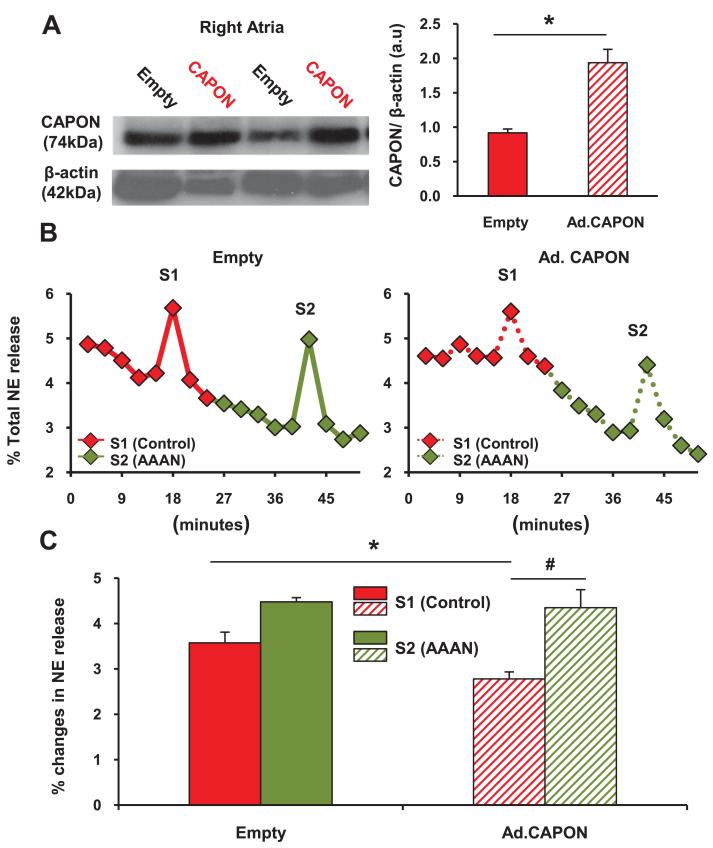
Cardiac Norepinephrine Release
Percutaneous gene transfer targeted to the right atrium of SHRs in vivo also increased right atrial CAPON protein expression as assessed by Western blot (Figure 6A). [3H]-norepinephrine release from isolated atrial preparations in response to 5 Hz field stimulation was significantly decreased by Ad.PRSx8-mCherry/CAPON by 22.1±0.97% when compared with the empty virus (Ad.PRSx8-mCherry/CAPON, 2.7±0.15%; n=6 versus Ad.PRSx8-mCherry, 3.5±0.24%; n=6; P<0.05; Figure 6B and 6C). The specific nNOS inhibitor AAAN reversed the effect of CAPON gene transfer on [3H]-norepinephrine release.
Figure 6.
Representative Western blot showing the upregulation of neuronal nitric oxide synthase adaptor protein (CAPON) expression in right atria by in vivo percutaneous injection with Ad.PRSx8-mCherry/CAPON (Ad.CAPON; n=6) when compared with Ad.PRSx8-mCherry (empty, n=6; *P<0.05, unpaired t test) in the 4-week-old spontaneously hypertensive rat. Representative raw data (B) and group mean data (C) showing that in vivo percutaneous transduction with Ad.PRSx8-mCherry/CAPON (Ad.CAPON, n=6) reduced 3H-norepinephrine (NE) release from isolated double atrial preparations in response to electric field stimulation (S1, 5 Hz) when compared with Ad.PRSx8-mCherry (empty, n=6; *P<0.05, unpaired t test; #P<0.05, paired t test). The effect of CAPON gene transfer can be abolished with a specific nNOS inhibitor (S2, AAAN, 10 μmol/L).
Discussion
The novel findings of our study are as follows. First, we demonstrate that CAPON is expressed in cardiac sympathetic neurons and is of a similar molecular mass to that first reported in hypothalamic neurons.13 Second, the expression of CAPON is reduced in the prohypertensive SHR compared with the WKY rat at 4 weeks, suggesting that it may be linked to the predisease neuronal phenotype. Third, upregulation of CAPON in the SHR restored its expression to similar levels seen in the WKY neurons. It also increased nNOS activity and the concentration of neuronal cGMP without changing the expression of nNOS itself. As a consequence, the neuronal calcium current and intracellular calcium transient was reduced in the SHR to levels observed in the WKY. This translated into reduced atrial norepinephrine release. These effects were reversed by nNOS inhibition, suggesting that CAPON modulation of sympathetic neurotransmission is coupled to an NO-dependent pathway.
CAPON Is Expressed in Cardiac Sympathetic Neurons
CAPON, a highly conserved protein, was first identified in rat brain neurons26 as a binding protein for nNOS.13 CAPON has been further discovered to be localized in ventricular myocytes,10 rat neural tissues including the facial nerve,27 sciatic nerve,28,29 dorsal root ganglion, and lumbar spinal cord.29 It has been implicated in neuronal pathogenesis, including peripheral nerve regeneration,28 neuron loss and survival,29 schizophrenia,30 pain,29 and inflammation.26 Here, we observed significant CAPON expression in both WKY and SHR stellate neurons; however, levels were significantly reduced in prehypertensive SHR neurons. Interestingly, CAPON is also present in choline acetyltransferase–positive intracardiac neurons (C.-J. Lu, N. Herring, D.J. Paterson, unpublished data, 2014) although it is unknown whether it affects the sympathetic phenotype reported here.
nNOS-CAPON Signaling in the Prehypertensive SHR
Sympathetic hyperactivity and parasympathetic insufficiency are correlated with mortality in patients with and without cardiovascular disease.31–33 In particular, overactivity of the sympathetic nervous system is implicated in the pathogenesis of human essential hypertension.34–40 This has also been observed in an animal of model of genetic hypertension, the SHR, as early as 4 weeks where arterial blood pressure and ventricular weight:body weight ratio19,20 is not different from age- and weight-matched WKY.19,41,42 At this age, however, these animals have a distinct autonomic phenotype of heightened cardiac sympathetic neurotransmission driven by enhanced calcium transients20,25 and reduced norepinephrine reuptake transporter activity.43 This translates into an enhanced tachycardia during right stellate ganglia simulation in vitro19 and elevated heart rates in vivo under anesthesia,19 as well as in telemetered animals.42 How sympathetic impairment occurs during cardiovascular disease is not fully understood, but it clearly involves changes at different sites in the neural-cardiac axis, from brain nuclei down to alterations in local neuronal circuits at the end organ that further add to the complexity of this regulation.44
The augmented neuronal calcium current and impaired nNOS/CAPON signaling observed here may account for a major part of altered calcium homeostasis,25 although defective mitochondrial buffering of [Ca2+]i may also contribute25 to this calcium impairment, and excessive adrenergic neurotransmission in the SHR.18 Dysregulated neuronal Ca2+ signaling has recently been reported in both stellate neurons and parasympathetic neurons (intracardiac) in animals with heart failure.45 Specifically, the N type Ca2+ current was enhanced in the sympathetic neuron, but impaired in the cholinergic neuron.45 These observations provide an electrophysiological basis for the cardiac sympatho-vagal phenotype seen in heart failure and hypertension. Of interest, we see that dyregulation of both the neuronal Ca2+ current and the intracellular Ca2+ transient are early cellular markers in the evolution of sympathetic hyperactivity. What is the cellular link to impaired intracellular Ca2+ handling and is it related oxidative stress as some have suggested?.20,26
nNOS acts through modulation of cGMP and PDE2 to reduce cAMP-protein kinase A–dependent regulation of neuronal calcium transients16 and norepinephrine release17 in sympathetic neurons. Because prehypertensive SHRs have reduced cardiac stellate expression of nNOS, the β1 subunit of soluble guanylate cyclase, cGMP,20 and given that CAPON acts as a modifier of nNOS in brain neurons, we suspected that CAPON might also play a role in calcium-handling and norepinephrine release in cardiac sympathetic neurons. Although the role of CAPON as an inhibitor or mediator of nNOS function in human disease has been widely debated,46 in our study, overexpression of CAPON increased neuronal nNOS activity and cGMP levels in the SHR, while stabilizing nNOS protein expression. This is similar to that seen in isolated ventricular myocytes where CAPON enhanced NOS enzymatic activity and NO release.10 Considering the brief half-life of NO and its high reactivity as a free radical gas, the biological and cellular effects of nNOS-NO signaling may be highly localized and dependent on the subcellular translocation of nNOS between membrane and cytosolic compartments as previously suggested.47 For example, CAPON plays an important role in directing nNOS to specific target proteins such as synpasin.14 However, like Chang et al,10 we cannot rule out the possibility that CAPON also acts through nNOS-independent pathways given that CAPON may compete with other PDZ-binding proteins through interaction via its C terminus.
Our electrophysiological experiments could not determine whether CAPON modulation was more important in the neuronal soma or axonal terminal that is more applicable to localized release. Nevertheless, increased CAPON/NOS-cGMP signaling restored the neuronal calcium current and calcium transient in the SHR to the levels seen in WKY neurons. The reduction in the neuronal calcium transient and norepinephrine release was reversed by nNOS inhibition, suggesting the involvement of an NO-dependent pathway. Interestingly, CAPON is colocalized not only with nNOS but also with L-type calcium channels (LTCa2+) and potassium channels (Kir3.1) in cardiomyocytes,11 suggesting that it may play a more widespread role in the modulation of ion channels. Moreover, given the localization of CAPON to the intercalated disc in human cardiomyocytes, it has been suggested that CAPON regulates ion flow through the cardiomyocyte gap junctions.48
Overexpression of CAPON in ventricular myocytes significantly shortens the APD by inhibiting ICa,L and activation of IKr.10 This may have implications in long-QT syndrome where genome-wide association studies have identified a common genetic variant in CAPON that might contribute to QT interval abnormalities.1–7 Although sympathetic stimulation can shorten the APD, it also stimulates myocyte calcium loading, and therefore it is conceivable that abnormal sympathetic activation because of CAPON impairment superimposed on a long-QT phenotype would further exacerbate the likelihood of afterdepolarizations.
Perspective
We present evidence that CAPON-nNOS modulate sympathetic neurotransmission and that this is dysregulated in the early stages of an animal model of sympathetic hyperactivity. Whether polymorphisms in the CAPON gene are associated with sympathetic hyperactivity remains to be established. However, sympathetic drive can modulate the QT interval and trigger life-threatening ventricular arrhythmias. Differences in CAPON expression or activity related to single-nucleotide polymorphisms in genome-wide association studies are associated with altered QT interval. It is, therefore, conceivable that abnormal sympathetic neurotransmission because of the same CAPON single-nucleotide polymorphisms could potentially further amplify the electrophysiological phenotype. Strategies that upregulate CAPON will shorten the APD10 and also decrease sympathetic drive, thereby providing a rationale for therapeutic targeting.
Supplementary Material
Novelty and Significance.
What Is New?
The neuronal calcium current and intracellular calcium transient are larger in the prohypertensive spontaneously hypertensive rat than in the Wistar–Kyoto rat, and this is associated with reduced expression of neuronal nitric oxide synthase adaptor protein (CAPON).
Targeted upregulation of CAPON reduced the intracellular neuronal calcium current and calcium transient by increasing nNOS activity and cGMP production, resulting in decreased adrenergic neurotransmission.
What Is Relevant?
Single-nucleotide polymorphisms in the CAPON gene have also been shown to be an important risk modifier for sudden cardiac death and QT variability in patients.
Summary
Artificial upregulation of CAPON decreases cardiac sympathetic hyperactivity. Dysregulation of this adaptor protein in sympathetic neurons might exacerbate the electrophysiological phenotype seen in patients with CAPON mutations.
Acknowledgments
Sources of Funding
This work was supported by a project grant from the British Heart Foundation (BHF). D.J. Paterson and N. Herring also acknowledge support from the BHF Centre of Research Excellence, Oxford.
Footnotes
Disclosures
None.
The online-only Data Supplement is available with this article at http://hyper.ahajournals.org/lookup/suppl/doi:10.1161/HYPERTENSIONAHA.115.05290/-/DC1.
References
- 1.Arking DE, Pfeufer A, Post W, et al. A common genetic variant in the NOS1 regulator NOS1AP modulates cardiac repolarization. Nat Genet. 2006;38:644–651. doi: 10.1038/ng1790. doi: 10.1038/ng1790. [DOI] [PubMed] [Google Scholar]
- 2.Eijgelsheim M, Newton-Cheh C, Aarnoudse AL, van Noord C, Witteman JC, Hofman A, Uitterlinden AG, Stricker BH. Genetic variation in NOS1AP is associated with sudden cardiac death: evidence from the Rotterdam Study. Hum Mol Genet. 2009;18:4213–4218. doi: 10.1093/hmg/ddp356. doi: 10.1093/hmg/ddp356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kao WH, Arking DE, Post W, et al. Genetic variations in nitric oxide synthase 1 adaptor protein are associated with sudden cardiac death in US white community-based populations. Circulation. 2009;119:940–951. doi: 10.1161/CIRCULATIONAHA.108.791723. doi: 10.1161/CIRCULATIONAHA.108.791723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aarnoudse AJ, Newton-Cheh C, de Bakker PI, Straus SM, Kors JA, Hofman A, Uitterlinden AG, Witteman JC, Stricker BH. Common NOS1AP variants are associated with a prolonged QTc interval in the Rotterdam Study. Circulation. 2007;116:10–16. doi: 10.1161/CIRCULATIONAHA.106.676783. doi: 10.1161/CIRCULATIONAHA.106.676783. [DOI] [PubMed] [Google Scholar]
- 5.Post W, Shen H, Damcott C, Arking DE, Kao WH, Sack PA, Ryan KA, Chakravarti A, Mitchell BD, Shuldiner AR. Associations between genetic variants in the NOS1AP (CAPON) gene and cardiac repolarization in the old order Amish. Hum Hered. 2007;64:214–219. doi: 10.1159/000103630. doi: 10.1159/000103630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crotti L, Monti MC, Insolia R, Peljto A, Goosen A, Brink PA, Greenberg DA, Schwartz PJ, George AL., Jr NOS1AP is a genetic modifier of the long-QT syndrome. Circulation. 2009;120:1657–1663. doi: 10.1161/CIRCULATIONAHA.109.879643. doi: 10.1161/CIRCULATIONAHA.109.879643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tomás M, Napolitano C, De Giuli L, Bloise R, Subirana I, Malovini A, Bellazzi R, Arking DE, Marban E, Chakravarti A, Spooner PM, Priori SG. Polymorphisms in the NOS1AP gene modulate QT interval duration and risk of arrhythmias in the long QT syndrome. J Am Coll Cardiol. 2010;55:2745–2752. doi: 10.1016/j.jacc.2009.12.065. doi: 10.1016/j.jacc.2009.12.065. [DOI] [PubMed] [Google Scholar]
- 8.Earle N, Yeo Han D, Pilbrow A, Crawford J, Smith W, Shelling AN, Cameron V, Love DR, Skinner JR. Single nucleotide polymorphisms in arrhythmia genes modify the risk of cardiac events and sudden death in long QT syndrome. Heart Rhythm. 2014;11:76–82. doi: 10.1016/j.hrthm.2013.10.005. doi: 10.1016/j.hrthm.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Shen MJ, Zipes DP. Role of the autonomic nervous system in modulating cardiac arrhythmias. Circ Res. 2014;114:1004–1021. doi: 10.1161/CIRCRESAHA.113.302549. doi: 10.1161/CIRCRESAHA.113.302549. [DOI] [PubMed] [Google Scholar]
- 10.Chang KC, Barth AS, Sasano T, Kizana E, Kashiwakura Y, Zhang Y, Foster DB, Marbán E. CAPON modulates cardiac repolarization via neuronal nitric oxide synthase signaling in the heart. Proc Natl Acad Sci U S A. 2008;105:4477–4482. doi: 10.1073/pnas.0709118105. doi: 10.1073/pnas.0709118105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Treuer AV, Gonzalez DR. NOS1AP modulates intracellular Ca(2+) in cardiac myocytes and is up-regulated in dystrophic cardiomyopathy. Int J Physiol Pathophysiol Pharmacol. 2014;6:37–46. [PMC free article] [PubMed] [Google Scholar]
- 12.Beigi F, Oskouei BN, Zheng M, Cooke CA, Lamirault G, Hare JM. Cardiac nitric oxide synthase-1 localization within the cardiomyocyte is accompanied by the adaptor protein, CAPON. Nitric Oxide. 2009;21:226–233. doi: 10.1016/j.niox.2009.09.005. doi: 10.1016/j.niox.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaffrey SR, Snowman AM, Eliasson MJ, Cohen NA, Snyder SH. CAPON: a protein associated with neuronal nitric oxide synthase that regulates its interactions with PSD95. Neuron. 1998;20:115–124. doi: 10.1016/s0896-6273(00)80439-0. [DOI] [PubMed] [Google Scholar]
- 14.Jaffrey SR, Benfenati F, Snowman AM, Czernik AJ, Snyder SH. Neuronal nitric-oxide synthase localization mediated by a ternary complex with synapsin and CAPON. Proc Natl Acad Sci U S A. 2002;99:3199–3204. doi: 10.1073/pnas.261705799. doi: 10.1073/pnas.261705799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fang M, Jaffrey SR, Sawa A, Ye K, Luo X, Snyder SH. Dexras1: a G protein specifically coupled to neuronal nitric oxide synthase via CAPON. Neuron. 2000;28:183–193. doi: 10.1016/s0896-6273(00)00095-7. [DOI] [PubMed] [Google Scholar]
- 16.Wang L, Henrich M, Buckler KJ, McMenamin M, Mee CJ, Sattelle DB, Paterson DJ. Neuronal nitric oxide synthase gene transfer decreases [Ca2+]i in cardiac sympathetic neurons. J Mol Cell Cardiol. 2007;43:717–725. doi: 10.1016/j.yjmcc.2007.09.005. doi: 10.1016/j.yjmcc.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Wang L, Li D, Plested CP, Dawson T, Teschemacher AG, Paterson DJ. Noradrenergic neuron-specific overexpression of nNOS in cardiac sympathetic nerves decreases neurotransmission. J Mol Cell Cardiol. 2006;41:364–370. doi: 10.1016/j.yjmcc.2006.05.007. doi: 10.1016/j.yjmcc.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Li D, Wang L, Lee CW, Dawson TA, Paterson DJ. Noradrenergic cell specific gene transfer with neuronal nitric oxide synthase reduces cardiac sympathetic neurotransmission in hypertensive rats. Hypertension. 2007;50:69–74. doi: 10.1161/HYPERTENSIONAHA.107.088591. doi: 10.1161/HYPERTENSIONAHA.107.088591. [DOI] [PubMed] [Google Scholar]
- 19.Shanks J, Manou-Stathopoulou S, Lu CJ, Li D, Paterson DJ, Herring N. Cardiac sympathetic dysfunction in the prehypertensive spontaneously hypertensive rat. Am J Physiol Heart Circ Physiol. 2013;305:H980–H986. doi: 10.1152/ajpheart.00255.2013. doi: 10.1152/ajpheart.00255.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li D, Nikiforova N, Lu CJ, Wannop K, McMenamin M, Lee CW, Buckler KJ, Paterson DJ. Targeted neuronal nitric oxide synthase transgene delivery into stellate neurons reverses impaired intracellular calcium transients in prehypertensive rats. Hypertension. 2013;61:202–207. doi: 10.1161/HYPERTENSIONAHA.111.00105. doi: 10.1161/HYPERTENSIONAHA.111.00105. [DOI] [PubMed] [Google Scholar]
- 21.He Y, Baas PW. Growing and working with peripheral neurons. Methods Cell Biol. 2003;71:17–35. doi: 10.1016/s0091-679x(03)01002-1. [DOI] [PubMed] [Google Scholar]
- 22.Mohan RM, Heaton DA, Danson EJ, Krishnan SP, Cai S, Channon KM, Paterson DJ. Neuronal nitric oxide synthase gene transfer promotes cardiac vagal gain of function. Circ Res. 2002;91:1089–1091. doi: 10.1161/01.res.0000047531.75030.b5. [DOI] [PubMed] [Google Scholar]
- 23.de Bono JP, Warrick N, Bendall JK, Channon KM, Alp NJ. Radiochemical HPLC detection of arginine metabolism: measurement of nitric oxide synthesis and arginase activity in vascular tissue. Nitric Oxide. 2007;16:1–9. doi: 10.1016/j.niox.2006.03.008. doi: 10.1016/j.niox.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 24.Crabtree MJ, Tatham AL, Al-Wakeel Y, Warrick N, Hale AB, Cai S, Channon KM, Alp NJ. Quantitative regulation of intracellular endothelial nitric-oxide synthase (eNOS) coupling by both tetrahydrobiopterin-eNOS stoichiometry and biopterin redox status: insights from cells with tet-regulated GTP cyclohydrolase I expression. J Biol Chem. 2009;284:1136–1144. doi: 10.1074/jbc.M805403200. doi: 10.1074/jbc.M805403200. [DOI] [PubMed] [Google Scholar]
- 25.Li D, Lee CW, Buckler K, Parekh A, Herring N, Paterson DJ. Abnormal intracellular calcium homeostasis in sympathetic neurons from young prehypertensive rats. Hypertension. 2012;59:642–649. doi: 10.1161/HYPERTENSIONAHA.111.186460. doi: 10.1161/HYPERTENSIONAHA.111.186460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shao B, Jiang J, Wu Q, Xu Y, Lv Q, Li X, Wang P, Shen A, Yan M. The nuclear localization of CAPON in hippocampus and cerebral cortex neurons after lipopolysaccharide stimulation. Neuroimmunomodulation. 2011;18:89–97. doi: 10.1159/000320419. doi: 10.1159/000320419. [DOI] [PubMed] [Google Scholar]
- 27.Che YH, Tamatani M, Tohyama M. Changes in mRNA for post-synaptic density-95 (PSD-95) and carboxy-terminal PDZ ligand of neuronal nitric oxide synthase following facial nerve transection. Brain Res Mol Brain Res. 2000;76:325–335. doi: 10.1016/s0169-328x(00)00013-9. [DOI] [PubMed] [Google Scholar]
- 28.Cui Z, Lv Q, Yan M, Cheng C, Guo Z, Yang J, Chen M, Xia Y, Zhang L, Shen A. Elevated expression of CAPON and neuronal nitric oxide synthase in the sciatic nerve of rats following constriction injury. Vet J. 2011;187:374–380. doi: 10.1016/j.tvjl.2010.01.014. doi: 10.1016/j.tvjl.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 29.Shen A, Chen M, Niu S, Sun L, Gao S, Shi S, Li X, Lv Q, Guo Z, Cheng C. Changes in mRNA for CAPON and Dexras1 in adult rat following sciatic nerve transection. J Chem Neuroanat. 2008;35:85–93. doi: 10.1016/j.jchemneu.2007.07.004. doi: 10.1016/j.jchemneu.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Xu B, Wratten N, Charych EI, Buyske S, Firestein BL, Brzustowicz LM. Increased expression in dorsolateral prefrontal cortex of CAPON in schizophrenia and bipolar disorder. PLoS Med. 2005;2:e263. doi: 10.1371/journal.pmed.0020263. doi: 10.1371/journal.pmed.0020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341:1351–1357. doi: 10.1056/NEJM199910283411804. doi: 10.1056/NEJM199910283411804. [DOI] [PubMed] [Google Scholar]
- 32.La Rovere MT, Bigger JT, Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet. 1998;351:478–484. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- 33.Nolan J, Batin PD, Andrews R, Lindsay SJ, Brooksby P, Mullen M, Baig W, Flapan AD, Cowley A, Prescott RJ, Neilson JM, Fox KA. Prospective study of heart rate variability and mortality in chronic heart failure: results of the United Kingdom heart failure evaluation and assessment of risk trial (UK-heart) Circulation. 1998;98:1510–1516. doi: 10.1161/01.cir.98.15.1510. [DOI] [PubMed] [Google Scholar]
- 34.Grassi G. Assessment of sympathetic cardiovascular drive in human hypertension: achievements and perspectives. Hypertension. 2009;54:690–697. doi: 10.1161/HYPERTENSIONAHA.108.119883. doi: 10.1161/HYPERTENSIONAHA.108.119883. [DOI] [PubMed] [Google Scholar]
- 35.Smith PA, Graham LN, Mackintosh AF, Stoker JB, Mary DA. Relationship between central sympathetic activity and stages of human hypertension. Am J Hypertens. 2004;17:217–222. doi: 10.1016/j.amjhyper.2003.10.010. doi: 10.1016/j.amjhyper.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 36.Esler M, Ferrier C, Lambert G, Eisenhofer G, Cox H, Jennings G. Biochemical evidence of sympathetic hyperactivity in human hypertension. Hypertension. 1991;17(4 suppl):III29–III35. doi: 10.1161/01.hyp.17.4_suppl.iii29. [DOI] [PubMed] [Google Scholar]
- 37.Davrath LR, Goren Y, Pinhas I, Toledo E, Akselrod S. Early autonomic malfunction in normotensive individuals with a genetic predisposition to essential hypertension. Am J Physiol Heart Circ Physiol. 2003;285:H1697–H1704. doi: 10.1152/ajpheart.00208.2003. doi: 10.1152/ajpheart.00208.2003. [DOI] [PubMed] [Google Scholar]
- 38.Anderson EA, Sinkey CA, Lawton WJ, Mark AL. Elevated sympathetic nerve activity in borderline hypertensive humans. Evidence from direct intraneural recordings. Hypertension. 1989;14:177–183. doi: 10.1161/01.hyp.14.2.177. [DOI] [PubMed] [Google Scholar]
- 39.Flaa A, Mundal HH, Eide I, Kjeldsen S, Rostrup M. Sympathetic activity and cardiovascular risk factors in young men in the low, normal, and high blood pressure ranges. Hypertension. 2006;47:396–402. doi: 10.1161/01.HYP.0000203952.27988.79. doi: 10.1161/01.HYP.0000203952.27988.79. [DOI] [PubMed] [Google Scholar]
- 40.Greenwood JP, Stoker JB, Mary DA. Single-unit sympathetic discharge: quantitative assessment in human hypertensive disease. Circulation. 1999;100:1305–1310. doi: 10.1161/01.cir.100.12.1305. [DOI] [PubMed] [Google Scholar]
- 41.Kokubo M, Uemura A, Matsubara T, Murohara T. Noninvasive evaluation of the time course of change in cardiac function in spontaneously hypertensive rats by echocardiography. Hypertens Res. 2005;28:601–609. doi: 10.1291/hypres.28.601. doi: 10.1291/hypres.28.601. [DOI] [PubMed] [Google Scholar]
- 42.Komolova M, Friberg P, Adams MA. Altered vascular resistance properties and acute pressure-natriuresis mechanism in neonatal and weaning spontaneously hypertensive rats. Hypertension. 2012;59:979–984. doi: 10.1161/HYPERTENSIONAHA.111.178194. doi: 10.1161/HYPERTENSIONAHA.111.178194. [DOI] [PubMed] [Google Scholar]
- 43.Shanks J, Mane S, Ryan R, Paterson DJ. Ganglion-specific impairment of the norepinephrine transporter in the hypertensive rat. Hypertension. 2013;61:187–193. doi: 10.1161/HYPERTENSIONAHA.112.202184. doi: 10.1161/HYPERTENSIONAHA.112.202184. [DOI] [PubMed] [Google Scholar]
- 44.Armour JA. The little brain on the heart. Cleve Clin J Med. 2007;74(Suppl 1):S48–S51. doi: 10.3949/ccjm.74.suppl_1.s48. [DOI] [PubMed] [Google Scholar]
- 45.Tu H, Liu J, Zhang D, Zheng H, Patel KP, Cornish KG, Wang WZ, Muelleman RL, Li YL. Heart failure-induced changes of voltage-gated Ca2+ channels and cell excitability in rat cardiac postganglionic neurons. Am J Physiol Cell Physiol. 2014;306:C132–C142. doi: 10.1152/ajpcell.00223.2013. doi: 10.1152/ajpcell.00223.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Courtney MJ, Li LL, Lai YY. Mechanisms of NOS1AP action on NMDA receptor-nNOS signaling. Front Cell Neurosci. 2014;8:252. doi: 10.3389/fncel.2014.00252. doi: 10.3389/fncel.2014.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paton JF, Kasparov S, Paterson DJ. Nitric oxide and autonomic control of heart rate: a question of specificity. Trends Neurosci. 2002;25:626–631. doi: 10.1016/s0166-2236(02)02261-0. [DOI] [PubMed] [Google Scholar]
- 48.Kapoor A, Sekar RB, Hansen NF, et al. QT Interval-International GWAS Consortium. An enhancer polymorphism at the cardiomyocyte intercalated disc protein NOS1AP locus is a major regulator of the QT interval. Am J Hum Genet. 2014;94:854–869. doi: 10.1016/j.ajhg.2014.05.001. doi: 10.1016/j.ajhg.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.