Abstract
Measuring lung function is an important component in the decision making process for patients with obstructive airways disease (OAD). Not only does it help in arriving at a specific diagnosis, but it also helps in evaluating severity so that appropriate pharmacotherapy can be instituted, it helps determine prognosis and it helps evaluate response to therapy. Spirometry is currently the most commonly performed lung function test in clinical practice and is considered to be the gold standard diagnostic test for asthma and COPD. However, spirometry is not an easy test to perform because the forceful expiratory and inspiratory manoeuvres require good patient co-operation. Children aged <5 years, elderly people and those with physical and cognitive limitations cannot perform spirometry easily.
Introduction
Measuring lung function is an important component in the decision making process for patients with obstructive airways disease (OAD). Not only does it help in arriving at a specific diagnosis, but it also helps in evaluating severity so that appropriate pharmacotherapy can be instituted, it helps determine prognosis and it helps evaluate response to therapy. Spirometry is currently the most commonly performed lung function test in clinical practice and is considered to be the gold standard diagnostic test for asthma and COPD. However, spirometry is not an easy test to perform because the forceful expiratory and inspiratory manoeuvres require good patient co-operation. Children aged <5 years, elderly people and those with physical and cognitive limitations cannot perform spirometry easily.
In 1956, DuBois et al. [1] described the forced oscillation technique (FOT) as a tool to measure lung function using sinusoidal sound waves of single frequencies generated by a loud speaker and passed into the lungs during tidal breathing. The output was a measure of respiratory impedance (Zrs), which included the respiratory resistance (Rrs) and respiratory reactance (Xrs) measured over a range of frequencies (usually from 3 to 35 Hz). These parameters provided valuable information about the mechanical properties of the airways and lung parenchyma. The main advantage of this device was that the procedure was easy to perform and provided information about the lung which was different from that given by the spirometer. The earlier FOT instruments allowed only one sound frequency to be passed at a time. To measure Zrs over a range of sound frequencies therefore took a long time. Some of the more recent FOTs now use sound waves of two or three different frequencies at one time. The main advantage of FOT is that it provides very good time resolution with measures of respiratory resistance.
In 1975, Michaelson et al. [2] developed a computer-driven loudspeaker output to apply bursts of square wave oscillatory pressures (5 times⋅s−1) of multiple sound frequencies and analysed the pressure–flow relationship using spectral analysis. This improvised technique of FOT that could use multiple sound frequencies at one time was called the impulse oscillometry system (IOS). The temporal resolution of IOS is slightly inferior to FOT and it sends pulses of pressure waves inside the lungs that can be a bit uncomfortable. However, the IOS provides extensive description of oscillatory pressure–flow relationships over a range of frequencies between 4 and 32 Hz and gives better mathematical analyses of resistance and reactance using the fast fourier transform (FFT) technique [3]. Moreover, the mixed multi-frequency waveform provides improved signal-to-noise characteristics [4]. This new technique was subsequently refined over the years by Jaeger and became commercially available in 1998 [5]. Both FOT and IOS are widely used in paediatric clinics across the world as well as in several lung physiology laboratories as a valuable clinical research tool.
The main advantage of FOT/IOS is that the patient needs to perform simple tidal breathing manoeuvres that require less effort and co-operation than spirometry, meaning that children and the elderly can therefore perform this test easily. Moreover, it can be performed in patients on ventilators and also during sleep. One of the most remarkable features of FOT/IOS in relation to spirometry is that it has much greater sensitivity to detect peripheral airways obstruction. In most cases, spirometry does not provide a clear indication of peripheral airway obstruction regardless of the information contained in the flow–volume curve and the forced expiratory flow at 25–75% of forced vital capacity (FEF25–75%). FOT/IOS are therefore more sensitive instruments to detect small airways obstruction in patients with asthma and chronic obstructive pulmonary disease (COPD). More recently, the within-breath analysis of Rrs and Xrs has been shown to help differentiate between asthma and COPD and also offer more useful information about the pathophysiology of asthma and COPD, which the spirometer does not. The differences between spirometry and FOT/IOS are described in table 1.
Table 1.
Differences between spirometry and FOT/IOS
| Parameter | Spirometry | FOT/IOS |
| Main principle | Flow sensor/volume displacement helps measure flow rates and lung volumes | Forced oscillations of single frequency sound waves (FOT) or impulses of multiple frequency sound waves (IOS) are pushed into the lungs as pressure waves to measure respiratory resistance and reactance |
| Main parameters | Volumes: FEV1, FVC | Zrs, Rrs, Xrs, Fres, Ax |
| Flows: PEFR, FEF25–75% | ||
| Patient co-operation required | +++ | + |
| Type of breathing manoeuvre | Forced exhalation | Tidal breathing |
| Variability (intra-subject) | 3–5% | 5–15% |
| Sensitivity to airway location | ||
| Central | + | +++ |
| Peripheral | ++ | +++ |
| Cut off for bronchodilator response | 12–15% for FEV1 | 40% for R5 or X5 |
| Cut off for bronchoconstrictor response | 20% for FEV1 | 50% for R5 |
| Insight into lung mechanics | + | +++ |
| Standardised methodology | +++ | ++ |
| Availability of robust reference values | +++ | + |
FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; PEFR: peak expiratory flow rate; FEF25–75%: forced expiratory flow at 25–75% of FVC; Zrs: respiratory impedance; Rrs: respiratory resistance; Xrs: respiratory reactance; Fres: resonant frequency; Ax: reactance area; R5: respiratory resistance at 5 Hz; X5: respiratory reactance at 5 Hz.
Choosing between FOT and IOS to measure respiratory resistance and reactance is like choosing between a volume–displacement spirometer and a flow sensor-based spirometer respectively. Although the volume–displacement spirometers offer more accurate measures of lung volumes than the flow sensor-based spirometers, they are bulky, difficult to maintain and do not offer important readouts that a flow sensor-based spirometer does. Both the FOT and IOS devices measure Rrs and Xrs at multiple frequencies, but they do not necessarily show similar values.
What are the parameters that FOT/IOS measure?
The impulses generated by the loudspeaker travel superimposed upon the normal tidal breathing through the large and small airways. Higher frequencies (>20 Hz) travel shorter distances (generally up to the large airways), while lower frequencies (<15 Hz) travel deeper into the lung and reach the small airways and lung parenchyma (fig. 1). A useful analogy here is radio waves: radio waves of high frequency, such as FM radio travel shorter distances, while radio waves of lower frequency, such as AM radio travel long distances. A pressure–flow transducer measures inspiratory and expiratory flow and pressure, which are then separated from the breathing pattern by “signal filtering”. Measured Zrs is the sum of all the forces (Rrs and Xrs) opposing the pressure impulses (oscillations) and is calculated from the ratio of pressure and flow at each frequency [6]. The FOT/IOS is therefore an accurate and powerful method that measures Rrs and Xrs from input Zrs measurements made over a range of frequencies.
Figure 1.
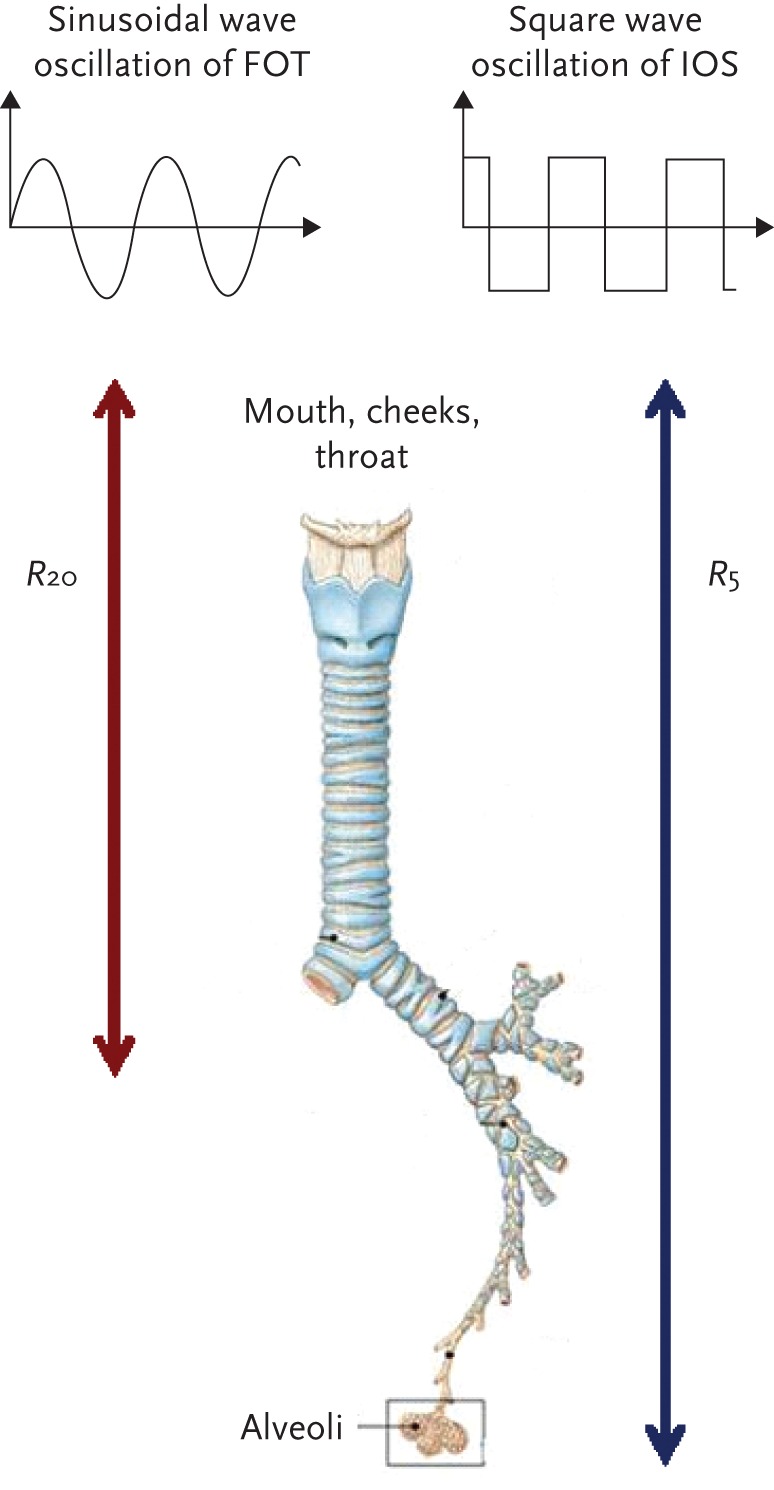
Type of sound waves in FOT and IOS and distances travelled by sound waves of different frequencies.
Respiratory resistance
Rrs measured by FOT and IOS includes the resistance of the oropharynx, larynx, trachea, large and small airways, lung and chest wall tissue. However, the use of multiple oscillation frequencies permits a dissection of large airway behaviour from that of peripheral small airways. Sound waves at frequency <15 Hz travel more distally and those >20 Hz are damped out in the intermediate sized airways.
The resistance at 5 Hz (R5) represents the total airway resistance, and the resistance at 20 Hz (R20) represents the resistance of the large airways. Subtracting R20 from R5 (R5−R20) reflects resistance in the small airways.
In healthy adult subjects, R is nearly independent of oscillation frequency (i.e. resistance is more or less the same at frequencies between 5 and 20 Hz). When airway obstruction occurs, either central or peripheral, R5 is increased above normal values. Central airway obstruction elevates R evenly independent of oscillation frequency, whereas peripheral airways obstruction increases R at low frequencies, an effect that diminishes with increasing frequency (fig. 2). Therefore, in small airways obstruction, R becomes frequency dependent and is considered to be a characteristic feature. Small children normally present frequency dependence of resistance, and this may be greater than in adults in the presence of peripheral airflow obstruction. Resistance is measured in cmH2O⋅L−1⋅s−1 or kPa⋅L−1⋅s−1.
Figure 2.
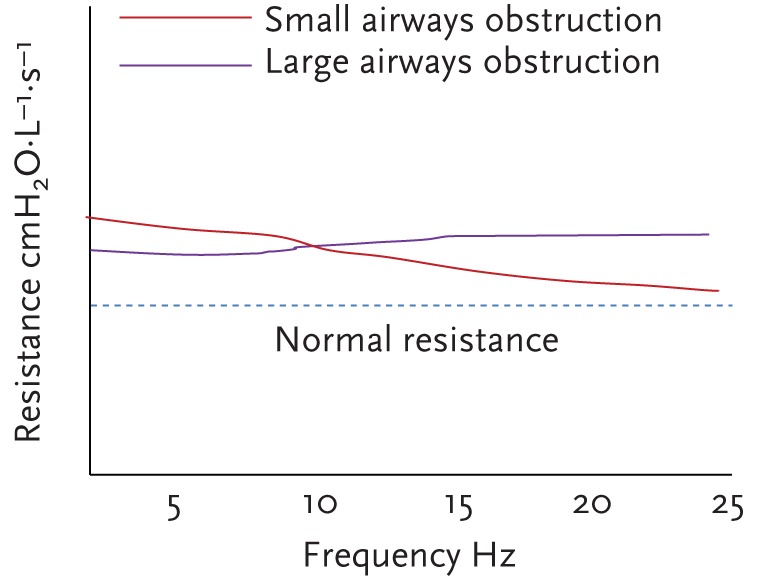
Respiratory resistance versus frequency.
Respiratory reactance
Xrs is the imaginary part of Zrs and includes the mass-inertive forces of the moving air column expressed in terms of inertance (I) and the elastic properties or compliance of lung periphery expressed as capacitance (C). Reactance can be viewed as the rebound resistance, or an echo, giving information about the distensible airways. C represents the ability of the respiratory system to store energy and is primarily located in the lung periphery.
C and I are in opposite phase with each other, and unlike resistive properties of the normal respiratory system, they are dependent on oscillation frequency. At low frequencies, the magnitude of the oscillatory capacitative pressure loss is relatively large and that of inertive pressure loss is relatively small. Therefore, at low frequencies, the capacitative properties of the small peripheral airways dominate. As oscillation frequency increases, the magnitude of the capacitative pressure dissipation decreases, while that of inertive pressure increases. Therefore, at high frequencies, the inertive properties of the large airways dominate. By convention, capacitative pressure losses are designated negative, and inertive pressure losses, positive [3]. Accordingly, the balance between the two is negative at low frequencies and positive at high frequencies (fig. 3). Like resistance values, reactance values are also measured in cmH2O⋅L−1⋅s−1 or kPa⋅L−1⋅s−1.
Figure 3.
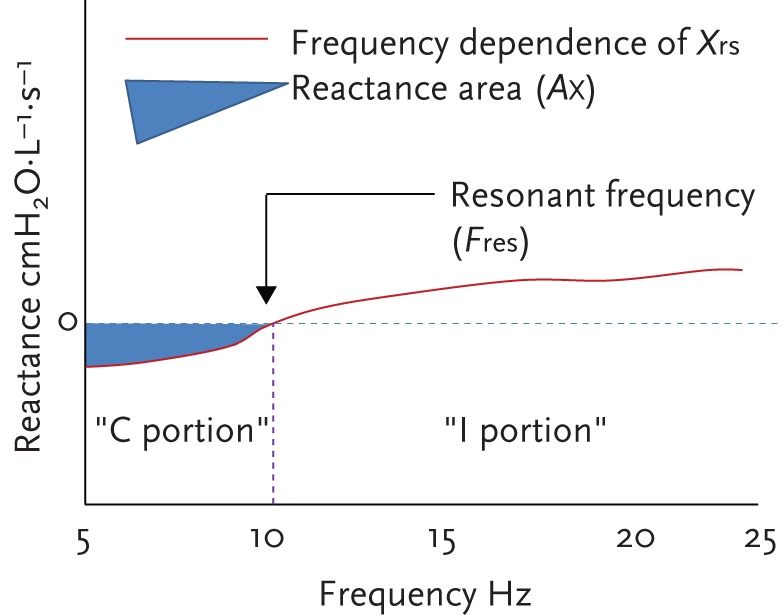
Reactance values in a healthy subject showing the “C” (compliance) and “I” (inertance) portions of reactance, area of reactance (AX) and resonant frequency (Fres).
The reactance at 5 Hz (X5) reflects the combined effect of tissue elastance and inertance, although at this lower frequency, the effect of tissue elastance would dominate. X5 therefore reflects elastic recoil of the peripheral airways [7]. Because the ability of the lungs to store capacitative energy is primarily manifest in the small airways, X5 can provide important information about the distal/small airways. States that reduce the elasticity of the lung, such as fibrosis and hyperinflation make the capacitance increasingly negative, i.e. more negative or higher X5 values.
Resonant frequency
At one intermediate frequency, the magnitudes of capacitative and inertive pressure components are equal. Since they are opposite in sign, the total reactance at this frequency is zero. This frequency is called the resonant frequency (Fres). Fres marks the transition from capacitative dominance at low frequencies to inertive dominance at high frequencies. This also helps conveniently categorise frequencies below Fres as low and above Fres as high, and indicates a frequency at which the total impedance to airflow is totally flow resistive. Normal Fres is approximately 6–11 Hz. Fres tends to be higher in children, decreases with age and increases in both obstructive and restrictive diseases.
Reactance area
Reactance area (AX), also called the “Goldman Triangle” (named after Michael Goldman who described it for the first time) is the integrated low frequency respiratory reactance magnitude between 5 Hz and Fres (fig. 3) and is measured in cmH2O⋅L−1 or kPa⋅L−1. AX is a useful index related to respiratory compliance and therefore of small airways patency. AX is a single quantity that reflects changes in the degree of peripheral airway obstruction and closely correlates with R5−R20. The normal AX is generally <0.33 kPa⋅L−1.
How to perform the test
The IOS instrument should be calibration checked every day for volume using a 3-L syringe and for resistance using a reference resistance of 0.2 kPa⋅L−1⋅s−1 to ensure that the sensors are working accurately.
The procedure should be explained to the patient and the sitting position is preferred. Legs must be kept uncrossed in order to reduce extra-thoracic pressure and a nose clip should be worn. The mouthpiece of the FOT/IOS should be at a comfortable height so that the neck is slightly extended. Ensure that there is a tight seal between the mouthpiece and lips to prevent air leak. The cheeks should be held firmly either by the patient with his/her hands or by an assistant who presses the cheeks firmly from behind (fig. 4).
Figure 4.
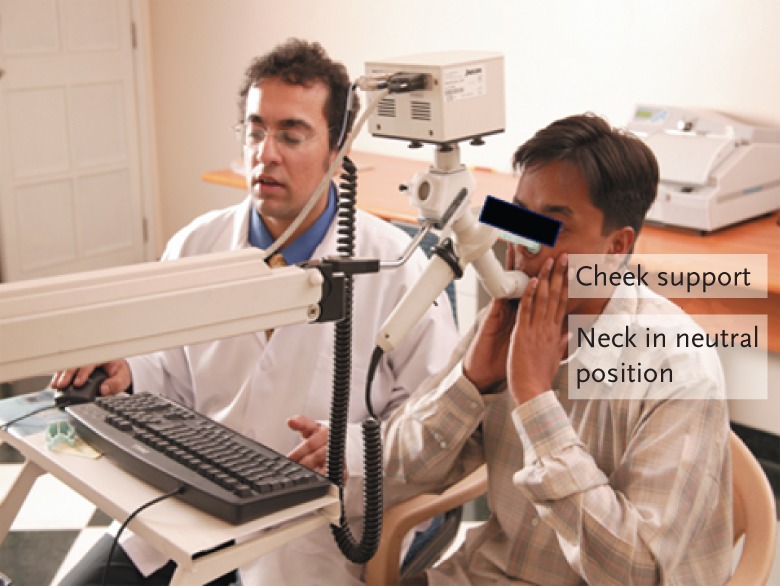
Position of the patient while performing IOS. Note how the cheeks are held firmly.
Ask the patient to perform normal tidal breathing in a relaxed state during the FOT/IOS procedure. The recording should be performed for at least 30–45 s. During this period, around 120–150 sound impulses are pushed into the lungs from which the mean reactance and resistance values are determined at frequencies from 5 to 20 Hz. A minimum of three such tests should be performed. Care should be taken to ensure reproducible results without any artefacts as mentioned below. If there are breathing segments which contain artefacts, they should be discarded.
If spirometry and FOT/IOS are going to be performed at the same visit, spirometry should be performed after FOT/IOS because the forced manoeuvres of spirometry have an impact on resistance and reactance values.
Some common artefacts include:
Poor cheek support. Because pressure oscillations are applied at the mouth, the impedance of extra-thoracic airway walls, including cheek, tongue, mouthpiece and upper airway affects the results of measurements. If the cheeks are not held firmly, R20 values reduce significantly and are therefore underestimated. Absence of cheek support affects the values of R5 and X5 significantly in patients with obstructive airways disease as well as interstitial lung disease, and has relatively little impact on healthy subjects [8]. Although the cheek support can be given by the patient or an assistant, it has been shown that cheeks supported by as assistant show slightly reduced R5 and increased X5 [8]. This is probably because of the position of arms and chest wall while holding the cheeks.
Use of bacterial filters. The use of a bacterial filter adds a dead space volume of around 60 mL and increases FOT/IOS resistance values by around 0.04 kPa⋅L−1⋅s−1. Resistance values measured with a bacterial filter will therefore be slightly greater than those without a filter.
Tongue position. If the tongue position interferes with free airflow through the mouthpiece, a uniform increase in resistance is seen at all frequencies. This cannot be identified by a single FOT/IOS test. Therefore, multiple tests need to be performed and looked for repeatable measures. The tongue position has little effect on the reactance values at all frequencies.
In addition to the above, air leaks, swallowing, breath holding and vocalisation are the other common artefacts that should be avoided.
For bronchodilator reversibility assessment, a short-acting bronchodilator is administered and an equal number of measurements are performed in the same fashion.
In IOS, the quality assurance is measured by “coherence”, which is an index that recognises the validity of the results. It is a value between 0 and 1 that reflects the reproducibility of impedance measurements. Coherence at 5 Hz should ideally be >0.8 cmH2O and coherence at 20 Hz between 0.9 and 1.0. These values are for adults and, so far, there are no validated values for children. Coherence is decreased by improper technique, swallowing, glottis closure, obstruction of airflow by the tongue or irregular breathing.
The day-to-day variability for IOS parameters has been shown to be 5–15% in adults and 16–17% in children. This degree of variability indicates that obtaining similar repeated measures is not difficult, and that IOS is a fairly reproducible test although not as much as the spirometric indices of forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC).
Predicted values
It must be emphasised that FOT and IOS do not produce equal measurements of resistance and reactance, therefore predicted values derived by an FOT machine may not necessarily be applicable for IOS machines. Tanimura et al. [9] compared the resistance and reactance values between MostGraph (FOT) and Jaeger (IOS) using phantom models. The resistance values varied by up to 10% from estimated values in both devices. Additionally, there was a difference in frequency dependence for the resistance between devices. The reactance values were higher with the FOT than IOS. Clearly, more studies are required to establish reliable device-specific predicted values.
Children
In children, age and height have been shown to have a significant impact on resistance and reactance values. As the lung grows, the airway calibre increases as well as the number and size of alveoli. Therefore, on one hand, respiratory resistance values at all frequencies decrease with growing age and increasing height. On the other hand, as age and height increase, X5 values become less negative with little change in X20. Height has been shown to be the strongest covariate, contributing to around 56–60% variance for impedance, resistance, reactance, Fres and AX values. Studies in Caucasian and Oriental children have shown more or less similar predicted values, suggesting that predicted values generated at one place can be used globally. There are at least seven studies that have reported predicted values for FOT/IOS values for children, although most of them have been for IOS. However, more studies are required to generate robust predicted values for different parts of the world.
Adults
Compared with children, there have been fewer attempts to develop normal predicted FOT/IOS values for adults. So far, there have been four published studies that have derived predicted values. Like in children, height has been shown to be the most influential predictor of FOT/IOS values. The KORA (Cooperative Health Research in the Augsburg Region) study cohort population from Germany among 154 and 243 nonsmoking men and women, respectively, was used to study the predicted values for IOS among Caucasian adults aged ≥ 45 years [10]. They showed that 1) females had higher resistance and lower reactance values than men, 2) R5-R20, AX and Fres showed age-related changes, 3) X5 values showed age-related changes only in females, 4) body weight was a significant predictor for most IOS parameters in females, but not males, and 5) obesity was shown to cause an elevation in X5 and AX values. Unfortunately, the predicted values in this study were quite different from those reported in earlier studies although they were from the same Caucasian population. There is an urgent need to develop robust predictive equations for the adult population for both FOT and IOS from different parts of the world.
FOT/IOS in respiratory disorders
FOT/IOS in asthma
Childhood asthma is often a clinical diagnosis because of the lack of a reliable and practical objective diagnostic tool. Ortiz et al. [11] were among the first to show that children aged 2–5 years with a suspected diagnosis of asthma in whom spirometry could not be performed, showed significant improvements in IOS parameters after giving bronchodilator treatment.
Although it is possible to perform spirometry in older children, often children presenting with asthma symptoms have normal spirometry, yet show abnormal changes in IOS parameters such as X5 and AX. Giving these children inhaled corticosteroids has produced marked improvements in their symptoms [12]. Abnormal IOS parameters therefore help improve confidence when making a diagnosis of asthma in children, even when spirometry reports are normal.
Many children with asthma have difficulty verbalising symptoms and some find it difficult to perceive changes in their respiratory status. Objective parameters, such as spirometry, peak flow measurement or even exhaled breath nitric oxide do not accurately reflect a decline in asthma control. Recent studies have suggested that those children who have currently controlled asthma but have increased peripheral airway IOS indices, often show a greater risk of losing asthma control over the next 2–3 months [13]. Children with an increased R5−R20 of >1.5 kPa⋅L−1⋅s−1 and AX values of ≥7.0 kPa⋅L−1 more often showed an increased risk of losing asthma control. These observations suggest that monitoring small airway function by IOS can be useful in identifying children who are at risk for losing asthma control.
More recently, three-dimensional analysis of resistance and reactance using FOT has shown unique patterns for controlled versus uncontrolled asthma in children [14].
In adult asthmatics, small airway dysfunction as evaluated by IOS has been shown to be associated with excessive bronchoconstriction and poor asthma control [15]. The IOS parameter R5−R20 has been shown to be a useful marker of fluctuation of the heterogeneity of airway constriction over time and has been shown to predict future asthma exacerbations [16].
Mori et al. [17] have reported that coloured three-dimensional analyses of respiratory resistance and reactance, especially ΔX5 (i.e. the difference between inspiratory and expiratory X5), can help differentiate asthma from COPD with confidence.
Furthermore, Shirai et al. [18] have recently reported that coloured three-dimensional images of respiratory impedance obtained using FOT show unique phenotypes of asthma. The typical asthma–asthma phenotype presents with moderately high Rrs over all frequencies with slight changes in Xrs, especially X5, while the asthma–COPD phenotype presents with much higher Rrs and Xrs with a marked respiratory cycle and frequency dependence, while the asthma–normal phenotype presents with low Rrs and Xrs and few within-breath changes. Although very exciting and promising, more work is required to understand the true value of these observations in clinical practice.
FOT/IOS in COPD
Pulmonary mechanics caused by airflow obstruction in COPD are better seen in reactance values than resistance values, unlike in asthma where resistance values are more impaired. Patients with self-reported symptoms suggestive of COPD have been shown to have reduced X5, irrespective of whether they have normal or abnormal spirometry [19]. X5 is the only parameter that has been shown to correlate significantly with decrements in FEV1 in patients with COPD over time [20].
In 1993, Peslin et al. [21] reported that some patients with COPD on mechanical ventilation developed large negative swings in Xrs during exhalation when measured by FOT. This was explained on the basis that low-frequency oscillatory signals cannot pass the choke point (caused by small airways collapse) and reach the alveoli during expiration. This leads to a marked reduction in respiratory compliance, i.e. a fall in Xrs. In 2004, Dellacà et al. [22] reported that the differences between inspiratory and expiratory phases of respiratory reactance (ΔXrs) measured by the FOT were due to expiratory flow limitation (EFL), which is a characteristic feature of patients with moderate-to-severe COPD that manifests clinically as dynamic hyperinflation. This within-breath difference between inspiratory and expiratory reactance at low frequencies, called ΔX5 was therefore found to provide valuable information regarding EFL in patients with COPD. The normal ΔXrs values are ≤0.07 kPa⋅L−1⋅s−1. In patients with asthma, this increases to around 0.10 kPa⋅L−1⋅s−1, while in patients with COPD it increases to more than 0.21 kPa⋅L−1⋅s−1 [23]. These cut off values are based on only one study, and more work needs to be done to identify robust cut-off values.
More recently, Mikamo et al. [24] reported that high EFL index (>0.55 kPa⋅L−1⋅s−1) measured by FOT was independently predicted by emphysema extent measured by high-resolution computed tomography (HRCT), peripheral airway obstruction expressed by FEF25–75%, hyperinflation as expressed by functional residual capacity, and airway calibre as expressed by whole breath R5. The ΔXrs also correlated well with the modified Medical Research Council scale for breathlessness. These results suggest that EFL or ΔX5 measured by FOT is a good global measure of COPD. It is hoped that, in the future, ΔXrs will help evaluate the severity of COPD as well as response to treatment in patients of COPD.
IOS and interstitial lung disease
Patients with interstitial lung disease (ILD) show reduced FVC with a normal FEV1/FVC ratio on spirometry, which is however not diagnostic of ILD. Total lung capacity measured by body plethysmography and lung diffusion measured by single-breath diffusing capacity of the lung for CO (DLCO) with a 10-s breath hold provide the most useful physiological measure of ILD. However, these tests are not easily available in most clinics and, quite often, patients with ILD find it difficult to perform a good-quality test.
In 1968, Fisher et al. [25] showed evidence of increased respiratory resistance on FOT in patients with ILD, but the sample size was small and the FOT analysis was only very basic. In 2009, van Noord et al. [26] reported increased Rrs and reduced Xrs in patients with advanced ILD, but these values were similar to those observed in patients with moderate-to-severe COPD. The authors therefore commented that the results of FOT cannot help differentiate between obstructive and restrictive disorders.
In 2013, Mori et al. [27] reported that although the total X5 values were lower in ILD and comparable to patients with COPD, the X5 values were smaller in the expiratory phases compared with the inspiratory phases in ILD, which is the reverse of what is found in patients with COPD. Sugiyama et al. [28] also reported that inspiratory X5 values were more negative than expiratory X5 values in patients with ILD and was exactly the opposite of what was found in patients with COPD (in which expiratory X5 values were more negative). The ΔX5 values were therefore negative in COPD (mean −0.08 kPa⋅L−1⋅s−1) and positive in ILD (+0.05 kPa⋅L−1⋅s−1). Mirror changes were seen in ΔAX values (fig. 5).
Figure 5.
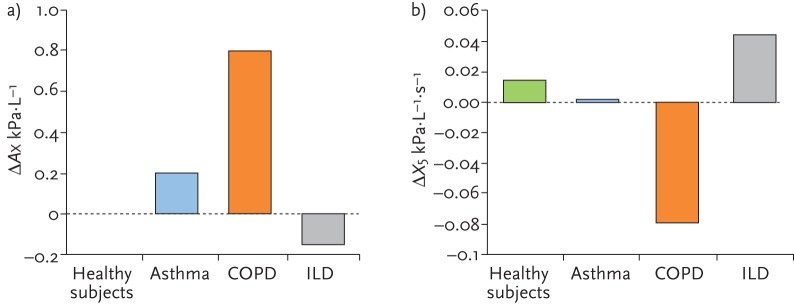
Mean a) ΔAX and b) ΔX5 values in healthy subjects, and patients with asthma, COPD and ILD. Adapted from [28] with permission from the publisher.
More recently, Fuji et al. [29] reported that inspiratory Fres values measured on FOT correlated independently with the fibrosis extent on HRCT as well as with the composite pulmonary index (FEV1, FVC and DLCO). The authors suggested that inspiratory Fres is a measure of increased lung elastic recoil resulting from fibrosis in ILD and may have prognostic value. The ability for FOT parameters to predict the composite pulmonary index and HRCT, if confirmed in multicentre studies, would be of immense clinical benefit because many patients with ILD are not able to perform good quality spirometry or even the single breath DLCO tests. However, prospective clinical trials are needed to evaluate the true benefit of FOT/IOS in these settings.
The future
Despite the advantages of FOT/IOS in terms of its noninvasiveness and lack of dependency on patient cooperation, the FOT has not yet become a standard methodology for the routine assessment of lung function in clinical practice. Although obtaining respiratory impedance values is easy, the interpretation of resistance and reactance curves and the derived parameters requires training and experience, and it is a difficult task for an untrained pulmonologist. This may be one of the main reasons why FOT/IOS has not progressed as much as it should have.
More recently, attempts have been made to develop machine learning algorithms that help make diagnosis easy and automated. Amaral et al. [30] have recently reported that using k-nearest neighbour and random forest classifiers, which are different types of machine learning algorithms, it was possible to diagnose and categorise COPD airway obstruction and also assist clinicians in tracking disease progression, evaluating risk of future disease exacerbations and guiding therapy. These are still early days, but in the future we are likely to see diagnostic algorithms being developed for asthma, COPD and other lung diseases for FOT and IOS which will help clinicians tremendously.
Footnotes
Conflict of interest None declared
References
- 1.Dubois AB, Brody AW, Lewis DH, et al. . Oscillation mechanics of lungs and chest in man. J Appl Physiol 1956; 8: 587–594. [DOI] [PubMed] [Google Scholar]
- 2.Michaelson ED, Grassman ED, Peters WR. Pulmonary mechanics by spectral analysis of forced random noise. J Clin Invest 1975; 56: 1210–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldman MD, Saadeh C, Ross D. Clinical applications of forced oscillation to assess peripheral airway function. Respir Physiol Neurobiol 2005; 148: 179–194. [DOI] [PubMed] [Google Scholar]
- 4.Làndsér F, Clément J, Van de Woestijne KP. Normal values of total respiratory resistance and reactance determined by forced oscillations: influence of smoking. Chest 1982; 81: 586–591. [DOI] [PubMed] [Google Scholar]
- 5.Klug B, Bisgaard H. Specific airway resistance, interrupter resistance, and respiratory impedance in healthy children aged 2-7 years. Pediatr Pulmonol 1998; 25: 322–331. [DOI] [PubMed] [Google Scholar]
- 6.Bickel S, Popler J, Lesnick B, et al. . Impulse oscillometry: interpretation and practical applications. Chest 2014; 146: 841–847. [DOI] [PubMed] [Google Scholar]
- 7.Komarow HD, Myles IA, Uzzaman A, et al. . Impulse oscillometry in the evaluation of diseases of the airways in children. Ann Allergy Asthma Immunol 2011; 106: 191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uchida A, Ito S, Suki B, et al. . Influence of cheek support on respiratory impedance measured by forced oscillation technique. Springer Plus 2013; 2: 342 doi: 10.1186/2193-1801-2-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanimura K, Hirai T, Sato S, et al. . Comparison of two devices for respiratory impedance measurement using a forced oscillation technique: basic study using phantom models. J Physiol Sci 2014; 64: 377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schulz H, Flexeder C, Behr J, et al. . Reference values of impulse oscillometric lung function indices in adults of advanced age. PLoS One 2013; 8: e63366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ortiz G, Menendez R. The effects of inhaled albuterol and salmeterol in 2- to 5-year-old asthmatic children as measured by impulse oscillometry. J Asthma 2002; 39: 531–536. [DOI] [PubMed] [Google Scholar]
- 12.Lee JY, Seo JH, Kim HY, et al. . Reference values of impulse oscillometry and its utility in the diagnosis of asthma in young Korean children. J Asthma 2012; 49: 811–816. [DOI] [PubMed] [Google Scholar]
- 13.Shi Y, Aledia AS, Galant SP, et al. . Peripheral airway impairment measured by oscillometry predicts loss of asthma control in children. J Allergy Clin Immunol 2013; 131: 718–723. [DOI] [PubMed] [Google Scholar]
- 14.Murakami K, Habukawa C, Kurosawa H, et al. . Evaluation of airway responsiveness using colored three-dimensional analyses of a new forced oscillation technique in controlled asthmatic and nonasthmatic children. Respir Investig 2014; 52: 57–64. [DOI] [PubMed] [Google Scholar]
- 15.Alfieri V, Aiello M, Pisi R, et al. . Small airway dysfunction is associated to excessive bronchoconstriction in asthmatic patients. Respir Res 2014; 15: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonem S, Umar I, Burke D, et al. . Airway impedance entropy and exacerbations in severe asthma. Eur Respir J 2012; 40: 1156–1163. [DOI] [PubMed] [Google Scholar]
- 17.Mori K, Shirai T, Mikamo M, et al. . Colored 3-dimensional analyses of respiratory resistance and reactance in COPD and asthma. COPD 2011; 8: 456–463. [DOI] [PubMed] [Google Scholar]
- 18.Shirai T, Mori K, Mikamo M, et al. . Usefulness of colored 3D imaging of respiratory impedance in asthma. Allergy Asthma Immunol Res 2013; 5: 322–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frantz S, Nihlén U, Dencker M, et al. . Impulse oscillometry may be of value in detecting early manifestations of COPD. Respir Med 2012; 106: 1116–1123. [DOI] [PubMed] [Google Scholar]
- 20.Gong SG, Yang WL, Zheng W, et al. . Evaluation of respiratory impedance in patients with chronic obstructive pulmonary disease by an impulse oscillation system. Mol Med Rep 2014; 10: 2694–2700. [DOI] [PubMed] [Google Scholar]
- 21.Peslin R, Felicio da Silva J, Duvivier C, et al. . Respiratory mechanics studied by forced oscillations during artificial ventilation. Eur Respir J 1993; 6: 772–784. [PubMed] [Google Scholar]
- 22.Dellacà RL, Santus P, Aliverti A, et al. . Detection of expiratory flow limitation in COPD using the forced oscillation technique. Eur Respir J 2004; 23: 232–240. [DOI] [PubMed] [Google Scholar]
- 23.Paredi P, Goldman M, Alamen A, et al. . Comparison of inspiratory and expiratory resistance and reactance in patients with asthma and chronic obstructive pulmonary disease. Thorax 2010; 65: 263–267. [DOI] [PubMed] [Google Scholar]
- 24.Mikamo M, Shirai T, Mori K, et al. . Predictors of expiratory flow limitation measured by forced oscillation technique in COPD. BMC Pulm Med 2014; 14: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fisher AB, DuBois AB, Hyde RW. Evaluation of the forced oscillation technique for the determination of resistance to breathing. J Clin Invest 1968; 47: 2045–2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Noord JA, Clément J, Cauberghs M, et al. . Total respiratory resistance and reactance in patients with diffuse interstitial lung disease. Eur Respir J 1989; 2: 846–852. [PubMed] [Google Scholar]
- 27.Mori K, Shirai T, Mikamo M, et al. . Respiratory mechanics measured by forced oscillation technique in combined pulmonary fibrosis and emphysema. Respir Physiol Neurobiol 2013; 185: 235–240. [DOI] [PubMed] [Google Scholar]
- 28.Sugiyama A, Hattori N, Haruta Y, et al. . Characteristics of inspiratory and expiratory reactance in interstitial lung disease. Respir Med 2013; 107: 875–882. [DOI] [PubMed] [Google Scholar]
- 29.Fujii M, Shirai T, Mori K, et al. . Inspiratory resonant frequency of forced oscillation technique as a predictor of the composite physiologic index in interstitial lung disease. Respir Physiol Neurobiol 2015; 207: 22–27. [DOI] [PubMed] [Google Scholar]
- 30.Amaral JL, Lopes AJ, Faria AC, et al. . Machine learning algorithms and forced oscillation measurements to categorise the airway obstruction severity in chronic obstructive pulmonary disease. Comput Methods Programs Biomed 2015; 118: 186–197. [DOI] [PubMed] [Google Scholar]


