Abstract
Mushrooms are known to complement chemotherapy and radiation therapy by countering the side effects of cancer. Recently, there has been great interest in isolation of novel bioactive compounds from mushrooms due to their numerous health beneficial effects. Chemically water-extractable polysaccharide (MFKF-AP1β), with a molecular weight of 12 kDa, was isolated from fruiting bodies of mushroom Fomes fomentarius. In this research, we investigated the anti-tumor effects of MFKF-AP1β on human lung carcinoma A549 cells. Results showed that MFKF-AP1β markedly inhibited A549 cell growth in a dose-dependent manner based on the amount of lactate dehydrogenase (LDH) released and morphological alterations. In addition, MFKF-AP1β induced cellular apoptosis by causing single-stranded DNA breakage, as evidenced by apoptosis assay. Furthermore, MFKF-AP1β (25–100 μg/ml) significantly induced single-stranded DNA breakage in A549 cells, as shown by comet assay. Taken together, our results demonstrate that MFKF-AP1β has strong anti-tumor effects mediated through induction of apoptosis. Therefore, MFKF-AP1β could be useful in lung chemotherapy.
Keywords: DNA damage, Anti-apoptosis, Comet assay, Anti-oxidant, DNA binding
1. Introduction
Lung cancer has become the most common cause of cancer-related death in both men and women, accounting for 28% of all cancer-related deaths in the U.S. (Cardenal et al., 1999). Although treatment of lung cancer has improved, mortality remains high in lung cancer patients. A growing body of research suggests that naturally occurring compounds can act as antioxidants as well as cancer preventative and therapeutic agents (Parkin et al., 1999; Rahman et al., 2005; Hashibe et al., 2007). Development of chemotherapeutic agents with maximal anti-tumor activity and minimal toxicity has become a favorable route for cancer management. In this context, mushrooms play a pivotal role as anti-tumor agents. Edible mushrooms have been used as health nutritional supplements for several centuries, and they complement chemotherapy and radiation therapy by countering the side effects of cancer (Mizuno et al., 1995).
The bioactive compounds in mushrooms responsible for their anti-tumor potential include polysaccharides, proteins, fats, ash, glycosides, alkaloids, volatile oils, tocopherols, phenolics, flavonoids, carotenoids, folates, ascorbic acid enzymes, and organic acids (Mizuno et al., 1992, 1995; Jong and Birmingham, 1993; Ferreira et al., 2009). Among these bioactive compounds, polysaccharides are the most well-known and most potent mushroom-derived substances with anti-tumor activity (Ferreira et al., 2010). In this study, we analyzed the anti-tumor activity of a polysaccharide (MFKF-AP1β) isolated from fruiting bodies of the medicinal mushroom Fomes fomentarius in human lung cancer A549 cells.
F. fomentarius, a basidiomycete fungus, has been used as a traditional Chinese and Korean medicine for many centuries for the treatment of various diseases, including oral ulcers, gastroenteric disorders, hepatocirrhosis, inflammation, and various cancers. Recent studies have shown that F. fomentarius has antioxidant, anti-inflammatory, and anti-diabetic activities (Lee, 2005; Park et al., 2004). However, there are few reports on the apoptotic activity of F. fomentarius (Ito et al., 1976; Chen et al., 2008).
To discover novel agents from natural products that improve the therapeutic outcome of cancer, we tested the anti-tumor effects of a polysaccharide (MFKF-AP1β) isolated from F. fomentarius on A549 lung cancer cells. Specifically, we tested the apoptotic activity of MFKF-AP1β in lung carcinomas.
2. Materials and methods
2.1. Reagents
RPMI 1640, HEPES, staurosporine, Hoechst 33342, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide were purchased from Sigma–Aldrich, St. Louis, USA. Fetal bovine serum was supplied by Gibco, USA. All solvents were HPLC grade and supplied by J.T. Baker (Phillipsburg, NJ, USA).
2.2. Materials
Chemically water-extractable polysaccharide (MFKF-AP1β) was isolated from hot water extracts of fruiting bodies of mushroom F. fomentarius by successive DEAE-Sepharose FF and concanavalin A-Sepharose 4B column chromatography. Molecular weight of MFKF-AP1β was estimated to be about 12 kDa by high performance liquid chromatography (HPLC) (Park et al., 2013). Solutions of MFKF-AP1β (25–100 μg/ml) were prepared in a water/methanol mixture (1:1, v/v).
2.3. Cell culture
Lung cancer cells A549 (KCLB) were cultivated in RPMI 1640 medium supplemented with 25 mM HEPES buffer, 25 mM sodium bicarbonate, 300 mM l-glutamate, and 10% heat-inactivated fetal bovine serum (Gibco, USA) in a CO2 incubator (5% CO2 in air) at 37 °C. For the experiment, cells were cultured in 12-well plates at 1 × 106 cells/well. Cells were treated with several concentrations of MFKF-AP1β (25, 50, and 100 μg/ml) for 24 h. As a positive control, 1 μM staurosporine (Sigma, St. Louis, USA) was added for 4 h to induce apoptosis. Cells were then centrifuged (600g × 3 min) and harvested.
2.4. MTT assay and cell morphology
Cell viability was measured by MTT bioassay. Briefly, A549 cells (1 × 106 cells/well) were seeded in each well of a 96-well plate for 24 h. After incubation with different concentrations of MFKF-AP1β for 24 h, 10 μl of MTT solution (5 mg/ml on PBS) dissolved in PBS was added and incubated for 4 h. After color development, 100 μl of DMSO was applied. Absorbance was measured using an ELISA plate reader (Bio-Tek Instrument Co., WA, USA) at 540 nm. The viability% was measured using formula-
2.5. Lactate dehydrogenase (LDH) assay
A549 cells (1 × 106 cells/well) were seeded in each well of a 96-well plate for 24 h. After incubation with different concentrations of MFKF-AP1β for 24 h, medium was collected and cleared by centrifugation. After supernatants were collected, cytotoxicity was quantified by measuring the amount of total LDH released by cells using an LDH assay kit (Sigma–Aldrich, St. Louis, USA) according to the manufacturer’s protocol. Briefly, LDH, a glycolytic enzyme, is concerned with the reduction of pyruvic acid in the presence of dihydronicotinamide adenine dinucleotide (NADH). LDH assay reagent was added to supernatants and incubated for up to 30 min at room temperature in the dark, after which the reaction was stopped by adding 1 N HCl. The absorbance of samples was measured at 450 nm.
2.6. Assay for nuclear apoptosis (Hoechst staining)
To determine DNA chromatin morphological features, cells were treated with Hoechst 33342 stain according to Diaz-Ruiz et al., 2001. Briefly, cells were cultured and treated for 24 h with different concentrations of MFKF-AP1β and staurosporine (1 μM) for 4 h. After washing twice in PBS, cells were fixed with cold 4% formaldehyde. Cells were then washed with PBS again and incubated with Hoechst 33342 (1 μg/ml) at 37 °C for 10 min. After washing with PBS, cells were analyzed under a fluorescence microscope (Nikon Eclipse TS100 Epi-fluorescence microscope, Japan).
2.7. Comet assay
To evaluate DNA damage, comet assay was performed according to Singh et al. (1988), with some modifications. A549 lung cancer cells treated with MFKF-AP1β and staurosporine were harvested by washing twice with phosphate-buffered saline (PBS) and then suspended in 70 μl of 1% (w/v) low melting point agarose in PBS, pH 7.4, at 37 °C. The cell solution was immediately pipetted onto frosted glass microscope slides pre-coated with a layer of 1% (w/v) normal melting point agarose, after which the third layer composed of 75 μl of 0.5% (w/v) low melting point agarose similarly prepared in PBS was applied. The agarose was allowed to set for 10 min on ice. To remove cellular proteins, slides were immersed in lysis solution (2.5 M NaCl, 0.1 M Na2EDTA, 10 mM Tris, pH 10.0, with NaOH, 10% v/v dimethyl sulfoxide, and 1% v/v Triton X-100) at 4 °C for 1 h. Slides were then placed in a electrophoresis tank containing electrophoresis buffer (0.3 M NaOH and 1 mM Na2EDTA) for 30 min before electrophoresis at 40 V for 40 min. The slides were then washed with neutralizing solution (0.4 M Tris–HCl, pH 7.5) for 20 min before staining with 50 μl of ethidium bromide (20 mg/ml), and analyzed visually with a Nikon EPI fluorescence microscope (Japan).
2.8. Measurement of single-stranded DNA
As DNA in apoptotic cells is sensitive to formamide, denatured DNA was detected by a monoclonal antibody against single-stranded DNA using an ApoStrand™ ELISA apoptosis detection kit (Enzo Life Sciences, Plymouth Meeting, PA, USA) according to the manufacturer’s protocol. Briefly, 1.0 × 106 cells were cultured in a 96-well microplate and treated with different concentrations of MFKF-AP1β. After 12 h, cells were fixed and dried for attachment to the plate surface. Cells were then treated with formamide and heated at 56 °C for 30 min, followed by incubation with an antibody mixture for 30 min after blocking non-specific binding sites. After washing, peroxidase substrate was added to each well, and the absorbance was measured at 405 nm using an ELISA reader (Bio-Tek Instrument Co., WA, USA).
2.9. Statistical analysis
Each experimental condition was analyzed at least in triplicate (n = 3). Data were expressed as the mean ± standard deviation (S.D.). Statistical analyses were carried out using Student’s t-test. Differences were considered statistically significant at ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
3. Results
3.1. Anti-proliferative effects of MFKF-AP1β on A549 cells
We characterized the anti-proliferative effects of MFKF-AP1β on A549 cells by measuring cell viability using MTT assay. Cultures of A549 cells were treated with MFKF-AP1β at concentrations of 25, 50, and 100 μg/ml for 24 h. As shown in Fig. 1, MFKF-AP1β inhibited proliferation of A549 cells. Cell viability was lowest (59.22 ± 5.17%) after 24 h of MFKF-AP1β treatment at a concentration of 100 μg/ml, as compared to MFKF-AP1β concentrations of 25 and 50 μg/ml (64.46 ± 1.48% and 65.73 ± 1.24%, respectively).
Figure 1.
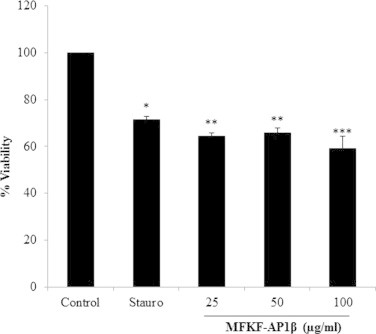
Effect of MFKF-AP1β on viability of A549 lung cancer cells. The viability of cells pretreated with 1 μM staurosporine (positive control) and MFKF-AP1β at different concentrations (25, 50, and 100 μg/ml) for 24 h was estimated using MTT assay after being cultured for 24 h. Data are represented as the mean ± SD of three independent experiments. An asterisk indicates ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
3.2. Cytotoxic effects of MFKF-AP1β
To better characterize the cytotoxicity of MFKF-AP1β in human lung cancer cells (A549), we performed cellular lactate dehydrogenase (LDH) release assay. In this experiment, intracellular LDH released into culture medium was an indicator of cytotoxicity. A549 cells treated with 25, 50, and 100 μg/ml of MFKF-AP1β for 24 h showed a dose-dependent increase in LDH activity, indicating cellular damage. Further, LDH release into culture medium was highest upon treatment with 100 μg/ml of MFKF-AP1β (43.74 ± 1.19%), which induced A549 cell death through cytotoxicity (Fig. 2). A significantly lower amount of LDH was released at concentrations of 25 and 50 μg/ml (39.91 ± 1.19% and 40.25 ± 1.29%, respectively).
Figure 2.
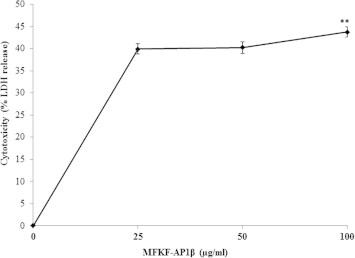
Lactate dehydrogenase (LDH) release assay. There was a significant increase in LDH release from lung cancer cells as measured by colorimetric assay. Pretreatment with 1 μM staurosporine (positive control) and MFKF-AP1β at different concentrations (25, 50, and 100 μg/ml) for 24 h significantly increased LDH release from A549 cells as a measure of cytotoxicity. Data are represented as the mean ± SD of three independent experiments. An asterisk indicates ∗∗p < 0.01.
3.3. MFKF-AP1β induces significant morphological alterations
The cytotoxic effects of MFKF-AP1β were also reflected in the morphological appearance of A549 cells after 24 h (Fig. 3). Treatment with 1 μM staurosporine and 100 μg/ml of MFKF-AP1β had a marked effect on cellular morphology, as cells became more round and refractile under a microscope. Cells treated with 100 μg/ml of MFKF-AP1β became apoptotic bodies and eventually detached from the surface, whereas untreated cells (control) remained well spread with a flattened morphology. Treatment with 50 μg/ml of MFKF-AP1β had a comparatively weaker effect, and 25 μg/ml of MFKF-AP1β had no discernible effect. These results suggest that MFKF-AP1β-treated human lung cancer cells undergo cell death and exhibit morphological features suggestive of apoptosis.
Figure 3.
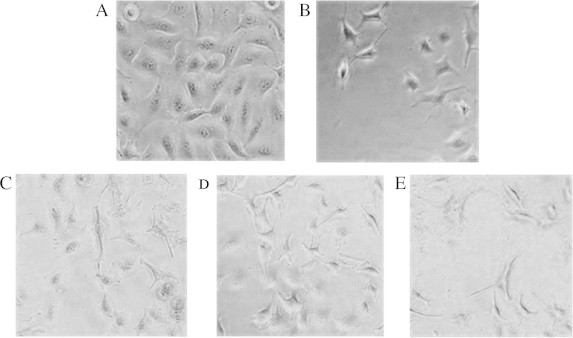
Morphological changes induced by (A) control, (B) 1 μM staurosporine, (C) 25 μg/ml of MFKF-AP1β, (D) 50 μg/ml of MFKF-AP1β, and (E) 100 μg/ml of MFKF-AP1β in A549 lung cancer cells after 24 h of treatment.
3.4. MFKF-AP1β potentiates cellular apoptosis
To determine whether or not the above results can be attributed to apoptosis of A549 cells, we performed chromatin condensation assay using Hoechst 33342 visualized by fluorescence microscopy. Hoechst staining assay (Fig. 4) revealed a greater number of apoptotic cells showing characteristic morphologic alterations such as nucleic shrinkage and formation of pycnonuclei following treatment with 100 μg/ml of MFKF-AP1β and staurosporine for 24 h compared to the control group.
Figure 4.
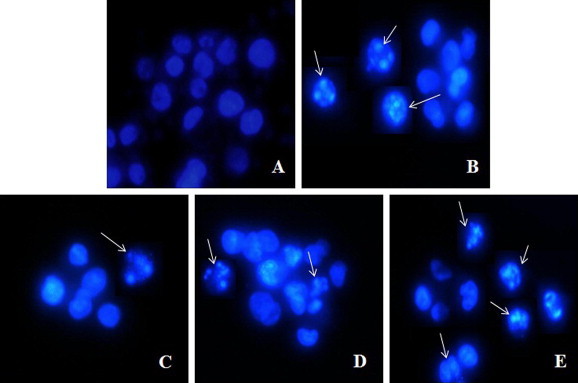
Representative nuclear stained images of A549 cells following treatment with different concentrations of MFKF-AP1β (25, 50, and 100 μg/ml) and 1 μM staurosporine observed under a fluorescence microscope at a magnification of 40×. (A) Control; (B) treated with staurosporine; (C–E) MFKF-AP1β 25, 50, and 100 μg/ml, respectively.
3.5. MFKF-AP1β induces DNA fragmentation in A549 cells
Degradation of DNA into multiple internucleosomal fragments of 180–200 base pairs is a distinct biochemical hallmark of apoptosis. To detect this, we performed single cell gel electrophoresis (comet assay), which is a sensitive technique that allows detection of DNA strand breaks. Cells treated with staurosporine and MFKF-AP1β showed comet formation in a dose-dependent manner, whereas untreated cells did not show any comet-like appearance (Fig. 5). To further determine whether or not DNA fragmentation is typical of apoptosis, we performed apoptosis detection assay using an ApoStrand™ ELISA apoptosis detection kit, which detects in vitro single-stranded DNA breaks induced by MFKF-AP1β treatment. The sensitivity of this assay reflects changes in chromatin structure associated with apoptosis, such as chromatin condensation and digestion of proteins stabilizing DNA. As shown in Fig. 6, treatment with MFKF-AP1β at concentrations of 25, 50, and 100 μg/ml for 24 h significantly increased the apoptotic rate of A549 cells in a dose-dependent manner. Taken together, these results indicate that, apart from its cytotoxic effects, MFKF-AP1β can induce apoptosis in A549 cells.
Figure 5.
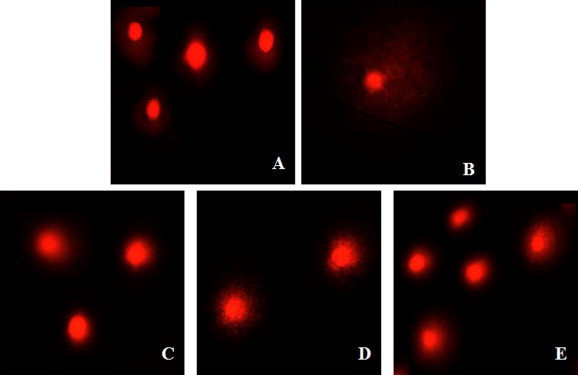
Representative Comet images of A549 cells following treatment with different concentrations of MFKF-AP1β (25, 50, and 100 μg/ml) and staurosporine (1 μM). (A) Control; (B) treated with staurosporine; (C–E) MFKF-AP1β (25, 50, and 100 μg/ml, respectively).
Figure 6.
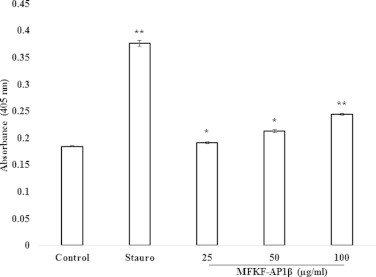
Induction of cell death by MFKF-AP1β. A549 cells were treated with 1 μM staurosporine and MFKF-AP1β (25, 50, and 100 μg/ml), after which amount of single-stranded DNA was detected by an ApoStrand™ ELISA apoptosis detection kit. An asterisk indicates ∗p < 0.05, ∗∗p < 0.01.
4. Discussion
Cancer remains one of the major causes of death worldwide. The most effective treatment strategy for cancer is cytotoxic agent-based chemotherapy, which increases patient survival but also has side effects that severely limit its clinical effectiveness such as acquisition of drug resistance (Appel et al., 2007; Rutkoski and Raines, 2008; Lal et al., 2011). Therefore, novel therapeutic strategies involving natural compounds could reduce the cumulative effects of chemotherapy by enhancing anti-tumor activities. The present paper evaluated the effects of MFKF-AP1β, a dietary polysaccharide from medicinal mushroom F. fomentarius, on a human lung adenocarcinoma cell line (A549) in relation to cell growth inhibition and apoptosis induction. The anti-tumor activities of polysaccharides isolated from medicinal fungi are very attractive due to their low toxicity in normal cells and their apparent lack of side effects in clinical patients (Fukushima, 1989).
In this study, we first reported a cell growth inhibitory effect for MFKF-AP1β in lung cancer cells. Exposure to MFKF-AP1β for 24 h resulted in dose-dependent inhibition of A549 cell growth, as evidenced by cell morphological alterations (Fig. 3). Further, MFKF-AP1β reduced cellular metabolic activity of A549 cells and potentiated cytotoxic activity via LDH release into the medium (Fig. 3). Up to this point, the mode of cell death responsible for these results was unconfirmed. Autophagy, apoptosis, and necrosis are all different types of cell death, and apoptosis is the desired mode of cell death in chemotherapy (Amos et al., 1998). Recently, studies have extensively focused on the apoptotic potential of compounds as anti-tumor agents (Rosse et al., 1998). In the current study, we investigated whether or not the cytotoxic effects of MFKF-AP1β on A549 cells were mediated via apoptosis by examining cellular changes associated with apoptosis using comet assay, Hoechst staining, and apoptosis assay.
Induction of apoptosis is a common mechanism of anti-tumor drugs (Ahmad et al., 2010). Here, we detected apoptotic cells by comet assay with ethidium bromide staining as well as apoptosis assay, which detects formation of single-stranded DNA breaks (Figs. 5 and 6, respectively). MFKF-AP1β-treated cells exhibited a dose-dependent increase in nuclear chromatin staining, as visualized by Hoechst 33342 (Fig. 4), including morphological characteristics of apoptotic cells such as increased nuclear condensation, nuclear shrinkage, and pycnonuclei formation. In the current study, both Hoechst staining and comet assay confirmed that proportions of apoptotic cells dramatically increased in a dose-dependent manner upon MFKF-AP1β treatment compared with staurosporine or control cells (Figs. 4 and 5). Therefore, MFKF-AP1β can potentiate cytotoxicity by promoting apoptosis in cancer cells.
The ability of polysaccharides to induce apoptosis in cancer cells can differ greatly according to their chemical composition and configuration as well as their physical properties. Although it is difficult to correlate the structure and anti-tumor activity of complex polysaccharides, a relationship can be inferred. It has been suggested that structural features in the main chain of the glucan such as β-(1→3) linkages as well as β-(1→6) branch points are needed for anti-tumor activity (Miyazaki and Nishijima, 1981; Chihara, 1992). High molecular weight glucans appear to be more effective than low molecular weight species (Mizuno, 1996, 1999a,b). The polysaccharide used in the present study was a partially purified polysaccharide fraction with an average molecular weight of over 12 kDa. Its exact mechanism of action for activating apoptosis remains to be identified.
5. Conclusion
In conclusion, our studies prove that MFKF-AP1β induces cell death in A549 lung carcinoma cells in a dose-dependent manner as well as apoptosis by elevation of LDH release, disruption of cellular morphology, and induction of nuclear damage. However, further investigation about the relationship between MFKF-AP1β-mediated cytotoxic and apoptotic pathways is needed.
Acknowledgement
This research was supported by the Daegu University Research Grant – Republic of Korea, 2013.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahmad A., Sakr W.A., Rahman M.W.K. Anticancer properties of indole compounds: mechanism of apoptosis induction and role in chemotherapy. Curr. Drug Targets. 2010;11:652–666. doi: 10.2174/138945010791170923. [DOI] [PubMed] [Google Scholar]
- Amos C.L., Woetmann A., Nielsen M., Geisler C., Odum N., Brown B.L., Dobson P.R. The role of caspase 3 and BclxL in the action of interleukin 7 (IL-7): a survival factor in activated human T cells. Cytokine. 1998;10:662–668. doi: 10.1006/cyto.1998.0351. [DOI] [PubMed] [Google Scholar]
- Appel J.M., Nielsen D., Zerahn B., Jensen B.V., Skagen K. Anthracycline-induced chronic cardiotoxicity and heart failure. Acta Oncol. 2007;46:576–580. doi: 10.1080/02841860601156165. [DOI] [PubMed] [Google Scholar]
- Cardenal F., López-Cabrerizo M.P., Antón A., Alberola V., Massuti B., Carrato A., Barneto I., Lomas M., García M., Lianes P., Montalar J., Vadell C., González-Larriba J.L., Nguyen B., Artal A., Rosell R. Randomized phase III study of gemcitabine-cisplatin versus etoposide-cisplatin in the treatment of locally advanced or metastatic non small-cell lung cancer. J. Clin. Oncol. 1999;17:12–18. doi: 10.1200/JCO.1999.17.1.12. [DOI] [PubMed] [Google Scholar]
- Chen W., Zhao Z., Chen S.F., Li Y.Q. Optimization for the production of exopolysaccharide from Fomes fomentarius in submerged culture and its antitumor effect in vitro. Bioresour. Technol. 2008;99:3187–3194. doi: 10.1016/j.biortech.2007.05.049. [DOI] [PubMed] [Google Scholar]
- Chihara G. Recent progress in immunopharmacology and therapeutic effects of polysaccharides. Dev. Biol. Stand. 1992;77:191–197. [PubMed] [Google Scholar]
- Diaz-Ruiz C., Montaner B., Perez-Tomas R. Prodigiosin induces cell death and morphological changes indicative of apoptosis in gastric cancer cell line HGT-1. Histol. Histopathol. 2001;16:415–421. doi: 10.14670/HH-16.415. [DOI] [PubMed] [Google Scholar]
- Ferreira I.C.F.R., Barros L., Abreu R.M.V. Antioxidant in Wild mushrooms. Curr. Med. Chem. 2009;16:1543–1560. doi: 10.2174/092986709787909587. [DOI] [PubMed] [Google Scholar]
- Ferreira I.C.F.R., Vaz J.A., Vasconcelos M.H., Martins A. Compounds from wild mushrooms with antitumor potential. Anti-Cancer Agents Med. Chem. 2010;10:424–436. doi: 10.2174/1871520611009050424. [DOI] [PubMed] [Google Scholar]
- Fukushima M. The overdose of drugs in Japan. Nature. 1989;342:850–851. doi: 10.1038/342850a0. [DOI] [PubMed] [Google Scholar]
- Hashibe M., Brennan P., Benhamou S., Castellsague X., Chen C., Curado M.P., Dal Maso L., Daudt A.W., Fabianova E., Fernandez L., Wunsch-Filho V., Franceschi S., Hayes R.B., Herrero R., Koifman S., La Vecchia C., Lazarus P., Levi F., Mates D., Matos E., Menezes A., Muscat J., Eluf-Neto J., Olshan A.F., Rudnai P., Schwartz S.M., Smith E., Sturgis E.M., Szeszenia-Dabrowska N., Talamini R., Wei Q., Winn D.M., Zaridze D., Zatonski W., Zhang Z.F., Berthiller J., Boffetta P. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. J. Natl Cancer Inst. 2007;99:777–789. doi: 10.1093/jnci/djk179. [DOI] [PubMed] [Google Scholar]
- Ito H., Sugiura M., Miyazaki T. Antitumor polysaccharide fraction from the culture filtrate of Fomes fomentarius. Chem. Pharm. Bull. (Tokyo) 1976;10:2575. doi: 10.1248/cpb.24.2575. [DOI] [PubMed] [Google Scholar]
- Jong S.C., Birmingham J.M. Medicinal and therapeutic value of the shiitake mushroom. Adv. Appl. Microbiol. 1993;39:153–184. doi: 10.1016/s0065-2164(08)70595-1. [DOI] [PubMed] [Google Scholar]
- Lal R., Enting D., Kristeleit H. Systemic treatment of non-small-cell lung cancer. Eur. J. Cancer. 2011;47:S375–S377. doi: 10.1016/S0959-8049(11)70209-X. [DOI] [PubMed] [Google Scholar]
- Lee J.S. Effects of Fomes fomentarius supplementation on antioxidant enzyme activities, blood glucose, and lipid profile in streptozotocin-induced diabetic rats. Nutr. Res. 2005;25:187–195. [Google Scholar]
- Miyazaki T., Nishijima M. Studies on fungal polysaccharides. XXVII. Structural examination of a water-soluble, antitumor polysaccharide of Ganoderma lucidum. Chem. Pharm. Bull. (Tokyo) 1981;29:3611–3616. doi: 10.1248/cpb.29.3611. [DOI] [PubMed] [Google Scholar]
- Mizuno T., Saito H., Nishitoba T., Kawagishi H. Antitumor-active substances from mushrooms. Food Rev. Int. 1995;11:23–61. [Google Scholar]
- Mizuno T., Waza T., Ito H., Suzuki C., Ukai N. Antitumor-active polysaccharides isolated from the fruiting body of Hericium erinaceum, an edible and medicinal mushroom called yamabushitake or houtou. Biosci. Biotechnol. Biochem. 1992;56:347–348. doi: 10.1271/bbb.56.347. [DOI] [PubMed] [Google Scholar]
- Mizuno T. Development of antitumor polysaccharides from mushroom fungi. Foods Ingred. J. Jpn. 1996;167:69–85. [Google Scholar]
- Mizuno T. Bioactive substances in Hericium erinaceus (Bull.: Fr.) Pers. (Yamabushitake), and its medicinal utilization. Int. J. Med. Mushrooms. 1999;1:105–119. [Google Scholar]
- Mizuno T. The extraction and development of antitumor active polysaccharides from medicinal mushrooms in Japan. Int. J. Med. Mushrooms. 1999;1:9–29. [Google Scholar]
- Park J.K., Park K.W., Shin K.S., Lee C.M., Seok S.J., Kim J.B., Koo B.S., Han B.S., Yoon S.H. Isolation and chemical analysis of potent anti-complementary polysaccharides from fruiting bodies of the Fomes fomentarius. Korean J. Microbiol. Biotechnol. 2013;41:198–206. [Google Scholar]
- Park Y.M., Kim I.T., Park H.J., Choi J.W., Park K.Y., Lee J.D., Nam B.H., Kim D.G., Lee J.Y., Lee K.T. Anti-inflammatory and anti-nociceptive effects of the methanol extract of Fomes fomentarius. Biol. Pharm. Bull. 2004;27:1588–1593. doi: 10.1248/bpb.27.1588. [DOI] [PubMed] [Google Scholar]
- Parkin D.M., Pisani P., Ferlay J. Estimates of the worldwide incidence of 25 major cancers in 1990. Int. J. Cancer. 1999;80:827–841. doi: 10.1002/(sici)1097-0215(19990315)80:6<827::aid-ijc6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Rahman M., Sakamoto J., Fukui T. Calculation of population attributable risk for bidi smoking and oral cancer in south Asia. Prev. Med. 2005;40:510–514. doi: 10.1016/j.ypmed.2004.09.030. [DOI] [PubMed] [Google Scholar]
- Rosse T., Olivier R., Monney L., Rager Conus S., Fellay I., Jansen B., Borner C. Bcl-2 prolongs cell survival after Bax-induced release of cytochrome c. Nature. 1998;391:496–499. doi: 10.1038/35160. [DOI] [PubMed] [Google Scholar]
- Rutkoski T.J., Raines R.T. Evasion of ribonuclease inhibitor as a determinant of ribonuclease cytotoxicity. Curr. Pharm. Biotechnol. 2008;9:185–199. doi: 10.2174/138920108784567344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N.P., McCoy M.T., Tice R.R., Schneider E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]


