Abstract
Numerous studies have investigated the effects of folic acid supplementation on colorectal cancer risk, but conflicting results were reported. We herein performed a meta-analysis based on relevant studies to reach a more definitive conclusion. The PubMed and Embase databases were searched for quality randomized controlled trials (RCTs) published before October 2014. Eight articles met the inclusion criteria and were subsequently analyzed. The results suggested that folic acid treatment was not associated with colorectal cancer risk in the total population (relative risk [RR] = 1.00, 95% confidence interval [CI] = 0.82–1.22, P = 0.974). Moreover, no statistical effect was identified in further subgroup analyses stratified by ethnicity, gender, body mass index (BMI) and potential confounding factors. No significant heterogeneity or publication bias was observed. In conclusion, our meta-analysis demonstrated that folic acid supplementation had no effect on colorectal cancer risk. However, this finding must be validated by further large studies.
Folic acid is a water-soluble vitamin first extracted and purified in 1941 from spinach leaves. Folic acid deficiency causes an imbalance in the one-carbon metabolic pathway, which is vital to hemoglobin synthesis as well as DNA synthesis, repair and methylation1,2. Research on folic acid treatment traces back to the last century when Metz et al. reported that pregnant women received iron from folic acid supplementation3. Dr. Laurence was a forerunner exploring the association between folic acid and neural tube defects4. Later, folic acid was reported to influence public health conditions, such as cardiovascular diseases, acute lymphoblastic leukemia, neuropathy and cancers, including colorectal cancer5,6,7,8,9.
Colorectal cancer is one of the most aggressive cancers worldwide, with mortality increasing in recent years10,11. Despite new therapeutic approaches, the prognosis of patients with colorectal cancer remains poor, and the median survival is only approximately 20 months for individuals with advanced disease12. Therefore, the need to discover proper chemopreventive agents to relieve disease burden is urgent. One potential target for therapy involves aberrant methylation, which is associated with the pathogenesis of early-stage colorectal cancer. Given that folic acid affects DNA methylation, it may play a role in carcinogenesis13.
Many researchers have examined the potential effects of folic acid supplementation in the prevention of colorectal cancer14. Various studies have focused on the association between folic acid and colorectal cancer for approximately two decades15, but existing epidemiological data are inconsistent. Folic acid fortification may increase the rate of colorectal cancer16. However, a meta-analysis of three randomized controlled trials (RCTs) observed no such effect17. Given that the results of the latest RCTs have been inconsistent, we performed this meta-analysis to provide a systematic evaluation.
Results
Study characteristics
A total of 1,229 relevant reports were retrieved from the PubMed and Embase databases, and 72 eligible studies were identified for further assessment. Eight RCTs ultimately met the inclusion criteria18,19,20,21,22,23,24,25 (Fig. 1), two of which were related to the prevention of cardiovascular disease18,23, four were related to the occurrence or recurrence of colorectal adenoma19,20,22,23 and two studies assessed cancer risk21,24. Each study was a population-based RCT, which ensured the methodological quality of the article. All trials were placebo-controlled except for the studies by Gao et al. and Logan et al.20,25. Seven studies were double-blinded studies, whereas the remaining one provided no details regarding this25. The dose of folic acid supplemented daily varied from 0.5 to 2.5 mg. Each article was of high quality based on our quality assessment, and all had received a score ranging from 3 to 5 out of a total of 5 points. Detailed characteristics of the relevant literature are presented in Table 1.
Figure 1. A flow chart of the study identification and selection.
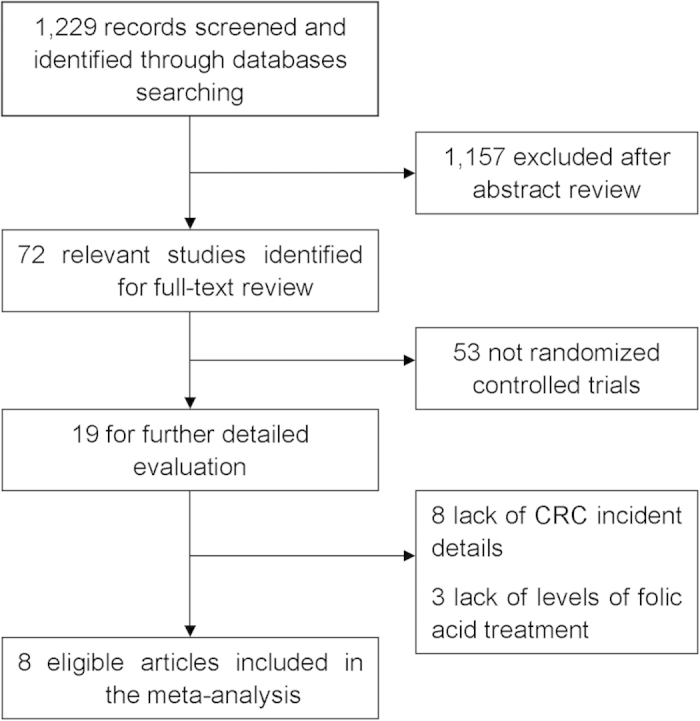
Table 1. Main characteristics of studies pooled in this meta-analysis.
| Sample size | CRC incidents | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Year | Ethnicity | Control source | Active | control | Age (year) | Male (%) | BMI (kg/m2) | Current smoker (%) | Prior disease (daily) | Folic acid | Additional treatment | Duration (months) | Active | control | Score of quality |
| Lonn et al.19 | 2006 | Mixed | HB | 2758 | 2764 | 68.9 | 71.8 | 29.7 | 11.5 | Vascular disease or diabetes | 2.5 mg | 50 mg vitamin B6 and 1 mg vitamin B12 | 60 | 50 | 37 | 5 |
| Cole et al.20 | 2007 | Mixed | HB | 516 | 505 | 57.0 | 63.8 | 27.5 | 14.5 | Colorectal adenoma | 1 mg | 81 mg or 325 mg aspirin or none | 75 | 3 | 4 | 5 |
| Logan et al.21 | 2008 | Caucasian | HB | 432 | 421 | NR | NR | NR | NR | Colorectal adenoma | 0.5 mg | 300 mg aspirin or none | 27 | 10 | 10 | 5 |
| Zhang et al.22 | 2008 | Caucasian | HB | 2721 | 2721 | 62.8 | 0 | 30.6 | 11.9 | CVD or 3 or more coronary risk factors | 2.5 mg | 50 mg vitamin B6 and 1 mg vitamin B12 | 88 | 18 | 22 | 3 |
| Wu et al.23 | 2009 | Caucasian | HB | 338 | 334 | 65.3 | 38.4 | 25.7 | 7.0 | Colorectal adenoma | 1 mg | None | 57 | 1 | 3 | 5 |
| Armitage et al.24 | 2010 | Caucasian | HB | 6033 | 6031 | 64.2 | 83.0 | NR | 12.0 | MI, other CHD, other vascular disease or diabetes | 2 mg | 1 mg vitamin B12 | 80 | 86 | 91 | 5 |
| Hankey et al.25 | 2012 | Mixed | HB | 4089 | 4075 | NR | 63.9 | NR | 23.3 | Stroke or transient ischemic attack | 2.5 mg | 50 mg vitamin B6 and 1 mg vitamin B12 | 41 | 21 | 21 | 5 |
| Gao et al.26 | 2013 | Asian | HB | 430 | 430 | 60.5 | 50.3 | NR | 17.3 | None | 1 mg | None | 36 | 2 | 2 | 4 |
HB, hospital based; BMI, body mass index; CVD, cardiovascular disease; MI, myocardial infarction; CHD, coronary heart disease; CRC, colorectal cancer; NR, not reported.
Quantitative synthesis
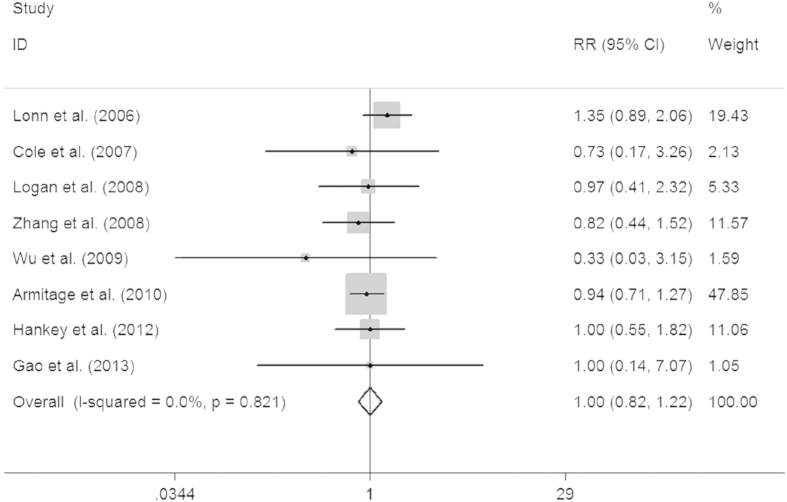
Our analysis revealed that supplementary folic acid lacked any association with the colorectal cancer incidence (relative risk [RR] = 1.00, 95% confidence interval [CI] = 0.82–1.22, P = 0.974; Fig. 2). A subgroup analysis based on ethnicity led to a similar conclusion (Caucasian RR = 0.91, 95% CI = 0.71–1.17, P = 0.463; mixed ethnicity RR = 1.19, 95% CI = 0.85–1.67, P = 0.303). In further analyses stratified by age, gender, body mass index (BMI), dose of folic acid, duration of the study or putative confounding factors, no significant effect was observed (Table 2).
Figure 2. Forest plot of the association between colorectal cancer risk and folic acid supplementation.
The squares and horizontal lines correspond to the study-specific RR and 95% CI, respectively. The areas of the squares reflect the weight. The diamond represents the summary RR and 95% CI.
Table 2. Summary of overall and subgroup analyses of the association between folic acid treatment and colorectal cancer risk.
| CRC incidents | |||||||
|---|---|---|---|---|---|---|---|
| Stratification variablesa | Active | Control | RR (95% CI) | Z | P> | Ph | I2(%) |
| Total population | 191 | 190 | 1.00 (0.82–1.22) | 0.03 | 0.974 | 0.821 | <0.001 |
| Ethnicity | |||||||
| Caucasian | 115 | 126 | 0.91 (0.71–1.17) | 0.73 | 0.463 | 0.807 | <0.001 |
| Mixed | 74 | 62 | 1.19 (0.85–1.67) | 1.03 | 0.303 | 0.578 | <0.001 |
| Total | 189 | 188 | 1.00 (0.82–1.23) | 0.03 | 0.974 | 0.727 | <0.001 |
| Age (year)b | |||||||
| <mean20,22,24,26 | 109 | 119 | 0.92 (0.71–1.19) | 0.67 | 0.501 | 0.967 | <0.001 |
| >mean19,23 | 51 | 40 | 1.28 (0.85–1.93) | 1.17 | 0.243 | 0.227 | 31.4 |
| Total | 160 | 159 | 1.01 (0.81–1.25) | 0.05 | 0.957 | 0.605 | <0.001 |
| Male (%)b | |||||||
| <mean22,23,25 | 22 | 27 | 0.78 (0.44–1.37) | 0.87 | 0.384 | 0.724 | <0.001 |
| >mean19,20,24,25 | 160 | 153 | 1.05 (0.84–1.30) | 0.39 | 0.694 | 0.542 | <0.001 |
| Total | 182 | 180 | 1.01 (0.82–1.23) | 0.05 | 0.962 | 0.727 | <0.001 |
| BMI (kg/m2 b | |||||||
| <mean20,23 | 4 | 7 | 0.56 (0.17–1.91) | 0.92 | 0.355 | 0.561 | <0.001 |
| >mean19,22 | 68 | 59 | 1.15 (0.82–1.63) | 0.81 | 0.417 | 0.188 | 42.3 |
| Total | 72 | 66 | 1.09 (0.78–1.52) | 0.51 | 0.608 | 0.364 | 5.9 |
| Dose of folic acidb | |||||||
| <mean20,21,23,24,26 | 102 | 110 | 0.92 (0.71–1.21) | 0.58 | 0.562 | 0.919 | <0.001 |
| >mean19,22,25 | 89 | 80 | 1.11 (0.82–1.50) | 0.70 | 0.485 | 0.386 | <0.001 |
| Total | 191 | 190 | 1.00 (0.82–1.22) | 0.03 | 0.974 | 0.821 | <0.001 |
| Durationb | |||||||
| <mean19,21,23,25,26 | 84 | 73 | 1.15 (0.84–1.57) | 0.86 | 0.389 | 0.711 | <0.001 |
| >mean20,22,24 | 107 | 117 | 0.91 (0.70–1.19) | 0.68 | 0.497 | 0.881 | <0.001 |
| Total | 191 | 190 | 1.00 (0.82–1.22) | 0.03 | 0.974 | 0.821 | <0.001 |
| Prior Disease | |||||||
| Colorectal adenoma19,20,23 | 14 | 17 | 0.81 (0.40–1.62) | 0.61 | 0.544 | 0.669 | <0.001 |
| CVD19,22,24,25 | 175 | 171 | 1.02 (0.83–1.26) | 0.22 | 0.829 | 0.477 | <0.001 |
| Possible confounding factors | |||||||
| Vitamin19,20,22,23,24,25 | 179 | 178 | 1.01 (0.82–1.24) | 0.05 | 0.962 | 0.605 | <0.001 |
| Antiplatelet drugs19,20,21,23,25 | 85 | 75 | 1.13 (0.83–1.54) | 0.77 | 0.442 | 0.653 | <0.001 |
| Lipid-lowering drugs19,24 | 136 | 128 | 1.06 (0.84–1.35) | 0.50 | 0.617 | 0.169 | 47.1 |
| Alcohol20,22,23,25,26 | 45 | 52 | 0.86 (0.58–1.28) | 0.73 | 0.466 | 0.908 | <0.001 |
| Diabetes19,22,24,25 | 175 | 171 | 1.02 (0.83–1.26) | 0.22 | 0.829 | 0.477 | <0.001 |
| Current smoker19,20,22,26 | 181 | 180 | 1.01 (0.82–1.23) | 0.05 | 0.962 | 0.727 | <0.001 |
| Hypertension19,22,24 | 154 | 150 | 1.03 (0.82–1.28) | 0.23 | 0.814 | 0.289 | 19.4 |
CVD, cardiovascular disease; CRC, colorectal cancer.
P, P value for association.
Ph, P value for heterogeneity.
aonly articles reporting the variables were analyzed.
bweighted mean of included articles.
Tests for heterogeneity and sensitivity
Fixed-effects models were utilized to analyze the association because no significant heterogeneity was observed (Table 2). The sensitivity analyses revealed that the RR with 95% CI was not obviously affected by removing one article at a time (data not shown).
Publication bias
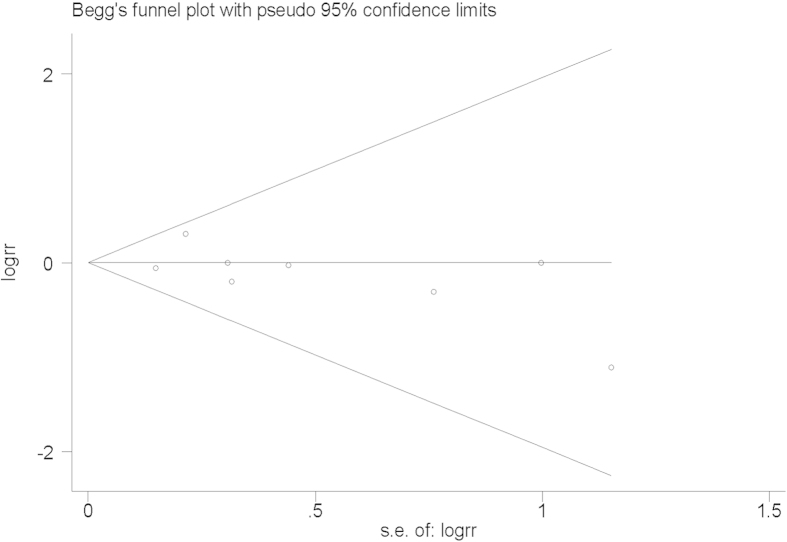
The shape of Begg’s funnel plot did not exhibit any obvious asymmetry (Fig. 3), and the Egger’s test revealed no evidence of publication bias (t = −1.05, P = 0.334).
Figure 3. Begg’s funnel plot for the publication bias test.
Each point represents a separate study for the indicated association.
Discussion
Folic acid was confirmed to protect against neural tube defects (NTDs) in the early 1990s. Since then, folic acid has been recommended to women of childbearing age to prevent birth defects26,27. Considerable attention has focused on the potential role of folic acid in preventing carcinogenesis, owing to its functions in DNA synthesis, repair and methylation28. For example, an association study by Lashner et al. explored the impact of folic acid treatment on cancer incidence in patients with chronic ulcerative colitis29. A meta-analysis by Sanjoaquin et al. suggested a small protective function of folic acid consumption on colorectal cancer30. Another meta-analysis by Kennedy et al. revealed a reduced cancer risk for subjects with increased folic acid intake31. In our analyses, however, no specific evidence for an overall relationship was detected. The inclusion criteria were potentially responsible for the difference, as only RCTs were included in our meta-analysis.
A high folic acid level was reported to break the homeostasis of the one-carbon metabolic pathway and increase cancer risk32, and fluorouracil misincorporation and DNA methylation disorders were postulated as possible mechanisms33. However, a subgroup analysis based on folic acid level did not change our conclusion in this study. The occurrence and development of colorectal cancer are associated with complex processes that may persist for 20 years or longer34, and longer duration trials would likely be needed to detect clinically detectable effects. In our analyses, however, a longer duration of treatment (>mean) did not exhibit a difference compared with a shorter (<mean) treatment period. Because the adenoma-carcinoma sequence is widely accepted as a gradual progression consisting of original normal epithelial mucosa, adenoma and ultimately carcinoma35, the existence of colorectal adenoma before the randomized trial could have been at least partly responsible for the formation of colorectal cancer in the above studies. In the present study, three articles were analyzed after stratification based on the prior existence of adenoma, but no statistically significant difference was observed based on a prior adenoma in these cases; however, the number of patients was relatively small19,20,22. We also observed no differences in the subgroup analysis based on gender. We hypothesized that the duration of follow-up influenced the apparent effect, but the corresponding analysis could not be performed given the lack of available information.
Previous animal experiments suggested that the effect of folic acid on carcinogenesis is dependent on the supplementary dose. In previous studies of normal cells, folic acid deficiency enhanced carcinogenesis, whereas supplementation enhanced tumor progression in tumor cells36,37. Based on our present results, both higher (>mean) and lower (<mean) doses did not affect the risk. Interestingly, one trial included in our meta-analysis suggested that folic acid plasma levels were more important than supplement levels25. In addition, a recent study demonstrated that the plasma folic acid concentration was associated with the risk of colorectal cancer, particularly for individuals with precancerous lesions38. However, details regarding dietary and plasma folic acid levels were not available for the studies included in our analyses, limiting our ability to assess the correlation. Elimination of subjects with risk factors (smoking, hypertension, alcohol intake, diabetes, etc.) would cause the data to be insufficient. Thus, these records were included, and subgroup analyses were conducted. However, all of these analyses yielded negative results (Table 2).
Despite the diversity of studies concerning different populations, family history, living environments, habits and customs, no significant heterogeneity or publication bias was observed in our analysis. In general, the articles included were compatible for this meta-analysis. To our knowledge, this meta-analysis is the most systematic examination of the association between folic acid supplementation and colorectal cancer based on all relevant RCTs. All chosen articles were of high quality, thus enhancing the reliability of our analyses and reducing the inherent bias. Such analyses may offer hypotheses for further functional studies and may shed light on the complexities of the pathways involved in colorectal cancer development. There are also some limitations that should be kept in mind when interpreting the results. First, two articles had relatively small samples, which may have affected the conclusion22,25. Second, potential heterogeneity may have been introduced due to methodological differences among trials. Finally, the possibility of publication bias existed in the review process, which could cause misleading results.
In conclusion, the present meta-analysis, which included the largest number of relevant RCTs to date, indicated that folic acid supplementation did not affect the colorectal cancer risk. However, questions remain regarding the role folic acid may play in colorectal cancer prevention, and larger studies with a rigorous design and strict methods are needed.
Methods
Publication search
We searched the PubMed and Embase databases for all studies published before October 2014. Combinations of the following MeSH terms were used for the search: “folic acid/folate,” “colorectal cancer,” “colon/rectal cancer” and “carcinoma.” Articles including association studies between folic acid fortification and colorectal cancer incidence were collected. The reference lists of relevant studies were also reviewed to identify any studies that were potentially missed. To be eligible for our analysis, the studies had to meet the following criteria: 1) exclusive RCT design; 2) explores the correlation between folic acid supplementation and colorectal cancer risk; 3) RR with a 95% CI or the number of colorectal cancer events was reported; 4) the supplementary folic acid level was stated; 5) published in English. Figure 1 provides a flow chart of the selection procedure.
Data extraction and quality assessment
Two authors screened the relevant publications and then extracted all data independently, complying with the selection criteria above. Discrepancies were resolved by another author after group discussion. The following data were extracted: first author’s last name, year of publication, ethnicity of the subjects, source of controls, sample size, age, gender, BMI, smoking status, prior disease, folic acid intake level, additional treatment, duration of the studies and colorectal cancer incidence. Ethnicity was categorized as Asian, African, Caucasian or mixed.
Quality assessments were performed based on the following features: randomization, double-blinding, generation of random numbers, reporting of dropouts and withdrawals and concealment of allocation39. Each feature was awarded one point, and all studies scored between 0 and 5 (see Supplementary Table S1 online). The publications that received a score greater than 2 were considered to be of high quality.
Statistical analysis
The relative risk (RR) with a 95% confidence interval (CI) was calculated to measure the strength of the association. The presence of between-trial heterogeneity was tested by the χ2-based Q test. The degree of variability was assessed by calculating the I2(inconsistency index) value. If the result of the Q test was P > 0.10, the RR was analyzed by the fixed-effects model. Otherwise, a random-effects model was used due to significant heterogeneity. A sensitivity analysis was performed to estimate the stability of the results by removing each study from the analysis, one at a time. Potential publication biases were also evaluated. In addition to visual inspection of the funnel plot, a value of P < 0.05 was considered to indicate the presence of significant publication bias40. All analyses were performed using the Stata software program (version 10.1; Stata Corporation, College Station, Texas) using two-sided P-values.
Additional Information
How to cite this article: Qin, T. et al. Folic acid supplements and colorectal cancer risk: meta-analysis of randomized controlled trials. Sci. Rep. 5, 12044; doi: 10.1038/srep12044 (2015).
Supplementary Material
Acknowledgments
This study was partially supported by the National Natural Science Foundation of China (NSFC 81472634), the Health Department Guidance Project of Jiangsu Province (Z201201), the Program for Development of Innovative Research Team in the First Affiliated Hospital of NJMU and the Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (JX10231801), the Jiangsu Province Clinical Science and Technology Projects (Clinical Research Center, BL2012008), the Summit of the Six Top Talents Program of Jiangsu Province (2013-WSN-034), and the Jiangsu Provincial Science and Technology Innovation Team and the Priority Academic Program Development of Jiangsu Higher Education Institutions (Public Health and Preventive Medicine).
Footnotes
Author Contributions Conceived and designed the experiments: M.D., T.Q. and Q.S. Analyzed the data: H.D. and M.W. Contributed analysis tools: L.Z., M.D. and T.Q. Wrote the first draft of the manuscript: T.Q., M.D. and H.D. Reviewed, edited and approved the manuscript: M.W. and L.Z.
References
- Sachdev H. P. & Gera T. Preventing childhood anemia in India: iron supplementation and beyond. European journal of clinical nutrition 67, 475–480, 10.1038/ejcn.2012.212 (2013). [DOI] [PubMed] [Google Scholar]
- Choi S. W. & Mason J. B. Folate and carcinogenesis: an integrated scheme. The Journal of nutrition 130, 129–132 (2000). [DOI] [PubMed] [Google Scholar]
- Metz J., Festenstein H. & Welch P. Effect of Folic Acid and Vitamin B12 Supplementation on Tests of Folate and Vitamin B12 Nutrition in Pregnancy. The American journal of clinical nutrition 16, 472–479 (1965). [DOI] [PubMed] [Google Scholar]
- Laurence K. M., James N., Miller M. H., Tennant G. B. & Campbell H. Double-blind randomised controlled trial of folate treatment before conception to prevent recurrence of neural-tube defects. Br Med J (Clin Res Ed) 282, 1509–1511 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange H. et al. Folate therapy and in-stent restenosis after coronary stenting. The New England journal of medicine 350, 2673–2681, 10.1056/NEJMoa032845 (2004). [DOI] [PubMed] [Google Scholar]
- Milne E. et al. Maternal folate and other vitamin supplementation during pregnancy and risk of acute lymphoblastic leukemia in the offspring. International journal of cancer. Journal international du cancer 126, 2690–2699, 10.1002/ijc.24969 (2010). [DOI] [PubMed] [Google Scholar]
- Reynolds E. H. The neurology of folic acid deficiency. Handbook of clinical neurology 120, 927–943, 10.1016/B978-0-7020-4087-0.00061-9 (2014). [DOI] [PubMed] [Google Scholar]
- de Vogel S. et al. Serum folate and vitamin B12 concentrations in relation to prostate cancer risk—a Norwegian population-based nested case-control study of 3000 cases and 3000 controls within the JANUS cohort. International journal of epidemiology 42, 201–210, 10.1093/ije/dys199 (2013). [DOI] [PubMed] [Google Scholar]
- Lazzeroni M. et al. The science behind vitamins and natural compounds for breast cancer prevention. Getting the most prevention out of it. Breast 20 Suppl 3, S36–41, 10.1016/S0960-9776(11)70292-2 (2011). [DOI] [PubMed] [Google Scholar]
- Yang Y., Gu X., Zhou M., Xiang J. & Chen Z. Serum microRNAs: A new diagnostic method for colorectal cancer. Biomedical reports 1, 495–498, 10.3892/br.2013.109 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peto J. Cancer epidemiology in the last century and the next decade. Nature 411, 390–395, 10.1038/35077256 (2001). [DOI] [PubMed] [Google Scholar]
- Hubner R. A. & Houlston R. S. Folate and colorectal cancer prevention. British journal of cancer 100, 233–239, 10.1038/sj.bjc.6604823 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Ohlsson R. & Henikoff S. The epigenetic progenitor origin of human cancer. Nature reviews. Genetics 7, 21–33, 10.1038/nrg1748 (2006). [DOI] [PubMed] [Google Scholar]
- Benito E. et al. Nutritional factors in colorectal cancer risk: a case-control study in Majorca. International journal of cancer. Journal international du cancer 49, 161–167 (1991). [DOI] [PubMed] [Google Scholar]
- Meyer F. & White E. Alcohol and nutrients in relation to colon cancer in middle-aged adults. American journal of epidemiology 138, 225–236 (1993). [DOI] [PubMed] [Google Scholar]
- Mason J. B. et al. A temporal association between folic acid fortification and an increase in colorectal cancer rates may be illuminating important biological principles: a hypothesis. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 16, 1325–1329, 10.1158/1055-9965.EPI-07-0329 (2007). [DOI] [PubMed] [Google Scholar]
- Carroll C. et al. Meta-analysis: folic acid in the chemoprevention of colorectal adenomas and colorectal cancer. Alimentary pharmacology & therapeutics 31, 708–718, 10.1111/j.1365-2036.2010.04238.x (2010). [DOI] [PubMed] [Google Scholar]
- Lonn E. et al. Homocysteine lowering with folic acid and B vitamins in vascular disease. The New England journal of medicine 354, 1567–1577, 10.1056/NEJMoa060900 (2006). [DOI] [PubMed] [Google Scholar]
- Cole B. F. et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA : the journal of the American Medical Association 297, 2351–2359, 10.1001/jama.297.21.2351 (2007). [DOI] [PubMed] [Google Scholar]
- Logan R. F., Grainge M. J., Shepherd V. C., Armitage N. C. & Muir K. R. Aspirin and folic acid for the prevention of recurrent colorectal adenomas. Gastroenterology 134, 29–38, 10.1053/j.gastro.2007.10.014 (2008). [DOI] [PubMed] [Google Scholar]
- Zhang S. M. et al. Effect of combined folic acid, vitamin B6, and vitamin B12 on cancer risk in women: a randomized trial. JAMA : the journal of the American Medical Association 300, 2012–2021, 10.1001/jama.2008.555 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K. et al. A randomized trial on folic acid supplementation and risk of recurrent colorectal adenoma. The American journal of clinical nutrition 90, 1623–1631, 10.3945/ajcn.2009.28319 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage J. M. et al. Effects of homocysteine-lowering with folic acid plus vitamin B12 vs placebo on mortality and major morbidity in myocardial infarction survivors: a randomized trial. JAMA : the journal of the American Medical Association 303, 2486–2494, 10.1001/jama.2010.840 (2010). [DOI] [PubMed] [Google Scholar]
- Hankey G. J. et al. Treatment with B vitamins and incidence of cancer in patients with previous stroke or transient ischemic attack: results of a randomized placebo-controlled trial. Stroke; a journal of cerebral circulation 43, 1572–1577, 10.1161/STROKEAHA.111.641613 (2012). [DOI] [PubMed] [Google Scholar]
- Gao Q. Y. et al. Folic acid prevents the initial occurrence of sporadic colorectal adenoma in Chinese older than 50 years of age: a randomized clinical trial. Cancer Prev Res (Phila) 6, 744–752, 10.1158/1940-6207.CAPR-13-0013 (2013). [DOI] [PubMed] [Google Scholar]
- Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. MRC Vitamin Study Research Group. Lancet 338, 131-137 (1991). [PubMed]
- Czeizel A. E. & Dudas I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. The New England journal of medicine 327, 1832–1835, 10.1056/NEJM199212243272602 (1992). [DOI] [PubMed] [Google Scholar]
- Kim Y. I. Folate and carcinogenesis: evidence, mechanisms, and implications. The Journal of nutritional biochemistry 10, 66–88 (1999). [DOI] [PubMed] [Google Scholar]
- Lashner B. A., Heidenreich P. A., Su G. L., Kane S. V. & Hanauer S. B. Effect of folate supplementation on the incidence of dysplasia and cancer in chronic ulcerative colitis. A case-control study. Gastroenterology 97, 255–259 (1989). [DOI] [PubMed] [Google Scholar]
- Sanjoaquin M. A., Allen N., Couto E., Roddam A. W. & Key T. J. Folate intake and colorectal cancer risk: a meta-analytical approach. International journal of cancer. Journal international du cancer 113, 825–828, 10.1002/ijc.20648 (2005). [DOI] [PubMed] [Google Scholar]
- Kennedy D. A. et al. Folate intake and the risk of colorectal cancer: a systematic review and meta-analysis. Cancer epidemiology 35, 2–10, 10.1016/j.canep.2010.11.004 (2011). [DOI] [PubMed] [Google Scholar]
- Sauer J., Mason J. B. & Choi S. W. Too much folate: a risk factor for cancer and cardiovascular disease? Current opinion in clinical nutrition and metabolic care 12, 30–36, 10.1097/MCO.0b013e32831cec62 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. I. Folate and DNA methylation: a mechanistic link between folate deficiency and colorectal cancer? Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 13, 511–519 (2004). [PubMed] [Google Scholar]
- Manzano A. & Perez-Segura P. Colorectal cancer chemoprevention: is this the future of colorectal cancer prevention? TheScientificWorldJournal 2012, 327341, 10.1100/2012/327341 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso J., Boer J., Morreau H. & Fodde R. Expression and genomic profiling of colorectal cancer. Biochimica et biophysica acta 1775, 103–137, 10.1016/j.bbcan.2006.08.004 (2007). [DOI] [PubMed] [Google Scholar]
- Kim Y. I. Role of folate in colon cancer development and progression. The Journal of nutrition 133, 3731S–3739S (2003). [DOI] [PubMed] [Google Scholar]
- Ulrich C. M. & Potter J. D. Folate and cancer—timing is everything. JAMA : the journal of the American Medical Association 297, 2408–2409, 10.1001/jama.297.21.2408 (2007). [DOI] [PubMed] [Google Scholar]
- Takata Y. et al. Plasma folate concentrations and colorectal cancer risk: a case-control study nested within the Shanghai Men’s Health Study. International journal of cancer. Journal international du cancer 135, 2191–2198, 10.1002/ijc.28871 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D. et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 352, 609–613, 10.1016/S0140-6736(98)01085-X (1998). [DOI] [PubMed] [Google Scholar]
- Egger M., Davey Smith G., Schneider M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.