Abstract
In mice, inhibition of both the fibroblast growth factor (FGF) mitogen-activated protein kinase kinase/extracellular-signal regulated kinase (MEK/Erk) and the Wnt signaling inhibitor glycogen synthase-3β (GSK3β) enables the derivation of mouse embryonic stem cells (mESCs) from nonpermissive strains in the presence of leukemia inhibitory factor (LIF). Whereas mESCs are in an uncommitted naïve state, human embryonic stem cells (hESCs) represent a more advanced state, denoted as primed pluripotency. This burdens hESCs with a series of characteristics, which, in contrast to naïve ESCs, makes them not ideal for key applications such as cell-based clinical therapies and human disease modeling. In this study, different small molecule combinations were applied during human ESC derivation. Hereby, we aimed to sustain the naïve pluripotent state, by interfering with various key signaling pathways. First, we tested several combinations on existing, 2i (PD0325901 and CHIR99021)-derived mESCs. All combinations were shown to be equally adequate to sustain the expression of naïve pluripotency markers. Second, these conditions were tested during hESC derivation. Overall, the best results were observed in the presence of medium supplemented with 2i, LIF, and the noncanonical Wnt signaling agonist Wnt5A, alone and combined with epinephrine. In these conditions, outgrowths repeatedly showed an ESC progenitor-like morphology, starting from day 3. Culturing these “progenitor cells” did not result in stable, naïve hESC lines in the current conditions. Although Wnt5A could not promote naïve hESC derivation, we found that it was sustaining the conversion of established hESCs toward a more naïve state. Future work should aim to distinct the effects of the various culture formulations, including our Wnt5A-supplemented medium, reported to promote stable naïve pluripotency in hESCs.
Introduction
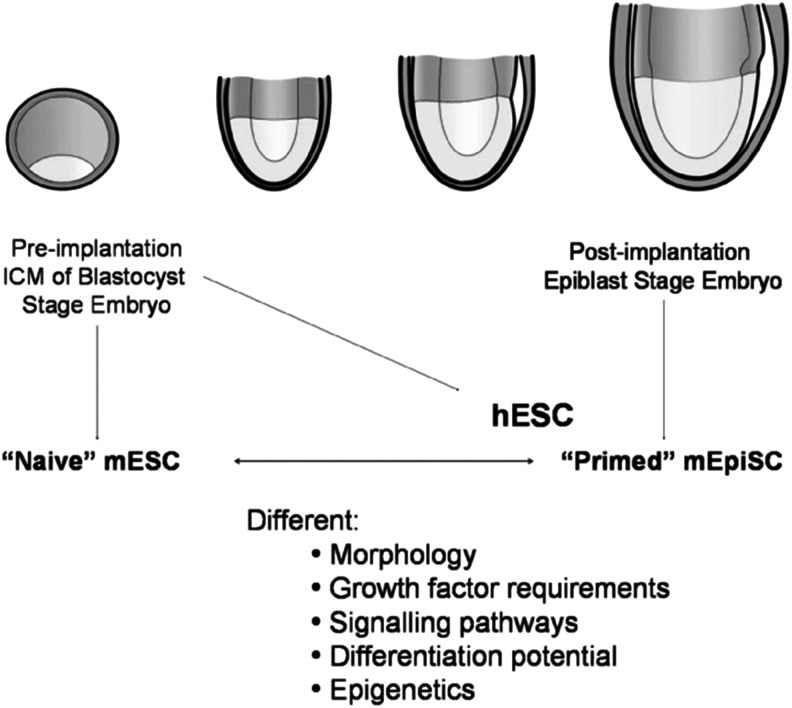
It has been shown that pluripotency is not confined to a fixed state, but exists at least in two distinct forms (Nichols and Smith, 2009; Hanna et al., 2010). The first embryonic stem cells (ESCs) were derived in 1981 from the inner cell mass (ICM) of the pre-implantation embryo in mouse strain 129 (Evans and Kaufman, 1981; Martin, 1981). The derivation of naïve murine ESCs (mESCs) from mice strains other than strain 129 was only successful by simultaneous inhibition of the glycogen synthase kinase-3β (GSK3β) and the mitogen-activated protein kinase (Erk1/2) pathway by CHIR99021 and PD0325901, respectively (the “2 inhibitor” or “2i” condition) (Silva and Smith, 2008; Ying et al., 2008; Nichols et al., 2009; Nichols and Smith, 2011). In 2007, two different groups succeeded in the derivation of primed mouse epiblast stem cells (mEpiSCs) from the post-implantation blastocyst (Brons et al., 2007; Tesar et al., 2007). Although human ESCs (hESCs) are derived from the pre-implantation embryo, similarly to naïve mESCs (Thomson et al., 1998), they more closely resemble the post-implantation epiblast-derived mEpiSCs (Fig. 1). This could be the result of developmental progression of the human ICM in vitro, during ESC derivation. In support of this, it was demonstrated that hESC outgrowth formation is preceded by a post-ICM intermediate (PICMI), showing expression of both early and late epiblast markers (O'Leary et al., 2012, 2013).
FIG. 1.
Origin of mESCs and mEpiSCs, in relation to hESCs.
The so-called naïve ESCs are considered to be more pluripotent compared to primed ESCs on the basis of their unique capacity to generate chimeras efficiently upon reintroduction into the pre-implantation blastocyst (Bradley et al. 1984). Female naïve stem cells maintain both X chromosomes active (XaXa) and exhibit a global reduction in DNA methylation (Bao et al. 2009, Han et al. 2010). Naïve ESCs are also more resistant toward primordial germ cell (PGC) differentiation in vitro (Rossant, 2008; Hayashi and Surani, 2009; Nichols and Smith, 2011). In contrast, primed mEpiSCs are very inefficient in the contribution to chimeras, female EpiSCs have already undergone X chromosome inactivation (XiXa) and they show an increase in DNA methylation. Primed EpiSCs are also poised for differentiation into PGC precursors in vitro (Rossant, 2008; Bao et al., 2009; Hayashi and Surani, 2009; Han et al., 2010; Nichols and Smith, 2011).
Naïve ESCs can be cloned from single cells with high efficiency, grow as domed colonies, and are stabilized by leukemia inhibitory factor (LIF)/Stat3 signaling (Smith, 2001), whereas fibroblast growth factor (FGF) and transforming growth factor-β (TGFβ)/activin signaling provoke lineage specification (Burdon et al., 1999; Greber et al., 2010; Kunath et al., 2007). Primed ESCs, on the other hand, are intolerant to single-cell passaging, which prevents bulk production, show a flattened morphology, and are dependent on FGF and TGFβ/activin signaling, whereas LIF/STAT3 signaling is dispensable for maintenance of hESC pluripotency and self-renewal (Daheron et al., 2004; Hanna et al., 2010; Humphrey et al., 2004; Nichols and Smith, 2011; Rossant, 2008).
Until recently, efforts to derive naïve hESCs directly from the human blastocyst did not meet with success (De Los Angeles et al., 2012; Lengner et al., 2010). Lengner et al. showed that derivation in hypoxic conditions results in hESCs with two active X chromosomes (Lengner et al., 2010), one of the hallmarks of the naïve state. Still, it was demonstrated that female hESC lines derived under hypoxic conditions do not inherit two active X chromosomes from the ICM or the PICMI, but rather stochastically reactivate an already inactivated X chromosome after passaging in vitro (O'Leary et al., 2012). The exact timing of X chromosome inactivation/reactivation during hESC generation is still under debate, and it has to be stressed that an active X chromosomal state is only one of the many parameters to define the naïve state of pluripotency.
Other efforts resulted in the successful conversion of primed hESCs toward a more mESC-like naïve hESCs (Gu et al., 2012; Hanna et al., 2010). Whereas Hanna et al. employed the ectopic induction of Oct4, Klf4, and Klf2 in the presence of 2i and LIF (Hanna et al., 2010), Gu et al. eliminated the need for this transgenic induction by combining 2i, ascorbic acid, and SB431542 (SB) (Gu et al., 2012). SB is an inhibitor of the type I receptors ALK4/5/7 of the TGFβ/activin pathway, shown to stimulate the derivation of mESCs (Hassani et al., 2012) and the formation of induced pluripotent stem cells (iPSCs) (Maherali and Hochedlinger, 2009). The converted cells were denoted as mESC-like hESCs (mhESCs) and displayed several characteristics similar to those of naïve mESCs. Yet they could only be maintained for a limited number of passages. This indicates that a mESC-like state is possible in human cells, but the appropriate conditions to stabilize naïve pluripotency were not yet achieved. A breakthrough came with the publication of Gafni et al., who succeeded in finding the conditions that allow hESCs to display the most important properties of the naïve ground state (Gafni et al., 2013).
The aim of the current study was to stimulate naïve hESC derivation by deploying several small molecule combinations, capable of engaging with specific signaling pathways. First, we tested the combination of PD0325901 and CHIR99021 (2i), known to stimulate naïve mESC derivation in nonpermissive mice strains, as well as SB, a TGFβ-signaling inhibitor known to have a positive effect on self-renewal of naïve mESCs. These small molecules were chosen for their positive effect on epiblast proliferation in human embryos (Van der Jeught et al., 2013, 2014). Second, the combination of SB and PD0325907 (PD) was tested, which had recently shown to support the ground state of pluripotency and to result in a more stable genomic integrity after long-term culture than the 2i condition (Hassani et al., 2014).
Next, as the early-to-late epiblast transition might already take place at the pre-implantation stage in the Rhesus model (Tachibana et al., 2012), blocking developmental progression from early to late epiblast represents a possible strategy for the derivation of early epiblast-like, naïve hESCs. Manipulation of the Wnt and Hippo signaling pathways, both active in the early epiblast (Nishioka et al., 2009; ten Berge et al., 2011), could promote self-renewal and developmental progression (Merrill, 2012; Sokol, 2011). Unlike in mESCs, active Wnt signaling in hESCs counteracts self-renewal and stimulates differentiation (Davidson et al., 2012; ten Berge et al., 2011). Habib et al. recently showed that a localized signal of the Wnt signaling agonist Wnt3A in mESCs results in asymmetric inheritance of pluripotency following cell division (Habib et al., 2013). However, global stimulation of noncanonical Wnt signaling by Wnt5A rescued the asymmetric effect of the local Wnt3A signal (Berndt and Moon, 2013; Habib et al., 2013). Therefore, we speculated that the application of Wnt5A could have a positive effect on hESC pluripotency and self-renewal. New evidence hints that self-renewal by Wnt stimulation may be favored when Hippo signaling is active, whereas the absence of Hippo signaling may lead to developmental progression by Wnt stimulation (Azzolin et al., 2012; Rosenbluh et al., 2012; Tsai et al., 2012). Therefore, we also tested the effect of the small molecule epinephrine, known for its potential capacity to sustain Hippo signaling (Codelia and Irvine, 2012; Yu et al., 2012).
Materials and Methods
Ethical aspects
Institutional Review Board approval was obtained from the Ethical Committee, Ghent University Hospital (2009/281) and the Belgian Federal Ethical Committee on Embryo Research (Adv-030). For patients to donate embryos for this study, they signed informed consents before they started their in vitro fertilization/ intracytoplasmic sperm injection (IVF/ICSI) cycle.
Embryo source
Fresh spare embryos were used that did not meet the cryopreservation criteria of the IVF laboratory following embryo transfer due to high fragmentation (≥35%) on day 2 or 3 of development (day of oocyte retrieval being day 0), multinucleated blastomeres on day 2, delayed development (<5 blastomeres on day 3 or a ≤1 blastomere increase from day 2 to 3), and/or abnormal fertilization (0, 1, or ≥3 pronuclei) on day 1. Only embryos that contained at least four blastomeres on day 3 and showed less than 50% fragmentation were included into the study. Although these embryos are of poor quality, we have previously shown that they are still useful for research purposes (O'Leary et al., 2011).
Thawing of 1,2-propanediol–frozen embryos
In addition to these fresh embryos, some 1,2-propanediol (PrOH)-frozen embryos were used as well. The thawing of these embryos was performed by exposure to sequential thawing solutions: Solution 1 contained 1 M PrOH, 0.2 M sucrose in Dulbecco's Phosphate-Buffered Saline (D-PBS) supplemented with 0.5% human serum albumin (HSA); solution 2 contained 0.5 M PrOH and 0.2 M sucrose in D-PBS/HSA; solution 3 contained 0.2 M sucrose in D-PBS/HSA; and solution 4 contained DPBS/HSA. Straws were removed from liquid nitrogen and kept at room temperature for 40 sec, followed by 30 sec on a 30°C warm plate. The embryos were expelled from the straw and quickly moved to solution 1 for 5 min at room temperature followed by solutions 2, 3, and 4 each for 5 min at room temperature. Finally, the embryos were placed in a fresh drop of solution 4 at 37°C for 5 min before they were cultured at 6% CO2 and 5% O2.
Embryo scoring and culture
The fresh spare embryos were cultured from the zygote stage in Cook Cleavage Medium (Cook Ireland LTD, Limerick, Ireland, www.cookmedical.com) at 37°C, 5% O2 and 6% CO2, and at day 3, they were transferred to Cook Blastocyst Medium. Depending on the derivation method, blastocysts were scored at day 5 or 6 at a fixed time interval using the grading system taken from Stephenson et al. (2006). ICM grades A and B are given to large and distinct ICMs, with grade A being more compact and B having larger, less compact cells. Grade C is used for small ICMs, grade D for degenerating ICMs, and grade E when no apparent ICM is visible. For the scoring of the trophectoderm, grades A, B, or C correspond to good, moderate, and poor quality, respectively. Blastocyst expansion was scored from 1 (no expansion of overall size) to 6 (fully hatched from zona pellucida).
hESC derivation
In summary, day 5 or day 6 blastocysts with both good- and poor-quality ICMs were exposed to prewarmed Acidic Tyrode's (cat. no. T1788, Sigma, Bornem, Belgium) or Pronase (cat. no. P6911, Sigma) to remove their zona pellucidae. After washing, full blastocysts were plated in individual culture dishes with a nearly confluent feeder layer of mitomycin C– (cat. no. M4287, Sigma) treated CD1 mouse embryonic fibroblasts (MEFs). Where mentioned, the ICM was isolated from the blastocyst before plating using laser-assisted microdissection. The culture environment from blastocyst stage to hESC level consisted of 37°C, 6% CO2, and 5% O2. The standard hESC culture medium contained the following ingredients (all purchased from Invitrogen, Merelbeke, Belgium; unless stated otherwise): Knockout Dulbecco's Modified Eagle Medium (KO-DMEM; cat. no. 10829-018), 20% Knockout Serum Replacement (KO-SR; cat. no. 10828-028), 1% nonessential amino acids (NEAA; cat. no. 11140-035), 0.1 mM l-glutamine (cat. no. 25030-024), 1% penicillin/streptomycin (cat. no. 15140-122), 0.1 mM β-mercaptoethanol (cat. no. M3148, Sigma), and 4 ng/mL basic fibroblast growth factor (bFGF or FGF2; cat no. 13256-029). N2B27 medium contained 48% DMEM/F12 (cat. no. 31331-028), 48% Neurobasal Medium (cat. no. 21103-049), 1% N2 Supplement (cat. no. 17502-048), 2% B27 Supplement (17504-044), 0.1 mM l-glutamine, 1% penicillin/streptomycin, 1% NEAA, 0.1 mM β-mercaptoethanol, and 0.25 gram bovine serum albumin (BSA).
The base medium was either supplemented with human LIF (cat. no. L5283, Sigma) and/or any of the following small molecules: MEK inhibitor PD0325901 (Cayman, 1 μM) and GSK3β inhibitor CHIR99021 (Axon Medchem, 3 βM), together called 2i, the TGFβ inhibitor SB431542 (SB, Tocris Bioscience, 10 μM), the potential Hippo activator epinephrine bitartrate (Epi; University Hospital Ghent, Pharmacy, 1 μM) or the noncanonical Wnt activator Wnt5A (R&D Systems, 200 ng/mL). In parallel, control hESCs derivation experiments were performed, plating day 6 blastocysts in standard hESC culture medium (UGent protocol; Van der Jeught et al., 2013).
Blastocyst outgrowths were observed and pictures were taken using an inverted microscope (Olympus 1X71-S8F-3) with a heated surface. The outgrowths were dissociated on day 3 or day 5 using 0.25% trypsin/EDTA (cat. no. 25200-056, Invitrogen) or Accutase (cat. no. A1110501, Invitrogen) to create a mixture of small cell aggregates (not fully single cell), and these were plated onto fresh MEF feeders. Where mentioned, the Rho kinase inhibitor (ROCK inhibitor) Y-27632 was added after dissociation. Following this, the cells were observed for another week, and medium was refreshed after 3 days. When possible, the observed colonies/cell aggregates were passaged further.
mESC culture in different conditions
To test the capacity of the different media combinations to sustain naïve ESC self-renewal, media were first applied at the mESC level. For this, mESCs were derived from B6/D2 mice in the presence of N2B27, 2i, and mouse LIF (cat no. L5158, Sigma) on gelatin-coated dishes. After five passages, the standard N2B27 medium was replaced by one of the derivation media, and mESC cultures were maintained for four more passages before the cells were collected for real-time quantitative reverse transcriptase PCR (RT-qPCR) analysis and immunocytochemistry.
hESC culture in presence of Wnt5A-containing conversion medium
Following routine passaging, cell suspensions of UG11 (XY) were plated on inactivated MEFs in naïve conversion medium composed of hESC medium supplemented with bFGF (Peprotech, 8 ng/mL), recombinant human LIF (Sigma, 1000U), and the small molecules PD0325901 (MEKi, 1 μM, Cayman), CHIR99021 (GSK3βi, 3 μM, Axon Medchem), and Wnt5A (200 ng/mL, R1D Systems). The resulting dome-shaped naïve colonies (within 4–6 days of plating) were passaged further as single cells using 0.05% trypsin/EDTA (Invitrogen) every 2–3 days and replated on fresh inactivated MEFs. The naïve hESCs were maintained in low O2 conditions, similar to primed hESC lines.
RNA isolation and RT-qPCR
Collected mESCs and hESCs were lysed in TRIzol (cat. no. 15596-026, Invitrogen,. Total RNA was purified from the inorganic phase using the RNeasy Mini Kit (cat. no. 74104. Qiagen, Venlo, The Netherlands). cDNA was synthesized using the iScript Advanced cDNA Synthesis Kit for RT-qPCR (cat. no. 170-8842, BioRad, Nazareth, Belgium,). cDNA concentration was determined with the Quant-iT Oligreen ssDNA Assay Kit (cat. no. O11492, BioRad). A total of 10 ng cDNA from each sample was used for RT-qPCR. Each reaction consisted of 20 μL of master mix and 5 μL of cDNA. The gene assay master mix comprised iTAQ Supermix with ROX (cat. no. 172-5855, BioRad) or iTAQ SYBR Green Supermix with ROX (cat. no. 172-5850, BioRad), depending on the gene assay.
For mESCs, gene expression analysis was performed for Sox2, Nanog, Oct4, Rex1, Pecam1, Stella (pluripotency markers); Nodal, Sox7, Sox17, Gata4, Gata6 (endoderm markers); Fgf5, Brachyury (T), Tbx6 (mesoderm markers); and Krt18 (ectoderm marker) (Life Technologies, Merelbeke, Belgium). Normalization was done using the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH). All primers were designed and validated in house. For analysis of hESCs, gene expression analysis was performed for SOX2, NANOG, REX1, KLF2, KLF4, and ESRRB. B2M, RLP13A, ACTB, and ALUSEQ were used as housekeeping genes. The PCR reaction was performed on the ABI Prism 7000 Sequence Detection System (Applied Biosystems). The following thermocycling conditions were applied: 2 min at 95°C, followed by 45 cycles of 15 sec at 95°C, and 1 min at 60°C. No cDNA samples or no RT samples were used as negative controls. All cycle threshold (Ct) values were normalized against the housekeeping gene, and the final values were described as fold change expression values compared to the control.
Results
mESC culture in the presence of different media
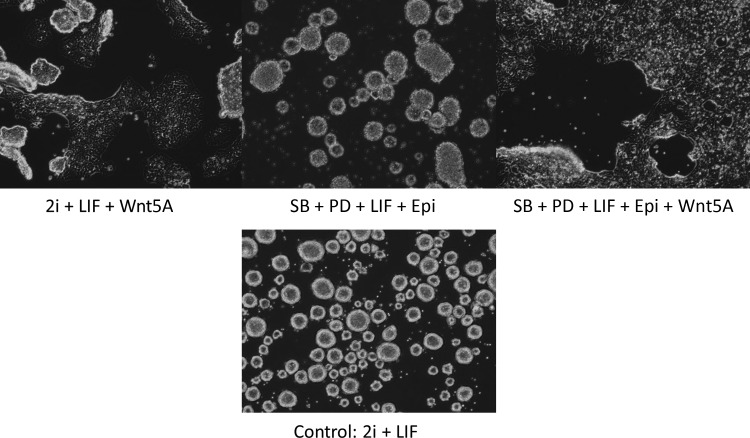
In a first experiment, we tested the effect of different culture combinations on established, 2i-derived mESC cultures. Therefore, mESCs were cultured in standard N2B27 medium, supplemented with 2i and LIF (control) or in one of the following conditions: (1) N2B27+2i+LIF+Wnt5A; (2) N2B27+SB+PD+LIF+Epi; and (3) N2B27+SB+PD+LIF+Epi+Wnt5A. Overall, we observed that condition (2) could sustain mESC self-renewal and proliferation with little signs of differentiation. In the presence of the noncanonical Wnt activator Wnt5A, mESCs showed some morphological signs of differentiation at the margins (Fig. 2). After four passages, cells were collected for RT-qPCR. We measured the expression of the pluripotent markers Sox2, Nanog, Oct4, Pecam1, Stella, and Rex1, as well as Nodal, Sox7, Sox17, Gata4, Gata6 (endoderm), Fgf5, Brachyury (T), Tbx6 (mesoderm), and Krt18 (ectoderm). RT-qPCR did not reveal any significant differences between the various culture methods (Fig. S1) (Supplementary Data are available at www.liebertpub.com/cell/). This implies that the media combinations are equally adequate in sustaining naïve mESC pluripotency.
FIG. 2.
Morphology of mESCs in presence of the different media, at day 4 after plating.
Human blastocyst plating in different conditions
After confirming the effect of different media on pluripotency of the established mESCs, these (and other) media were applied for hESC derivation. Because it is believed that the culture condition is not the only factor determining the success of naïve stem cell derivation, the day of blastocyst plating (day 5 versus day 6), the day of blastocyst outgrowth dissociation (day 3 or day 5 after plating), and the enzyme used for outgrowth dissociation (0.25% trypsin/EDTA or Accutase) were varied as well (Table 1). The anti-apoptotic Rho-associated kinase inhibitor Y27632 was applied to avoid cell death after outgrowth dissociation in some conditions (Table 1). In total, eight methods were tested for supporting the naïve state of pluripotency in human cells. Synchronous, standard hESC derivation according to established protocols (O'Leary et al., 2012: Van der Jeught et al., 2013) was performed as a control (not shown in Table 1).
Table 1.
Different Methods Tested for Their Capacity to Sustain Naïve hESCs Derivation
| Method | Blastocyst stage | Number | Medium 1 | PICMI? | Day of outgrowth fragmentation | ROCKi? | Enzyme | Medium 2 |
|---|---|---|---|---|---|---|---|---|
| 1 | Day 6 | 21 | hESC+bFGF | Yes (4/21) | Day 5 | Yes | Trypsin | N2B27+2i+LIF |
| 2 | Day 6 | 15 | 50% N2B27+2i+ LIF+50% hESC+ bFGF |
No | Day 5 | Yes | Trypsin | N2B27+2i+LIF |
| 3 | Day 6 | 13 | N2B27+2i+LIF | No | Day 5 | Yes | Trypsin | Same as medium 1 |
| 4 | Day 5 | 19 | N2B27+2i+LIF | No | Day 5 | No | Trypsin | Same as medium 1 |
| 5 | Day 6 | 14 | N2B27+2i+SB+ LIF |
No | Day 5 | No | Trypsin | Same as medium 1 |
| 6 | Day 6 | 13 | N2B27+2i+Wnt5a+LIF | No | Day 5 | No | Trypsin | Same as medium 1 |
| 7 | Day 5 | 9 | N2B27+SB+PD+ LIF+Epia |
No | Day 3 | No | Accutase | Same as medium 1 |
| 8 | Day 5 | 10 | N2B27+SB+PD+ LIF+Epia+Wnt5a |
No | Day 3 | No | Accutase | Same as medium 1 |
Epinephrine was also added during embryo culture, from day 4 to day 5.
hESC, human embryonic stem cells; bFGF, basic fibroblast growth factor; 2i, PD0325901 and CHIR99021; LIF, leukemia inhibitory factor; Epi, epinephrine.
In the first method, primed pathways, like the FGF/Erk pathway, were activated from the blastocyst until the outgrowth stage, whereas a combination of pathways supporting the naïve and primed state medium was applied in the second method. Because these conditions (partly) stimulate primed pluripotency, they were not tested on established mESC cultures in the first experiment. In methods three and four, blastocysts of different developmental stages (early day 5 versus late day 6) were plated in standard 2i-supplemented N2B27 medium, known to sustain derivation of mESCs. In method five, SB and 2i were added to the N2B27 medium, in combination with LIF. Methods six, seven, and eight are the conditions that were already tested on established mESC cultures in the first experiment (Table 1).
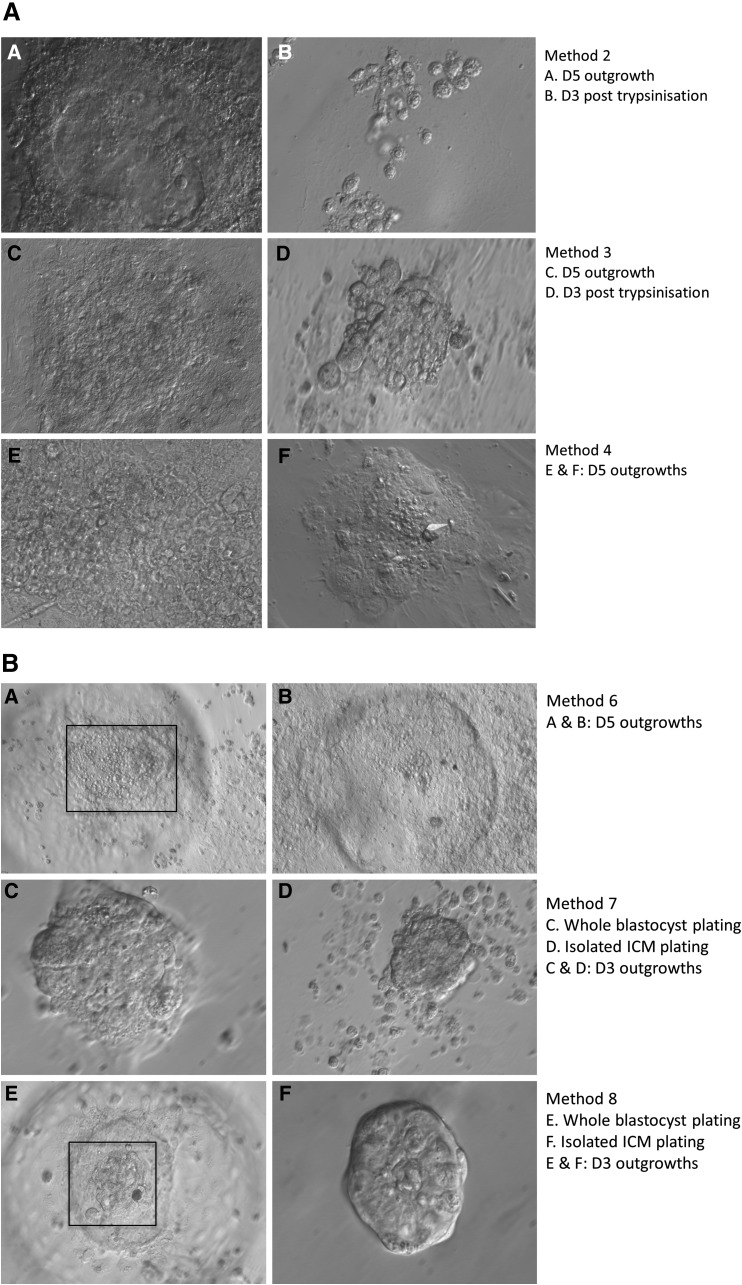
Figure 3, A and B, shows the morphology of the outgrowths observed in different culture conditions. Interestingly, in conditions six and eight, some ICM outgrowths displayed a ball-shaped morphology, with ESC progenitor-like cells (cobblestone-like morphology) present at day 3 of development. This might result from the shared presence of Wnt5A in these cultures. However, after single-cell dissociation, these potential ESC progenitors failed to survive and to proliferate further. Methods one and five did not result in potential blastocyst outgrowths, and are therefore not presented in Figure 3.
FIG. 3.
(A) Outgrowth morphology observed after applying methods 2, 3, and 4. (A and B) Blastocyst outgrowth at day 5 (A), and day 3 post trypsinization of the outgrowth (B) in a mixture of primed hESCs and naïve N2B27 medium. (C and D) Day 5 after plating a day 6 blastocyst in N2B27+2i+LIF (C), and day 3 after trypsinization of the outgrowth (D). (E and F) Day 5 after plating two day 5 blastocysts in N2B27+2i+LIF. D5, day 5; D3, day 3. (B) Outgrowth morphology observed after applying methods 6, 7, and 8. Notice the progenitor-like cells in the squares. (A and B) Day 5 after plating two day 6 blastocysts in presence of Wnt5A. (C and D) Day 3 outgrowth in presence of epinephrine after plating a whole day 5 blastocyst (C) or an isolated ICM (D). (E and F) Day 3 outgrowth in presence of Wnt5A and epinephrine after plating a whole day 5 blastocyst (E) or an isolated ICM (F). D5, day 5; D3, day 3.
Induction of naïve-like pluripotency in established hESC culture using Wnt5A-containing medium
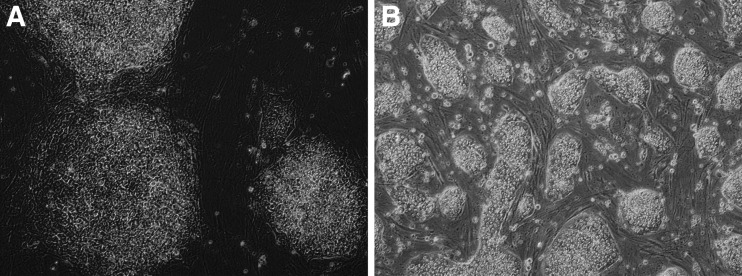
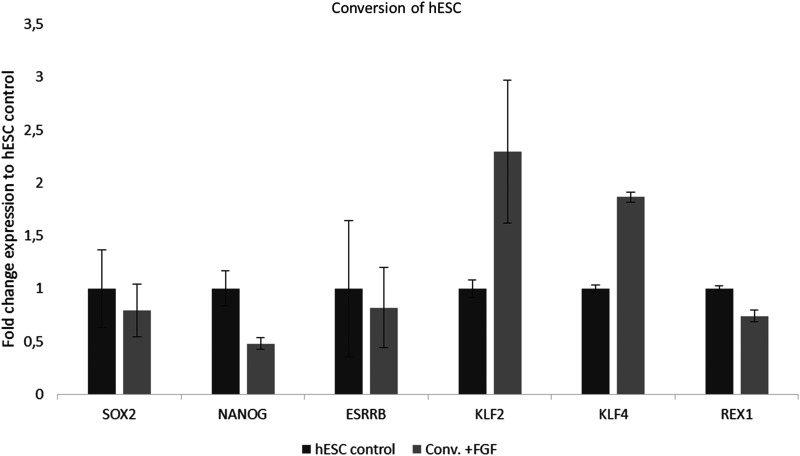
On the basis of the initial survival of the blastocyst outgrowths in the presence of Wnt5A, we wanted to investigate if Wnt5A-supplemented medium could convert established primed hESCs (UG11; XY) toward a more naïve state of pluripotency. Therefore, hESC medium containing 8 ng/mL bFGF was supplemented with 2i, hLIF, and the noncanonical Wnt activator Wnt5A. The resulting cells could be passaged as single cells starting from the first passage number. Moreover, they performed a naïve-like morphology, represented by small, dome-shaped colonies (Fig. 4). After four passages, cells were collected for RT-qPCR. Gene expression analysis revealed an increased expression of KLF4 (p=0.0006) and KLF2 (p=0.054) and a similar expression of ESRRB, SOX2, and NANOG, compared to their primed counterparts. However, REX1 was expressed significantly lower in the Wnt5a condition compared to the primed hESCs (Fig. 5).
FIG. 4.
(A) Morphology of hESCs cultured in control hESC medium. (B) Wnt5A supplemented conversion medium, after four passages.
FIG. 5.
Fold change expression (±SD) of SOX2, NANOG, ESRRB, KLF2, KLF4, and REX1 in hESCs cultured for four passages in Wnt5A supplemented medium (conv.+FaF) compared to control hESCs.
Discussion
As future reliable hESC-based clinical applications are compromised by the current inherent unfavorable properties of primed hESCs, naïve hESC derivation is of great interest. Only recently, Gafni et al. succeeded in the direct derivation of this type of mESC-like hESCs from the blastocyst stage onward using chemically defined conditions (Gafni et al., 2013). This major breakthrough was achieved by supplementing the culture media with 2i/LIF, the p38i MAPK inhibitor SB203580, the noncanonical Wnt/c-Jun-N-terminal kinase inhibitor (JNKi) SP600125, and by FGF2 and TGFβ1 cytokine supplementation. Moreover, Rho-associated coiled-coil kinases (ROCK) and protein kinase C (PKC) inhibitors were identified as optional boosters of naïve cell viability and growth, resulting in the so-called naïve human stem cell medium (NHSM; Gafni et al., 2013) condition. The successful creation of four newly derived naïve hESC lines was reported, as well as the conversion of several already established primed hESC and iPSC lines in NHSM medium. The human naïve pluripotent lines exhibited a typical dome-shaped morphology, displayed gene expression patterns typically seen in ground state naïve mESCs, and maintained a normal karyotype after extended passaging. Importantly, cross-species chimeric mouse embryos could be generated following microinjection of human naïve iPSCs into mouse morulae.
Ware et al. also succeeded in the establishment of hESCs with naïve pluripotency traits (Ware et al., 2014). The conversion of existing hESC lines was achieved by applying deacetylase inhibitors in combination with 2i, LIF, and FGF2, whereas direct derivation from frozen human blastocysts needed the application of 2i and FGF2 only. The persisting requirement for FGF2 in both publications for optimally attaining the naïve state for human cell populations does not comply with the requirements in mouse. Therefore, it is likely that FGF signaling mechanisms differ between the two species, and FGF might activate additional, pluripotency-promoting pathways next to the differentiation-imposing MEK-ERK branch. Importantly, it should be noted that the naïve derivation efficiency reported by Ware et al. was very low (0.78%; 1/128 donated embryos), which questions the robustness and reproducibility of this attempt. In the study of Gafni et al., details concerning naïve derivation efficiency were not reported. The fact that different culture cocktails can give rise to the naïve state of human pluripotency, as well as the current low efficiency, demands further investigation regarding which culture niche should be considered as the gold standard for attaining naïve hESCs at a high efficiency rate, similar to naïve mESCs (Ware et al., 2014). While this manuscript was under revision, two more groups succeeded in the conversion of primed hESCs (Takashima et al., 2014; Valamehr et al., 2014), whereas Theunissen et al. reported the successful derivation of naïve hESCs in 5i/L/FA medium, but mostly with abnormal karyotypes (Theunissen et al., 2014).
In this study, we aimed to stimulate naïve hESC derivation by deploying several small molecule combinations capable of engaging with specific signaling pathways. In a first experiment, we tested selected small molecule combinations on the maintenance of the naïve pluripotent state of established mESCs. In all conditions, a sustained level of naïve pluripotency markers was revealed by gene expression analysis. However, this was inconsistent with the morphological observation of differentiated cells in the presence of Wnt5A in mESCs. We observed that the 2i condition, known to stimulate naïve mESCs derivation in nonpermissive mice strains (Silva and Smith, 2008; Nichols et al., 2009, 2011; Ying et al., 2008), does not support the derivation of hESCs from day 5 nor day 6 blastocysts (methods 3 and 4).
Moreover, in method 1, this 2i medium was applied after outgrowth dissociation only, whereas a mixture of primed and naïve medium was applied from the blastocyst stage on in method 2. These methods represent a rather gentle approach, supporting the concept that human blastocysts should be exposed gradually to the naïve environment. However, they did not result in stable naïve hESC derivation. Recently, Hassani et al. showed that simultaneous suppression of TGFβ and FGF signaling pathways sustains the efficient derivation of mESCs from refractory and nonpermissive strains (Hassani et al., 2014). Moreover, they showed that their employed combination of small molecules (SB and PD) allows the establishment of ground state pluripotency, while maintaining higher genome stability after long-term culture compared to the 2i condition (Hassani et al., 2014; Nichols et al., 2009). This novel approach to establish ground state pluripotency shields the cells from differentiation by activating BMP4 signaling, whereas the 2i condition activates pluripotency genes and the Wnt/β-catenin pathway.
CHIR99021, one of the key components of 2i, is known to activate Wnt signaling through the inhibition of GSK3β. However, the complete mechanism of action of 2i is still under debate, mainly due to the complexity of GSK3β signaling. Although activation of EsRRβ is the main outcome of CHIR99021, GSK3β affects also many other intracellular signaling pathways, targeting over 40 substrates (Valvezan and Klein, 2012). These compounds are not exclusively active in sustaining self-renewal, because some are also involved in differentiation. Additionally, it has been shown that the genomic integrity of stem cells is compromised by suppression of GSK3β (Acevedo et al., 2007; Tighe et al., 2007). Therefore, we replaced the standard 2i condition by SB and PD in methods 7 and 8, however without successful acquisition of naïve pluripotency. In method 5, SB was added in combination with 2i. Although both small molecules are known to sustain naïve pluripotency in mice and to support the proliferation of epiblast progenitor cells in human blastocysts (Van der Jeught et al., 2013, 2014), this condition did not yield good blastocyst outgrowths.
To artificially stabilize a naïve ground state equivalent to an early epiblast, it would be necessary to block developmental progression toward a late epiblast stage. Because both Wnt and Hippo signaling are active during the early epiblast stage (Nishioka et al., 2009; ten Berge et al., 2011), we investigated to what extent manipulation of these pathways could promote self-renewal and naïve pluripotency.
Recent studies on molecular interactions between Wnt and Hippo pathway components hint toward a positive regulatory role for Hippo signaling over Wnt-controlled self-renewal (Azzolin et al., 2012; Rosenbluh et al., 2012; Tsai et al., 2012). To investigate this further, we employed the hormone epinephrine, which potentially promotes Hippo signaling (Codelia and Irvine, 2012; Yu et al., 2012). Epinephrine was added alone (condition 7) or in combination with Wnt5A (condition 8). To increase the effect of this Hippo activator, it was also added during embryo culture, from day 4 to day 5. In conditions 6 and 8, Wnt5A was shown to have a positive influence on the morphology of the outgrowths. In these conditions, regions of cells were observed with a cobblestone-like morphology, suggesting a more naïve ESC-like progenitor nature.
Because epinephrine is a hormone produced in response to stress, it could provide an interesting link between Hippo signaling and stress-induced diapause, which is characterized by delayed implantation of the blastocyst and delayed developmental progression. Intriguingly, diapaused blastocysts improve the derivation success of mESCs (Brook and Gardner, 1997; Nichols and Smith, 2012). Moreover, the common dependence of mESCs and diapaused embryos on the cytokine LIF could mean that the presence of a diapause-like program establishes naïve pluripotency (Welling and Geijsen, 2013; Zhang et al., 2013). Accordingly, artificial induction of diapause in human blastocysts might be used in future attempts to promote naïve hESC derivation, for example, by testing the effect of other stress hormones.
The plating of blastocysts in standard hESC culture medium resulted in control hESC lines with an efficiency of 16% (results not shown); thus, it was shown that the blastocysts themselves are not the main cause of hESC derivation failure in the experimental conditions. Also, it should be noted that the limited availability of human embryos for research limited the scale of these experiments.
On the basis of the positive effect of Wnt5A on the blastocyst outgrowths in conditions 6 and 8, we tested whether Wnt5A-supplemented hESC medium could induce the conversion of hESCs toward a more naïve state of pluripotency. After a few passages, we observed a change in colony morphology and a higher self-renewal (reduced time-to-passage). Moreover, colonies could be passaged repeatedly as single cells. RT-qPCR showed an increased expression of the naïve markers KLF4 (p=0.0006) and KLF2 (p=0.054), and a similar expression level of ESRRB, SOX2, and NANOG in this condition compared to the standard hESCs. However, REX1 expression was significantly lower compared to the primed hESCs. Altogether, these observations suggest that Wnt5A, together with 2i, bFGF, and LIF, promotes induction of a naïve-like pluripotent state in established hESCs through Krüppel-like factors 2 and 4 (KLF2/4).
In this study, different small molecule combinations were tested for their capacity to induce the naïve state of pluripotency in the human. Although these small molecules had been shown to maintain mESC pluripotency by interfering with various key signaling pathways, they could not promote naïve hESC derivation. However, application of the noncanonical Wnt agonist Wnt5A, in combination with 2i, bFGF, and LIF, could support the conversion of primed hESCs toward a more naïve state of pluripotency. This was supported by the higher expression level of KLF2/4, known to support ground state pluripotency (Yeo et al., 2014; Zhang et al., 2010).
In conclusion, we have excluded the effectiveness of several medium formulations for the derivation of naïve hESCs, while we elucidated an important role for the noncanonical Wnt activator Wnt5A in the conversion of primed hESCs toward a more naïve state. This will be investigated further. Because several groups recently succeeded in naïve hESC derivation using different small molecule cocktails, it will be important to compare and comprehend the action of these formulations, as well as the cells they are creating.
Supplementary Material
Acknowledgments
M.V.d.J. is holder of a PhD grant of the Agency for Innovation by Science and Technology (IWT, grant no. SB093128), Belgium. J.T. is holder of a PhD grant of the Agency for Innovation by Science and Technology (IWT, grant no. 131673), Belgium. G.D. and this research are supported by the Flemish Foundation for Scientific Research (FWO-Vlaanderen, grant no. FWO-3G062910) and a Concerted Research Actions funding from BOF (Bijzonder Onderzoeksfonds University Ghent, grant no. BOF GOA 01G01112). S.M.C.d.S.L. is supported by the Netherlands Organization of Scientific Research (NWO) (ASPASIA 015.007.037) and the Interuniversity Attraction Poles (PAI) (no. P7/07). P.D.S. is holder of a fundamental clinical research mandate by the Flemish Foundation of Scientific Research (FWO-Vlaanderen), Belgium.
We would like to thank Ferring Company (Aalst, Belgium) for financial support of this study, and David van Bruggen for creation of Figure 1.
Author Disclosure Statement
The authors declare that there are no conflicts of interest.
References
- Acevedo N., Wang X., Dunn R.L., and Smith G.D. (2007). Glycogen synthase kinase-3 regulation of chromatin segregation and cytokinesis in mouse preimplantation embryos. Mol. Reprod. Dev. 74, 178–188 [DOI] [PubMed] [Google Scholar]
- Azzolin L., Zanconato F., Bresolin S., Forcato M., Basso G., Bicciato S., Cordenonsi M., and Piccolo S. (2012). Role of TAZ as mediator of Wnt signaling. Cell 151, 1443–1456 [DOI] [PubMed] [Google Scholar]
- Bao S., Tang F., Li X., Hayashi K., Gillich A., Lao K., and Surani M.A. (2009). Epigenetic reversion of post-implantation epiblast to pluripotent embryonic stem cells. Nature 461, 1292–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt J.D., and Moon R.T. (2013). Cell biology. Making a point with Wnt signals. Science 339, 1388–1389 [DOI] [PubMed] [Google Scholar]
- Bradley A., Evans M., Kaufman M.H., and Robertson E. (1984). Formation of germ-line chimaeras from embryo-derived teratocarcinoma cell lines. Nature 309, 255–256 [DOI] [PubMed] [Google Scholar]
- Brons I.G., Smithers L.E., Trotter M.W. Rugg-Gunn P., Sun B., Chuva de Sousa Lopes S.M., Howlett S.K., Clarkson A., Ahrlund-Richter L., Pedersen R.A., and Vallier L. (2007). Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature 448, 191–195 [DOI] [PubMed] [Google Scholar]
- Brook F.A., and Gardner R.L. (1997). The origin and efficient derivation of embryonic stem cells in the mouse. Proc. Natl. Acad. Sci. USA 94, 5709–5712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdon T., Chambers I., Stracey C., Niwa H., and Smith A. (1999). Signaling mechanisms regulating self-renewal and differentiation of pluripotent embryonic stem cells. Cells Tissues Organs 165, 131–143 [DOI] [PubMed] [Google Scholar]
- Codelia V.A., and Irvine K.D. (2012). Hippo signaling goes long range. Cell 150, 669–670 [DOI] [PubMed] [Google Scholar]
- Daheron L., Opitz S.L., Zaehres H., Lensch M.W., Andrews P.W., Itskovitz-Eldor J., and Daley G.Q. (2004). LIF/STAT3 signaling fails to maintain self-renewal of human embryonic stem cells. Stem Cells 22, 770–778 [DOI] [PubMed] [Google Scholar]
- Davidson K.C., Adams A.M., Goodson J.M., McDonald C.E., Potter J.C., Berndt J.D., Biechele T.L., Taylor R.J., and Moon R.T. (2012). Wnt/beta-catenin signaling promotes differentiation, not self-renewal, of human embryonic stem cells and is repressed by Oct4. Proc. Natl. Acad. Sci. USA 109, 4485–4490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Los Angeles A., Loh Y.H., Tesar P.J., and Daley G.Q. (2012). Accessing naïve human pluripotency. Curr. Opin. Genet. Dev. 22, 272–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M.J., and Kaufman M.H. (1981). Establishment in culture of pluripotential cells from mouse embryos. Nature 292, 154–156 [DOI] [PubMed] [Google Scholar]
- Gafni O., Weinberger L., Mansour A.A., Manor Y.S., Chomsky E., Ben-Yosef D., Kalma Y., Viukov S., Maza I., Zviran A., Rais Y., Shipony Z., Mukamel Z., Krupalnik V., Zerbib M., Geula S., Caspi I., Schneir D., Shwartz T., Gilad S., Amann-Zalcenstein D., Benjamin S., Amit I., Tanay A., Massarwa R., Novershtern N., and Hanna J.H. (2013). Derivation of novel human ground state naive pluripotent stem cells. Nature 504, 282–286 [DOI] [PubMed] [Google Scholar]
- Greber B., Wu G., Bernemann C., Joo J.Y., Han D.W., Ko K., Tapia N., Sabour D., Sterneckert J., Tesar P., and Scholer H.R. (2010). Conserved and divergent roles of FGF signaling in mouse epiblast stem cells and human embryonic stem cells. Cell Stem Cell 6, 215–226 [DOI] [PubMed] [Google Scholar]
- Gu Q., Hao J., Zhao X. Y., Li W., Liu L., Wang L., Liu Z. H. and Zhou Q. (2012). Rapid conversion of human ESCs into mouse ESC-like pluripotent state by optimizing culture conditions. Protein Cell 3, 71p–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib S.J., Chen B.C., Tsai F.C., Anastassiadis K., Meyer T., Betzig E., and Nusse R. (2013). A localized Wnt signal orients asymmetric stem cell division in vitro. Science 339, 1445–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han D.W., Tapia N., Joo J.Y., Greber B., Arauzo-Bravo M.J., Bernemann C., Ko K., Wu G., Stehling M., Do J.T., and Scholer H.R. (2010). Epiblast stem cell subpopulations represent mouse embryos of distinct pregastrulation stages. Cell 143, 617–627 [DOI] [PubMed] [Google Scholar]
- Hanna J., Cheng A.W., Saha K., Kim J., Lengner C.J., Soldner F., Cassady J.P., Muffat J., Carey B.W., and Jaenisch R. (2010). Human embryonic stem cells with biological and epigenetic characteristics similar to those of mouse ESCs. Proc. Natl. Acad. Sci. USA 107, 9222–9227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassani S.N., Totonchi M., Farrokhi A., Taei A., Larijani M.R., Gourabi H., and Baharvand H. (2012). Simultaneous suppression of TGF-beta and ERK signaling contributes to the highly efficient and reproducible generation of mouse embryonic stem cells from previously considered refractory and non-permissive strains. Stem Cell Rev 8, 472–481 [DOI] [PubMed] [Google Scholar]
- Hassani S.N., Totonchi M., Sharifi-Zarchi A., Mollamohammadi S., Pakzad M., Moradi S., Samadian A., Masoudi N., Mirshahvaladi S., Farrokhi A., Greber B., Arauzo-Bravo M.J., Sabour D., Sadeghi M., Salekdeh G.H., Gourabi H., Scholer H.R., and Baharvand H. (2014). Inhibition of TGFbeta signaling promotes ground state pluripotency. Stem Cell Rev. 10, 16–30 [DOI] [PubMed] [Google Scholar]
- Hayashi K., and Surani M.A. (2009). Self-renewing epiblast stem cells exhibit continual delineation of germ cells with epigenetic reprogramming in vitro. Development 136, 3549–3556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey R.K., Beattie G.M., Lopez A.D., Bucay N., King C.C., Firpo M.T., Rose-John S., and Hayek A. (2004). Maintenance of pluripotency in human embryonic stem cells is STAT3 independent. Stem Cells 22, 522–530 [DOI] [PubMed] [Google Scholar]
- Kunath T., Saba-El-Leil M.K., Almousailleakh M., Wray J., Meloche S., and Smith A. (2007). FGF stimulation of the Erk1/2 signalling cascade triggers transition of pluripotent embryonic stem cells from self-renewal to lineage commitment. Development 134, 2895–2902 [DOI] [PubMed] [Google Scholar]
- Lengner C.J., Gimelbrant A.A., Erwin J.A., Cheng A.W., Guenther M.G., Welstead G.G., Alagappan R., Frampton G.M., Xu P., Muffat J., Santagata S., Powers D., Barrett C.B., Young R.A., Lee J.T., Jaenisch R. and Mitalipova M. (2010). Derivation of pre-X inactivation human embryonic stem cells under physiological oxygen concentrations. Cell 141, 872–883 [DOI] [PubMed] [Google Scholar]
- Maherali N., and Hochedlinger K. (2009). Tgfbeta signal inhibition cooperates in the induction of iPSCs and replaces Sox2 and cMyc. Curr. Biol. 19, 1718–1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G.R. (1981). Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. USA 78, 7634–7638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill B.J. (2012). Wnt pathway regulation of embryonic stem cell self-renewal. Cold Spring Harb. Perspect. Biol. 4, a007971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols J., and Smith A. (2009). Naive and primed pluripotent states. Cell Stem Cell 4, 487–492 [DOI] [PubMed] [Google Scholar]
- Nichols J., and Smith A. (2011). The origin and identity of embryonic stem cells. Development 138, 3–8 [DOI] [PubMed] [Google Scholar]
- Nichols J., and Smith A. (2012). Pluripotency in the embryo and in culture. Cold Spring Harb. Perspect. Biol. 4, a008128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols J., Silva J., Roode M., and Smith A. (2009). Suppression of Erk signalling promotes ground state pluripotency in the mouse embryo. Development 136, 3215–3222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka N., Inoue K., Adachi K., Kiyonari H., Ota M., Ralston A., Yabuta N., Hirahara S., Stephenson R.O., Ogonuki N., Makita R., Kurihara H., Morin-Kensicki E.M., Nojima H., Rossant J., Nakao K., Niwa H., and Sasaki H. (2009). The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev. Cell 16, 398–410 [DOI] [PubMed] [Google Scholar]
- O'Leary T., Heindryckx B., Lierman S., Van der Jeught M., Menten B., Deforce D., Cornelissen R., de Sousa Lopes S.C., and De Sutter P. (2011). The influence of early embryo traits on human embryonic stem cell derivation efficiency. Stem Cells Dev. 20, 785–793 [DOI] [PubMed] [Google Scholar]
- O'Leary T., Heindryckx B., Lierman S., van Bruggen D., Goeman J.J., Vandewoestyne M., Deforce D., de Sousa Lopes S.M., and De Sutter P. (2012). Tracking the progression of the human inner cell mass during embryonic stem cell derivation. Nat. Biotechnol. 30, 278–282 [DOI] [PubMed] [Google Scholar]
- O'Leary T., Heindryckx B., Lierman S., Van der Jeught M., Duggal G., De Sutter P., and Chuva de Sousa Lopes S.M. (2013). Derivation of human embryonic stem cells using a post-inner cell mass intermediate. Nat. Protoc. 8, 254–264 [DOI] [PubMed] [Google Scholar]
- Rosenbluh J., Nijhawan D., Cox A.G., Li X., Neal J.T., Schafer E.J., Zack T.I., Wang X., Tsherniak A., Schinzel A.C., Shao D.D., Schumacher S.E., Weir B.A., Vazquez F., Cowley G.S., Root D.E., Mesirov J.P., Beroukhim R., Kuo C.J., Goessling W., and Hahn W.C. (2012). Beta-catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell 151, 1457–1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossant J. (2008). Stem cells and early lineage development. Cell 132, 527–531 [DOI] [PubMed] [Google Scholar]
- Silva J., and Smith A. (2008). Capturing pluripotency. Cell 132, 532–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. (2001). Embryonic Stem Cells. In Stem Cell Biology. Marshak D.R.G., Gardner R.L., and Gottlieb D., eds. (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY: ) pp. 205–230 [Google Scholar]
- Sokol S.Y. (2011). Maintaining embryonic stem cell pluripotency with Wnt signaling. Development 138, 4341–4350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson E.L., Braude P.R., and Mason C. (2006). Proposal for a universal minimum information convention for the reporting on the derivation of human embryonic stem cell lines. Regen. Med. 1, 739–750 [DOI] [PubMed] [Google Scholar]
- Tachibana M., Sparman M., Ramsey C., Ma H., Lee H.S., Penedo M.C., and Mitalipov S. (2012). Generation of chimeric rhesus monkeys. Cell 148, 285–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima Y., Guo G., Loos R., Nichols J., Ficz G., Oxley D., Santos F., Clarke J., Mansfield W., Reik W., Bertone P., and Smith A. (2014). Resetting transcription factor control circuitry toward ground-state pluripotency in human. Cell 158, 1254–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Berge D., Kurek D., Blauwkamp T., Koole W., Maas A., Eroglu E., Siu R.K., and Nusse R. (2011). Embryonic stem cells require Wnt proteins to prevent differentiation to epiblast stem cells. Nat. Cell Biol. 13, 1070–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesar P.J., Chenoweth J.G., Brook F.A., Davies T.J., Evans E.P., Mack D.L., Gardner R.L., and McKay R.D. (2007). New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature 448, 196–199 [DOI] [PubMed] [Google Scholar]
- Theunissen T.W., Powell B.E., Wang H., Mitalipova M., Faddah D.A., Reddy J., Fan Z.P., Maetzel D., Ganz K., Shi L., Lunglangwa T., Imsoonthomruksa S., Stelzer Y., Rangarajan S., D'Alessio A., Zhang J., Gao Q., Dawlaty M.M., Young R.A., Gray N.S., and Jaenisch R. (2014). Systematic identification of culture conditions for induction and maintenance of naïve human pluripotency. Cell Stem Cell 15, 471–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson J.A., Itskovitz-Eldor J., Shapiro S.S., Waknitz M.A., Swiergiel J.J., Marshall V.S., and Jones J.M. (1998). Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147 [DOI] [PubMed] [Google Scholar]
- Tighe A., Ray-Sinha A., Staples O.D., and Taylor S.S. (2007). GSK-3 inhibitors induce chromosome instability. BMC Cell Biol. 8, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai B.P., Hoverter N.P., and Waterman M.L. (2012). Blending hippo and WNT: Sharing messengers and regulation. Cell 151, 1401–1403 [DOI] [PubMed] [Google Scholar]
- Valamehr B., Robinson M., Abujarour R., Rezner B., Vranceanu F., Le T., Medcalf A., Lee T.T., Fitch M., Robbins D., and Flynn P. (2014). Platform for induction and maintenance of transgene-free hiPSCs resembling ground state pluripotent stem cells. Stem Cell Rep. 2, 366–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valvezan A.J., and Klein P.S. (2012). GSK-3 and Wnt signaling in neurogenesis and bipolar disorder. Front. Mol. Neurosci. 5, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Jeught M., O'Leary T., Ghimire S., Lierman S., Duggal G., Versieren K., Deforce D., Chuva de Sousa Lopes S., Heindryckx B., and De Sutter P. (2013). The combination of inhibitors of FGF/MEK/Erk and GSK3beta signaling increases the number of OCT3/4- and NANOG-positive cells in the human inner cell mass, but does not improve stem cell derivation. Stem Cells Dev. 22, 296–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Jeught M., Heindryckx B., O'Leary T., Duggal G., Ghimire S., Lierman S., Van Roy N., Chuva de Sousa Lopes S.M., Deroo T., Deforce D., and De Sutter P. (2014). Treatment of human embryos with the TGFbeta inhibitor SB431542 increases epiblast proliferation and permits successful human embryonic stem cell derivation. Hum. Reprod. 29, 41–48 [DOI] [PubMed] [Google Scholar]
- Ware C.B., Nelson A.M., Mecham B., Hesson J., Zhou W., Jonlin E.C., Jimenez-Caliani A.J., Deng X., Cavanaugh C., Cook S., Tesar P.J., Okada J., Margaretha L., Sperber H., Choi M., Blau C.A., Treuting P.M., Hawkins R.D., Cirulli V., and Ruohola-Baker H. (2014). Derivation of naive human embryonic stem cells. Proc. Natl. Acad. Sci. USA 111, 4484–4489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welling M., and Geijsen N. (2013). Uncovering the true identity of naive pluripotent stem cells. Trends Cell Biol. 23, 442–448 [DOI] [PubMed] [Google Scholar]
- Yeo J.C., Jiang J., Tan Z.Y., Yim G.R., Ng J.H., Göke J., Kraus P., Liang H., Gonzales K.A., Chong H.C., Tan C.P., Lim Y.S., Tan N.S., Lufkin T., Ng H.H. (2014). Klf2 is an essential factor that sustains ground state pluripotency. Cell Stem Cell 14, 864–872 [DOI] [PubMed] [Google Scholar]
- Ying Q. L., Wray J., Nichols J., Batlle-Morera L., Doble B., Woodgett J., Cohen P., and Smith A. (2008). The ground state of embryonic stem cell self-renewal. Nature 453, 519–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F.X., Zhao B., Panupinthu N., Jewell J.L., Lian I., Wang L.H., Zhao J., Yuan H., Tumaneng K., Li H., Fu X.D., Mills G.B., and Guan K.L. (2012). Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell 150, 780–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Krawetz R., and Rancourt D.E. (2013). Would the real human embryonic stem cell please stand up? Bioessays 35, 632–638 [DOI] [PubMed] [Google Scholar]
- Zhang P., Andrianakos R., Yang Y., Liu C., and Lu W. (2010). Kruppel-like factor 4 (Klf4) prevents embryonic stem (ES) cell differentiation by regulating Nanog gene expression. J. Biol. Chem. 285, 9180–9189 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.