Abstract
Abstract
There has been remarkable progress in the treatment of cystic fibrosis (CF) patients over the past 20 years. However, limitations of standard therapies have highlighted the need for a convenient alternative treatment to effectively target the pathophysiologic basis of CF-related disease by improving mucociliary clearance of airway secretions and consequently improve lung function and reduce respiratory exacerbations. Mannitol is an osmotic agent available as a dry powder, dispensed in a convenient disposable inhaler device for the treatment of adult patients with CF. Inhalation of mannitol as a dry powder is thought to change the viscoelastic properties of airway secretions, increase the hydration of the airway surface liquid and contribute to increased mucociliary and cough clearance of retained secretions. In two large phase 3 studies [1, 2], long-term use of inhaled mannitol resulted in a significant and clinically meaningful improvement in lung function relative to control in adult CF subjects and had an acceptable safety profile. Clinical experience with inhaled mannitol confirms that it is safe and effective. A minority of patients are unable to tolerate the medication. However, through training in proper inhaler technique and setting clear expectations regarding therapeutic effects, both the tolerance and adherence necessary for long term efficacy can be positively influenced.
Educational aims
To discuss the importance of airway clearance treatments in the management of cystic fibrosis.
To describe the clinical data that supports the use of mannitol in adult patients with cystic fibrosis.
To highlight the role of mannitol tolerance testing in screening for hyperresponsiveness.
To provide practical considerations for patient education in use of mannitol inhaler.
Key points
Inhaled mannitol is a safe and effective option in adult patients with cystic fibrosis.
Mannitol tolerance testing effectively screens for hyperresponsiveness prior to initiation of therapy.
Physiotherapists and respiratory therapists play an integral role in the introduction and maintenance of dry powder inhalation therapy.
Patient training and follow-up is important for optimising longer term adherence.
Introduction
Chronic airways infection and inflammation is associated with considerable morbidity and early mortality in patients with cystic fibrosis (CF) [3]. In CF, the lung airway surface liquid is depleted, resulting in a defective mucociliary clearance. This contributes to retained airways phlegm, which promotes infection and inflammation within the airway lumen and wall, leading to airway remodelling. Initial reversible injury eventually results in refractory bronchiectasis and progressive loss of lung function [4].
Mechanical clearance of secretions from the airways is a primary therapy for CF patients, with a number of airway clearance therapies (ACTs) recommended in current guidelines [5]. There are recommended medications given by inhalation, including hypertonic saline and recombinant human deoxyribonuclease (rhDNase), that are used as an adjunct to ACTs. These agents alter the rheological properties of airway phlegm, thereby easing its clearance from the airways [5]. Inhaled rhDNase improves lung function by digesting high molecular weight DNA that is typically released in the CF airways by dead neutrophils and which contributes to the secretion viscosity [6, 7]. Inhaled hypertonic saline has been investigated as both an alternative and parallel treatment to rhDNase [8]. It has been demonstrated to increase airway surface liquid volume in vitro and modestly improve lung function in patients with CF [9–12]. Although recommended in guidelines, hypertonic saline is not a regulatory approved treatment and there are widely recognised tolerability issues in some patients [13, 14]. In addition, the administration of nebulised saline is time consuming and is associated with poor adherence [8]. This highlights the need for a convenient alternative treatment to effectively improve clearance of airway secretions and lung function.
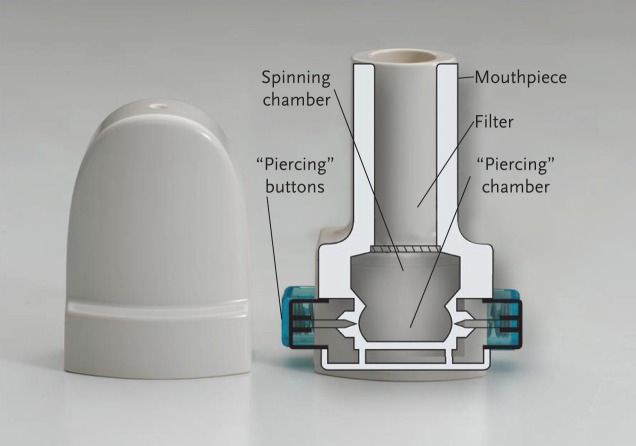
The naturally occurring sugar alcohol, mannitol, is an osmotic agent that has been studied as a dry powder formulated as 3-micron spheres for optimised inhaled use through a convenient disposable inhaler device for the treatment of patients with CF [15]. Inhalation of mannitol as a dry powder is thought to change the viscoelastic properties of airway phlegm, increase the hydration of the airway surface liquid and contribute to increased clearance of the retained secretions through mucociliary activity and productive cough (see video showing mannitol's mode of action, presented in the supplementary material alongside the online version of this article at breathe.ersjournals.com) [16]. As it does not require refrigeration, nebulisation, routine equipment cleaning or sterilisation, mannitol may provide a convenient treatment for CF patients, or an alternative option for adults who already have a high treatment burden (see fig. 1) [17].
Figure 1.
Mannitol inhaler.
Two recent, near identical, randomised, multicentre, double-blind, controlled, parallel-group phase 3 studies (CF301 and CF302) investigated the safety and efficacy of inhaled mannitol in subjects with CF over a period of 6 months [1, 2, 18]. These studies demonstrated clinically relevant benefits of inhaled mannitol even in study populations that were heavily treated with standard therapies. Inhaled mannitol was recently approved for use in adults in Europe. It is therefore relevant to present data specific to adult patients, distinct from the paediatric subjects who were included in previous publications. The pooled data from adult subjects (aged ≥18 years) in CF301 and CF302 are presented here and are derived using the statistical methodologies applied to the overall populations [18].
Methods
Upon passing a mannitol tolerance test given to subjects at screening (sequential administrations of incremental doses of mannitol up to a maximum dose of 400 mg to assess hyperresponsiveness), subjects were randomised 3:2 to receive either mannitol 400 mg or control medication (mannitol 50 mg), respectively, twice daily for 26 weeks. A 50 mg subtherapeutic dose of mannitol was selected as control for the phase 3 studies using capsules indistinguishable from the active ones. The use of a placebo, such as inhalation-grade lactose was not feasible because safety data were not available for the quantities required for these clinical trials and blinding would be further complicated by the different taste of lactose. Similarly, nonrespirable mannitol was also considered unsuitable for use as a control due to concern that the large particle size might act as an irritant and any resultant lung function deterioration would make assessment problematic. Both studies had an open-label phase, in which participating subjects received mannitol for an additional 26 weeks or longer. Detailed methodologies of the studies have been published previously [1, 2, 18]. Enrolled subjects had a diagnosis of CF with a defined range of baseline forced expiration volume in 1 s (FEV1) between 30 and 90% of predicted (study CF301), and between 40 and 90% of predicted (study CF302). Continuation of all approved CF therapies aside from hypertonic saline was permitted. The primary efficacy outcome for both studies was FEV1 [18]. Additional efficacy outcomes assessed included the post-dose sputum weight and frequency of pulmonary exacerbations. Subgroups were analysed based on rhDNase use and safety was assessed based on adverse events and monitoring of laboratory parameters.
Results
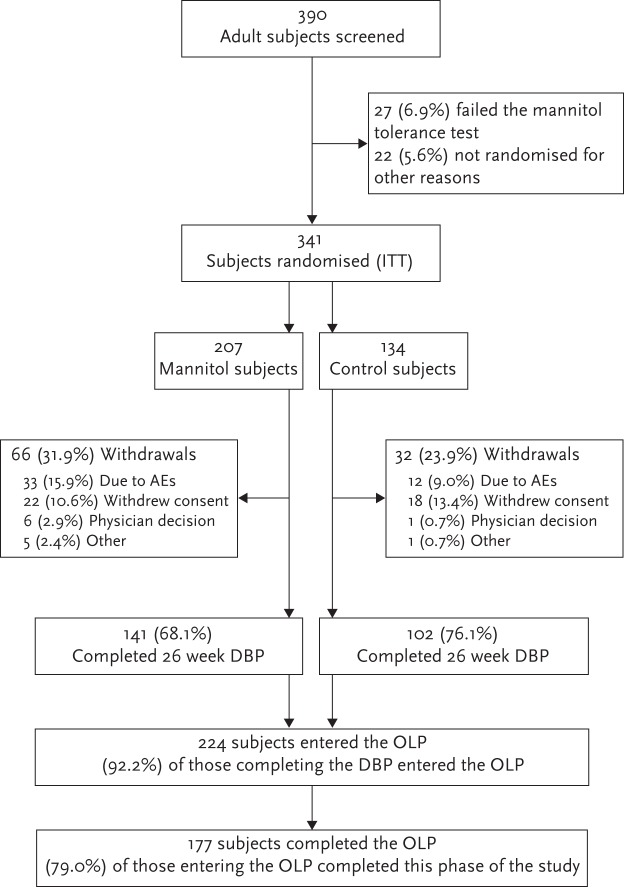
A total of 390 adult subjects were screened for inclusion, of whom 341 were randomised to receive either mannitol (n=207) or control (n=134). Overall, 141 subjects from the mannitol group and 102 subjects from the control group completed the 26-week double-blind phase. As shown in figure 2, a total of 130 and 94 adult subjects from the mannitol and control groups, respectively, consented to enter the optional open-label phase and received 400 mg mannitol twice daily.
Figure 2.
Disposition of adult subjects in studies CF-301 and CF-302. AE: adverse event; OLP: open-label phase; DBP: double-blind phase; ITT: intention-to-treat.
Baseline characteristics of the adult subjects were generally comparable between treatment groups (table 1). However, there were more male subjects in the mannitol group (61.4 versus 53.0% in the control group), and more subjects receiving rhDNase treatment in the control group (63.4 versus 58.9% in the mannitol group).
Table 1.
Baseline characteristics of adult subjects enrolled in CF-301 and CF-302
| Characteristic | Mannitol 400 mg twice daily (n=207) | Control 50 mg twice daily (n=134) |
| Age years | ||
| Mean±sd | 28.3±8.75 | 28.8±8.46 |
| Range | 18–56 | 18–53 |
| Sex n (%) | ||
| Male | 127 (61.4) | 71 (53.0) |
| FEV1 % predicted | ||
| Mean±sd | 59.8±15.6 | 58.4±15.8 |
| Median (range) | 60.4 (26.4–92.6) | 54.2 (29.2–92.3) |
| FEV1 L | ||
| Mean±sd | 2.34 (0.80) | 2.16 (0.70) |
| Median (range) | 2.20 (0.88–4.92) | 2.02 (1.03–4.12) |
| BMI kg·m−2 | ||
| Mean±sd | 22.6±3.73 | 22.1±3.25 |
| Range | 15.3–44.6 | 14.9–33.4 |
| rhDNase treatment n (%) | 122 (58.9) | 85 (63.4) |
| Inhaled antibiotics n (%) | ||
| Tobramycin | 72 (34.8) | 47 (35.1) |
| Colistin | 62 (30.0) | 39 (29.1) |
| Systemic antibodies n (%) | ||
| Azithromcyin | 121 (58.5) | 75 (56.0) |
| Drugs for obstructive airway disease n (%) | ||
| Short-acting bronchodilator# | 201 (97.1) | 126 (94.0) |
| Inhaled corticosteroids¶ | 135 (65.2) | 93 (69.4) |
BMI: body mass index; FEV1: forced expiration volume in 1 s. #: salbutamol (albuterol) or terbutaline; ¶: includes subjects on combination of an inhaled corticosteroid and a β agonist.
Effects of mannitol on lung function
Study CF301 and study CF302 both demonstrated a statistically significant improvement in lung function (FEV1 and forced vital capacity (FVC)) in the subgroup of adult patients compared with control (difference in FEV1 for CF301 and CF302 respectively was 108.46 mL (95% CI 47.56–169.35; p<0.001) and 85.94 mL (95% CI 4.63–167.26; p=0.038)).
For the pooled analysis, 317 adult subjects (188 mannitol and 129 control) had FEV1 data at baseline and one post-baseline visit. Over 26 weeks, mannitol improved lung function as demonstrated by a mean absolute change in FEV1 from baseline compared with control of +99.5 mL (95% CI 49.1–149.9; p=0.001) and a relative change in % predicted for normal compared with control of +4.72% (95% CI 1.86–7.59; p=0.001). Statistically significant improvements in FEV1 were sustained over the duration of the double-blind phase (fig. 3). Improvements in lung function were maintained for an additional 26 weeks in mannitol-treated subjects who consented to enter the open-label phase, indicating that the beneficial effects of mannitol on lung function can be sustained with prolonged treatment (fig. 4).
Figure 3.
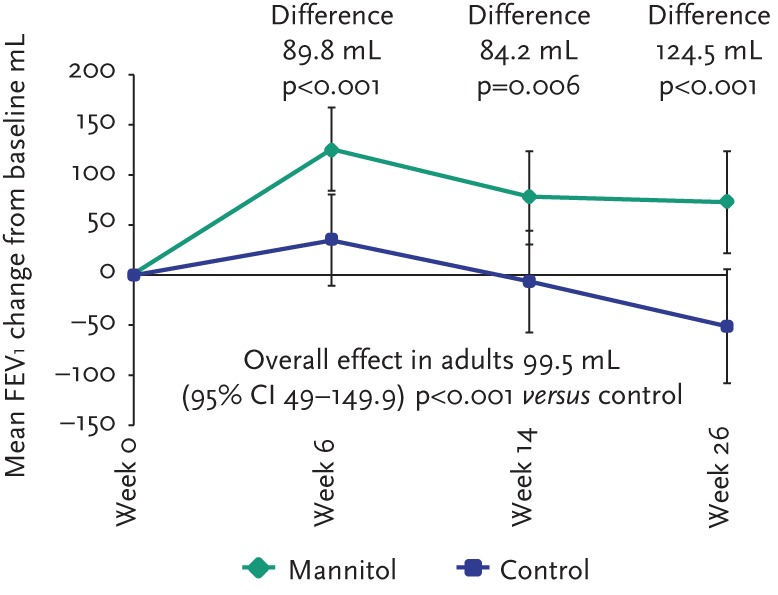
Change in FEV1 from baseline sustained during double-blind phase of studies (intention-to-treat).
Figure 4.
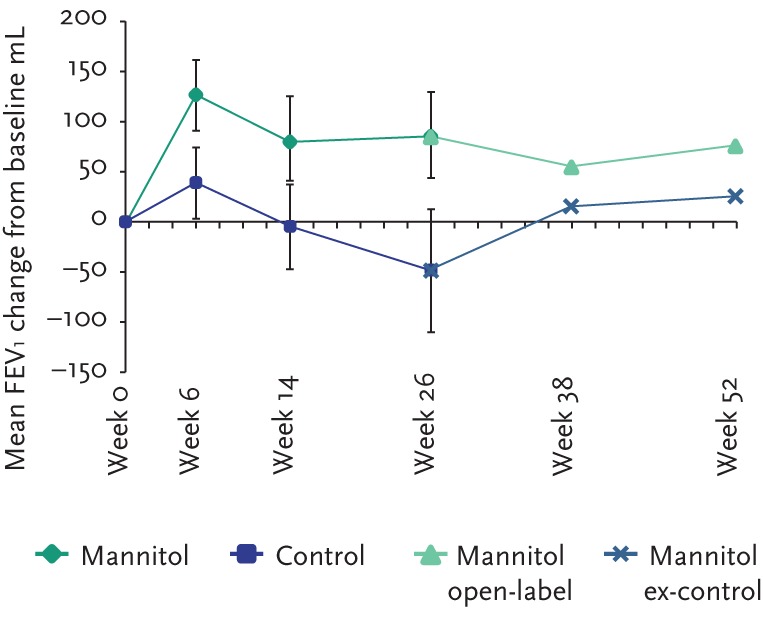
Sustained improvements in lung function from baseline in patients who completed the double-blind phase and entered the open-label extension phase.
The individual subject response at week 6 was highly predictive of longer-term FEV1 changes as shown in figure 5. Furthermore, subjects in whom FEV1 improved over 26 weeks suffered fewer exacerbations (see fig. 6). In subjects who had received control medication (mannitol 50 mg twice daily) in the double-blind phase, the mean FEV1 increased upon switching treatment to mannitol 400 mg twice daily in the open-label phase. At week 52, the mean FEV1 had increased by 74.7 mL in these subjects.
Figure 5.
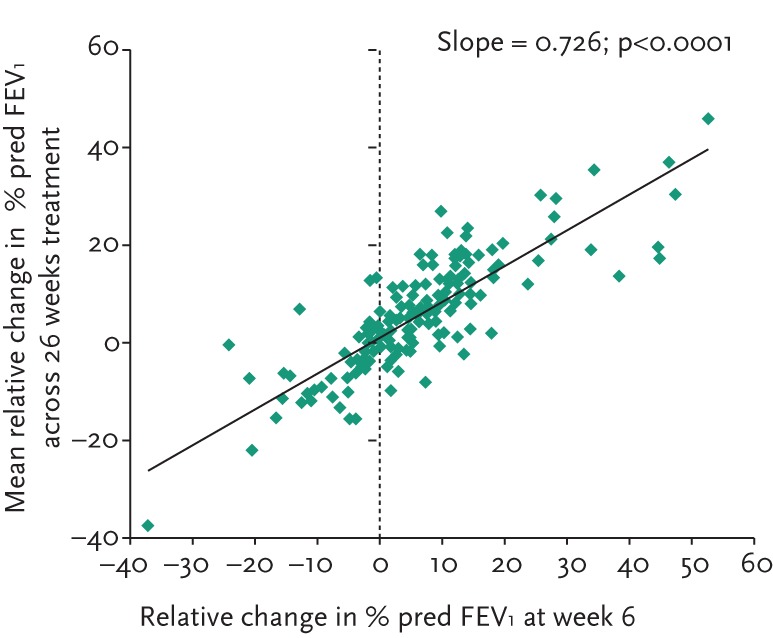
Scatter plot showing correlation between FEV1 response at week 6 and over 26 weeks.
Figure 6.
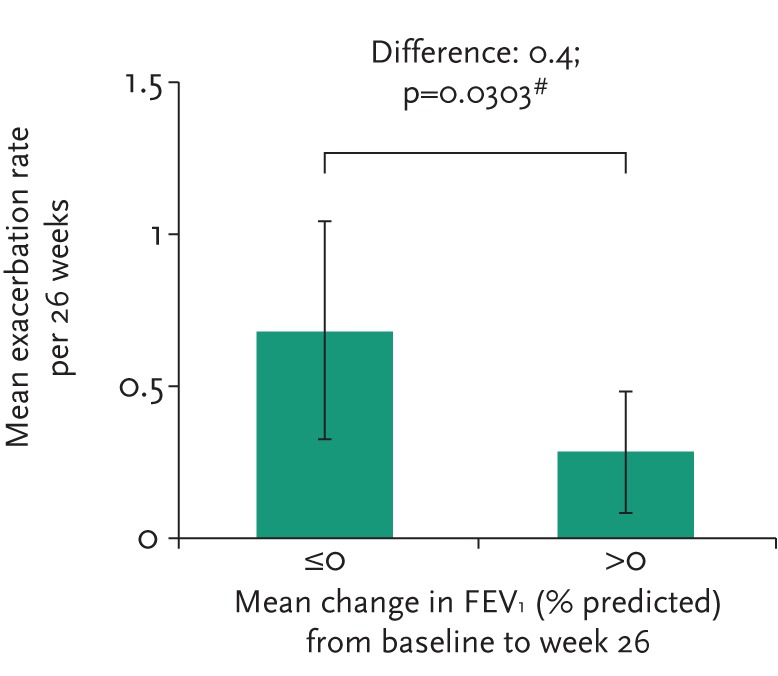
Exacerbation rate by change from baseline in FEV1 during study period.
Lung function in other subgroups of adult subjects
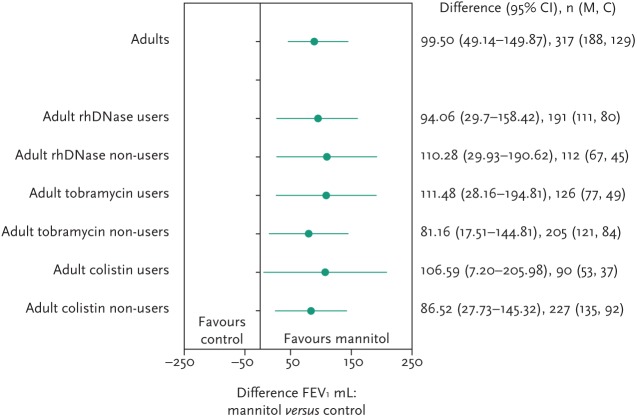
To assess the efficacy of inhaled mannitol when used in conjunction with best standard care, lung function scores were compared in various treatment groups. Improvements were seen irrespective of rhDNase, tobramycin or colistin use, as shown in figure 7.
Figure 7.
FEV1 (mL) changes in mannitol treated patients by concomitant therapy.
Effects of mannitol on the frequency of pulmonary exacerbations
Overall, adult subjects treated with inhaled mannitol had a meaningful trend in reduction in risk for pulmonary exacerbations during the 26-week double-blind phase, with a relative risk versus control of 0.76 (95% CI 0.51–1.13). This appeared to be driven by mannitol-treated subjects who had improved FEV1 between baseline and week 26. These subjects had a significantly reduced frequency of pulmonary exacerbations relative to those whose lung function did not respond (relative risk 0.4; p=0.03).
Withdrawals, education and compliance
There are challenges associated with the introduction of an inhaled dry powder, particularly at large doses. In study CF301, there was a dropout rate of 35.3%, which led to additional instruction of study coordinators to teach subjects proper inhaler technique with reinforcement and additional training provided during study CF302. The withdrawal rate in study CF302 was 20.5%, a 41.9% reduction compared with CF301. Overall, median treatment compliance as measured by capsule returns in adult subjects who stayed on medication for the duration of the study was over 88% and 91% in the mannitol and control groups, respectively. Overall, mannitol was well tolerated by adult subjects with CF, although there was a higher drop-out rate overall in the mannitol group compared with the control group
Safety
Adverse events in adults were experienced by similar proportions of adult subjects in both treatment groups (see table 2). Adverse events were generally mild or moderate, and consistent with CF and its standard therapies. There was a greater incidence of adverse events leading to discontinuation in mannitol-treated subjects compared with those on control (15.5% and 9.0% respectively). The most frequently reported adverse event in both treatment groups was “condition aggravated” (pulmonary exacerbation). Serious adverse events that were considered related to treatment were infrequent (3.4% for mannitol, 1.5% for control). Respiratory adverse events are of special interest with inhaled therapies. The most commonly reported respiratory adverse events occurring more frequently in mannitol-treated subjects were cough, haemoptysis and pharyngeal pain.
Table 2.
Summary of adverse events in pooled adult analysis
| Mannitol 400 mg twice daily (n=207) | Control 50 mg twice daily (n=134) | |
| Patients with ≥1 AE | 86.0 | 85.8 |
| Patients with ≥1 AE leading to discontinuation from the study | 15.5 | 9.0 |
| AEs by MedDRA preferred term (occurring in ≥10% patients overall)# | ||
| Condition aggravated | 37.2 | 41.0 |
| Headache | 15.5 | 19.4 |
| Cough | 18.8 | 11.9 |
| Lower respiratory tract infection | 8.2 | 12.7 |
| Bacteria sputum identified | 12.1 | 9.0 |
| Nasopharyngitis | 8.7 | 11.2 |
| Haemoptysis¶ | 10.6 | 8.2 |
| Patients with ≥1 SAE | 22.7 | 26.9 |
| SAEs by MedDRA preferred term (occurring in ≥1% patients overall)# | ||
| Condition aggravated | 16.9 | 17.9 |
| Haemoptysis | 2.4 | 0.7 |
| Lower respiratory tract infection | 1.9 | 2.2 |
| Constipation | 0.0 | 1.5 |
| Pneumonia | 0.0 | 1.5 |
| Intestinal obstruction | 0.0 | 1.5 |
| Catheterisation venous | 1.0 | 0.0 |
AE: adverse event; SAE: serious adverse event; MedDRA: Medical Dictionary for Regulatory Activities.
#: patients are counted once for each unique preferred term identified from the CRF verbatim text; ¶: refers to haemoptysis reported as an AE, but does not include all haemoptysis events that were recorded as part of an exacerbation. Overall incidence of haemoptysis including events reported exclusively as part of an exacerbation was 15.5% in mannitol arm and 17.9% in control.
As haemoptysis is frequently a component of pulmonary exacerbations, haemoptysis occurring as a component of a pulmonary exacerbation was noted if it occurred, regardless of whether it was reported separately as a haemoptysis adverse event. The incidence of exacerbation-related haemoptysis episodes in mannitol treated subjects was lower than the incidence in the control group (mannitol 4.8% versus control 9.7%). The total incidence of haemoptysis, including all events reported as a haemoptysis adverse event or those occurring as part of an exacerbation was marginally lower in the mannitol arm (15.5% and 17.9%, for mannitol and control, respectively).
There was no change in growth of CF organisms with mannitol and no difference in growth between mannitol and control over 26 weeks.
Clinical experience with inhaled mannitol
Clinical experience with inhaled mannitol in 20 patients was recently presented [19]. Positive and negative factors that may influence adherence were reported. Positive factors identified included the brevity and simplicity of administration with inhalation of 10 capsules taking about 5 min. The inhaler is breath actuated, portable and disposable. It requires no special care and does not require sterilisation. All of these features are intended to improve acceptability of the preparation. Negative factors that were identified included excessive coughing on inhalation in some patients and no perceived short-term benefit. These responses highlight the importance of education, particularly on correct inhalation technique, to the acceptance and effectiveness of this therapy as part of daily care. A trial of approximately 6 weeks is therefore encouraged before efficacy is reviewed meaning that an early review of response can be used to inform a decision whether to continue and so help manage adherence/treatment burden. For the management of hacking, dry and irritated cough, patients were advised to slow down the speed of inhalation, take sips of water between capsules and take time to settle the cough. Productive cough was managed by patients taking a moment between capsules to get control of their coughing and by practicing controlled huffing and coughing to clear secretions between inhalations. Patients tend to accommodate cough well, provided expectations are set, and the positive implication of a productive cough is understood.
In this clinical experience, ACT was generally more effective following inhaled mannitol administration. Cough that was productive of phlegm was often increased and some patients reported a reduction in non-productive paroxysmal coughing. The change in cough character was described as less intrusive and disruptive to daily life.
Discussion
Inhaled mannitol has been demonstrated to be a safe, effective treatment for adult patients with CF, and it can be used in addition to best standard care. This form of therapy addresses many of the complaints patients have with other aerosol therapies which often lead to reduced adherence. That is, the disposable capsule-based dry-powder inhaler is portable and easy to use, greatly reducing the time of treatment. The portable inhaler is also convenient for adults, enabling them to maintain an active lifestyle.
It appears from published studies that inhaled mannitol was not well tolerated by some subjects. This may have contributed to a significant withdrawal rate related to difficulties with inhaling the dry powder. The differences in the withdrawal rates between studies CF301 and CF302 however, seem to highlight the importance of education and training in achieving good treatment adherence. These data suggest that demonstration and reinforcement of proper inhaler technique and setting clear expectations with respect to treatment effects may increase the proportion of patients who could benefit from this novel medication.
The physiotherapist or respiratory therapist will play a key role in the introduction and maintenance of inhaled mannitol therapy for patients with CF. Patients who are prescribed mannitol will first undergo a mannitol tolerance test to be certain that clinically significant bronchospasm will not occur, and the mannitol tolerance test has been shown to be an effective screening procedure to identify those patients at risk for bronchospasm. The mannitol tolerance test also provides a valuable opportunity to educate patients about the proper use of the inhaler, including loading capsules, inhaler technique and the appropriate inspiratory flow rate. Patients should be informed about the possible side effects of inhaled mannitol including cough, which is considered part of its therapeutic effect. As this is the first opportunity for patients to experience taking inhaled mannitol, it would be ideally conducted by a CF healthcare professional (such as a physiotherapist or respiratory therapist) who has experience with the product.
Patients may be encouraged by findings from the phase 3 studies, in which not only was the response at week 6 highly predictive of long-term FEV1 improvement, but also subjects in whom the FEV1 improved over 26 weeks suffered fewer exacerbations. Therefore, all patients should be informed of this milestone and they should be evaluated with spirometry after approximately 6 weeks of treatment. As chronic therapy is necessary for long-term treatment benefit, follow-up of patients is important and should be used to monitor treatment adherence, and to encourage correct dosing (400 mg twice daily) and inhaler technique.
Tips for optimal use of inhaled mannitol
When preparing for a dose, patients should breathe out away from the inhaler, to prevent caking of the mannitol powder in the device and optimal dispersal of the dry powder. The capsule should be pierced a single time and the inhaler tilted downward to allow the capsule to fall into the spinning chamber. The patient should inhale with the head tilted slightly upward, breathing in deeply at an even, steady rate sufficient to make the capsule spin (see table 3). The rotating capsule will make an audible “rattle” indicating satisfactory inspiratory flow. Rapid inhalation may result in cough, in which case the patient should slow down their inhalation rate. The capsule should be checked after inspiration and if powder is still present, the patient should inhale a second time. After taking in the dose from each capsule, the patient should hold their breath for 5 s. There should not be a long delay between inhalations. The 10 capsules used during a dosing session should be inhaled closely together over about 5 min and, with practice, a full dose can be taken in 3–5 min (see supplementary video for overview of inhalation technique).
Table 3.
Tips for use of inhaled mannitol
|
See the supplementary material for video showing mannitol inhalation technique.
Conclusion
Inhaled mannitol is now available for adult patients with CF as an adjunctive therapy to augment airway clearance. Designated CF care team members will play vital roles in the evaluation and education of the patient who is prescribed inhaled mannitol. There are clear lessons that have been learned about this new dry powder therapy which should prove useful to the therapists in their discussions with patients about inhaled mannitol. Follow-up of patients is important to help ensure long-term benefit, and should be used to monitor adherence to treatment and encourage correct dosing.
Acknowledgements
We thank staff at Insight Medical Writing (Oxford, UK) for their assistance in preparing a first draft of this manuscript and Joanne Leadbetter (Pharmaxis Ltd) for her assistance with statistical analysis.
All authors helped to interpret data, write the manuscript and have seen and approved the final version. Diana Bilton and Moira L. Aitken were the Global Principal Investigators for CF301/CF302, respectively, and had full access to all the data in the study and had final responsibility to submit for publication. John Kolbe was New Zealand Lead Regional Investigator for CF301. Moira L Aitken, Patrick A Flume and Jonathan Zuckerman were on the CF302 Steering Committee. Helge Hebestreit was Lead Regional Investigator for Germany for CF302. Brett Charlton designed the CF301 and CF302 studies, approved the statistical plans, and was the Sponsor’s Responsible Medical Officer. Howard Fox approved the statistical plans and assisted in the design of CF302.
Footnotes
As a result of an erratum, the online version of this article has been revised
Conflict of interest Pharmaxis Limited sponsored the study. The study sponsor participated in the study design, data collection, data analysis, data interpretation and writing of the reports. Following completion of the trials, the data were held and analysed by the sponsor. The corresponding author had full access to all of the data, and had final responsibility for publication.
Diana Bilton has received fees for chairing an advisory board for Pharmaxis. Diana Bilton, John Kolbe, Moira Aitken, Patrick Flume, Jonathan Zucherman and Helge Hebestreit were all investigators during the studies and their institutions received standard clinical trial support from Pharmaxis. Investigators received travel support for the investigator meetings. No Investigator received any personal funding to participate in the study. Brett Charlton is the Medical Director of and holds stock options in Pharmaxis Ltd. Howard Fox is Chief Medical Officer of and holds stock options in Pharmaxis Ltd.
References
- 1.Bilton D, Robinson P, Cooper P, et al. Inhaled dry powder mannitol in cystic fibrosis: an efficacy and safety study. Eur Respir J 2011; 38: 1071–1080. [DOI] [PubMed] [Google Scholar]
- 2.Aitken ML, Bellon G, De Boeck K, et al. Long-term inhaled dry powder mannitol in cystic fibrosis: an international randomized study. Am J Respir Crit Care Med 2012; 185: 645–652. [DOI] [PubMed] [Google Scholar]
- 3.Flume PA. Pulmonary complications of cystic fibrosis. Respir Care 2009; 54: 618–627. [DOI] [PubMed] [Google Scholar]
- 4.Boucher RC. Evidence for airway surface dehydration as the initiating event in CF airway disease. J Int Med 2007; 261: 5–16. [DOI] [PubMed] [Google Scholar]
- 5.Flume PA, Robinson KA, O’Sullivan BP, et al. Cystic fibrosis pulmonary guidelines: airway clearance therapies. Respir Care 2009; 54: 522–537. [PubMed] [Google Scholar]
- 6.Flume PA, O’Sullivan BP, Robinson KA, et al. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Am J Respir Crit Care Med 2007; 176: 957–969. [DOI] [PubMed] [Google Scholar]
- 7.Shak S, Capon DJ, Hellmiss R, et al. Recombinant human DNase I reduces the viscosity of cystic fibrosis sputum. Proc Natl Acad Sci USA 1990; 87: 9188–9192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ratjen F. Restoring airway surface liquid in cystic fibrosis. N Engl J Med 2006; 354: 291–293. [DOI] [PubMed] [Google Scholar]
- 9.Donaldson SH, Bennett WD, Zeman KL, et al. Mucus clearance and lung function in cystic fibrosis with hypertonic saline. N Engl J Med 2006; 354: 241–250. [DOI] [PubMed] [Google Scholar]
- 10.Robinson M, Hemming AL, Regnis JA, et al. Effect of increasing doses of hypertonic saline on mucociliary clearance in patients with cystic fibrosis. Thorax 1997; 52: 900–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elkins MR, Bye PT. Inhaled hypertonic saline as a therapy for cystic fibrosis. Curr Opin Pulm Med 2006; 12: 445–452. [DOI] [PubMed] [Google Scholar]
- 12.Wark P, McDonald VM. Nebulised hypertonic saline for cystic fibrosis. Cochrane Database Syst Rev 2009; CD001506. [DOI] [PubMed] [Google Scholar]
- 13.Suri R, Metcalfe C, Lees B, et al. Comparison of hypertonic saline and alternate-day or daily recombinant human deoxyribonuclease in children with cystic fibrosis: a randomised trial. Lancet 2001; 358: 1316–1321. [DOI] [PubMed] [Google Scholar]
- 14.Robinson M, Daviskas E, Eberl S, et al. The effect of inhaled mannitol on bronchial mucus clearance in cystic fibrosis patients: a pilot study. Eur Respir J 1999; 14: 678–685. [DOI] [PubMed] [Google Scholar]
- 15.Hurt K, Bilton D. Inhaled mannitol for the treatment of cystic fibrosis. Expert Rev Respir Med 2012; 6: 19–26. [DOI] [PubMed] [Google Scholar]
- 16.Daviskas E, Rubin BK. Effect of inhaled dry powder mannitol on mucus and its clearance. Expert Rev Respir Med 2013; 7: 65–75. [DOI] [PubMed] [Google Scholar]
- 17.Pettit RS, Johnson CE. Airway-rehydrating agents for the treatment of cystic fibrosis: past, present, and future. Ann Pharmacother 2011; 45: 49–59. [DOI] [PubMed] [Google Scholar]
- 18.Bilton D, Bellon G, Charlton B, et al. Pooled analysis of two large randomised phase III inhaled mannitol studies in cystic fibrosis. J Cyst Fibros 2013; 12: 367–376. [DOI] [PubMed] [Google Scholar]
- 19.Button BM, Finlayson F, Borg B, et al. Clinical impact of inhaled mannitol in an adult cystic fibrosis population. J Cyst Fibros 2013; 1: S98. [Google Scholar]