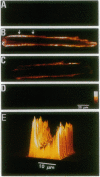
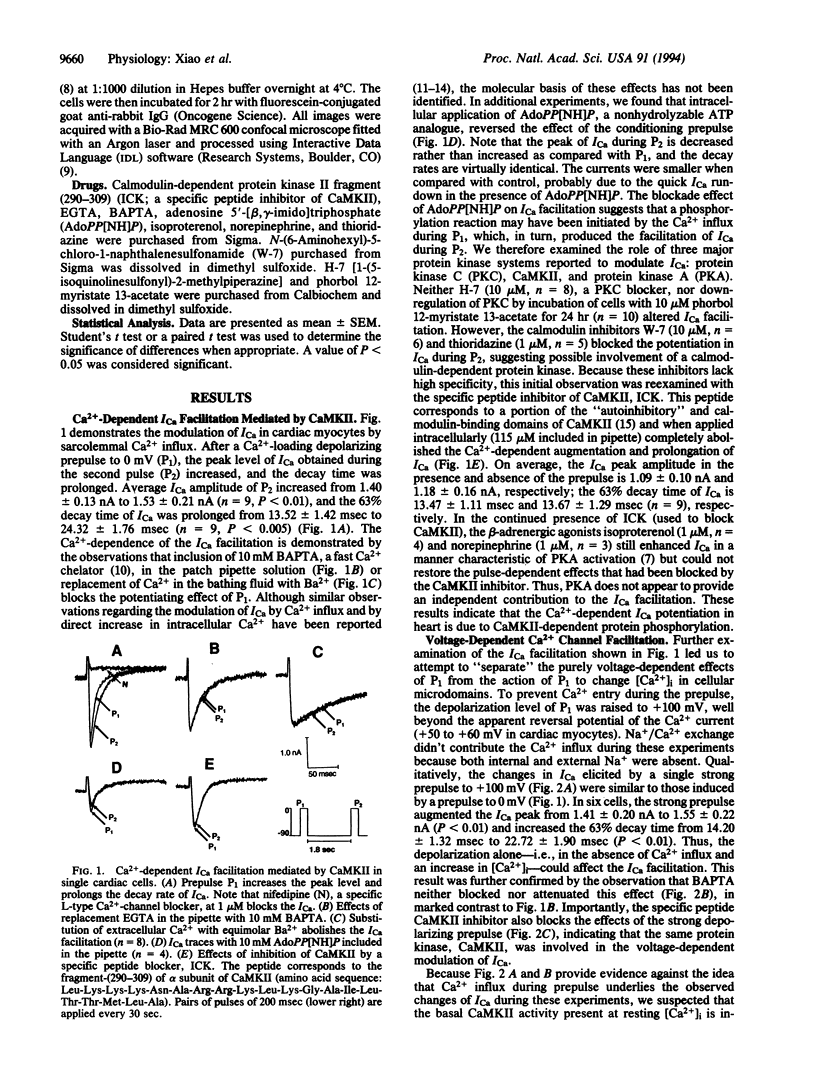
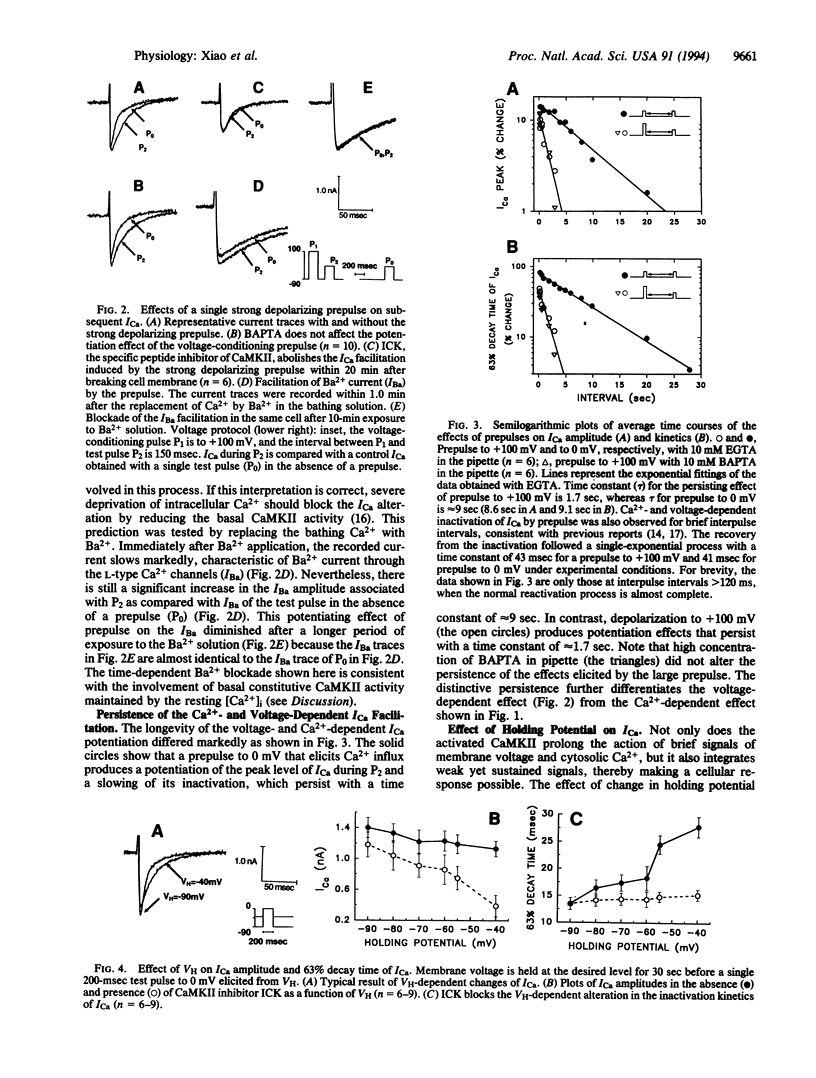
Abstract
Calcium entry through voltage-gated Ca2+ channels is critical in cardiac excitation-contraction coupling and calcium metabolism. In this report, we demonstrate both spatially resolved and temporally distinct effects of Ca2+/calmodulin-dependent protein kinase II (CaMKII) on L-type Ca2+ channel current (ICa) in rat cardiac myocytes. Either depolarization alone or calcium influx can increase the amplitude and slow the inactivation of ICa. The distinct voltage- and Ca(2+)-dependent effects persist with time constants of approximately 1.7 sec and 9 sec, respectively. Both effects are completely abolished by a specific peptide inhibitor of CaMKII. This CaMKII inhibitor also suppresses the prolongation of ICa induced by depolarizing holding potentials. Furthermore, using an antibody specific for the autophosphorylated (activated) CaMKII, we find that this kinase is localized close to sarcolemmal membranes and that the profile of CaMKII activation correlates qualitatively with the changes in ICa under various conditions. Therefore, we conclude that the action of CaMKII on ICa is dually regulated by membrane depolarization and by Ca2+ influx; the latter directly activates CaMKII, whereas the former likely promotes the interaction between constitutive CaMKII and the membrane-channel proteins. These regulatory mechanisms provide positive-feedback control of Ca2+ channels and are probably important in the regulation of cardiac contractility and other intracellular Ca(2+)-regulated processes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheng H., Lederer W. J., Cannell M. B. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993 Oct 29;262(5134):740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- Chu A., Sumbilla C., Inesi G., Jay S. D., Campbell K. P. Specific association of calmodulin-dependent protein kinase and related substrates with the junctional sarcoplasmic reticulum of skeletal muscle. Biochemistry. 1990 Jun 26;29(25):5899–5905. doi: 10.1021/bi00477a003. [DOI] [PubMed] [Google Scholar]
- Colbran R. J., Fong Y. L., Schworer C. M., Soderling T. R. Regulatory interactions of the calmodulin-binding, inhibitory, and autophosphorylation domains of Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 1988 Dec 5;263(34):18145–18151. [PubMed] [Google Scholar]
- Edelman A. M., Hunter D. D., Hendrickson A. E., Krebs E. G. Subcellular distribution of calcium- and calmodulin-dependent myosin light chain phosphorylating activity in rat cerebral cortex. J Neurosci. 1985 Oct;5(10):2609–2617. doi: 10.1523/JNEUROSCI.05-10-02609.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga K., Rich D. P., Soderling T. R. Generation of the Ca2(+)-independent form of Ca2+/calmodulin-dependent protein kinase II in cerebellar granule cells. J Biol Chem. 1989 Dec 25;264(36):21830–21836. [PubMed] [Google Scholar]
- Gorelick F. S., Wang J. K., Lai Y., Nairn A. C., Greengard P. Autophosphorylation and activation of Ca2+/calmodulin-dependent protein kinase II in intact nerve terminals. J Biol Chem. 1988 Nov 25;263(33):17209–17212. [PubMed] [Google Scholar]
- Gurney A. M., Charnet P., Pye J. M., Nargeot J. Augmentation of cardiac calcium current by flash photolysis of intracellular caged-Ca2+ molecules. Nature. 1989 Sep 7;341(6237):65–68. doi: 10.1038/341065a0. [DOI] [PubMed] [Google Scholar]
- Hadley R. W., Hume J. R. An intrinsic potential-dependent inactivation mechanism associated with calcium channels in guinea-pig myocytes. J Physiol. 1987 Aug;389:205–222. doi: 10.1113/jphysiol.1987.sp016654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S. Potentiation of the calcium-channel currents of internally perfused mammalian heart cells by repetitive depolarization. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3941–3945. doi: 10.1073/pnas.84.11.3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinow R., Madison D. V., Tsien R. W. Persistent protein kinase activity underlying long-term potentiation. Nature. 1988 Oct 27;335(6193):820–824. doi: 10.1038/335820a0. [DOI] [PubMed] [Google Scholar]
- Marban E., Tsien R. W. Enhancement of calcium current during digitalis inotropy in mammalian heart: positive feed-back regulation by intracellular calcium? J Physiol. 1982 Aug;329:589–614. doi: 10.1113/jphysiol.1982.sp014321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarron J. G., McGeown J. G., Reardon S., Ikebe M., Fay F. S., Walsh J. V., Jr Calcium-dependent enhancement of calcium current in smooth muscle by calmodulin-dependent protein kinase II. Nature. 1992 May 7;357(6373):74–77. doi: 10.1038/357074a0. [DOI] [PubMed] [Google Scholar]
- Meyer T., Hanson P. I., Stryer L., Schulman H. Calmodulin trapping by calcium-calmodulin-dependent protein kinase. Science. 1992 May 22;256(5060):1199–1202. doi: 10.1126/science.256.5060.1199. [DOI] [PubMed] [Google Scholar]
- Ocorr K. A., Schulman H. Activation of multifunctional Ca2+/calmodulin-dependent kinase in intact hippocampal slices. Neuron. 1991 Jun;6(6):907–914. doi: 10.1016/0896-6273(91)90231-n. [DOI] [PubMed] [Google Scholar]
- Sculptoreanu A., Scheuer T., Catterall W. A. Voltage-dependent potentiation of L-type Ca2+ channels due to phosphorylation by cAMP-dependent protein kinase. Nature. 1993 Jul 15;364(6434):240–243. doi: 10.1038/364240a0. [DOI] [PubMed] [Google Scholar]
- Silva A. J., Paylor R., Wehner J. M., Tonegawa S. Impaired spatial learning in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992 Jul 10;257(5067):206–211. doi: 10.1126/science.1321493. [DOI] [PubMed] [Google Scholar]
- Sollott S. J., Ziman B. D., Lakatta E. G. Novel technique to load indo-1 free acid into single adult cardiac myocytes to assess cytosolic Ca2+. Am J Physiol. 1992 Jun;262(6 Pt 2):H1941–H1949. doi: 10.1152/ajpheart.1992.262.6.H1941. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Okumura-Noji K., Ogura A., Kudo Y., Tanaka R. Antibody specific for the Thr-286-autophosphorylated alpha subunit of Ca2+/calmodulin-dependent protein kinase II. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):109–113. doi: 10.1073/pnas.89.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980 May 27;19(11):2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- Wang J., Best P. M. Inactivation of the sarcoplasmic reticulum calcium channel by protein kinase. Nature. 1992 Oct 22;359(6397):739–741. doi: 10.1038/359739a0. [DOI] [PubMed] [Google Scholar]
- Wegener A. D., Simmerman H. K., Lindemann J. P., Jones L. R. Phospholamban phosphorylation in intact ventricles. Phosphorylation of serine 16 and threonine 17 in response to beta-adrenergic stimulation. J Biol Chem. 1989 Jul 5;264(19):11468–11474. [PubMed] [Google Scholar]
- Witcher D. R., Kovacs R. J., Schulman H., Cefali D. C., Jones L. R. Unique phosphorylation site on the cardiac ryanodine receptor regulates calcium channel activity. J Biol Chem. 1991 Jun 15;266(17):11144–11152. [PubMed] [Google Scholar]
- Xiao R. P., Lakatta E. G. Beta 1-adrenoceptor stimulation and beta 2-adrenoceptor stimulation differ in their effects on contraction, cytosolic Ca2+, and Ca2+ current in single rat ventricular cells. Circ Res. 1993 Aug;73(2):286–300. doi: 10.1161/01.res.73.2.286. [DOI] [PubMed] [Google Scholar]