Abstract
Significance: The presence of a “pathogenic” or “highly virulent” biofilm is a fundamental risk factor that prevents a chronic wound from healing and increases the risk of the wound becoming clinically infected. There is presently no unequivocal gold standard method available for clinicians to confirm the presence of biofilms in a wound. Thus, to help support clinician practice, we devised an algorithm intended to demonstrate evidence of the presence of a biofilm in a wound to assist with wound management.
Recent Advances: A variety of histological and microscopic methods applied to tissue biopsies are currently the most informative techniques available for demonstrating the presence of generic (not classified as pathogenic or commensal) biofilms and the effect they are having in promoting inflammation and downregulating cellular functions.
Critical Issues: Even as we rely on microscopic techniques to visualize biofilms, they are entities which are patchy and dispersed rather than confluent, particularly on biotic surfaces. Consequently, detection of biofilms by microscopic techniques alone can lead to frequent false-negative results. Furthermore, visual identification using the naked eye of a pathogenic biofilm on a macroscopic level on the wound will not be possible, unlike with biofilms on abiotic surfaces.
Future Direction: Lacking specific biomarkers to demonstrate microscopic, nonconfluent, virulent biofilms in wounds, the present focus on biofilm research should be placed on changing clinical practice. This is best done by utilizing an anti-biofilm toolbox approach, rather than speculating on unscientific approaches to identifying biofilms, with or without staining, in wounds with the naked eye. The approach to controlling biofilm should include initial wound cleansing, periodic debridement, followed by the application of appropriate antimicrobial wound dressings. This approach appears to be effective in removing pathogenic biofilms.

Steven L. Percival, PhD
Scope and Significance
In chronic wounds an underlying and fundamental risk factor preventing healing, and increasing the propensity for infection, is the development of a “pathogenic” or “highly virulent” biofilm. Such a biofilm is one that is upregulated genetically and biochemically, compared to more “dormant,” “commensal,” and “relatively nonpathogenic” mature biofilms, such as those found on the skin or in the gastrointestinal tract. These nonpathogenic biofilms help protect the human body from infection and disease i.e., they provide colonization resistance, but they can revert quickly to pathogenic or virulent biofilm during periods of stress. Compared to nonpathogenic biofilms, the higher number of upregulated genes in pathogenic biofilms lead to: excessive development of degrading enzymes (matrix metalloproteinases); enhanced production of extracellular polymeric substance (EPS); greater generation of quorum sensing molecules; and, increased microbial proliferation and dissemination. These genetic and biochemical effects within a pathogenic biofilm result in more vigorous immune responses, thus leading to chronic inflammation.
These two forms of biofilms require different management strategies. Although biofilms develop in all chronic wounds, it is the pathogenic biofilms that constitute the true risk to nonhealing chronic wounds. With this in mind, the essential, but as of yet unanswered, question remains why some biofilm-infected wounds heal whereas others do not. The most basic response is that all biofilms are not the same, whether they are in a wound or in any other anatomical site of the human body. The fundamental characteristics inherent to biofilms have helped to seed hypothetical and conceptual theories.1 In spite of some initial convincing clinical evidence, there is still no consensus that pathogenic biofilms delay wound healing. This has delayed positive clinical practices and outcomes that might ensue from understanding this principle. Thus, we believe it would be useful to provide an algorithm to help support the clinician to identify biofilms, specifically basing this on the pathogenic biofilm model hypothesis. We hope that the algorithm provided in this article will help to guide the clinician regarding when an appropriate anti-biofilm management approach should be applied to a nonhealing chronic wound.
Translational Relevance
Biofilms have been observed to date in a variety of different anatomical areas. They are composed of only 10–20% microorganisms, the other 80–90% being EPS. EPS is composed of an array of chemical and biological agents, including polysaccharides, proteins, lipids, glycolipids, metals, and extracellular DNA.2–5 Determining the components of EPS and quantifying them helps researchers to establish the biofilm's maturity and complexity; thus, it is one of the fundamental markers used to demonstrate evidence of biofilms in wounds. Despite this, there has been surprisingly little discussion about the composition and identification of EPS in published articles on wound biofilms. This may be explained by the difficulty in differentiating between biofilm EPS and components of the extracellular matrix. Furthermore, the diversity of microbes within wound biofilms vary greatly, depending on the anatomic environment in which the biofilm resides and the patient's own microbiota. This diversity, together with synergistic interactions between the different microbes, will determine the biofilm's virulent and pathogenic state.6 The study of microbial interactions, the science known as sociomicrobiology, will play an important role in understanding the more problematic and pathogenic biofilms relevant to wound biofilmology.
Clinical Relevance
Many published articles provide evidence of the role of biofilms in wounds, in both animals and humans.7–13 Pathogenic biofilms in wounds have been found to delay healing, which has important consequences in terms of patient quality of life and costs of medical care. Data from both animal and human wound studies have identified that biofilms exist as discrete and patchy microcolonies of microbes.7,9,10,14 However, even if microscopic staining techniques are employed to visualize biofilms there is a substantial likelihood that they will not be observed in a wound biopsy. This relates to both the microscopical size of biofilms and the fact that on biological surfaces they grow in a nonrandom and patchy arrangement. Most published articles on wound biofilms have defined them as microcolonies or cellular aggregates.15 For both acute and chronic wounds we prefer to simply define biofilms as: “A community of microorganisms that are attached to each other, and/or a surface, encased within a matrix of extracellular polymeric substance, appearing as microcolonies or aggregates of cells.”
Background
About 80% of all infections in humans, and 65% of those that are healthcare-associated, are related to biofilms. Specifically, James et al.7 reported that 60% of chronic wounds contained a biofilm, but they did not specify whether these were pathogenic or not. While biofilms have been clinically identified in both chronic and acute wounds,7,9,16 most published studies reported on only small cohorts of patients and relatively few wound samples. Further studies on a larger number of patients are needed to help establish how pathogenic biofilms contribute to delayed healing. We believe, however, that the currently available scientific evidence and clinical experience are sufficient to posit the hypothesis that “all chronic wounds contain biofilms.” This hypothesis seems supportable by the key observation that 99.99% of all microbes are associated with a surface (abiotic or biotic).17
Discussion
A chronic wound represents “a safe haven for microorganisms,” as it provides a moist and highly nutritious environment. Furthermore, within the biofilm state the microorganisms will be protected from immunological attack. The slough, which is largely composed of devitalized tissue, within a chronic wound will provide an environment in which resident microorganisms easily proliferate and develop into a biofilm. This will also serve as a protective entity for the microorganisms. Thus, chronic wounds with large amounts of slough may be at a high risk of proliferation of diverse, pathogenic, and antimicrobial-tolerant biofilms.
Presently, only crude methods are available to measure biofilms, i.e., using microbiological staining techniques on wound biopsy samples.18 However, as previously mentioned biofilms are not confluent on a surface, it is highly probable that they will not be recovered or identified in biopsies of many chronic wounds. Furthermore, many microbes in biofilms reside in a viable, but nonculturable state, impairing their recovery. Variations in the biofilm-forming potential of bacterial isolates obtained from wounds may not be easily observed because culture techniques are heavily weighted toward easily cultivable microorganisms, and the incidence of false-negative cultures may increase where bacteria are encased within the biofilm matrix.10 Also, using swabbing techniques may result in the over-representation of surface bacteria and an under-representation of bacteria that reside deep within the wound tissue.10,19 Microbes in biofilms that otherwise may not be recovered utilizing standard culture techniques on agar may be identified with fluorescent in situ hybridization or other molecular based techniques.20
Relevant basic science context
In addition to its effect on host defenses as noted above, wound biofilm impairs the formation of granulation tissue and reduces epithelialization.13,14,20–28 Furthermore, it is likely that the presence of pathogenic biofilms substantially contribute to antimicrobial tolerance. Thus, identifying biofilms in wounds is a clinically important issue, particularly when a tissue biopsy specimen is not available. Unfortunately, there is currently no existing clinical method for the identification of pathogenic biofilms and no helpful treatment algorithm.
Identification of a biofilm
Abiotic surfaces
The visualization of biofilms by the naked eye on nonbiological surfaces is relatively easy when the biofilm has grown to a certain size, especially if it contains color. Visible biofilms have been observed on orthopedic devices,29 catheters,30 and other medical devices.31 As in the case of biofilm (plaque) on a tooth surface, the principal visual markers in these contexts include the presence of a shiny, gel-like, yellow-colored slime.32 However, in the absence of any color, biofilms (plaque) on the teeth have been disclosed using dyes. Biofilm disclosing agents have been in use since the early 20th century, with basic Fuchsine and Erythrosine being used as early as the 1960s.32 Fluorescein disclosing systems, such as Plak-Check® (Sunstar Butler) are used extensively to stain biofilms.
Intraluminally, for example, within endotracheal tubes or intravascular catheters that have been removed from a patient, biofilms appear as a gel-like, nonconfluent patch, often black colored, and opaque.
Biotic surfaces
Unlike abiotic surfaces, the macroscopic identification of biofilms on biotic surfaces, particularly chronic wounds, is speculative and often based on a color induced by the overpopulating dominant sessile or planktonic aggregates of microbes. For example, in cystic fibrosis patients biofilm aggregates are dispersed within mucus samples and, therefore, cannot be visualized. A similar scenario occurs in the slough of wounds. In these situations, biofilms can only be recognized by microscopic techniques with appropriate staining methods. In some situations, evidence of Pseudomonas aeruginosa, a common cause of infections in cystic fibrosis patients, is highlighted by a slight blue-green tinge in tissue or mucus samples, due to the production of a pigment called pyocyanin. However, this approach to demonstrate evidence of a biofilm is not universally helpful, and can be misleading in the presence of polymicrobial biofilms.
As biofilms are unique and bespoke to the patient and site, there is currently no single marker that can be applied to all biofilms. Furthermore, the likelihood of finding such a marker is low, considering that biofilms do not comply with basic medical principles, such as Koch's postulate.6 Despite over 30 years of biofilm research, there are no definitive, nondestructive, and noninvasive markers that can conclusively reveal the presence of a biofilm, especially a virulent pathogenic biofilm. Diagnosing the presence of biofilms is rendered more difficult by the presence of slow-growing bacterial cells residing in the biofilm state.33 Additionally, the presence of small colony variants and persister cells within the biofilm hampers the ability to diagnose a biofilm infection; standard microbiological techniques often miss these slow-growing phenotypic variants.34
There are some markers that are useful for the diagnosis of a biofilm infection. These include the tolerance to antimicrobials to which the planktonic bacteria are susceptible and finding culture-negative results when clinical observations suggest a high suspicion of infection.35
Proposed visual markers for wound biofilm
A relatively small number of researchers and clinicians suggest that biofilms may reveal themselves as a slimy, translucent, and confluent film on a wound. However, without solid scientific and clinical evidence, we consider this to be a crude and often misleading visual marker for biofilm infection in a wound.19 Unless clear evidence emerges to support this idea, it should be viewed with caution.
Even as the biofilm name implies a confluent film many, if not all, biofilms, as discussed previously, are very patchy and not confluent on a surface. This is particularly evident in young biofilms, but also occurs in mature biofilms. Markers that provide evidence of a biofilm include the presence of distinctive microcolonies, often only 10–100 μm in width, and EPS, often with surrounding polymorphonucleocytes.36 Consequently, for now, scientists and clinicians should avoid the temptation to consider slimy translucent film on a wound as constituting evidence of a biofilm. Employing this approach, without scientific evidence, could be detrimental to wound care practice and advancement of this important clinical area.
Microorganisms will attach onto any surface, whether liquid or solid. Within a wound, slough (largely composed of denatured proteins37), necrotic tissue, and even nonbiological components such as fibers from a dressing, are all surfaces. In the mucus or sputum of cystic fibrosis patients biofilms are referred to as microbial aggregates and are dispersed within the mucus itself.38 These “microbial aggregates” are dispersed rather than confluent entities. These biofilm aggregates often appear in wound slough and may also be part of the dislodged or debrided biofilm that still have the general characteristics of the mother biofilm, from which they detached. However, pathogenic biofilm must always be considered to play a role in wound healing, no matter how small the entity. In some situations they can result in dehiscence of a wound.
An array of different biofilms can be present within a wound environment, such as: on the wound surface; as aggregates of cells dispersed within the wound exudate; in slough or on necrotic tissue; on the wound dressing; or anything that may have fallen into the wound. Consequently, in a wound bed with both abiotic and biotic biofilms, as well as non-pathogenic and pathogenic biofilms, it is more complicated to determine if biofilm is present than the literature suggests. Thus, when a biofilm is suspected in a wound that is showing delayed healing, clinicians should focus on management strategies to combat these multicellular organisms rather than speculating on their presence. We have, therefore, developed an algorithm that we believe will provide clinicians assistance in identifying biofilms.
Potential algorithm for biofilms
An algorithm to help identify biofilms in chronic wounds may be useful when wound biopsies are available (allowing preliminary identification of biofilm), but also when they are not. We have developed the simple algorithm shown in Fig. 1 that may help to guide the clinician toward more appropriate management strategies for stalled or nonhealing wounds. The proposed algorithm should, of course, only be applied when all other wound-related factors have been addressed. It is only intended as an aid and should always be used in conjunction with clinical judgment and local protocols of care.
Figure 1.
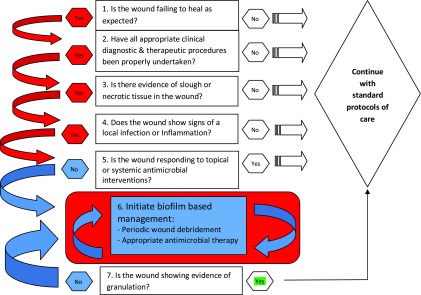
A potential algorithm for the detection and treatment of biofilms without the necessity to undertake biopsy samples. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Treatment of biofilms
To date there are few effective anti-biofilm strategies applied to chronic wounds. This is despite the application of biofilms management now being incorporated into the “Tissue-Inflammation/Infection-Moisture-Edge” (TIME) concept proposed by Leaper et al.39 for use in wound bed preparation. Another clinically useful management procedure for hard-to-heal wounds, called biofilm-based wound management,40 has been reported to achieve positive clinical outcomes. Biofilm management algorithms have also been suggested by Hess41 and by Wolcott et al.42 Approaches for nonhealing wounds have included the use of debridement, dressings that contain antimicrobials, anti-biofilm agents, antibiotics, antimicrobial and anti-biofilm cleansers.
Debridement is a key procedure in chronic wounds as it helps to remove problematic slough, necrotic tissue and, therefore, the pathogenic biofilm. Debridement of a wound may be performed with ultrasound,43 lavage, autolytic agents, enzymes, chemicals, sharp surgical procedures, and irrigating/cleansing solutions. Regular debridement of nonhealing chronic wounds is a key method to remove the biofilm,40,42,44 with the biofilm becoming dispersed into the wound exudates.45
However, biofilms have adapted an ability to regenerate quickly and the microbial cells dispersed into the wound exudate are able to rapidly reattach to a surface. This has led to the as yet unproven belief that following debridement appropriate antimicrobials (preferably antiseptics) should also be applied to the wound on a regular basis to reduce the microbial bioburden. This may ensure that the conditions become unfavorable for the detached biofilm to reform. Several different antiseptic agents can be employed for reducing microbial bioburden. Topical antimicrobials that are considered effective on planktonic microbes include polyhexamethylene biguanide hydrochloride, benzalkonium chloride, silver, iodine, and chlorhexidine.46 In general, it is best to avoid employing antibiotics, either topically or systemically, unless there are clinical findings compatible with a wound infection. Another approach is to use a wound dressing that is able to sequester and immobilize microbes. The dressing must also have the ability to sequester enzymes and toxins.
No anti-biofilm technologies developed thus far have received regulatory acceptance to make instructions for use claims. Therefore, the approach above is currently based on selected clinical experience for wounds that are nonhealing due to biofilms. However, it is very unlikely that one anti-biofilm agent will provide the answer for all universal wound care needs.
Like biofilm removal in dentistry, it may be helpful for chronic wounds that demonstrate nonhealing to first be conditioned with an antimicrobial cleansing agent, incorporating antimicrobials and surfactants, and then debrided. Surfactants have the ability to dislodge the biofilm, as reported in the food industry and dentistry. This in turn disperses the microbes in the biofilm and increases the susceptibility of the microorganisms to antimicrobial agents by disrupting the EPS. Additionally, as reported by Percival and colleagues,47,48 ethylenediaminetetraacetic acid (EDTA) has the ability to permeate microbes, increasing their susceptibility to antimicrobials. Furthermore, a biofilm's entirety is maintained by its use of metal ions in the EPS, including magnesium, calcium, and iron. Consequently, removing these molecules by using EDTA or lactoferrin may help to reduce the biofilm's stability and increase its susceptibility to interventions.
Summary
We have discussed how two types of biofilm may exist in a chronic wound: those that are nonpathogenic or commensal and potentially beneficial for wound and skin healing and those that are pathogenic and detrimental to healing. The pathogenic biofilms impair the host's immune response, reduce epithelialization, and dysregulate enzymatic systems. Single-species biofilms generally do not exist in a chronic wound; thus, we are dealing with a more complex polymicrobial biofilm. There is unscientific speculation around visualization of a biofilm in a wound through the naked eye. However, biofilms are not films per se, but are patchy aggregates of microcolonies covering small areas. At present, no one point of care device will be able to assist the clinician in the visualization and diagnosis of biofilms in a wound. Rather, combinations of biofilm diagnostic tools will be required to identify problematic biofilms, not just biofilms per se. Biosensors that measure subtle changes in the wound that are induced by a biofilm may be one approach for a biofilm marker.
Slough has not yet been adequately defined or analyzed for evidence of biofilms. As slough is composed of devitalized proteinaceous tissue in conjunction with viable tissue, this will provide an ideal environment for microbial adhesion and biofilm development. Slough will constitute a potential bioreactor system, which will serve to disseminate microbes further into the wound bed. Consequently, the removal of slough will help to remove the majority of the microbial biofilms before employing antimicrobial interventions. Furthermore, EPS is vital to the nature of the biofilm and may also be useful as a biomarker, in conjunction with microbial genomics, to highlight the evidence of a virulent biofilm in a wound.
Investigations to date have made it clear that the management of biofilms requires further technological and clinical advancements. However, more evidence on the role that pathogenic biofilms play in wound healing is fundamental to guiding anti-biofilm approaches to wound management. Despite this, effective anti-biofilm management practices and technologies must be combined with currently useful clinical practices to attempt to achieve higher rates of healing of chronic wounds.
Biofilm-based wound management should be employed routinely as positive clinical outcomes have been achieved with this approach. Going forward, it is imperative that some new wound dressings being developed have anti-biofilm capabilities or characteristics. However, there is an important balance between necessity and overkill. In order for a clinician to make a decision as to when more expensive techniques are required to get rid off, or reduce, the pathogenic biofilm, a simple algorithm could be employed. We have offered such an algorithm to help guide clinical practice, but it has not been subjected to validation to date.
In conclusion, it is important to realize that biofilms, like microorganisms, are not all bad; some, the commensal biofilm, may actually provide benefit to wound healing. It is the virulent or pathogenic biofilms that represent a major long-term cause for concern for nonhealing and development of infection in a wound.
Take-Home Messages.
• The presence of “Pathogenic” or “highly virulent” biofilm is a fundamental risk factor that often prevents a chronic wound from healing.
• No gold standard method is currently available for clinicians to confirm evidence of biofilms in a wound.
• Biofilms are not films per se, but are patchy aggregates of microorganisms called microcolonies.
• Wound slough and dressings constitute potential bioreactor systems, that can serve to disseminate microbes further into the wound bed.
• The removal of slough will help to remove the majority of the microbial biofilms before employing antimicrobial interventions.
• Unlike the common misconception, biofilms are not slimy, translucent, and confluent films on a wound. Using microscopic appearance as evidence for or against the presence of biofilm can be detrimental to wound care practice.
Abbreviations and Acronyms
- EDTA
ethylenediaminetetraacetic acid
- EPS
extracellular polymeric substance
Acknowledgments and Funding Sources
No funding sources were obtained for this review article.
Author Disclosure and Ghostwriting
No competing financial interests exist. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
About the Authors
Steven L. Percival holds a PhD in microbiology and biofilms, a BSc in Applied Biological Sciences, Postgraduate Certificate in Education, diploma in Business Administration, an MSc in Public Health and an MSc in Medical and Molecular Microbiology. Early in his career, Steven held R&D and commercial positions in the Department of Biotechnology, British Textile Technology Group (BTTG) Plc and phenomenex Ltd., followed then by 6 years as a senior university lecturer and later the positions of Director of R&D and Chief Scientific Officer at Aseptica, Inc. and senior clinical fellowships at the Centers for Disease Control, Atlanta and Leeds Teaching Hospitals Trust, Leeds. More recently, Steven held senior R&D manager positions at Bristol Myers Squibb, ConvaTec, Advanced Medical Solutions Plc and held an honorary Professorship in the Medical School at West Virginia University. In 2011, Steven joined Scapa Healthcare Plc as Vice President of Global Healthcare R&D. He also holds the position of honorary Professor at the University of Liverpool, UK. He has written over 300 scientific publications and conference abstracts on biofilms, antimicrobials, wounds, infection control, and has authored or edited seven textbooks.
Benjamin A. Lipsky is Teaching Associate at Green Templeton College and affiliated with the University of Oxford Division of Medical Sciences, Visiting Professor of Medicine at the University of Geneva and Professor of Medicine Emeritus at the University of Washington. He joined the faculty of the University of Washington in the Department of Medicine, rising to full Professor of Medicine. He served as consultant in infectious diseases and internal medicine, Director of the Primary Care Clinic, and ran an Antibiotic and Wound Infection Research Clinic. Professor Lipsky has authored over 200 peer-reviewed research articles (H-index 63), as well as over 100 other medical articles and textbook chapters, 120 research abstract presentations, and three books on infectious diseases. He has chaired the guideline committees on diabetic foot infections of both the Infectious Diseases Society of American and the International Working Group on the Diabetic Foot since their inception.
Gianfranco Donelli obtained his masters degree in Biological Sciences from the Sapienza University of Rome in 1966 and since 1967 he worked as researcher at the Istituto Superiore di Sanità (ISS - Italian National Institute of Health) in Rome. In 1975 he was appointed Director of the Electron Microscopy Unit and in 1979 he was the winner of a public competition for a position of Research Director in molecular biology. In 1982 Professor Donelli founded the Laboratory of Ultrastructures of ISS, which he led till 1996. In the last two decades, Professor Donelli has been appointed as Coordinator of the Infectious Diseases Research Project of ISS (1991–1995); Vice-President of the Advisory Committee for Biological and Medical Sciences of the Italian National Research Council (1994–1999); International Affairs Officer of the Italian Society of Microbiology and FEMS delegate (2002- present); President of the Society for Microbial Ecology and Disease (2006–2009); Vice-Chairperson of the Executive Committee of ESCMID Study Group for Biofilms (2009–2013). Since 1999 Professor Donelli's research activity has been mainly focused on biofilm-based healthcare-associated infections and possible strategies to prevent and counteract the development of microbial biofilms in the human body. Professor Donelli is author of over 250 full-length articles published on international scientific journals, (H-index=37) and since 2010 he has become Director of the Microbial Biofilm Laboratory at the Fondazione Santa Lucia research hospital in Rome.
Claudia Vuotto is currently a Ph.D. student at the Polytechnic University of Marche – Faculty of Medicine and Surgery – Biomedical sciences, drugs, and public health course. She obtained the MSc in Applied Cell Biology from Sapienza University of Rome in 2010. The title of the graduation experimental thesis was “Biofilms of anaerobic bacteria obtained from the intestinal tract.” Both traineeships for her experimental theses have been carried out at the Italian National Institute of Health (Istituto Superiore di Sanità-ISS) in Rome under the supervision of Professor Gianfranco Donelli. During her theses work she applied microbial methods to grow bacteria in biofilm, ultrastructural techniques (SEM) for biofilm surface characterization, and molecular techniques (DGGE) to identify biofilm-growing bacterial species isolated from biliary stents.
In 2010 she obtained a fellowship at the Department of Technology and Health, ISS, in the field of biofilm-based infections related to the insertion of prosthetic implants. From 2011 to the present, she is research assistant at the Microbial Biofilm Laboratory, Fondazione Santa Lucia Research Hospital in Rome, where she performs further investigations on the microbial biofilms. Dr. Vuotto is co-author of 10 original full-length articles and 1 Springer book chapter.
References
- 1.Neu TR, Lawrence JR. Advanced techniques for in situ analysis of the biofilm matrix (structure, composition, dynamics) by means of laser scanning microscopy. Methods Mol Biol 2014;1147:43–64 [DOI] [PubMed] [Google Scholar]
- 2.Branda SS, Vik A, Friedman L, et al. Biofilms: the matrix revisited. Trends Microbiol 2005;13:20. [DOI] [PubMed] [Google Scholar]
- 3.Flemming HC, Wingender J. The biofilm matrix. Nat Rev Microbiol 2010;8:623–633 [DOI] [PubMed] [Google Scholar]
- 4.Donlan RM. Biofilms: microbial life on surfaces. Emerg Infect Dis 2002;8:881–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flemming HC. The perfect slime. Colloids Surf B Biointerfaces 2011;86:251–259 [DOI] [PubMed] [Google Scholar]
- 6.Percival SL, Thomas JG, Williams DW. Biofilms and bacterial imbalances in chronic wounds: anti-Koch. Int Wound J 2010;7:169–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.James GA, Swogger E, Wolcott R, et al. Biofilms in chronic wounds. Wound Repair Regen 2008;16:37–44 [DOI] [PubMed] [Google Scholar]
- 8.Kirketerp-Møller K, Jenson PO, Fazli M, Madsen KG, Pedersen J, Moser C, et al. Distribution, organization, and ecology of bacteria in chronic wounds. J Clin Microbiol 2008;46:2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kennedy P, Brammah S, Wills E. Burns, biofilm and a new appraisal of burn wound sepsis. Burns 2010;36:49–56 [DOI] [PubMed] [Google Scholar]
- 10.Westgate SJ, Percival SL, Knottenbelt DC, Clegg PD, Cochrane CA. Microbiology of equine wounds and evidence of bacterial biofilms. Vet Microbiol 2011;150:152–159 [DOI] [PubMed] [Google Scholar]
- 11.Swanson EA, Freeman LJ, Seleem MN, Snyder PW. Biofilm-infected wounds in a dog. J Am Vet Med Assoc 2014;244:699–707 [DOI] [PubMed] [Google Scholar]
- 12.Freeman K, Woods E, Welsby S, Percival SL, Cochrane CA. Biofilm evidence and the microbial diversity of horse wounds. Can J Microbiol 2009;55:197. [DOI] [PubMed] [Google Scholar]
- 13.Dalton T, Dowd SE, Wolcott RD, Sun Y, Watters C, Griswold JA, et al. An in vivo polymicrobial biofilm wound infection model to study interspecies interactions. PLoS One 2011;6:e27317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis SC, Ricotti C, Cazzaniga A, Welsh E, Eaglstein WH, Mertz PM. Microscopic and physiologic evidence for biofilm-associated wound colonization in vivo. Wound Repair Regen 2008;16:23–29 [DOI] [PubMed] [Google Scholar]
- 15.Roche ED, Renick PJ, Tetens SP, Ramsay SJ, Daniels EQ, Carson DL. Increasing the presence of biofilm and healing delay in a porcine model of MRSA-infected wounds. Wound Repair Regen 2012;20:537–543 [DOI] [PubMed] [Google Scholar]
- 16.Krom BP, Oskam J. Microbial biofilms and wound healing: an ecological hypothesis. Phlebology 2014;29:168–173 [DOI] [PubMed] [Google Scholar]
- 17.Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 2002;15:167–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Q, Phillips PL, Sampson EM, et al. Development of a novel ex vivo porcine skin explant model for the assessment of mature bacterial biofilms. Wound Repair Regen 2013;21:704–714 [DOI] [PubMed] [Google Scholar]
- 19.Fazli M, Bjarnsholt T, Kirketerp-Møller K, et al. Quantitative analysis of the cellular inflammatory response against biofilm bacteria in chronic wounds. Wound Repair Regen 2011;19:387. [DOI] [PubMed] [Google Scholar]
- 20.Malic S, Hill KE, Hayes A, Percival SL, Thomas DW, Williams DW. Detection and identification of specific bacteria in wound biofilms using peptide nucleic acid fluorescent in situ hybridization (PNA FISH). Microbiology 2009;155(Pt 8):2603–2611 [DOI] [PubMed] [Google Scholar]
- 21.Schierle CF, de la Garza M, Mustoe TA, et al. Staphylococcal biofilms impair wound healing by delaying reepithelialization in a murine cutaneous wound model. Wound Repair Regen 2009;17:354–359 [DOI] [PubMed] [Google Scholar]
- 22.Geringer MR, Gurjala AN, et al. Treatment of Pseudomonas aeruginosa biofilm-infected wounds with clinical wound care strategies: a quantitative study using an in vivo rabbit ear model. Plast Reconstr Surg 2012;2;262e–274e [DOI] [PubMed] [Google Scholar]
- 23.Pastar I, Nusbaum AG, Gil J, et al. Interactions of methicillin resistant Staphylococcus aureus USA300 and Pseudomonas aeruginosa in polymicrobial wound infection. PLoS One 2013;8:e56846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao G, Hochwalt PC, Usui ML, et al. Delayed wound healing in diabetic (db/db) mice with Pseudomonas aeruginosa biofilm challenge: a model for the study of chronic wounds. Wound Repair Regen 2010;18:467–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gurjala AN, Geringer MR, Seth AK, et al. Development of a novel, highly quantitative in vivo model for the study of biofilm-impaired cutaneous wound healing. Wound Repair Regen 2011;19:400–410 [DOI] [PubMed] [Google Scholar]
- 26.Seth AK, Geringer MR, Galiano RD. Quantitative comparison and analysis of species-specific wound biofilm virulence using an in vivo, rabbit ear model. J Am Coll Surg 2012;215:388–399 [DOI] [PubMed] [Google Scholar]
- 27.Seth AK, Geringer MR, Hong SJ, et al. (2012). Comparative analysis of single-species and polybacterial wound biofilms using a quantitative, in vivo, rabbit ear model. PLoS One 2012;7:e42897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen KT, Seth AK, Hong SJ, et al. Deficient cytokine expression and neutrophil oxidative burst contribute to impaired cutaneous wound healing in diabetic, biofilm-containing chronic wounds. Wound Repair Regen 2013;21(6):833–841 [DOI] [PubMed] [Google Scholar]
- 29.Zimmerli W. Clinical presentation and treatment of orthopaedic implant-associated infection. J Intern Med 2014;276:111–119 [DOI] [PubMed] [Google Scholar]
- 30.Pradeep Kumar SS, Easwer HV, Maya N. and kumar A. Multiple drug resistant bacterial biofilms on implanted catheters - a reservoir of infection. J Assoc Physicians India 2013;61:702–707 [PubMed] [Google Scholar]
- 31.Martins M, Rodrigues A, Pedrosa JM, Carvalho MJ, Cabrita A, Oliveira R. Update on the challenging role of biofilms in peritoneal dialysis. Biofouling 2013;29:1015–1027 [DOI] [PubMed] [Google Scholar]
- 32.Squillaro RC, Cohen WD, Laster L. A comparison of microbial plaque disclosants after personal oral hygiene instruction and prophylaxis. J Prev Dent 1975;2:3–7 [PubMed] [Google Scholar]
- 33.Fux CA, Stoodley P, Hall-Stoodley L, Costerton JW. Bacterial biofilms: a diagnostic and therapeutic challenge. Expert Rev Anti Infect Ther 2003;1:667–668 [DOI] [PubMed] [Google Scholar]
- 34.Neut D, Tijdens-Creusen EJ, Bulstra SK, van der Mei HC, Busscher HJ. Biofilms in chronic diabetic foot ulcers—a study of 2 cases. Acta Orthop 2011;82:383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parsek MR, Singh PK. Bacterial biofilms: an emerging link to disease pathogenesis. Annu Rev Microbiol 2003;57:677–701 [DOI] [PubMed] [Google Scholar]
- 36.Woods E, Davis P, Barnett J, Percival SL. Wound healing, immunology and biofilms. In: Percival SL, Cutting K, eds. Microbiology of Wounds. 2010:271–292 [Google Scholar]
- 37.Cutting KF. Wound exudate: composition and functions. Br J Community Nurs 2003;8:4–9 [DOI] [PubMed] [Google Scholar]
- 38.Yu Q, Griffin EF, Moreau-Marquis S, Schwartzman JD, Stanton BA, O'Toole GA. In vitro evaluation of tobramycin and aztreonam versus Pseudomonas aeruginosa biofilms on cystic fibrosis-derived human airway epithelial cells. J Antimicrob Chemother 2012;67:2673–2681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leaper DJ, Schultz G, Carville K, Fletcher J, Swanson T, Drake R. Extending the TIME concept: What have we learned in the past 10 years? Int Wound J 2012;9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolcott RD, Rhoads DD. A study of biofilm-based wound management in subjects with critical limb ischaemia. J Wound Care 2008;17:145. [DOI] [PubMed] [Google Scholar]
- 41.Hess CT. Biofilm intervention pathway: an idealized algorithm. Adv Skin Wound Care 2012;25:480. [DOI] [PubMed] [Google Scholar]
- 42.Wolcott RD, Rumbaugh KP, James G, et al. Biofilm maturity studies indicate sharp debridement opens a time- dependent therapeutic window. J Wound Care 2010;19:320. [DOI] [PubMed] [Google Scholar]
- 43.Stanisic MM, Provo BJ, Larson DL, Kloth LC. Wound debridement with 25 kHz ultrasound. Adv Skin Wound Care 2005;18:484–490 [DOI] [PubMed] [Google Scholar]
- 44.Attinger C, Wolcott R. Clinically Addressing Biofilm in Chronic Wounds. Adv Wound Care (New Rochelle) 2012;1:127–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rhoads DD, Wolcott RD, Percival SL. Biofilms in wounds: management strategies. J Wound Care 2008;17:502–508 [DOI] [PubMed] [Google Scholar]
- 46.Percival SL, Finnegan S, Donelli G, Vuotto C, Rimmer S, Lipsky BA. Antiseptics for treating infected wounds: efficacy on biofilms and effect of pH. Criti Rev Microbiol, 2014. (August) [DOI] [PubMed] [Google Scholar]
- 47.Kite P, Eastwood K, Percival SL. Assessing the effectiveness of EDTA formulations for use as a novel catheter lock solution for the eradication of biofilms. In: McBain A, Allison D, Pratten J, Spratt D, Upton M, Verran J, eds. Biofilms, Persistence and Ubiquity. Cardiff, Wales: Bioline, September 6–8th 2005:181–190 [Google Scholar]
- 48.Kite P, Eastwood K, Hatton D, Sugden S, Percival SL. MBEC of catheter related bacteria to tetra sodium EDTA using a modified Calgary Biofilm Device. British Colombia, Canada: ASM Biofilms, November 1–6 2003:162, 164 [Google Scholar]


