Abstract
The chemokine fractalkine (CX3CL1) recently attracted increasing attention in the field of placenta research due to its dual nature, acting both as membrane-bound and soluble form. While the membrane-bound form mediates flow resistant adhesion of leukocytes to endothelial and epithelial cells via its corresponding receptor CX3CR1, the soluble form arises from metalloprotease dependent shedding and bears chemoattractive activity for monocytes, natural killer cells and T-cells. In human placenta, fractalkine is expressed at the apical microvillous plasma membrane of the syncytiotrophoblast, which may enable close physical contact with circulating maternal leukocytes. Based on these observations we tested the hypothesis that fractalkine mediates adhesion of monocytes to the villous trophoblast. Forskolin-induced differentiation and syncytialization of the trophoblast cell line BeWo was accompanied with a substantial upregulation in fractalkine expression and led to increased adhesion of the monocyte cell line THP-1, which preferentially bound to syncytia. Blocking as well as silencing of the fractalkine receptor CX3CR1 proved involvement of the fractalkine/CX3CR1 system in adherence of THP-1 monocytes to villous trophoblast. Pre-incubation of THP-1 monocytes with human recombinant fractalkine as well as silencing of CX3CR1 expression in THP-1 monocytes significantly impaired their adherence to BeWo cells and primary term trophoblasts. The present study suggests fractalkine as another candidate amongst the panel of adhesion molecules enabling stable interaction between leukocytes and the syncytiotrophoblast.
Keywords: placental fractalkine, chemokine, trophoblast, monocyte, adhesion
Introduction
Onset of maternal blood flow into the intervillous space of the human placenta at the end of the first trimester of pregnancy enables direct physical interaction between maternal circulating blood cells and the placental syncytiotrophoblast (Huppertz et al. 2012; Jauniaux et al. 2000). This physical interaction between circulating maternal cells and the syncytiotrophoblast, which completely covers placental villi towards the intervillous space and thereby contributes to the placental barrier, could be regulated by cytokines, chemokines and adherence molecules. Amongst these, the chemokine fractalkine (CX3CL1) recently attracted increasing attention due to its dual nature, existing both as membrane-bound and soluble form. While the membrane-bound form mediates flow resistant adhesion of leukocytes to endothelial and epithelial cells via its corresponding G protein-coupled, 7-transmembrane receptor CX3CR1, the soluble form arises from metalloprotease dependent shedding and bears chemoattractive activity for monocytes, natural killer cells and T-cells (Imai et al. 1997). Thus, fractalkine mediates different steps of leukocyte recruitment, depending on whether it is cleaved or not. Moreover, the fractalkine/CX3CR1 system is suggested to facilitate transendothelial or transepithelial migration (or transmigration) of leukocytes (Schwarz et al. 2010). From a pathophysiologic point of view, the fractalkine/CX3CR1 system is involved in critical events including fibrogenesis, neuropathic pain sensation, the pathogenesis of certain cancers and neurodegenerative diseases (D’Haese et al. 2012).
The knowledge regarding a role of the fractalkine/CX3CR1 system in human placental development and physiology is restricted to a very small number of studies. Initial expression analyses and migration assays with trophoblast cell lines suggest a role of fractalkine in establishing first contact between the trophoblast and the uterine wall, and to promote trophoblast migration through the maternal decidua (Cammas et al. 2005; Hannan et al. 2006; Hannan and Salamonsen 2008). Recently, upregulation of placental fractalkine was suggested to contribute to increased fetal microvessel density in placentas from pregnancies complicated by diabetes mellitus (Szukiewicz et al. 2013). Another study demonstrated increased release of soluble fractalkine into perfusion fluids of perfused placental lobules in response to lipopolysaccharide (LPS) and hypoxia (Szukiewicz et al. 2014b), indicating upregulation of fractalkine in the placental endothelium under pro-inflammatory conditions. Moreover, increased fractalkine expression has recently been described for human amniotic epithelial cells from pregnancies complicated by chorioamnionitis (Szukiewicz et al. 2014a).
We have recently shown that placental fractalkine is expressed at the apical microvillous plasma membrane of the syncytiotrophoblast, from where it is released into the maternal circulation by constitutive metalloprotease dependent shedding (Siwetz et al. 2014). Inhibition of metalloprotease activity not only impairs the release of soluble fractalkine, but also leads to accumulation of the membrane-bound form. While increased expression and release of placental fractalkine was suggested to contribute to low grade systemic inflammatory responses in third trimester of normal pregnancy, aberrant placental metalloprotease activity may affect the abundance of the membrane-bound form of fractalkine, giving rise to abnormal interaction of the syncytiotrophoblast with maternal leukocytes. However, a putative role of the fractalkine/CX3CR1 system in the interaction of maternal leukocytes and the syncytiotrophoblast has not been considered so far. Thus, the hypothesis was tested whether or not placental fractalkine can mediate adhesion of monocytes to the villous trophoblast.
Material and methods
Culture of BeWo cells
BeWo cells were purchased from the European Collection of Cell Cultures (ECACC) and cultured in DMEM/F12 (1:1, Gibco), supplemented with 10% FCS (v/v), streptomycin (100 mg/ml), penicillin (100 IU/ml), and L-glutamine (2mM), at 37°C in a humidified atmosphere containing 5% CO2 in air. Cells between passage 10 and 20 were used for in vitro experiments. BeWo cell differentiation was induced with Forskolin (Sigma), which was supplemented to the culture medium at a final concentration of 20μM as previously described (Gauster et al. 2010; Gauster et al. 2011).
Culture of THP-1 cells
THP-1 cell line was obtained from ECACC and was cultured in RPMI 1640 supplemented with 10 % FCS (v/v), 100 mg/ml streptomycin and 100 IU/ml penicillin (Gibco, liefetechnologies).
Isolation and culture of primary term trophoblasts
Primary trophoblasts were isolated from chorionic villi of three term placentas with informed consent from the women and approval by the ethical committee of the Medical University of Graz. Isolation was performed by enzymatic digestion and Percoll density gradient centrifugation as described previously (Cervar et al. 1999). Trophoblasts were cultured in DMEM (Gibco, lifetechnologies) with 10 % FCS (v/v), 100 mg/ml streptomycin and 100 IU/ml penicillin (Gibco, lifetechnologies). A representative proportion of primary trophoblasts was scrutinized for purity by immunocytochemistry and viability/differentiation was monitored by measurements of secreted human chorionic gonadotropin (hCG) levels as previously described (Blaschitz et al. 2000; Cervar et al. 1999; Gauster et al. 2011).
Immunocytochemistry
BeWo cells (8 × 104 per well) were seeded in chamber-slides (Nunc; Roskilde, Denmark). Next day BeWo cells were incubated in culture medium supplemented either with Forskolin (20μM) or with vehicle control DMSO (0.2%) for 48h. After incubation, cells were washed with PBS, dried and fixed for 10min in acetone. Chamber slides were rehydrated in PBS and background blocking was performed with Ultra Vision Protein Block supplemented with 10% human AB-serum for 10min. Mouse monoclonal anti-human CX3CL1/fractalkine antibody (R&D Systems, clone 81513, 2μg/ml working concentration) and mouse monoclonal anti-βhCG (biologo, clone H-298-12, diluted 1:10) were diluted in antibody diluent (DAKO) and incubated on slides for 30min at RT. After PBS washing steps, slides were incubated with Primary Antibody Enhancer (10min). After another washing step detection was achieved by incubation with UltraVision HRP-labelled polymer (15min) and 3-amino-9-ethylcarbacole (AEC, Dako, Denmark), according to the manufacturer’s instructions. For immunocytochemistry of THP-1 cells, cytospins were prepared by spinning 1 × 105 THP-1 cells for 5min at 300 × g onto glass slides (Menzel, Braunschweig, Germany). Cytospins were air dried and fixed for 10min in acetone. Staining was performed with polyclonal anti-CX3CR1 antibody (C8354, Sigma-Aldrich, 2μg/ml working concentration) as described above for BeWo cells. For negative controls, slides were incubated with mouse IgG1 (DAK-GO1, DAKO) or rabbit IgG (Negative Control for Rabbit IgG Ab-1, Thermo Scientific), and revealed no staining. Nuclei were stained with hemalaun and slides were mounted with Kaiser’s glycerol gelatine.
RT-PCR
For RT-PCR a commercially available RT-PCR Kit (OneStep RT-PCR Kit, Qiagen, Hilden, Germany) was used as previously described (Gauster et al. 2007). In brief, 100ng total RNA of each sample was mixed with kit components in a total volume of 20μl. One step RT-PCR was performed including reverse transcription at 50°C for 30min and a PCR activation step at 95°C for 15min. Subsequent three-step cycling was performed with denaturation at 94°C for 30s, annealing at 60°C for 30s and extension at 72°C for 1min using 28 cycles for all used primers. Primers targeting human fractalkine (GGCTCCGATATCTCTGTCGT and CTGTGCTGTCTCGTCTCCAA) and CX3CR1 (TCATCACCGTCATCAGCATT and GGCTTTCTTGTGGTTCTTGC) were purchased from Microsynth AG (Wolfurt, Austria). Primers for human chorionic gonadotropin beta subunit (βhCG, AGGTCACTTCACCGTGGTCT and GCACAGATGGTGGTGTTGAC) and ribosomal protein L30 (RPL30: GAAAGTACGTGCTCGGGTACAAACAGACTC and ATCGGAATCACCTGGGTCAATGATAGCCAG) were obtained from Ingenetix (Vienna, Austria). PCR products were separated on 1.5 % (w/v) agarose gels (SeaKem LE Agarose, Cambrex) and images acquired with FluorChem Q System (Alpha Innotech, Cell Bioscienes, Santa Clara, CA, USA).
THP-1 adhesion assay
For THP-1 adhesion experiments, BeWo cells (2×105 cells/well) and primary term trophoblasts (2×106 cells/well) were seeded in 12 well culture dishes and 2 ml/well culture medium. One day after seeding, BeWo cells were incubated with culture medium supplemented with either Forskolin (20μM) or solvent control DMSO (0.2%) for another 48h. Primary term trophoblasts were cultured without treatment for 72h. Thereafter, culture media were replaced by 1ml RPMI medium containing green fluorescence (CellTracker GreenCMFDA, lifetechnologies) labelled THP-1 cells (4×105 cells/well), and cells were co-cultured at 37°C for 90min. Co-culture was followed by three PBS washing steps and subsequent fixation with 4% formalin (1ml/well) for 30min at RT. Acquisition of co-culture images was performed in PBS (2ml/well). For CX3CR1 blocking experiments, green fluorescence THP-1 cells (1×106/ml) were pre-incubated with recombinant human full length fractalkine (rhCX3CL1/Fractalkine, R&D Systems, 5 and 10ng/ml) prior to co-culture at 37°C for 1h. After pre-incubation with rhCX3CL1, THP-1 cells were washed and cell number adjusted to 4×105 cells/well in RPMI. Aliquots of THP-1 cells were pre-incubated in parallel without rhCX3CL1 and served as control. THP-1 adhesion assays were performed as described above. THP-1 adhesion was assessed by acquisition of trophoblast monolayer areas and bound THP-1 cells in phase contrast and green fluorescence channel, respectively, in a Cell-IQ system (chipman technologies, Tampere, Finland). Pixel areas of bound THP-1 cells were related to pixel areas of trophoblast monolayers in 16 images per well using the Cell-IQ analyzer software.
Assessment of BeWo syncytialization
BeWo cells were seeded in chamber slides (8 × 104 per well) and treated with or without Forskolin as described for THP-1 adhesion assay. After co-culture cells were washed trice in PBS, air dried and fixed in acetone for 7min. Fixed co-cultures were subjected to immunofluorescence using a monoclonal anti-α-fodrin antibody (clone AA6, BioTrend, 1:100) to visualize cell borders as previously described (Gauster et al. 2010). Ten cell layer areas per chamber were systematically randomly selected and microphotographed using a microscope (Leica DM6000B), equipped with a motorized stage, a digital camera (Olympus DP72) and the newCAST stereology software (Visiopharm). BeWo nuclei in syncytia were counted and related to the total number of BeWo nuclei in the selected cell layer areas. In the same images green fluorescence labelled THP-1 cells were counted and related to the total number of BeWo nuclei. Moreover, THP-1 cells bound to BeWo syncytia were related to those bound to mononucleated BeWo cells.
CX3CR1 silencing in THP-1 cells
THP-1 cells (1×106) were seeded in 0.85ml RPMI without supplements per well in 6 well dishes and starved for 2h. siRNA targeting CX3CR1 (5′ GAA AGC CAA AGC CAU UAA A dTdT 3′, Microsynth AG) and a pool of non targeting control siRNA (ON-TARGETplus control pool, Dharmacon, Thermo Scientific) was used for siRNA-lipid complex preparation by mixing transfection reagent (lipofectamine RNAiMAX, lifetechnologies) and siRNA (10μM) in a ratio of 3:1 (v/v) according to the manufacturer’s protocol. After starving, 0.15ml siRNA-lipid complexes were added to each well and cells incubated for another 4h. Thereafter, 1ml RPMI including supplements and 20% FCS was added to each well to achieve a final FCS concentration of 10%. Transfected THP-1 cells incubated for 24h until they were subjected to green fluorescence labelling and adhesion assays described above.
Quantitative real-time PCR (qPCR) analysis of CX3CR1 expression
Total RNA from THP-1 cells was isolated with the Tri Reagent (Molecular Research Center, Cincinnati, OH, USA) and reversely transcribed to cDNA, using High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems; Foster City, CA, USA) according to the manufacturer’s instructions. cDNA was subsequently subjected to quantitative real time PCR using TaqMan Gene Expression Assays for CX3CR1 (Hs00365842_m1) and ribosomal protein L30 (RPL30, Hs00265497_m1) and the TaqMan Universal PCR Mastermix (Applied Biosystems). Components were mixed according to the manufacturer’s instructions and amplified in 20μl total volume/well (96 well plates, Roche, Mannheim, Germany) using a Bio-Rad CFX96 Real-Time PCR System. Ct values were automatically generated by the CFX Manager 2.0 Software (Bio-Rad) and relative quantification of gene expression was calculated by standard ΔΔCt method using the expression of RPL30 as reference.
Western blotting
THP-1 cells treated with either CX3CR1 siRNA or non targeting control siRNA were collected, washed in PBS and lysed in RIPA buffer (Sigma Aldrich) including Protease Inhibitor Cocktail (Roche Diagnostics, Mannheim, Germany) 24h after transfection. Protein concentration was determined according to Lowry et al. and 40μg total protein were applied and separated on precast 10% Bis-Tris gels (NuPAGE, Novex; Invitrogen). After electrophoresis protein was blotted by semi-dry blotting on 0.2μm nitrocellulose membranes (Trans-Blot, Bio-Rad Laboratories) and blotting efficiency was determined by staining membranes with Ponceau S solution (Sigma Aldrich). Immunodetection was conducted with a chemiluminescent immunodetection kit (Western Breeze; Invitrogen) according to the manufacturer’s instructions. Polyclonal anti-human CX3CR1 antibody (SP7048P, Acris) was diluted in blocking solution 1:500 (2μg/ml working concentration) and applied to the membrane overnight at 4°C. For normalization membranes were incubated with monoclonal anti-beta actin antibody (1:20.000; clone AC-15, Abcam, Cambridge, UK). Images were acquired with FluorChem Q System (Alpha Innotech, Cell Bioscienes, Santa Clara, CA, USA) and band densities were analyzed with Alpha View SA software 3.4.0. Cell lysates were analyzed in duplicate from four independent experiments. Results are presented as a ratio of relative CX3CR1 and beta-actin band densities, with non targeting control siRNA treated cells set to one.
Statistical analysis
Data were analyzed using SigmaPlot 12.5 and are presented as means ± SEM. Data were subjected to Normality Test (Shapiro-Wilk test) and Equal Variance Test. In case of normally distributed data differences between groups were tested using two-tailed t-test. Otherwise Mann-Whitney Rank Sum Test was applied. For multiple comparison procedure One Way Repeated Measures Analysis of Variance was followed by Holm-Sidak method to isolate groups that differ from the others. A p-value of less than 0.05 was considered statistically significant.
Results
Expression of the fractalkine/CX3CR1 system in trophoblast and monocyte cell lines
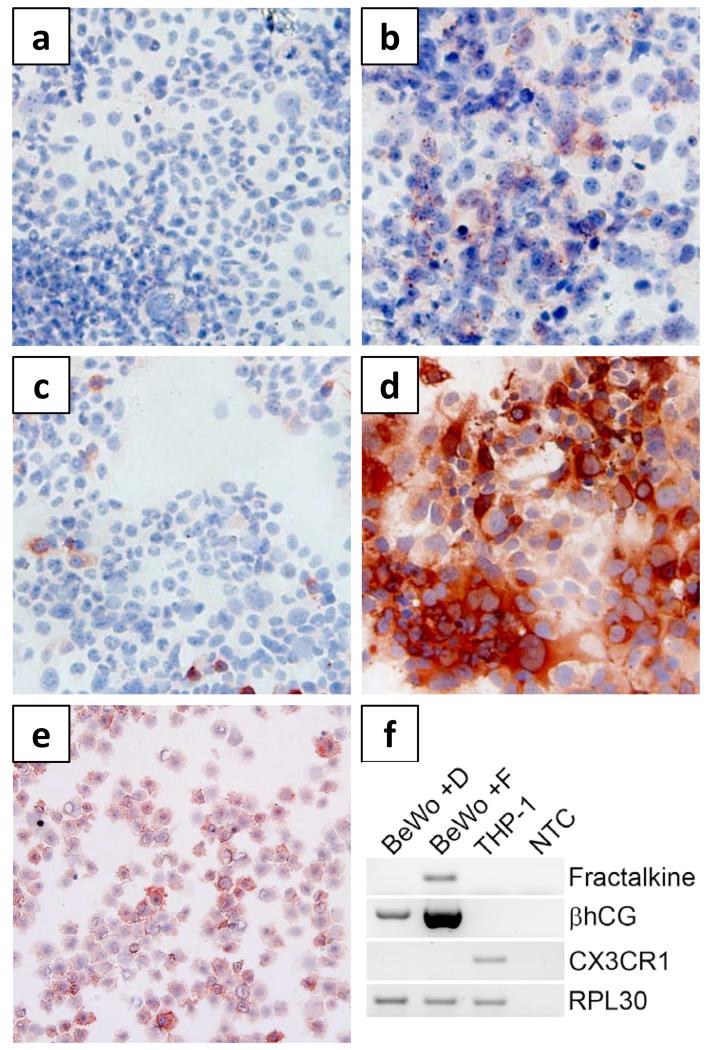
Immunocytochemistry of the trophoblast cell line BeWo, a model for the villous trophoblast population, offering good syncytialization and secretion of pregnancy-specific hormones in vitro (Wolfe 2006), showed virtually no fractalkine expression under basal culture condition (Fig. 1a). However, in the presence of Forskolin, a reagent known to induce trophoblast differentiation (Wice et al. 1990), immunocytochemistry showed fractalkine staining in a considerable proportion of BeWo cells (Fig. 1b). Differentiation of BeWo cells was confirmed by staining for the beta-subunit of human chorionic gonadotropin (βhCG), which was only sporadically detected under basal culture condition (Fig. 1c) and substantially increased upon Forskolin stimulation (Fig. 1d). Immunocytochemistry of the monocyte cell line THP-1 revealed a heterogeneous pattern of CX3CR1 expression, with intense staining of a subset of cells and moderate expression in the remaining population (Fig. 1e). RT-PCR analysis confirmed immunocytochemistry data (Fig. 1f). Furthermore, no CX3CR1 expression was shown in BeWo cells regardless of stimulation by Forskolin.
Figure 1. Fractalkine and CX3CR1 expression in the trophoblast cell line BeWo and THP-1 monocytes.
BeWo cells treated either with (a, c) solvent control DMSO (0.2%, 48h) or (b, d) Forskolin (20μM, 48h), were immunohistochemically stained for fractalkine (a, b) or βhCG (c, d). Cytospins of THP-1 monocytes were stained for CX3CR1 (e). RT-PCR revealed fractalkine expression in Forskolin treated BeWo cells (BeWo +F), while no expression was detected in cells treated with solvent control DMSO (BeWo +D) (f). Forskolin induced differentiation of BeWo cells was confirmed by increased expression of βhCG. CX3CR1 expression was detected in THP-1 monocytes, but not in BeWo cells. The expression of ribosomal protein L30 (RPL30) served as endogenous reference. No template control (NTC) did not show any amplification products.
Trophoblast syncytialization is accompanied by increased monocyte adhesion
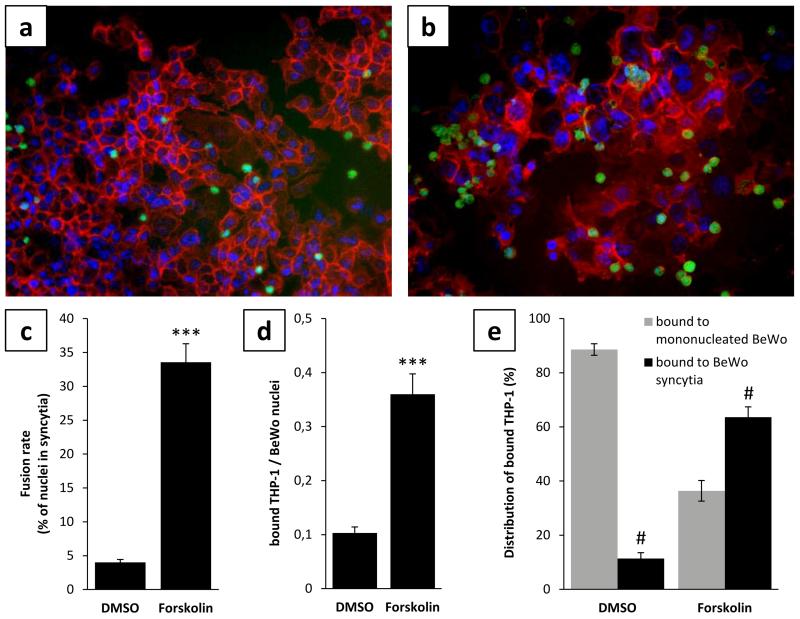
Beside biochemical differentiation of villous trophoblasts resulting in increasing hCG synthesis, morphological differentiation from the mononucleated to the syncytial state can be detected by remodelling of the membrane-associated spectrin-like alpha-fodrin (Gauster et al. 2010). Accordingly, BeWo cells under basal culture conditions showed distinct fodrin staining and appeared in a mononucleated state (Fig. 2a). However, when BeWo cells were undergoing differentiation induced by Forskolin, cell borders and the associated fodrin network vanished in areas of syncytialization (Fig. 2b) and 33.6% (± 2.72) of nuclei were present in syncytia after 48h (Fig. 2c).
Figure 2. BeWo syncytialization is accompanied by increased monocyte adhesion.
BeWo cells treated either with (a) solvent control DMSO (0.2%, 48h) or (b) Forskolin (20μM, 48h) were co-cultured with pre-labeled THP-1 monocytes (green) and stained by immunofluorescence for membrane-associated α-fodrin (red) to visualize syncytialization of BeWo and binding preference of THP-1 cells. Forskolin treatment induced syncytialization of BeWo cells with a fusion rate of 33.6%, whereas DMSO treated cells showed only 4.0% of nuclei in syncytia after 48h (c). THP-1 monocyte adherence to BeWo cells increased 3.5 fold after Forskolin treatment, when compared to control (d). THP-1 monocytes preferentially adhered to syncytia in Forskolin treated cells, whereas the majority of THP-1 bound mononucleated BeWo after DMSO treatment (e). Data are presented as mean ± SEM from three independent experiments performed in triplicates. ***p<0.001 (Forskolin vs DMSO treatment), #p<0.001 (bound to syncytia vs bound to mononucleated BeWo)
Differentiation and syncytialization of BeWo cells led to 3.5 fold increased binding of THP-1 monocytes. While the ratio of adhered monocytes to BeWo nuclei was only 0.10 (± 0.01) under basal culture conditions, monocyte adhesion increased to a ratio of 0.36 (± 0.04) when BeWo cells underwent Forskolin induced differentiation before (Fig. 2d). Assessment of the proportion of monocytes bound to syncytia and those bound to mononucleated BeWo, showed preferential adhesion of monocytes (63.6% ± 3.81) to syncytia in Forskolin stimulated BeWo cells (Fig. 2e). However, when BeWo cells were not stimulated before co-culture with THP-1 monocytes, the majority of monocytes (88.6% ± 2.13) adhered to mononucleated cells (Fig. 2e).
Monocyte adhesion to differentiated trophoblasts is mediated by fractalkine
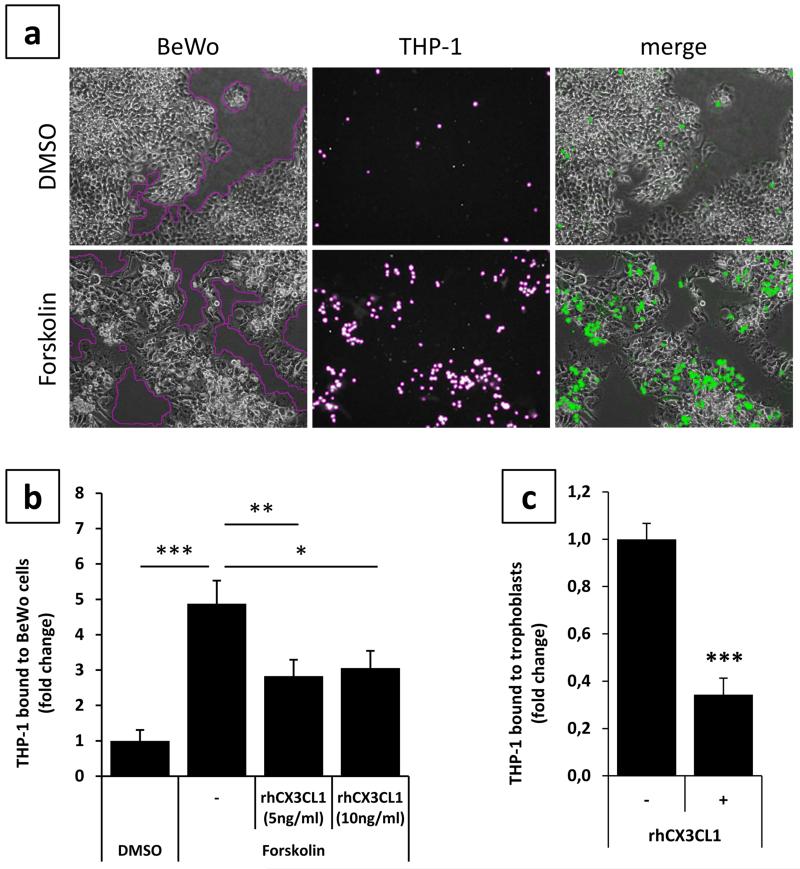
Differentiation and syncytialization of villous trophoblasts gives rise to reduced proliferation and morphological changes (Baczyk et al. 2009; Ramos et al. 2008). To ensure that obtained adhesion results were not due to a bias in evaluation of adhesion assays and different numbers of trophoblast nuclei in differentiated and non differentiated cells, a second strategy for analyzing adhesion was performed. This strategy was based on automatic measurement of trophoblast areas in relation to areas of adhered monocytes. For this purpose, phase contrast images (for trophoblast cell layers) and corresponding green fluorescence channel images (for adhered, green fluorescent labeled THP-1 cells) were taken (Fig. 3a). Automatic measurement of adhered THP-1 monocytes and trophoblast areas with subsequent relation to each other confirmed initial adhesion assays. If BeWo cells were stimulated with Forskolin for 48h prior to co-culture, adhesion of THP-1 monocytes increased 4.9 fold (± 0.65), compared to control (Fig. 3b).
Figure 3. CX3CR1 blocking on THP-1 monocytes impairs adherence to trophoblasts.
Monocyte binding was assessed by incubation of green fluorescence pre-labeled THP-1 monocytes with BeWo cells, pre-treated with either DMSO (0.2%, 48h, upper panel) or Forskolin (20μM, 48h, lower panel). Areas of trophoblast monolayers (phase contrast, first column) and bound THP-1 monocytes (fluorescence channel, second column) were measured by the Cell-IQ analyzer software and related to each other. Overlays (merge) of phase contrast images and corresponding fluorescence channel images are shown in the third column (a). Areas of bound THP-1 monocytes were related to areas of BeWo cells and data for solvent control DMSO was set as one. While Forskolin treatment of BeWo cells increased adherence of THP-1 monocytes 4.9 fold, pre-incubation of THP-1 monocytes with indicated concentrations of human recombinant fractalkine (rhCX3CL1) significantly impaired binding to Forskolin treated BeWo cells (b). Pre-incubation of THP-1 monocytes with 10ng/ml of rhCX3CL1 significantly decreased adherence to primary term trophoblasts (c). Data are presented as mean ± SEM from four independent BeWo cell experiments and three different term trophoblast isolations all performed in triplicates. *p<0.05, **p<0.01, ***p<0.001
In order to test whether or not THP-1 monocyte to trophoblast adhesion was mediated by the fractalkine/CX3CR1 system, the interaction of fractalkine and its receptor was blocked by addition of human recombinant fractalkine (rhCX3CL1). Pre-incubation of THP-1 monocytes with 5ng/ml and 10ng/ml rhCX3CL1 significantly impaired adherence to Forskolin stimulated BeWo cells by 41.9% and 37.3%, respectively (Fig. 3b). A similar effect was observed in co-culture experiments with THP-1 monocytes and primary term trophoblasts. Pre-incubation of THP-1 monocytes with 10ng/ml rhCX3CL1 markedly reduced adhesion of monocytes by 65.7% (± 0.07), compared to control (Fig. 3c).
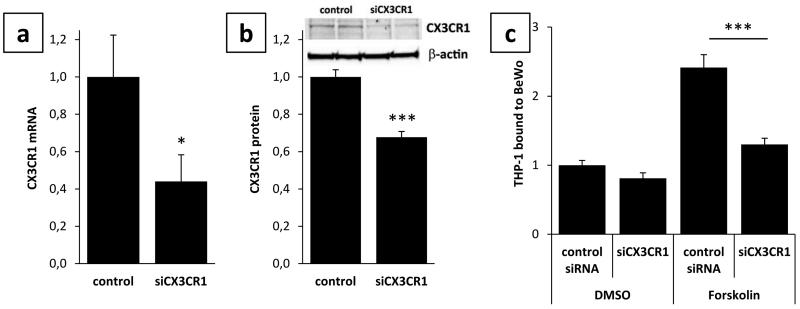
Besides blocking experiments, silencing of CX3CR1 expression in THP-1 monocytes was performed as a second strategy to elucidate the involvement of the fractalkine/CX3CR1 system in monocyte to trophoblast adhesion. qPCR analysis revealed a mean silencing efficiency of CX3CR1 expression by 55.9% (± 14.2) in THP-1 monocytes transfected with siRNA targeting CX3CR1, compared to non targeting control (Fig. 4a) 24h after transfection. Western Blot analysis confirmed qPCR data and showed a significant decrease of CX3CR1 protein to 62.6% (± 0.03) in cells transfected with siCX3CR1, compared to control (Fig. 4b). Silencing of CX3CR1 expression in THP-1 monocytes significantly reduced their adhesion to Forskolin stimulated BeWo cells by 46.3%, compared to control (Fig. 4c). In contrast, adhesion of CX3CR1 silenced THP-1 monocytes to undifferentiated BeWo cells decreased by only 18.9% (Fig. 4c), which did not reach statistical significance and may be explained by virtually no fractalkine expression in undifferentiated BeWo cells.
Figure 4. CX3CR1 silencing impairs THP-1 monocyte adherence to BeWo cells.
Transfection of BeWo cells with CX3CR1 targeting siRNA (siCX3CR1) significantly reduced CX3CR1 mRNA (a) and protein (b) levels after 24h, when compared to cells transfected with non targeting control. Silencing of CX3CR1 expression in THP-1 monocytes significantly decreased adhesion to Forskolin stimulated BeWo cells, but only marginally reduced binding to DMSO treated BeWo cells (c). Data are presented as mean ± SEM from three independent BeWo cell experiments performed in triplicates. *p<0.05, ***p<0.001
Discussion
Close cellular interaction and a synergistic dialogue between fetal and maternal cells is a prerequisite for successful pregnancy. This interaction is not restricted to very early processes of pregnancy, including implantation of the blastocyst or invasion of the extravillous trophoblast population into the maternal decidua, but also takes place later during ongoing gestation. Indeed, dissolution of the endovascular trophoblast plugs within the lumen of invaded spiral arteries at the end of first trimester results in onset of maternal blood flow into the intervillous space, enabling direct contact of circulating maternal blood cells and trophoblastic surfaces, i.e. the syncytiotrophoblast. However, under inflammatory conditions the interaction and dialogue between circulating maternal blood cells, in particular leukocytes, and the syncytiotrophoblast may get unbalanced and give rise to damage of the epithelial integrity. Adhesive properties of the syncytiotrophoblast are mediated through expression of molecules such as E-selectin, intercellular adhesion molecule (ICAM)-1 and ICAM-3 (Dye et al. 2001; Labarrere et al. 2014; Xiao et al. 1997).
The present study suggests fractalkine as another candidate amongst the panel of adhesion molecules enabling stable interaction between leukocytes and the syncytiotrophoblast. Our previous and current experiments with BeWo cells and primary term trophoblasts provide strong evidence that fractalkine expression is induced during differentiation and syncytialization from the mononucleated to the multinucleated state of villous trophoblast (Siwetz et al. 2014). This assumption is in good agreement with previous immunohistological observations, showing placental fractalkine located at the apical microvillous plasma membrane of the syncytiotrophoblast, but not in villous cytotrophoblasts (Siwetz et al. 2014). Differentiation of BeWo cells does not seem to be accompanied with induction of CX3CR1 expression, which was neither detected in undifferentiated nor differentiated BeWo cells. This observation is in contrast to a previous study by Hannan et al., who detected CX3CR1 expression in the trophoblast cell lines JEG-3, AC1M-32 and AC1M-88. Moreover, immunohistochemistry localized weak CX3CR1 expression in extravillous trophoblasts, the syncytiotrophoblast and the cell column of first trimester tissue, suggesting a role of fractalkine and its receptor CX3CR1 in trophoblast differentiation and directed trophoblast invasion (Hannan et al. 2006). However, with ongoing pregnancy CX3CR1 expression may be down-regulated in the villous trophoblast, as CX3CR1 was only detected in the fetal endothelium but not the villous trophoblast compartment at term (Joerink et al. 2011; Szukiewicz et al. 2013).
Here, two experimental strategies, including blocking as well as silencing of the fractalkine receptor CX3CR1, proved involvement of the fractalkine/CX3CR1 system in monocyte to villous trophoblast adherence in vitro. Our results also clearly demonstrate that stable monocyte to trophoblast interaction may be driven by more than fractalkine alone. First, monocytes not only adhered to differentiated, but also to undifferentiated BeWo cells, where fractalkine is virtually not expressed. Secondly, blocking and silencing of CX3CR1 in THP-1 monocytes did not completely abolish their adherence to differentiated BeWo and primary term trophoblasts, suggesting involvement of additional adherence mediators in our cell co-culture models. One putative candidate molecule may be ICAM-1, which was previously demonstrated to mediate monocyte adhesion to BeWo cells and primary trophoblasts in response to pro-inflammatory cytokines (Pfaff et al. 2005; Xiao et al. 1997). Pro-inflammatory cytokines, such as interleukin (IL)-1β, IL-17A, tumor necrosis factor (TNF)-α, and interferon (IFN)-gamma, are described to upregulate fractalkine expression in various epithelial cell types (Bhavsar et al. 2008; Dudas et al. 2011; Turner et al. 2010). Thus, it is tempting to speculate about upregulation of placental fractalkine under pro-inflammatory conditions and its implications on adhesion of monocytes to the syncytiotrophoblast. Tightly adherent monocytes are suggested to facilitate transmission of cell-associated infectious pathogens across the placental barrier by either mediating direct infection of the trophoblast or by transmigration of infected cells into the villous stroma (Labarrere et al. 2014; Xiao et al. 1997). Transmigration of maternal leukocytes could be enabled by local disruption of the syncytiotrophoblast by adhering monocytes, which release TNF-α and thereby contribute to the initiation of villitis (Garcia-Lloret et al. 2000). Moreover, upregulation of placental fractalkine, in response to pro-inflammatory cytokines, may also facilitate intervillous monocyte recruitment in cases of massive chronic intervillositis, which is characterized by the presence of a CD45 and CD68 reactive monocyte/macrophage infiltrate located almost exclusively between the villi (Labarrere et al. 2014).
In contrast, physiological implications of stable adhesion of maternal monocytes to the syncytiotrophoblast in healthy pregnancy are rather speculative. Xiao et al. suggested that adhesion of monocytes to the syncytiotrophoblast may be a normal mechanism of trophoblast renewal (Xiao et al. 1997). Interestingly, fractalkine expression as well as trophoblast apoptosis is induced by TNF-α and thus could be coordinated events (Bazan et al. 1997; Yui et al. 1994). Whether placental fractalkine mediates detection and elimination of aged trophoblasts by monocytes remains open. Another interesting hypothesis has recently been raised by Grasso et al., suggesting a bidirectional modulation of chemoattractant signals upon monocyte-trophoblast interaction. Accordingly, trophoblasts may be able to attract and prime monocytes to release a particular set of cytokines supporting their growth and survival (Grasso et al. 2014).
The fact that fractalkine is detected almost all over the microvillous plasma membrane of the syncytiotrophoblast in healthy placenta raises, however, the question by which mechanisms excessive adherence of CX3CR1 expressing maternal monocytes to the syncytiotrophoblast is prevented. One potential explanation may be sequestration of the CX3CR1 receptor on leukocytes by soluble fractalkine, which can be released from the maternal endothelium, but also from the syncytiotrophoblast itself (Siwetz et al. 2014). Another explanation as to how CX3CR1 expressing maternal monocytes are prevented from stable adherence to the syncytiotrophoblast may be detachment of transiently adhering monocytes by metalloprotease dependent cleavage of transmembrane fractalkine. Metalloprotease dependent shedding of transmembrane fractalkine has been suggested to generally decrease the adhesiveness of endothelial or epithelial cells for leukocytes, whereas absence of shedding activity mediates enhanced leukocyte adhesion to fractalkine expressing cells (Hundhausen et al. 2007; Schwarz et al. 2010). Shedding was also suggested to play an instrumental role after adherence of leukocytes to transmembrane fractalkine, resulting in detachment of adhered cells. Shedding of placental fractalkine from the syncytiotrophoblast microvillous plasma membrane occurs constitutively and may even be enhanced by stimulation. Interestingly, expression of both involved metalloproteases, a disintegrin and metalloprotease (ADAM)10 and ADAM17, has been shown to be increased in placentas from pregnancies complicated by pre-eclampsia (Yang et al. 2012; Zhao et al. 2010), suggesting a mechanism preventing fractalkine mediated leukocyte to syncytiotrophoblast adherence in this pregnancy pathology. Concerning the fractalkine/CX3CR1 system in human pregnancy, a well controlled balance between CX3CR1 expressing maternal leukocytes, placental transmembrane fractalkine and maternal serum level of soluble fractalkine may thus influence the degree of leukocyte to syncytiotrophoblast adhesion.
Acknowledgements
The authors are indebted to Renate Michelmayr (Department of Obstetrics and Gynaecology, Medical University of Graz, Austria) for her excellent technical assistance in cell isolation and cell culture work. M. Gauster is supported by the Austrian Science Fund (FWF): P23859-B19. The Deutsche Forschungsgemeinschaft (DFG) supported F. Herse (HE6249/1-1).
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- Baczyk D, Drewlo S, Proctor L, Dunk C, Lye S, Kingdom J. Glial cell missing-1 transcription factor is required for the differentiation of the human trophoblast. Cell Death Differ. 2009;16:719–727. doi: 10.1038/cdd.2009.1. [DOI] [PubMed] [Google Scholar]
- Bazan JF, Bacon KB, Hardiman G, Wang W, Soo K, Rossi D, Greaves DR, Zlotnik A, Schall TJ. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385:640–644. doi: 10.1038/385640a0. [DOI] [PubMed] [Google Scholar]
- Bhavsar PK, Sukkar MB, Khorasani N, Lee KY, Chung KF. Glucocorticoid suppression of CX3CL1 (fractalkine) by reduced gene promoter recruitment of NF-kappaB. FASEB J. 2008;22:1807–1816. doi: 10.1096/fj.07-094235. [DOI] [PubMed] [Google Scholar]
- Blaschitz A, Weiss U, Dohr G, Desoye G. Antibody reaction patterns in first trimester placenta: implications for trophoblast isolation and purity screening. Placenta. 2000;21:733–741. doi: 10.1053/plac.2000.0559. [DOI] [PubMed] [Google Scholar]
- Cammas L, Reinaud P, Dubois O, Bordas N, Germain G, Charpigny G. Identification of differentially regulated genes during elongation and early implantation in the ovine trophoblast using complementary DNA array screening. Biol Reprod. 2005;72:960–967. doi: 10.1095/biolreprod.104.034801. [DOI] [PubMed] [Google Scholar]
- Cervar M, Blaschitz A, Dohr G, Desoye G. Paracrine regulation of distinct trophoblast functions in vitro by placental macrophages. Cell Tissue Res. 1999;295:297–305. doi: 10.1007/s004410051236. [DOI] [PubMed] [Google Scholar]
- D’Haese JG, Friess H, Ceyhan GO. Therapeutic potential of the chemokine-receptor duo fractalkine/CX3CR1: an update. Expert Opin Ther Targets. 2012;16:613–618. doi: 10.1517/14728222.2012.682574. [DOI] [PubMed] [Google Scholar]
- Dudas PL, Sague SL, Elloso MM, Farrell FX. Proinflammatory/profibrotic effects of interleukin-17A on human proximal tubule epithelium. Nephron Exp Nephrol. 2011;117:e114–23. doi: 10.1159/000320177. [DOI] [PubMed] [Google Scholar]
- Dye JF, Jablenska R, Donnelly JL, Lawrence L, Leach L, Clark P, Firth JA. Phenotype of the endothelium in the human term placenta. Placenta. 2001;22:32–43. doi: 10.1053/plac.2000.0579. [DOI] [PubMed] [Google Scholar]
- Garcia-Lloret MI, Winkler-Lowen B, Guilbert LJ. Monocytes adhering by LFA-1 to placental syncytiotrophoblasts induce local apoptosis via release of TNF-alpha. A model for hematogenous initiation of placental inflammations. J Leukoc Biol. 2000;68:903–908. [PubMed] [Google Scholar]
- Gauster M, Berghold VM, Moser G, Orendi K, Siwetz M, Huppertz B. Fibulin-5 expression in the human placenta. Histochem Cell Biol. 2011;135:203–213. doi: 10.1007/s00418-011-0784-4. [DOI] [PubMed] [Google Scholar]
- Gauster M, Hiden U, Blaschitz A, Frank S, Lang U, Alvino G, Cetin I, Desoye G, Wadsack C. Dysregulation of placental endothelial lipase and lipoprotein lipase in intrauterine growth-restricted pregnancies. J Clin Endocrinol Metab. 2007;92:2256–2263. doi: 10.1210/jc.2006-2403. [DOI] [PubMed] [Google Scholar]
- Gauster M, Hiden U, van Poppel M, Frank S, Wadsack C, Hauguel-de Mouzon S, Desoye G. Dysregulation of placental endothelial lipase in obese women with gestational diabetes mellitus. Diabetes. 2011;60:2457–2464. doi: 10.2337/db10-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauster M, Siwetz M, Orendi K, Moser G, Desoye G, Huppertz B. Caspases rather than calpains mediate remodelling of the fodrin skeleton during human placental trophoblast fusion. Cell Death Differ. 2010;17:336–345. doi: 10.1038/cdd.2009.133. [DOI] [PubMed] [Google Scholar]
- Grasso E, Paparini D, Hauk V, Salamone G, Leiros CP, Ramhorst R. Differential migration and activation profile of monocytes after trophoblast interaction. PLoS One. 2014;9:e97147. doi: 10.1371/journal.pone.0097147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannan NJ, Jones RL, White CA, Salamonsen LA. The chemokines, CX3CL1, CCL14, and CCL4, promote human trophoblast migration at the feto-maternal interface. Biol Reprod. 2006;74:896–904. doi: 10.1095/biolreprod.105.045518. [DOI] [PubMed] [Google Scholar]
- Hannan NJ, Salamonsen LA. CX3CL1 and CCL14 regulate extracellular matrix and adhesion molecules in the trophoblast: potential roles in human embryo implantation. Biol Reprod. 2008;79:58–65. doi: 10.1095/biolreprod.107.066480. [DOI] [PubMed] [Google Scholar]
- Hundhausen C, Schulte A, Schulz B, Andrzejewski MG, Schwarz N, von Hundelshausen P, Winter U, Paliga K, Reiss K, Saftig P, Weber C, Ludwig A. Regulated shedding of transmembrane chemokines by the disintegrin and metalloproteinase 10 facilitates detachment of adherent leukocytes. J Immunol. 2007;178:8064–8072. doi: 10.4049/jimmunol.178.12.8064. [DOI] [PubMed] [Google Scholar]
- Huppertz B, Berghold VM, Kawaguchi R, Gauster M. A variety of opportunities for immune interactions during trophoblast development and invasion. Am J Reprod Immunol. 2012;67:349–357. doi: 10.1111/j.1600-0897.2012.01124.x. [DOI] [PubMed] [Google Scholar]
- Imai T, Hieshima K, Haskell C, Baba M, Nagira M, Nishimura M, Kakizaki M, Takagi S, Nomiyama H, Schall TJ, Yoshie O. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell. 1997;91:521–530. doi: 10.1016/s0092-8674(00)80438-9. [DOI] [PubMed] [Google Scholar]
- Jauniaux E, Watson AL, Hempstock J, Bao YP, Skepper JN, Burton GJ. Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am J Pathol. 2000;157:2111–2122. doi: 10.1016/S0002-9440(10)64849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joerink M, Rindsjo E, van Riel B, Alm J, Papadogiannakis N. Placental macrophage (Hofbauer cell) polarization is independent of maternal allergen-sensitization and presence of chorioamnionitis. Placenta. 2011;32:380–385. doi: 10.1016/j.placenta.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Labarrere CA, Bammerlin E, Hardin JW, Dicarlo HL. Intercellular adhesion molecule-1 expression in massive chronic intervillositis: Implications for the invasion of maternal cells into fetal tissues. Placenta. 2014;35:311–317. doi: 10.1016/j.placenta.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Pfaff AW, Georges S, Abou-Bacar A, Letscher-Bru V, Klein JP, Mousli M, Candolfi E. Toxoplasma gondii regulates ICAM-1 mediated monocyte adhesion to trophoblasts. Immunol Cell Biol. 2005;83:483–489. doi: 10.1111/j.1440-1711.2005.01356.x. [DOI] [PubMed] [Google Scholar]
- Ramos AJ, Cantero MR, Zhang P, Raychowdhury MK, Green A, MacPhee D, Cantiello HF. Morphological and electrical properties of human trophoblast choriocarcinoma, BeWo cells. Placenta. 2008;29:492–502. doi: 10.1016/j.placenta.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Schwarz N, Pruessmeyer J, Hess FM, Dreymueller D, Pantaler E, Koelsch A, Windoffer R, Voss M, Sarabi A, Weber C, Sechi AS, Uhlig S, Ludwig A. Requirements for leukocyte transmigration via the transmembrane chemokine CX3CL1. Cell Mol Life Sci. 2010;67:4233–4248. doi: 10.1007/s00018-010-0433-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siwetz M, Blaschitz A, Kremshofer J, Bilic J, Desoye G, Huppertz B, Gauster M. Metalloprotease dependent release of placenta derived fractalkine. Mediators Inflamm. 2014;2014:839290. doi: 10.1155/2014/839290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szukiewicz D, Kochanowski J, Mittal TK, Pyzlak M, Szewczyk G, Cendrowski K. Chorioamnionitis (ChA) modifies CX3CL1 (fractalkine) production by human amniotic epithelial cells (HAEC) under normoxic and hypoxic conditions. J Inflamm (Lond) 2014a;11 doi: 10.1186/1476-9255-11-12. 12-9255-11-12. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szukiewicz D, Kochanowski J, Mittal TK, Pyzlak M, Szewczyk G, Cendrowski K. CX3CL1 (fractalkine) and TNFalpha production by perfused human placental lobules under normoxic and hypoxic conditions in vitro: the importance of CX3CR1 signaling. Inflamm Res. 2014b;63:179–189. doi: 10.1007/s00011-013-0687-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szukiewicz D, Kochanowski J, Pyzlak M, Szewczyk G, Stangret A, Mittal TK. Fractalkine (CX3CL1) and Its Receptor CX3CR1 May Contribute to Increased Angiogenesis in Diabetic Placenta. Mediators Inflamm. 2013;2013:437576. doi: 10.1155/2013/437576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner SL, Mangnall D, Bird NC, Blair-Zajdel ME, Bunning RA. Effects of pro-inflammatory cytokines on the production of soluble fractalkine and ADAM17 by HepG2 cells. J Gastrointestin Liver Dis. 2010;19:265–271. [PubMed] [Google Scholar]
- Wice B, Menton D, Geuze H, Schwartz AL. Modulators of cyclic AMP metabolism induce syncytiotrophoblast formation in vitro. Exp Cell Res. 1990;186:306–316. doi: 10.1016/0014-4827(90)90310-7. [DOI] [PubMed] [Google Scholar]
- Wolfe MW. Culture and transfection of human choriocarcinoma cells. Methods Mol Med. 2006;121:229–239. doi: 10.1385/1-59259-983-4:227. [DOI] [PubMed] [Google Scholar]
- Xiao J, Garcia-Lloret M, Winkler-Lowen B, Miller R, Simpson K, Guilbert LJ. ICAM-1-mediated adhesion of peripheral blood monocytes to the maternal surface of placental syncytiotrophoblasts: implications for placental villitis. Am J Pathol. 1997;150:1845–1860. [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Wang Y, Zeng X, Ma XJ, Zhao Y, Qiao J, Cao B, Li YX, Ji L, Wang YL. Self-control of HGF regulation on human trophoblast cell invasion via enhancing c-Met receptor shedding by ADAM10 and ADAM17. J Clin Endocrinol Metab. 2012;97:E1390–401. doi: 10.1210/jc.2012-1150. [DOI] [PubMed] [Google Scholar]
- Yui J, Garcia-Lloret M, Wegmann TG, Guilbert LJ. Cytotoxicity of tumour necrosis factor-alpha and gamma-interferon against primary human placental trophoblasts. Placenta. 1994;15:819–835. doi: 10.1016/s0143-4004(05)80184-5. [DOI] [PubMed] [Google Scholar]
- Zhao S, Gu Y, Fan R, Groome LJ, Cooper D, Wang Y. Proteases and sFlt-1 release in the human placenta. Placenta. 2010;31:512–518. doi: 10.1016/j.placenta.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]