Abstract
Activation of the inflammasome, a protein complex responsible for many cellular functions, including the activation of the pro-inflammatory cytokines IL-1β and IL-18, has been identified as a key participant in many rheumatic diseases including autoimmune, inflammatory and autoinflammatory syndromes. This review will discuss the recent advances in understanding the role of this complex in various rheumatic diseases. Further, it will focus on available therapies which directly and indirectly target the inflammasome and its downstream cytokines to quiet inflammation and possibly dampen autoimmune processes.
Introduction
Over the past few decades, the treatment of rheumatic diseases has evolved from blanket suppression of inflammation with medications like glucocorticoids to specific targeting of inflammatory pathways with sophisticated biologic therapies. This therapeutic revolution has dramatically improved a physician’s armamentarium and patient outcomes. One inflammatory complex, termed the inflammasome, has been an exciting area of investigation in rheumatic disease. Targeting of this complex and its downstream cytokines has resulted in several FDA approved therapies, and more indications are in the pipeline. This review will summarize the mechanisms by with the inflammasome is involved in rheumatic disease and describe the impact targeting the inflammasome has had on patient care for autoimmune and autoinflammatory diseases.
The inflammasome
The inflammasome is a signaling platform which, when activated, results in the oligomerization and activation of inflammatory caspases. Caspase-1 is the quintessential inflammatory caspase and is the primary enzyme responsible for cleavage and activation of interleukin-beta (IL-1β) and IL-18, (reviewed in (1)). Various “danger signals,” including cellular stress, microbial products and crystalline material can trigger inflammasome activation. Some inflammasome activators, particularly during intracellular infections, may also result in an inflammatory form of cell death, termed pyroptosis(2). The inflammasome platform utilizes a central scaffold, such as NOD-like receptor (NLR) family members to assemble after activation. NLRP3 is the best characterized member of this family and is activated downstream of many physiologically relevant inflammatory triggers. NLRP3 interacts with an adaptor molecule, apoptosis-associated speck-like protein containing a CARD (ASC), which then interacts with the CARD domain on caspase-1, resulting in caspase-1 oligomerization and activation (reviewed in(3), summarized in Figure 1). Other inflammasome scaffolds not related to the NOD-like receptor also serve similar functions in inflammasome assembly but their triggers for activation primarily include cytosolic nucleic acids. These include Absent in Melanoma 2 (AIM2), IFN-γ inducible protein 16 (IFI16) and retinoic acid inducible gene I (RIG-I)(4). Intriguingly, these proteins also contribute to cellular antiviral responses, which may integrate inflammation and chronic interferon production in certain autoimmune diseases (3).
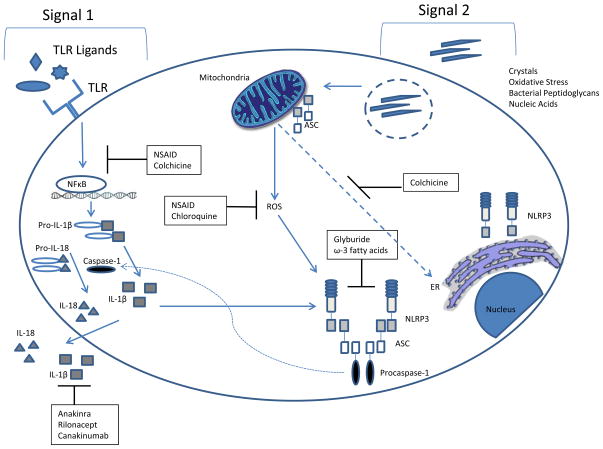
Figure 1. NLRP3 inflammasome activation and targeted therapy.
Signal one of inflammasome activation requires priming, often by toll-like receptor ligands. Signal two, which activates the inflammasome, stems from a wide variety of diverse stimuli such as crystals, oxidative stress, bacterial peptidoglycans, and nucleic acids. This cartoon depicts the mode of action of various medications and their impact on the inflammasome. Abbreviations ASC, apoptosis-related speck-like protein; CARD, caspase recruitment domain; ER, endoplasmic reticulum; NFκB, nucleaer factor kappa B; NSAID, nonsteroidal anti-inflammatory drug.
Because of its important inflammatory role, inflammasome activity is tightly regulated at multiple levels. Priming or “signal 1”, typically via NFκB activation by toll-like receptor (TLR) ligands, stimulates NLRP3 and IL-1β transcription and prepares the cell for a vigorous response upon activation (5, 6). De-ubiquitination of NLRP3 has also been reported as an important intermediate step for inflammasome priming(7). Subcellular compartmentalization contributes to inflammasome regulation, and anti-inflammatory signals may block inflammasome assembly by preventing co-localization of inflammasome components(8). Other adaptor and signaling molecules, such as caspase 8, also participate in inflammasome regulation (reviewed in(9)). Ultimate assembly and activation of the inflammasome requires ligands specific for different inflammasome scaffolds or cellular metabolic changes. These “signal 2” stimuli are as diverse as bacterial peptidoglycans, crystalline materials, oxidative stress and nucleic acids (10–13).
Targeting Inflammasome Activation in vivo
Thus far, medications used to target the inflammasome have primarily focused on inhibition of the pro-inflammatory cytokine IL-1β (see Table 1). IL-18 blockade is feasible, but trials in rheumatic diseases have not yet been undertaken for this target. Current approved medications include anakinra, a soluble receptor antagonist for IL-1R1, rilonacept, a soluble IL-1 receptor with preference for IL-1β>IL-1α, and canakinumab, a neutralizing antibody for IL-1β(14). These medications are approved for use in cryopyrin-associated periodic syndrome (CAPS) patients, and anakinra is also approved for use in rheumatoid arthritis(15). However, as described below, many indications are currently being explored for these medications.
Table 1.
Approved and experimental uses of available medications with inflammasome modulating activities.
| Medication | Mechanism | Approved uses | Experimental uses |
|---|---|---|---|
| Anakinra | IL-1R1 antagonist | CAPS, RA | Gout, Pseudogout, sJIA, AOSD, HIDS, TRAPS |
| Canakinumab | Neutralizing IL-1β antibody | CAPS, sJIA | FMF, Gout, AOSD, HIDS, TRAPS |
| Rilonacept | Soluble IL-1 receptor | CAPS | FMF, sJIA, AOSD, HIDS, TRAPS |
| Colchicine | Inhibits microtubule assembly which disrupts inflammasome activation. | Gout | FMF |
| Fish Oil/ω-FA | Inflammasome inhibition | ---- | SLE, RA |
| Hydroxychloroquine | Blockade of immune complex activation of the inflammasome | SLE, RA | |
| Glyburide | Inhibition of NLRP3 | Type II diabetes | SLE |
| Thalidomide | Inhibition of Caspase-1 | Multiple Myeloma | SLE, AS |
Abbreviations: AOSD=adult onset Still’s disease; AS=ankylosing spondylitis; CAPS=cyropyrin-associated periodic syndrome; FA=fatty acids; FMF=familial Mediterranean fever; HIDS=hyper-IgD syndrome; RA=rheumatoid arthritis; sJIA=systemic juvenile idiopathic arthritis; SLE=systemic lupus erythematosus; TRAPS=tnf-receptor associated periodic syndrome.
Inhibitors of activation of the inflammasome complex itself are also in development. Small molecule caspase-1 inhibitors have been developed and have shown improvement in inflammation in murine models and small human studies(15, 16). Recently, an orally available small molecule inhibitor of NLRP3 inflammasome activation has shown benefit in murine models of multiple sclerosis and Muckle-Wells Syndrome(17). Inhibitors of ASC oligomerization are also in development(18). This pipeline of inflammasome inhibitors is expanding and will hopefully increase therapeutic options for rheumatic disease patients.
Other medications that were initially developed for non-inflammasome-related indications have been found to have inflammasome inhibiting activity. Glyburide, which is prescribed for type II diabetes, has inhibitory effects on NLRP3 inflammasome activation(19). Omega-3 fatty acids, a component of fish oil, have also been shown to inhibit the NLRP3 inflammasome(20). Caspase-1 is inhibited by thalidomide, a medication used for multiple myeloma and for refractory rheumatic disease such as cutaneous lupus(21, 22). Antimalarial medications, such as hydroxychloroquine and chloroquine, which interferes with toll-like receptor (TLR) 7 and 9 activation in the lysosomal compartment, blocks inflammasome activation and IL-1β release following certain inflammasome activating triggers(13, 23). Colchicine, which inhibits microtubule assembly, is able to block inflammasome activation by preventing co-localization of ASC with NLRP3(24, 25). Non-steroidal anti-inflammatory drugs (NSAIDs) inhibit the NLRP3 inflammasome via blockade of Cox-2 effects on this complex(26). Some of these listed medications have been shown to have effects in rheumatic diseases, but further research is needed to determine the extent to which these effects are inflammasome-dependent.
Systemic Lupus Erythematosus
Systemic lupus erythematosus (SLE) is a systemic autoimmune disorder characterized by elevated type I interferon production(27) and immune complex deposition within the tissues leading to generation of an inflammatory response and subsequent organ damage. Presentations are varied between patients, and affected organs can include diverse areas including brain, bone marrow, skin, kidney and lungs. Also contributing to mortality in SLE is a striking increased risk of cardiovascular disease(28).
Recently, there is accumulating evidence for a role of the inflammasome in SLE pathogenesis. Upregulation of inflammasome components has been noted in lupus nephritis biopsies(29), and overexpression of inflammasome scaffolds such as IFI16 and AIM2 have also been reported in leukocytes from SLE patients(30). Immune complexes containing DNA or RNA antigens, can prime the inflammasome for activation and subsequently trigger assembly of NLRP3 inflammasome complexes (13, 23). The activated complement component, C3a, promotes ATP secretion and thus serves as a trigger for inflammasome activation(31). Neutrophil extracellular traps (NETs), which are increased in SLE patients and likely contribute to pathogenesis(32, 33) stimulate NLRP3 inflammasome activation and promotion of IL-1β and IL-18 activation and release(34). Genetic polymorphisms associated with RIG-I signaling have also been reported to have associations with SLE, but whether this relates to inflammasome activity of RIG-I or other interferon-related signaling has not been determined(35).
Murine studies have also suggested a role for the inflammasome in lupus pathogenesis. Nonspecific inhibitors of NLRP3 have demonstrated protective effects in several murine models of lupus(36–38). Inhibition of a well-described activator of NLRP3 inflammasome assembly, the P2X7 receptor, also benefits lupus nephritis(39). Absence of caspase-1 is also protective from autoantibody generation and lupus nephritis in the pristane model of lupus(40). Blockade of other activators of the NLRP3 inflammasome, such as high-mobility group box 1 protein (HMGB1), abrogates caspase-1 activation and improves lupus nephritis in BXSB mice(41). The role of other inflammasome complexes, such as that driven by AIM2 activation is less clear in SLE. Absence of AIM2 is protective in some models(42), but others have shown that inhibition of AIM2 may promote lupus pathogenesis. (43, 44). Stimulation of RIG-I has been shown to exacerbate lupus nephritis(45). The inflammasome-activated cytokines IL-1β and IL-18 also has an important role in murine lupus. Absence of IL-1β is protective in an anti-double stranded DNA induced lupus model(46) and inhibition of IL-1β downregulated autoantibody production from B cells isolated from mice with active lupus(47). Inhibition of IL-18 in the MRL-Faslpr model improved nephritis and skin disease(48, 49), and reductions in IL-18 transcripts are associated with improvement in nephritis(50).
Targeting of the inflammasome has not risen to the clinical forefront in SLE, but there are some reports that suggest it may be worthwhile. A very small, non-placebo controlled trial of lupus patients with refractory arthritis suggested that there may be a small benefit to the use of anakinra(24). Antimalarial therapy, a cornerstone of lupus treatment has inhibitory effects on inflammasome activation (13, 23) and has been shown to reduce IL-18 concentrations in SLE serum(51). Consumption of ω-3 FA have shown substantial benefit in murine models of lupus; they reduce inflammatory cytokine production, including lowering levels of IL-1β and IL-18(52, 53). One short-term, placebo controlled trial of ω-3FA did not show significant benefit to disease activity over 12 weeks(54), but the baseline SELENA-SLEDAI disease activity was low in these patients (mean of 1.5). Another placebo-controlled trial of ω-3FA in lupus patients with higher baseline activity (mean SLAM-R 10.2) showed improvement in both BILAG and SLAM-R scores(55). Whether the effects of ω-3FA are related to their inhibition of the inflammasome in this disease remains to be determined. Another way inflammasome inhibition may benefit lupus patients is by ameliorating complications of treatment. Cyclophosphamide-induced bladder inflammation is mediated by the inflammasome and treatment with glyburide improved this side effect in murine models(56).
Rheumatoid Arthritis
Rheumatoid arthritis is a systemic autoimmune disease characterized by symmetric, small joint inflammation that affects approximately 1% of the US population. Patient sera and joint fluids are characterized by increased concentrations of inflammatory cytokines including IL-1β, Il-6, IL-17 and TNFα(57). When left unchecked, progressive inflammation will result in joint destruction, disfigurement and disability(58). Despite major advances in therapy, there is still a great need for additional therapeutic development as many patients are unable to achieve complete remission with the use of current medications.
There are robust data from murine models which argue for a role for inflammasome activation in rheumatoid arthritis. Mutations in A20/Tnfaip3, which predispose mice to an RA-like disease, result in enhanced activation of the inflammasome, and disease is ameliorated in the absence of a functional NLRP3 inflammasome(59). In collagen-induced arthritis, another murine model of RA, inhibition of NLRP1 inflammasome activation is protective(60), and a functional ASC molecule is required for disease activity(61). Caspase-1 has also been shown to be required for chronic arthritis development in response to streptococcal cell wall antigens(62).
In human studies, evidence also exists to support a role for the inflammasome in RA. Components of the NLRP3 inflammasome are increased in peripheral blood mononuclear cells (PBMCs) of untreated RA patients(63), and myeloid and endothelial cells are likely sources of production in RA synovium(64). Elevated circulating levels of IL-18 and caspase-1 (although the active form was not determined) were seen in the sera of RA patients(63). Genetic evidence has linked polymorphisms and subsequent overexpression of NLRP1 to risk of RA(65). Presence of minor polymorphisms in both NLRP3 and an inflammasome regulating protein CARD8 were associated with increased disease severity and seropositivity(66). A NLRP3 polymorphism (rs4612666) is associated with poor response to anti-TNF therapy(67). These studies suggest that the inflammasome is upregulated and active in RA.
Evidence also supports a role for the inflammasome-activated cytokine IL-18 in RA. IL-18 polymorphisms have also been associated with RA risk(68). IL-18 promotes inflammation and production of pro-osteoclastogenic cytokines from fibroblast-like synoviocytes(69). Angiogenesis within the arthritic joint is also promoted by IL-18(70). Animal models suggest that IL-18 is required for induction of arthritis in mice by zymosan(71) and for collagen-induced arthritis in rats(72). However, no significant studies evaluating the role of IL-18 blockade in human arthritis have been conducted to date, so the role of IL-18 in human RA remains untested.
One of the first biologic medications to show benefit in rheumatoid arthritis was the IL-1 receptor antagonist, anakinra, and this resulted in FDA approval for this medication in 2001. However, the effectiveness of this medication is not as robust as other biologic therapies, and significant injection site reactions has limited its utility in RA(73). Thus, the use of anakinra has fallen to a second-line therapeutic in this disease. A small trial has shown some efficacy for canakinumab in improving RA disease activity, but this remains experimental(74).
Given that the inflammasome can have roles in addition to facilitating IL-1β activation, it is unknown whether targeted inhibition of the inflammasome itself would have more impact on arthritis activity in this disease. Some indirect evidence stems from recent studies which demonstrated that addition of fish oil/ω-3 fatty acids to triple therapy with hydroxychloroquine, sulfasalazine and methotrexate improved remission rates and lowered rates of treatment failure in a cohort of newly diagnosed rheumatoid arthritis patients(75). Further, consumption of one serving of fish per week was associated with a 29% relative risk reduction for RA(76). However, it is unknown whether the effects of fish oil are related to inflammasome inhibition. A phase II clinical trial was completed in 2002 for the caspase-1 inhibitor, pralnacasan, in rheumatoid arthritis. Abstracts suggested improved clinical activity, but the trial was formally withdrawn secondary to possible fibrosis in the liver(77). No further follow up was reported.
Other types of inflammatory arthropathies have not been well studied with regard to the role of the inflammasome. A small trial did not find evidence for NLRP3 polymorphisms associated with ankylosing spondylitis(78). However, another small trial suggested that thalidomide may have some benefit in ankylosing spondylitis(79), but whether this was related to thalidomide’s effects on caspase-1 was not explored.
Systemic-Onset Juvenile Idiopathic Arthritis and Adult-Onset Stills Disease
Systemic onset juvenile idiopathic arthritis (s-JIA) has been defined by the International League of Associations for Rheumatology (ILAR) as: “arthritis in one or more joints with or preceded by fever of at least two weeks duration which is documented to be quotidian for at least three days, accompanied by one or more of the following: evanescent erythematous rash; lymphadenopathy, hepatomegaly, and/or splenomegaly; and serositis” (80). The disease accounts for ~5–9% of JIA cases (81). Onset of symptoms typically occurs between 1 and 5 years of age, with a peak incidence at 2 years (82). Historically, s-JIA was a disease associated with significant morbidity, with ~30% of patients developing destructive polyarthritis (83). Patients with s-JIA are additionally at increased risk for macrophage activation syndrome (MAS), a form of secondary hemophagocytic lymphohistiocytosis manifested by fever, hepatosplenomegaly, lymphadenopathy, cytopenias, coagulopathies, and CNS inflammation. Morbidity and mortality is quite high with this complication, with one study citing mortality rates as high as 22% (84). Adult-Onset Stills Disease (AOSD) is often considered part of the spectrum of s-JIA, with later age onset (85). It too is manifested by spiking fevers, polyarthritis, evanescent rash, lymphadenopathy and pharyngitis, and can be complicated by MAS.
Polymorphims in NLRP3 may be associated with s-JIA(86), but study of the inflammasome itself has been limited in s-JIA and AOSD. However, the downstream cytokines IL-1β and IL-18 have been well characterized. IL-1β plays a key role in the pathogenesis of s-JIA and AOSD. IL-1β production is significantly increased in activated peripheral blood mononuclear cells isolated from patients with s-JIA (87) and in patients with untreated AOSD (88, 89). Serum from s-JIA patients can induce transcription of the IL-1 locus in PBMCs from healthy controls (87). The exact cellular source of IL-1β in s-JIA and AOSD has yet to be definitively identified, but clinical response to IL-1 blocking agents highlights the key role of the cytokine in disease pathogenesis. IL-18 levels also appear to correlate with disease activity in s-JIA (90). IL-18 induces IFN-γ, IL-17, and TNF-α, and likely plays a crucial role in macrophage activation. IL-18 levels are elevated in children with active JIA regardless of type; however, levels are markedly higher in children with s-JIA (91). IL-18 levels measured in serum and synovial fluid from s-JIA patients have been shown to increase with CRP level, IL-6 level, IL-1 level, number of active joints, and radiological score. It has additionally been shown that children in which IL-18 levels predominate over IL-6 levels are more likely to develop MAS(92).
Advances in understanding the role of the inflammasome, and particularly the role of IL-1β, in the pathogenesis of s-JIA and AOSD, have revolutionized therapy for affected patients. The IL-1 inhibitor, anakinra, was the first biologic agent to demonstrate significant efficacy, and has become a cornerstone in the treatment of s-JIA. Effectiveness in treating s-JIA was first reported in 2004, when anakinra produced a prompt and full response in two patients with refractory disease (93). Two larger and more comprehensive trials evaluating the efficacy of anakinra were released in 2011. The first of these was a multicenter, double-blind, placebo-controlled trial which included 24 patients. Treatment and control groups each had 12 patients and were given one month of anakinra vs. placebo. Primary outcome was defined as a 30% improvement in criteria of American College of Rheumatology for pediatric response (ACR Pedi 30), resolution of symptoms, and at least 50% decrease in CRP and ESR. After one month, 67% of anakinra-treated patients achieved primary endpoint compared with 8% of patients in the placebo group (94). The second study was a multicenter, international, observational trial looking at 46 s-JIA patients undergoing treatment with anakinra. 90% of study participants experienced resolution of systemic symptoms and refractory arthritis. Additionally, at two months, the majority of patients no longer required corticosteroids (95). These studies demonstrate the effectiveness of IL-1 antagonism with anakinra in treatment of s-JIA. Similar dramatic response to treatment with anakinra has additionally been demonstrated in AOSD, and the drug has been proposed as a treatment of choice for patients with refractory disease, but is not yet FDA approved for this indication (96).
Two phase III trials demonstrating efficacy of canakinumab for treatment of s-JIA were described in 2012. The first study was a double-blind, placebo-controlled trial with a primary outcome measure of adapted JIA ACR 30 response and resolution of fever within 15 days of starting treatment. 84% of patients in the treatment group achieved ACR 30 response, compared with 10% in the placebo group. The second study was a two-phase trial, in which all subjects received canakinumab as part of an open-label first phase. Forty five percent of patients were able to taper glucocorticoids during this phase, and 33% of patients were able to discontinue them. The second phase consisted of a double-blind, placebo-controlled withdrawal study in which the primary outcome was time to flare. 75% of patients in the placebo group experienced a flare of their disease, compared with 25% of patients in the canakinumab group, a statistically significant difference(97). Successful treatment of AOSD has additionally been reported with canakinumab, even in patients with disease refractory to treatment with anakinra(98). More recently, several studies have demonstrated good response in both AOSD and sJIA to treatment with rilonacept (99, 100).
Autoinflammatory Disorders
Abnormalities in the NLRP3-inflammasome appear to be responsible for many monogenic autoinflammatory diseases. Mutations in NLRC4 have also been identified to contribute to autoinflammatory disorders(101, 102). These diseases are the results of defects either in the inflammasome itself, pathways leading to its activation, or downstream steps in the activation of IL-1. Autoinflammatory disorders have been classified as IL-1-mediated (cryopyrin associated periodic syndromes (CAPS) and deficiency of interleukin 1 receptor agonist (DIRA)), partially IL-1 mediated (familial Mediterranean fever (FMF), hyperimmunoglobulinemia D syndrome (HIDS), pyogenic sterile arthritis, pyoderma gangrenosum and acne (PAPA)), and mediated by other pathways (Blau-Syndrome, cherubism, IL-10 receptor deficiency, Majeed’s Syndrome, TRAPS)(103).
CAPS comprises a clinical spectrum of three diseases: familial cold autoinflammatory syndrome (FCAS), Muckle-Wells Syndrome (MWS) and neonatal-onset multisystem inflammatory disease (NOMID). These diseases are all caused by an autosomal dominant mutation in NLRP3(104). Clinical presentation includes fever, urticarial rash, arthritis, conjunctivitis, and significantly elevated inflammatory markers (105). Severity of the disease ranges from episodes that are triggered by cold exposure and resolve within hours in FCAS, to continuous fever, permanent organ damage and death in untreated NOMID (106, 107).
DIRA is caused by autosomal recessive loss of function mutations in the IL1RN gene, which encodes the IL-1 receptor antagonist (IL-1Ra). These patients typically present with multifocal osteomyelitis and periostitis, pustular dermatitis, and elevated inflammatory markers. Fever is often low grade or can be absent (107, 108). FMF is an autosomal recessive autoinflammatory condition, the result of largely missense mutations in the MEFV gene (109). This gene encodes a protein known as pyrin, a major regulatory component of the inflammasome (110). The disease typically presents with recurrent febrile episodes marked by large joint arthritis and acute abdominal pain secondary to peritonitis (111, 112). Patients typically have leukocytosis, elevated ESR and CRP during acute flares (113). Type AA secondary amyloidosis, often leading to renal impairment, is the most frequent complication, occurring in ~13% of patients in a 2005 Turkish study (114). The frequency of amyloidosis is impacted by the use of colchicine, however(115).
HIDS is an autosomal recessive disease resulting from mutations in MVK (mevalonate kinase gene) (116). The disease is manifested by recurrent fevers (typically occurring every 4–6 weeks), rashes, abdominal pain, diarrhea, vomiting, tender lymphadenopathy, nonerosive arthritis, and elevated inflammatory markers (8, 117). IgD levels are persistently elevated (118). In most instances, the disease has a benign course and self-resolves in adulthood.
PAPA syndrome is caused by autosomal dominant mutations in the PSTPIP1 gene. Patients present with early-onset, deforming pyogenic arthritis, cutaneous ulcers, and pathergy. ESR and CRP are elevated during flares (119, 120).
TNF receptor-associated periodic syndrome (TRAPS) is an autoinflammatory syndrome resulting from autosomal dominant mutations in the TNFRSF1A gene, which encodes the TNF receptor (TNFR1). It is the second most common autoinflammatory disease (121, 122). Mean age of disease onset is 10 years, although this varies from 1 to 63 years. The disease manifests as recurrent fevers, acute abdominal pain, myalgias, and arthritis. Ocular involvement including conjunctivitis and anterior uveitis is seen in roughly half of patients (123–125).
IL-1 inhibition is currently the most successful targeted therapy in autoinflammatory disorders, and is utilized in the treatment of CAPS, colchicine-resistant FMF, TRAPS, HIDS, and DIRA (126). IL-1 blockade is now considered standard treatment for patients with CAPS. Treatment with anakinra, canakinumab, and rilonacept has been shown to result in complete resolution of symptoms in most cases (106). Anakinra has been used to treat NOMID for over 10 years and virtually all patients show a rapid response with resolution of fever, rash, and improvement inflammatory markers. However, doses needed to control symptoms in NOMID patients are significantly higher than those needed to treat FCAS patients (106). DIRA patients additionally have a striking response to treatment with anakinra, with rapid resolution of symptoms and normalization of inflammatory markers(107, 108).
Colchicine remains the standard therapy in patients with FMF, in many cases inducing complete remission. Colchicine has additionally been shown to be effective in preventing amyloidosis in many patients (127). Importantly, a role for colchicine in blocking inflammasome activation has recently been noted(25), but whether this is the mechanism by which colchicine works in FMF has yet to be determined. However, a minority of patients are unresponsive to or unable to tolerate colchicine. IL-1 blockade is a promising treatment approach in these patients. Successful treatment with anakinra has been demonstrated (128). Additionally, in a randomized, placebo-controlled trial published in 2012, rilonacept was effective at treating the disease (129). Case reports for the effectiveness of canakinumab are also noted.
Treatment of HIDS is challenging and typical therapies such as NSAIDs, corticosteroids, and colchicine are not effective. Etanercept and anakinra have been used with satisfactory response in some cases, and early treatment of attacks with anakinra may be a successful treatment strategy(130, 131). PAPA syndrome is additionally quite challenging to treat. Corticosteroids, cyclosporine, tacrolimus, IVIG, TNF inhibitors and thalidomide have been used with varying response. Response to IL-1 blockade is currently limited to case reports(132), but in general, IL-1 blockade is more effective at controlling arthritis and anti-TNF therapies are more effective for skin manifestations.
One would expect, based on the genetic defect causing TRAPS, that TNF blockade would be the treatment of choice for these patients. Interestingly, treatment with TNF agents can reduce frequency and severity of flares, but it does not eliminate them and efficacy of these agents often decreases over time(133, 134). More recently, anti-IL-1 therapy with anakinra or canakinumab has been used with promising results, and is becoming the treatment of choice in some centers (135, 136).
Crystal Arthropathies
Gout and pseudogout are characterized clinically by erythema, edema, warmth, and severe pain, reflecting a highly inflammatory response driven by monosodium urate (MSU) or calcium pyrophosphate dehydrate (CPPD) crystals, respectively. The prevalence of gout alone is estimated to occur in up to 3.9 percent of the population of the United States(137) and has a rising incidence(138). Evidence of CPPD is present in 15 - 44 percent of the population, with increasing prevalence with age(139). The inflammasome and subsequent IL-1β production, is centrally implicated in the pathogenesis of crystal-induced inflammatory arthritis.
Priming of the inflammasome for a gouty attack may occur through toll-like receptor recognition of the crystals themselves or by engagement of TLRs by fatty acids or other microbial patterns(47, 140, 141). MSU may also activate dendritic cells in a receptor-independent mechanism by which MSU electrostatically binds cholesterol-within the lipid rich membrane leading to lipid sorting and activation of an intracellular signaling cascade involving Syk(142). MSU and CPPD crystals are then able to stimulate NLRP3 inflammasome activation resulting in production of activated IL-1β(143). Reactive oxygen species (ROS), potassium efflux, and p62 induced autophagosomal inhibition have all been identified as mechanisms of activation of the inflammasome upon MSU exposure(144–147). Thus, MSU and CPPD act by at least two mechanisms to promote IL-1β activation; 1) priming resulting in induction of NFκB activation and IL-1β transcription and 2) inflammasome activation and cleavage of pro IL-1β to its biologically active form.
Murine models have shown supportive evidence for the role of the inflammasome in crystal-induced inflammation. Mice deficient in ASC, caspase-1 or the IL-1R had attenuated inflammatory response to intraperitoneal injection of MSU crystals(143). Further, lack of caspase-1 led to a moderate reduction of joint inflammation following joint co-injection with long-chain fatty acids(148). Other models have not demonstrated a profound effect of caspase-1 deficiency on the inflammation in MSU-injected joints(143, 149). These findings suggest that there may be additional pathways which contribute to IL-1β activation in gouty arthritis.
CPPD crystals are implicated in the inflammatory arthritis more commonly known as pseudogout. CPPD demonstrates similar activation of inflammatory caspases and subsequent formation of IL-1β as seen with MSU(143). NLRP3 deficient mice demonstrate impaired IL-1β release upon CPPD stimulation, indicating the integral role NLRP3 has on inflammasome activation. Similar to the findings in MSU, CPPD also recruits neutrophils when injected intraperitoneally in mouse models; a finding attenuated in mice deficient in caspase-1 or ASC(74).
Basic calcium phosphate has been studied in murine models and does not appear to have an inflammasome-dependent inflammatory effect(150). The inflammasome’s role in cholesterol crystal-driven arthritis has not been studied, however cholesterol crystals do trigger IL-1β in an NLRP3 dependent manner(151).
NSAIDs are a mainstay of therapy for gout and CPPD. NSAIDs impact inflammation through COX-1/COX-2 inhibition. COX-2 activation increases LPS-induced NFκB activation and promotes mitochondrial damage and ROS generation, which contribute to inflammasome activation(26, 152–155). Blockade of these pathways via NSAIDs reduces LPS-driven IL-1β production(29). Thus, NSAIDs may contribute to control of inflammation through inflammasome-modulating effect in addition to their effect on prostaglandin formation(156).
Colchicine, an alkaloid that prevents microtubule organization, is commonly used to treat acute flares of gout. Pre-treatment with colchicine prior to intra-articular MSU injection leads to reduced processing of IL-1β and inflammation(143). Colchicine prevents inflammasome activation by disrupting colocalization of NLRP3 and ASC, which is mediated by microtubules(25). Colchicine has also been found to increase AMPK activity, which thus inhibits NFκB activation and subsequent production of IL-1β(157).
IL-1 inhibition is a novel therapy for treatment of gout and has demonstrated clinical efficacy. Anakinra, a recombinant human IL-1R antagonist, has been utilized in uncontrolled studies of patients with contraindications to or failure of therapy with NSAIDs and colchicine. Although no randomized control trial has been performed, anakinra has been studied in a small open-labeled proof-of-concept study as well as several case reports and retrospective studies(158–160). These reports have demonstrated a favorable clinical response. The American College of Rheumatology recommends anakinra for treatment of a severe flare of gout or for treatment of gout refractory to traditional therapy modalities(161). Rilonacept has also demonstrated preliminary efficacy in gout. A double-blind active-controlled study of 225 patients with gout did not demonstrate improvement in pain relief compared to indomethacin alone(162). However, patients on rilonacept demonstrated a significant reduction in risk of gout attacks in patient initiating allopurinol versus those maintained on placebo alone(163–165). Rilonacept’s application for FDA approval for gout was denied in 2012, and the committee requested additional clinical information. Canakinumab has been investigated in several randomized control trials and is approved in Europe for treatment of acute gout flares. Canakinumab performed favorably compared to intramuscular triamcinolone for reduction of pain symptoms as well for reducing the risk of a new gout flare(166–168). Additional randomized control trials have studied canakinumab compared to colchicine for prophylaxis against gout flare in patients who recently started allopurinol. Canakinumab demonstrated lower mean number of flares, greater time to the first new gout flare, and shorter duration of flares than the colchicine group(168).
Treatment of pseudogout with IL-1 inhibition is limited to case reports(169–171). Although European League Against Rheumatism (EULAR) guidelines mention IL-1 inhibition as a possible future therapy, it is currently not recommended as standard therapy by EULAR for treatment of CPPD(172).
Conclusion
The importance of the inflammasome in the pathogenesis of autoimmune and autoinflammatory disease has become apparent over the last decade. Continued investigation in this arena will likely lead to additional indications for anti-IL-1 therapies. Novel usages for medications with effects on the inflammasome, such as ω-3FA, will also expand as our understanding of this complex and its role in disease broadens. Further, novel inflammasome inhibitors are in development and will hopefully have similar beneficial effects on a multitude of rheumatic conditions.
Acknowledgments
All authors have read the journal’s policy on conflicts of interest and authorship agreement. Drs. McCoy and Stannard have no conflicts to disclose. Dr. Kahlenberg receives research support from Novartis. For this work, Dr. McCoy and Dr. Stannard were supported in part by the Department of Veterans Affairs. Dr. Kahlenberg was partially supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number K08AR063668.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sara S. McCoy, Email: ssmccoy@med.umich.edu.
Jasmine Stannard, Email: jstannar@med.umich.edu.
J. Michelle Kahlenberg, Email: mkahlenb@med.umich.edu.
Cited References
- 1.Lamkanfi M, Dixit VM. Inflammasomes and Their Roles in Health and Disease. Annual Review of Cell and Developmental Biology. 2012;28(1):137–61. doi: 10.1146/annurev-cellbio-101011-155745. [DOI] [PubMed] [Google Scholar]
- 2.Miao EA, Leaf IA, Treuting PM, Mao DP, Dors M, Sarkar A, et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010;11(12):1136–42. doi: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013 Jun;13(6):397–411. doi: 10.1038/nri3452. //print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poeck H, Bscheider M, Gross O, Finger K, Roth S, Rebsamen M, et al. Recognition of RNA virus by RIG-I results in activation of CARD9 and inflammasome signaling for interleukin 1 beta production. Nat Immunol. 2010 Jan;11(1):63–9. doi: 10.1038/ni.1824. Epub 2009/11/17. eng. [DOI] [PubMed] [Google Scholar]
- 5.Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, et al. Cutting Edge: NF-κB Activating Pattern Recognition and Cytokine Receptors License NLRP3 Inflammasome Activation by Regulating NLRP3 Expression. The Journal of Immunology 2009. 2009 Jul 15;183(2):787–91. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kahlenberg JM, Lundberg KC, Kertesy SB, Qu Y, Dubyak GR. Potentiation of caspase-1 activation by the P2X7 receptor is dependent on TLR signals and requires NF-kappaB-driven protein synthesis. J Immunol. 2005 Dec 1;175(11):7611–22. doi: 10.4049/jimmunol.175.11.7611. Epub 2005/11/23. eng. [DOI] [PubMed] [Google Scholar]
- 7.Juliana C, Fernandes-Alnemri T, Kang S, Farias A, Qin F, Alnemri ES. Non-transcriptional Priming and Deubiquitination Regulate NLRP3 Inflammasome Activation. Journal of Biological Chemistry 2012. 2012 Oct 19;287(43):36617–22. doi: 10.1074/jbc.M112.407130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hwang I, Yang J, Hong S, Ju Lee E, Lee SH, Fernandes-Alnemri T, et al. Non-transcriptional regulation of NLRP3 inflammasome signaling by IL-4. Immunol Cell Biol. 2015 Jan 20; doi: 10.1038/icb.2014.125. Epub 2015/01/21. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Man SM, Kanneganti TD. Regulation of inflammasome activation. Immunological reviews. 2015 May;265(1):6–21. doi: 10.1111/imr.12296. Epub 2015/04/17. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franchi L, Kanneganti T-D, Dubyak GR, Núñez G. Differential Requirement of P2X7 Receptor and Intracellular K+ for Caspase-1 Activation Induced by Intracellular and Extracellular Bacteria. Journal of Biological Chemistry 2007. 2007 Jun 29;282(26):18810–8. doi: 10.1074/jbc.M610762200. [DOI] [PubMed] [Google Scholar]
- 11.Franchi L, Muñoz-Planillo R, Reimer T, Eigenbrod T, Núñez G. Inflammasomes as microbial sensors. European Journal of Immunology. 2010;40(3):611–5. doi: 10.1002/eji.200940180. [DOI] [PubMed] [Google Scholar]
- 12.Rock KL, Kataoka H, Lai J-J. Uric acid as a danger signal in gout and its comorbidities. Nature reviews. 2013 Jan;9(1):13–23. doi: 10.1038/nrrheum.2012.143. //print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shin MS, Kang Y, Lee N, Wahl ER, Kim SH, Kang KS, et al. Self Double-Stranded (ds)DNA Induces IL-1β Production from Human Monocytes by Activating NLRP3 Inflammasome in the Presence of Anti–dsDNA Antibodies. The journal of immunology. 2013 Jan 11;2013 doi: 10.4049/jimmunol.1201195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanchez Rodriguez R, Pauli ML, Neuhaus IM, Yu SS, Arron ST, Harris HW, et al. Memory regulatory T cells reside in human skin. The Journal of Clinical Investigation. 2014;124(3):1027–36. doi: 10.1172/JCI72932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012 Aug;11(8):633–52. doi: 10.1038/nrd3800. Epub 2012/08/02. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rudolphi K, Gerwin N, Verzijl N, van der Kraan P, van den Berg W. Pralnacasan, an inhibitor of interleukin-1beta converting enzyme, reduces joint damage in two murine models of osteoarthritis. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2003 Oct;11(10):738–46. doi: 10.1016/s1063-4584(03)00153-5. Epub 2003/09/18. eng. [DOI] [PubMed] [Google Scholar]
- 17.Coll RC, Robertson AA, Chae JJ, Higgins SC, Munoz-Planillo R, Inserra MC, et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med. 2015 Mar;21(3):248–55. doi: 10.1038/nm.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coll RC, Robertson A, Butler M, Cooper M, O’Neill LA. The cytokine release inhibitory drug CRID3 targets ASC oligomerisation in the NLRP3 and AIM2 inflammasomes. PLoS One. 2011;6(12):e29539. doi: 10.1371/journal.pone.0029539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lamkanfi M, Mueller JL, Vitari AC, Misaghi S, Fedorova A, Deshayes K, et al. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J Cell Biol. 2009 Oct 5;187(1):61–70. doi: 10.1083/jcb.200903124. Epub 2009/10/07. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan Y, Jiang W, Spinetti T, Tardivel A, Castillo R, Bourquin C, et al. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity. 2013 Jun 27;38(6):1154–63. doi: 10.1016/j.immuni.2013.05.015. Epub 2013/07/03. eng. [DOI] [PubMed] [Google Scholar]
- 21.Keller M, Sollberger G, Beer H-D. Thalidomide inhibits activation of caspase-1. The journal of immunology. 2009;183(9):5593–9. doi: 10.4049/jimmunol.0900476. [DOI] [PubMed] [Google Scholar]
- 22.Baret I, De Haes P. Thalidomide: Still an important second-line treatment in refractory cutaneous lupus erythematosus? The Journal of dermatological treatment. 2014 Apr 14; doi: 10.3109/09546634.2014.906036. Epub 2014/04/16. Eng. [DOI] [PubMed] [Google Scholar]
- 23.Shin MS, Kang Y, Lee N, Kim SH, Kang KS, Lazova R, et al. U1-Small Nuclear Ribonucleoprotein Activates the NLRP3 Inflammasome in Human Monocytes. The journal of immunology 2012. 2012 May 15;188(10):4769–75. doi: 10.4049/jimmunol.1103355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ostendorf B, Iking-Konert C, Kurz K, Jung G, Sander O, Schneider M. Preliminary results of safety and efficacy of the interleukin 1 receptor antagonist anakinra in patients with severe lupus arthritis. Ann Rheum Dis. 2005 Apr;64(4):630–3. doi: 10.1136/ard.2004.025858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Misawa T, Takahama M, Kozaki T, Lee H, Zou J, Saitoh T, et al. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat Immunol. 2013 May;14(5):454–60. doi: 10.1038/ni.2550. Epub 2013/03/19. eng. [DOI] [PubMed] [Google Scholar]
- 26.Hua KF, Chou JC, Ka SM, Tasi YL, Chen A, Wu SH, et al. Cyclooxygenase-2 regulates NLRP3 inflammasome-derived IL-1beta production. Journal of cellular physiology. 2015 Apr;230(4):863–74. doi: 10.1002/jcp.24815. Epub 2014/10/09. eng. [DOI] [PubMed] [Google Scholar]
- 27.Crow MK, Kirou KA. Interferon-alpha in systemic lupus erythematosus. Curr Opin Rheumatol. 2004 Sep;16(5):541–7. doi: 10.1097/01.bor.0000135453.70424.1b. [DOI] [PubMed] [Google Scholar]
- 28.Manzi S, Meilahn EN, Rairie JE, Conte CG, Medsger TA, Jansen-McWilliams L, et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. American Journal of Epidemiology. 1997;145(5):408–15. doi: 10.1093/oxfordjournals.aje.a009122. [DOI] [PubMed] [Google Scholar]
- 29.Kahlenberg JM, Thacker SG, Berthier CC, Cohen CD, Kretzler M, Kaplan MJ. Inflammasome activation of IL-18 results in endothelial progenitor cell dysfunction in systemic lupus erythematosus. J Immunol. 2011 Dec 1;187(11):6143–56. doi: 10.4049/jimmunol.1101284. Epub 2011/11/08. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kimkong I, Avihingsanon Y, Hirankarn N. Expression profile of HIN200 in leukocytes and renal biopsy of SLE patients by real-time RT-PCR. Lupus. 2009;18(12):1066–72. doi: 10.1177/0961203309106699. [DOI] [PubMed] [Google Scholar]
- 31.Asgari E, Le Friec G, Yamamoto H, Perucha E, Sacks SS, Köhl J, et al. C3a modulates IL-1β secretion in human monocytes by regulating ATP efflux and subsequent NLRP3 inflammasome activation. Blood 2013. 2013 Nov 14;122(20):3473–81. doi: 10.1182/blood-2013-05-502229. [DOI] [PubMed] [Google Scholar]
- 32.Villanueva E, Yalavarthi S, Berthier CC, Hodgin JB, Khandpur R, Lin AM, et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol. 2011 Jul 1;187(1):538–52. doi: 10.4049/jimmunol.1100450. Epub 2011/05/27. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J, et al. Neutrophils Activate Plasmacytoid Dendritic Cells by Releasing Self-DNA-Peptide Complexes in Systemic Lupus Erythematosus. Sci Transl Med. 2011 Mar 9;3(73):73ra19. doi: 10.1126/scitranslmed.3001180. Epub 2011/03/11. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kahlenberg JM, Carmona-Rivera C, Smith CK, Kaplan MJ. Neutrophil Extracellular Trap–Associated Protein Activation of the NLRP3 Inflammasome Is Enhanced in Lupus Macrophages. The journal of immunology 2013. 2013 Feb 1;190(3):1217–26. doi: 10.4049/jimmunol.1202388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith S, Jefferies C. Role of DNA/RNA sensors and contribution to autoimmunity. Cytokine & Growth Factor Reviews. 2014;25(6):745–57. doi: 10.1016/j.cytogfr.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 36.Tsai PY, Ka SM, Chang JM, Chen HC, Shui HA, Li CY, et al. Epigallocatechin-3-gallate prevents lupus nephritis development in mice via enhancing the Nrf2 antioxidant pathway and inhibiting NLRP3 inflammasome activation. Free Radic Biol Med. 2011 Aug 1;51(3):744–54. doi: 10.1016/j.freeradbiomed.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 37.Zhu FGJW, Bhagat L, Wang D, Yu D, Tang JX, Kandimalla ER, La Monica N, Agrawal S. A novel antagonist of Toll-like receptors 7, 8 and 9 suppresses lupus disease-associated parameters in NZBW/F1 mice. Autoimmunity. 2013;46(7):419–28. doi: 10.3109/08916934.2013.798651. [DOI] [PubMed] [Google Scholar]
- 38.Zhao J, Zhang H, Huang Y, Wang H, Wang S, Zhao C, et al. Bay11-7082 attenuates murine lupus nephritis via inhibiting NLRP3 inflammasome and NF-κB activation. International Immunopharmacology. 2013;17(1):116–22. doi: 10.1016/j.intimp.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 39.Zhao J, Wang H, Dai C, Wang H, Zhang H, Huang Y, et al. P2X7 Blockade Attenuates Murine Lupus Nephritis by Inhibiting Activation of the NLRP3/ASC/Caspase 1 Pathway. Arthritis & Rheumatism. 2013;65(12):3176–85. doi: 10.1002/art.38174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kahlenberg JM, Yalavarthi S, Zhao W, Hodgin JB, Reed TJ, Tsuji NM, et al. An essential role for caspase-1 in the induction of murine lupus and its associated vascular damage. Arthritis Rheum. 2014 Oct 14;66(1):153–62. doi: 10.1002/art.38225. Epub 2013/10/16. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang C, Li C, Jia S, Yao P, Yang Q, Zhang Y. High mobility group box 1 inhibition alleviates lupus-like disease in BXSB mice. Scandinavian journal of immunology. 2014 doi: 10.1111/sji.12165. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 42.Zhang W, Cai Y, Xu W, Yin Z, Gao X, Xiong S. AIM2 Facilitates the Apoptotic DNA-induced Systemic Lupus Erythematosus via Arbitrating Macrophage Functional Maturation. Journal of clinical immunology 2013. 2013 Jul 01;33(5):925–37. doi: 10.1007/s10875-013-9881-6. English. [DOI] [PubMed] [Google Scholar]
- 43.Yin Q, Sester DP, Tian Y, Hsiao YS, Lu A, Cridland JA, et al. Molecular mechanism for p202-mediated specific inhibition of AIM2 inflammasome activation. Cell reports. 2013 Jul 25;4(2):327–39. doi: 10.1016/j.celrep.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Panchanathan R, Duan X, Shen H, Rathinam VAK, Erickson LD, Fitzgerald KA, et al. Aim2 Deficiency Stimulates the Expression of IFN-Inducible Ifi202, a Lupus Susceptibility Murine Gene within the Nba2 Autoimmune Susceptibility Locus. The Journal of Immunology 2010. 2010 Dec 15;185(12):7385–93. doi: 10.4049/jimmunol.1002468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Allam R, Pawar RD, Kulkarni OP, Hornung V, Hartmann G, Segerer S, et al. Viral 5′-triphosphate RNA and non-CpG DNA aggravate autoimmunity and lupus nephritis via distinctTLR-independent immune responses. European Journal of Immunology. 2008;38(12):3487–98. doi: 10.1002/eji.200838604. [DOI] [PubMed] [Google Scholar]
- 46.Voronov E, Dayan M, Zinger H, Gayvoronsky L, Lin JP, Iwakura Y, et al. IL-1 beta-deficient mice are resistant to induction of experimental SLE. Eur Cytokine Netw. 2006 Jun;17(2):109–16. Epub 2006/07/15. eng. [PubMed] [Google Scholar]
- 47.Scott P, Ma H, Viriyakosol S, Terkeltaub R, Liu-Bryan R. Engagement of CD14 mediates the inflammatory potential of monosodium urate crystals. J Immunol. 2006 Nov 1;177(9):6370–8. doi: 10.4049/jimmunol.177.9.6370. Epub 2006/10/24. eng. [DOI] [PubMed] [Google Scholar]
- 48.Menke J, Bork T, Kutska B, Byrne K, Blanfeld M, Relle M, et al. Targeting transcription factor Stat4 uncovers a role for interleukin-18 in the pathogenesis of severe lupus nephritis in mice. Kidney international. 2011;79(4):452–63. doi: 10.1038/ki.2010.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kinoshita K, Yamagata T, Nozaki Y, Sugiyama M, Ikoma S, Funauchi M, et al. Blockade of IL-18 Receptor Signaling Delays the Onset of Autoimmune Disease in MRL-Faslpr Mice. The journal of immunology 2004. 2004 Oct 15;173(8):5312–8. doi: 10.4049/jimmunol.173.8.5312. [DOI] [PubMed] [Google Scholar]
- 50.Tsai P-Y, Ka S-M, Chang J-M, Lai J-H, Dai M-S, Jheng H-L, et al. Antroquinonol differentially modulates T cell activity and reduces interleukin-18 production, but enhances Nrf2 activation, in murine accelerated severe lupus nephritis. Arthritis & Rheumatism. 2012;64(1):232–42. doi: 10.1002/art.33328. [DOI] [PubMed] [Google Scholar]
- 51.Wozniacka A, Lesiak A, Narbutt J, McCauliffe DP, Sysa-Jedrzejowska A. Chloroquine treatment influences proinflammatory cytokine levels in systemic lupus erythematosus patients. Lupus 2006. 2006 May 1;15(5):268–75. doi: 10.1191/0961203306lu2299oa. [DOI] [PubMed] [Google Scholar]
- 52.Pestka JJ, Vines LL, Bates MA, He K, Langohr I. Comparative effects of n-3, n-6 and n-9 unsaturated fatty acid-rich diet consumption on lupus nephritis, autoantibody production and CD4+ T cell-related gene responses in the autoimmune NZBWF1 mouse. PLoS One. 2014;9(6):e100255. doi: 10.1371/journal.pone.0100255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Halade GV, Williams PJ, Veigas JM, Barnes JL, Fernandes G. Concentrated fish oil (Lovaza(R)) extends lifespan and attenuates kidney disease in lupus-prone short-lived (NZBxNZW)F1 mice. Exp Biol Med (Maywood) 2013 Jun;238(6):610–22. doi: 10.1177/1535370213489485. Epub 2013/08/07. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bello KJ, Fang H, Fazeli P, Bolad W, Corretti M, Magder LS, et al. Omega-3 in SLE: a double-blind, placebo-controlled randomized clinical trial of endothelial dysfunction and disease activity in systemic lupus erythematosus. Rheumatology international. 2013 Nov;33(11):2789–96. doi: 10.1007/s00296-013-2811-3. Epub 2013/07/03. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wright SA, O’Prey FM, McHenry MT, Leahey WJ, Devine AB, Duffy EM, et al. A randomised interventional trial of omega-3-polyunsaturated fatty acids on endothelial function and disease activity in systemic lupus erythematosus. Ann Rheum Dis. 2008 Jun;67(6):841–8. doi: 10.1136/ard.2007.077156. Epub 2007/09/19. eng. [DOI] [PubMed] [Google Scholar]
- 56.Hughes FM, Jr, Vivar NP, Kennis JG, Pratt-Thomas JD, Lowe DW, Shaner BE, et al. Inflammasomes are important mediators of cyclophosphamide-induced bladder inflammation. American journal of physiology. 2014 Feb 1;306(3):F299–308. doi: 10.1152/ajprenal.00297.2013. Epub 2013/11/29. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Burska A, Boissinot M, Ponchel F. Cytokines as biomarkers in rheumatoid arthritis. Mediators of inflammation. 2014;2014:545493. doi: 10.1155/2014/545493. Epub 2014/04/16. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Molina E, del Rincon I, Restrepo JF, Battafarano DF, Escalante A. Association of socioeconomic status with treatment delays, disease activity, joint damage and disability in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2015 Jan 7; doi: 10.1002/acr.22542. Epub 2015/01/13. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Walle LV, Van Opdenbosch N, Jacques P, Fossoul A, Verheugen E, Vogel P, et al. Negative regulation of the NLRP3 inflammasome by A20 protects against arthritis. Nature. 2014 Jun 29; doi: 10.1038/nature13322. online. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li F, Guo N, Ma Y, Ning B, Wang Y, Kou L. Inhibition of P2X4 suppresses joint inflammation and damage in collagen-induced arthritis. Inflammation. 2014 Feb;37(1):146–53. doi: 10.1007/s10753-013-9723-y. Epub 2013/09/26. eng. [DOI] [PubMed] [Google Scholar]
- 61.Ippagunta S, Brand D, Luo J, Boyd K, Calabrese C, Stienstra R, et al. Inflammasome-independent role of apoptosis-associated speck-like protein containing a CARD (ASC) in T cell priming is critical for collagen-induced arthritis. The Journal of biological chemistry. 2010;285(16):12454–62. doi: 10.1074/jbc.M109.093252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Joosten LA, Netea MG, Fantuzzi G, Koenders MI, Helsen MM, Sparrer H, et al. Inflammatory arthritis in caspase 1 gene-deficient mice: contribution of proteinase 3 to caspase 1-independent production of bioactive interleukin-1beta. Arthritis Rheum. 2009 Dec;60(12):3651–62. doi: 10.1002/art.25006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mathews RJ, Robinson JI, Battellino M, Wong C, Taylor JC, Eyre S, et al. Evidence of NLRP3-inflammasome activation in rheumatoid arthritis (RA); genetic variants within the NLRP3-inflammasome complex in relation to susceptibility to RA and response to anti-TNF treatment. Ann Rheum Dis. 2014 Jun;73(6):1202–10. doi: 10.1136/annrheumdis-2013-203276. Epub 2013/05/21. eng. [DOI] [PubMed] [Google Scholar]
- 64.Kolly L, Busso N, Palmer G, Talabot-Ayer D, Chobaz V, So A. Expression and function of the NALP3 inflammasome in rheumatoid synovium. Immunology. 2010 Feb;129(2):178–85. doi: 10.1111/j.1365-2567.2009.03174.x. Epub 2009/10/15. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sui J, Li H, Fang Y, Liu Y, Li M, Zhong B, et al. NLRP1 gene polymorphism influences gene transcription and is a risk factor for rheumatoid arthritis in han chinese. Arthritis Rheum. 2012 Mar;64(3):647–54. doi: 10.1002/art.33370. Epub 2011/10/07. eng. [DOI] [PubMed] [Google Scholar]
- 66.Kastbom A, Verma D, Eriksson P, Skogh T, Wingren G, Soderkvist P. Genetic variation in proteins of the cryopyrin inflammasome influences susceptibility and severity of rheumatoid arthritis (the Swedish TIRA project) Rheumatology (Oxford, England) 2008 Apr;47(4):415–7. doi: 10.1093/rheumatology/kem372. Epub 2008/02/12. eng. [DOI] [PubMed] [Google Scholar]
- 67.Sode J, Vogel U, Bank S, Andersen PS, Thomsen MK, Hetland ML, et al. Anti-TNF treatment response in rheumatoid arthritis patients is associated with genetic variation in the NLRP3-inflammasome. PLoS One. 2014;9(6):e100361. doi: 10.1371/journal.pone.0100361. Epub 2014/06/27. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cai LP, Zhou LJ, Lu SY, Liang YE, Chen XY, Liu L, et al. Association of IL-18 promoter gene polymorphisms with rheumatoid arthritis: a meta-analysis. Mol Biol Rep. 2014 Dec;41(12):8211–7. doi: 10.1007/s11033-014-3723-3. Epub 2014/09/07. eng. [DOI] [PubMed] [Google Scholar]
- 69.Zhang W, Cong XL, Qin YH, He ZW, He DY, Dai SM. IL-18 upregulates the production of key regulators of osteoclastogenesis from fibroblast-like synoviocytes in rheumatoid arthritis. Inflammation. 2013 Feb;36(1):103–9. doi: 10.1007/s10753-012-9524-8. Epub 2012/09/05. eng. [DOI] [PubMed] [Google Scholar]
- 70.Amin MA, Rabquer BJ, Mansfield PJ, Ruth JH, Marotte H, Haas CS, et al. Interleukin 18 induces angiogenesis in vitro and in vivo via Src and Jnk kinases. Ann Rheum Dis. 2010 Dec;69(12):2204–12. doi: 10.1136/ard.2009.127241. Epub 2010/08/04. eng. [DOI] [PubMed] [Google Scholar]
- 71.Ruth JH, Park CC, Amin MA, Lesch C, Marotte H, Shahrara S, et al. Interleukin-18 as an in vivo mediator of monocyte recruitment in rodent models of rheumatoid arthritis. Arthritis Res Ther. 2010;12(3):R118. doi: 10.1186/ar3055. Epub 2010/06/23. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ye XJ, Tang B, Ma Z, Kang AH, Myers LK, Cremer MA. The roles of interleukin-18 in collagen-induced arthritis in the BB rat. Clin Exp Immunol. 2004 Jun;136(3):440–7. doi: 10.1111/j.1365-2249.2004.02430.x. Epub 2004/05/19. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cohen SB. The use of anakinra, an interleukin-1 receptor antagonist, in the treatment of rheumatoid arthritis. Rheum Dis Clin North Am. 2004 May;30(2):365–80. vii. doi: 10.1016/j.rdc.2004.01.005. Epub 2004/06/03. eng. [DOI] [PubMed] [Google Scholar]
- 74.Alten R, Gomez-Reino J, Durez P, Beaulieu A, Sebba A, Krammer G, et al. Efficacy and safety of the human anti-IL-1beta monoclonal antibody canakinumab in rheumatoid arthritis: results of a 12-week, Phase II, dose-finding study. BMC musculoskeletal disorders. 2011;12:153. doi: 10.1186/1471-2474-12-153. Epub 2011/07/09. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Proudman SM, James MJ, Spargo LD, Metcalf RG, Sullivan TR, Rischmueller M, et al. Fish oil in recent onset rheumatoid arthritis: a randomised, double-blind controlled trial within algorithm-based drug use. Ann Rheum Dis. 2015 Jan;74(1):89–95. doi: 10.1136/annrheumdis-2013-204145. Epub 2013/10/02. eng. [DOI] [PubMed] [Google Scholar]
- 76.Di Giuseppe D, Wallin A, Bottai M, Askling J, Wolk A. Long-term intake of dietary long-chain n-3 polyunsaturated fatty acids and risk of rheumatoid arthritis: a prospective cohort study of women. Ann Rheum Dis. 2014 Nov;73(11):1949–53. doi: 10.1136/annrheumdis-2013-203338. Epub 2013/08/14. eng. [DOI] [PubMed] [Google Scholar]
- 77.GK, PF, SAR, MWH, JCR Selective Interleukin-1b converting enzyme (ICE/Caspase-1) inhibition with pralnacasan. Arthritis & Rheumatism supplement. 2001:Abstract #1134. [Google Scholar]
- 78.Kastbom A, Klingberg E, Verma D, Carlsten H, Forsblad-d’Elia H, Wesamaa J, et al. Genetic variants in CARD8 but not in NLRP3 are associated with ankylosing spondylitis. Scand J Rheumatol. 2013;42(6):465–8. doi: 10.3109/03009742.2013.779020. Epub 2013/04/04. eng. [DOI] [PubMed] [Google Scholar]
- 79.Deng X, Zhang J, Zhang J, Huang F. Thalidomide reduces recurrence of ankylosing spondylitis in patients following discontinuation of etanercept. Rheumatology international. 2013 Jun;33(6):1409–13. doi: 10.1007/s00296-012-2571-5. Epub 2012/11/13.eng. [DOI] [PubMed] [Google Scholar]
- 80.Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. The Journal of rheumatology. 2004 Feb;31(2):390–2. Epub 2004/02/05. eng. [PubMed] [Google Scholar]
- 81.Danner S, Sordet C, Terzic J, Donato L, Velten M, Fischbach M, et al. Epidemiology of juvenile idiopathic arthritis in Alsace, France. The Journal of rheumatology. 2006 Jul;33(7):1377–81. Epub 2006/07/06. eng. [PubMed] [Google Scholar]
- 82.Behrens EM, Beukelman T, Gallo L, Spangler J, Rosenkranz M, Arkachaisri T, et al. Evaluation of the presentation of systemic onset juvenile rheumatoid arthritis: data from the Pennsylvania Systemic Onset Juvenile Arthritis Registry (PASOJAR) The Journal of rheumatology. 2008 Feb;35(2):343–8. Epub 2007/12/19. eng. [PubMed] [Google Scholar]
- 83.Spiegel LR, Schneider R, Lang BA, Birdi N, Silverman ED, Laxer RM, et al. Early predictors of poor functional outcome in systemic-onset juvenile rheumatoid arthritis: a multicenter cohort study. Arthritis and rheumatism. 2000 Nov;43(11):2402–9. doi: 10.1002/1529-0131(200011)43:11<2402::AID-ANR5>3.0.CO;2-C. Epub 2000/11/18. eng. [DOI] [PubMed] [Google Scholar]
- 84.Sawhney S, Woo P, Murray KJ. Macrophage activation syndrome: a potentially fatal complication of rheumatic disorders. Archives of disease in childhood. 2001 Nov;85(5):421–6. doi: 10.1136/adc.85.5.421. Epub 2001/10/23. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kadavath S, Efthimiou P. Adult-onset Still’s disease-pathogenesis, clinical manifestations, and new treatment options. Annals of medicine. 2015 Jan;22:1–9. doi: 10.3109/07853890.2014.971052. [DOI] [PubMed] [Google Scholar]
- 86.Yang CA, Huang ST, Chiang BL. Association of NLRP3 and CARD8 genetic polymorphisms with juvenile idiopathic arthritis in a Taiwanese population. Scand J Rheumatol. 2014;43(2):146–52. doi: 10.3109/03009742.2013.834962. Epub 2013/12/04. eng. [DOI] [PubMed] [Google Scholar]
- 87.Pascual V, Allantaz F, Arce E, Punaro M, Banchereau J. Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. The Journal of experimental medicine. 2005 May 2;201(9):1479–86. doi: 10.1084/jem.20050473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fall N, Barnes M, Thornton S, Luyrink L, Olson J, Ilowite NT, et al. Gene expression profiling of peripheral blood from patients with untreated new-onset systemic juvenile idiopathic arthritis reveals molecular heterogeneity that may predict macrophage activation syndrome. Arthritis and rheumatism. 2007 Nov;56(11):3793–804. doi: 10.1002/art.22981. [DOI] [PubMed] [Google Scholar]
- 89.Ogilvie EM, Khan A, Hubank M, Kellam P, Woo P. Specific gene expression profiles in systemic juvenile idiopathic arthritis. Arthritis and rheumatism. 2007 Jun;56(6):1954–65. doi: 10.1002/art.22644. [DOI] [PubMed] [Google Scholar]
- 90.de Jager W, Hoppenreijs EP, Wulffraat NM, Wedderburn LR, Kuis W, Prakken BJ. Blood and synovial fluid cytokine signatures in patients with juvenile idiopathic arthritis: a cross-sectional study. Annals of the rheumatic diseases. 2007 May;66(5):589–98. doi: 10.1136/ard.2006.061853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lotito AP, Campa A, Silva CA, Kiss MH, Mello SB. Interleukin 18 as a marker of disease activity and severity in patients with juvenile idiopathic arthritis. The Journal of rheumatology. 2007 Apr;34(4):823–30. Epub 2007/03/09. eng. [PubMed] [Google Scholar]
- 92.Shimizu M, Nakagishi Y, Yachie A. Distinct subsets of patients with systemic juvenile idiopathic arthritis based on their cytokine profiles. Cytokine. 2013 Feb;61(2):345–8. doi: 10.1016/j.cyto.2012.11.025. [DOI] [PubMed] [Google Scholar]
- 93.Verbsky JW, White AJ. Effective use of the recombinant interleukin 1 receptor antagonist anakinra in therapy resistant systemic onset juvenile rheumatoid arthritis. The Journal of rheumatology. 2004 Oct;31(10):2071–5. Epub 2004/10/07. eng. [PubMed] [Google Scholar]
- 94.Quartier P, Allantaz F, Cimaz R, Pillet P, Messiaen C, Bardin C, et al. A multicentre, randomised, double-blind, placebo-controlled trial with the interleukin-1 receptor antagonist anakinra in patients with systemic-onset juvenile idiopathic arthritis (ANAJIS trial) Annals of the rheumatic diseases. 2011 May;70(5):747–54. doi: 10.1136/ard.2010.134254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nigrovic PA, Mannion M, Prince FH, Zeft A, Rabinovich CE, van Rossum MA, et al. Anakinra as first-line disease-modifying therapy in systemic juvenile idiopathic arthritis: report of forty-six patients from an international multicenter series. Arthritis and rheumatism. 2011 Feb;63(2):545–55. doi: 10.1002/art.30128. [DOI] [PubMed] [Google Scholar]
- 96.Laskari K, Tzioufas AG, Moutsopoulos HM. Efficacy and long-term follow-up of IL-1R inhibitor anakinra in adults with Still’s disease: a case-series study. Arthritis research & therapy. 2011;13(3):R91. doi: 10.1186/ar3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ruperto N, Brunner HI, Quartier P, Constantin T, Wulffraat N, Horneff G, et al. Two randomized trials of canakinumab in systemic juvenile idiopathic arthritis. The New England journal of medicine. 2012 Dec 20;367(25):2396–406. doi: 10.1056/NEJMoa1205099. [DOI] [PubMed] [Google Scholar]
- 98.Kontzias A, Efthimiou P. The use of Canakinumab, a novel IL-1beta long-acting inhibitor, in refractory adult-onset Still’s disease. Seminars in arthritis and rheumatism. 2012 Oct;42(2):201–5. doi: 10.1016/j.semarthrit.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 99.Petryna O, Cush JJ, Efthimiou P. IL-1 Trap rilonacept in refractory adult onset Still’s disease. Annals of the rheumatic diseases. 2012 Dec;71(12):2056–7. doi: 10.1136/annrheumdis-2012-201409. [DOI] [PubMed] [Google Scholar]
- 100.Ilowite NT, Prather K, Lokhnygina Y, Schanberg LE, Elder M, Milojevic D, et al. Randomized, double-blind, placebo-controlled trial of the efficacy and safety of rilonacept in the treatment of systemic juvenile idiopathic arthritis. Arthritis & rheumatology. 2014 Sep;66(9):2570–9. doi: 10.1002/art.38699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kitamura A, Sasaki Y, Abe T, Kano H, Yasutomo K. An inherited mutation in NLRC4 causes autoinflammation in human and mice. J Exp Med. 2014 Nov 17;211(12):2385–96. doi: 10.1084/jem.20141091. Epub 2014/11/12. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Romberg N, Al Moussawi K, Nelson-Williams C, Stiegler AL, Loring E, Choi M, et al. Mutation of NLRC4 causes a syndrome of enterocolitis and autoinflammation. Nat Genet. 2014 Oct;46(10):1135–9. doi: 10.1038/ng.3066. Epub 2014/09/15. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Henderson C, Goldbach-Mansky R. Monogenic IL-1 mediated autoinflammatory and immunodeficiency syndromes: finding the right balance in response to danger signals. Clinical immunology. 2010 May;135(2):210–22. doi: 10.1016/j.clim.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Aksentijevich I, Nowak M, Mallah M, Chae JJ, Watford WT, Hofmann SR, et al. De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): a new member of the expanding family of pyrin-associated autoinflammatory diseases. Arthritis and rheumatism. 2002 Dec;46(12):3340–8. doi: 10.1002/art.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Arostegui JI, Aldea A, Modesto C, Rua MJ, Arguelles F, Gonzalez-Ensenat MA, et al. Clinical and genetic heterogeneity among Spanish patients with recurrent autoinflammatory syndromes associated with the CIAS1/PYPAF1/NALP3 gene. Arthritis and rheumatism. 2004 Dec;50(12):4045–50. doi: 10.1002/art.20633. [DOI] [PubMed] [Google Scholar]
- 106.Goldbach-Mansky R, Dailey NJ, Canna SW, Gelabert A, Jones J, Rubin BI, et al. Neonatal-onset multisystem inflammatory disease responsive to interleukin-1beta inhibition. The New England journal of medicine. 2006 Aug 10;355(6):581–92. doi: 10.1056/NEJMoa055137. Epub 2006/08/11. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Reddy S, Jia S, Geoffrey R, Lorier R, Suchi M, Broeckel U, et al. An autoinflammatory disease due to homozygous deletion of the IL1RN locus. The New England journal of medicine. 2009 Jun 4;360(23):2438–44. doi: 10.1056/NEJMoa0809568. Epub 2009/06/06. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Aksentijevich I, Masters SL, Ferguson PJ, Dancey P, Frenkel J, van Royen-Kerkhoff A, et al. An autoinflammatory disease with deficiency of the interleukin-1-receptor antagonist. The New England journal of medicine. 2009 Jun 4;360(23):2426–37. doi: 10.1056/NEJMoa0807865. Epub 2009/06/06. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ancient missense mutations in a new member of the RoRet gene family are likely to cause familial Mediterranean fever. The International FMF Consortium. Cell. 1997 Aug 22;90(4):797–807. doi: 10.1016/s0092-8674(00)80539-5. Epub 1997/08/22. eng. [DOI] [PubMed] [Google Scholar]
- 110.Drenth JP, van der Meer JW. The inflammasome--a linebacker of innate defense. The New England journal of medicine. 2006 Aug 17;355(7):730–2. doi: 10.1056/NEJMcibr063500. Epub 2006/08/18. eng. [DOI] [PubMed] [Google Scholar]
- 111.Simon A, van der Meer JW, Drenth JP. Familial Mediterranean fever--a not so unusual cause of abdominal pain. Best practice & research Clinical gastroenterology. 2005 Apr;19(2):199–213. doi: 10.1016/j.bpg.2004.11.009. Epub 2005/04/19. eng. [DOI] [PubMed] [Google Scholar]
- 112.Ben-Chetrit E, Levy M. Familial Mediterranean fever. Lancet. 1998 Feb 28;351(9103):659–64. doi: 10.1016/S0140-6736(97)09408-7. Epub 1998/03/21. eng. [DOI] [PubMed] [Google Scholar]
- 113.Heller H, Sohar E, Pras M. Ethnic distribution and amyloidosis in familial Mediterranean fever (FMF) Pathologia et microbiologia. 1961;24:718–23. doi: 10.1159/000161188. Epub 1961/01/01. eng. [DOI] [PubMed] [Google Scholar]
- 114.Tunca M, Akar S, Onen F, Ozdogan H, Kasapcopur O, Yalcinkaya F, et al. Familial Mediterranean fever (FMF) in Turkey: results of a nationwide multicenter study. Medicine. 2005 Jan;84(1):1–11. doi: 10.1097/01.md.0000152370.84628.0c. Epub 2005/01/12. eng. [DOI] [PubMed] [Google Scholar]
- 115.Grattagliano I, Bonfrate L, Ruggiero V, Scaccianoce G, Palasciano G, Portincasa P. Novel therapeutics for the treatment of familial Mediterranean fever: from colchicine to biologics. Clinical pharmacology and therapeutics. 2014 Jan;95(1):89–97. doi: 10.1038/clpt.2013.148. Epub 2013/07/23. eng. [DOI] [PubMed] [Google Scholar]
- 116.Houten SM, Kuis W, Duran M, de Koning TJ, van Royen-Kerkhof A, Romeijn GJ, et al. Mutations in MVK, encoding mevalonate kinase, cause hyperimmunoglobulinaemia D and periodic fever syndrome. Nature genetics. 1999 Jun;22(2):175–7. doi: 10.1038/9691. Epub 1999/06/16. eng. [DOI] [PubMed] [Google Scholar]
- 117.Drenth JP, Boom BW, Toonstra J, Van der Meer JW. Cutaneous manifestations and histologic findings in the hyperimmunoglobulinemia D syndrome. International Hyper IgD Study Group. Archives of dermatology. 1994 Jan;130(1):59–65. Epub 1994/01/01. eng. [PubMed] [Google Scholar]
- 118.Ammouri W, Cuisset L, Rouaghe S, Rolland MO, Delpech M, Grateau G, et al. Diagnostic value of serum immunoglobulinaemia D level in patients with a clinical suspicion of hyper IgD syndrome. Rheumatology (Oxford, England) 2007 Oct;46(10):1597–600. doi: 10.1093/rheumatology/kem200. Epub 2007/09/07. eng. [DOI] [PubMed] [Google Scholar]
- 119.Wise CA, Gillum JD, Seidman CE, Lindor NM, Veile R, Bashiardes S, et al. Mutations in CD2BP1 disrupt binding to PTP PEST and are responsible for PAPA syndrome, an autoinflammatory disorder. Human molecular genetics. 2002 Apr 15;11(8):961–9. doi: 10.1093/hmg/11.8.961. Epub 2002/04/25. eng. [DOI] [PubMed] [Google Scholar]
- 120.Smith EJ, Allantaz F, Bennett L, Zhang D, Gao X, Wood G, et al. Clinical, Molecular, and Genetic Characteristics of PAPA Syndrome: A Review. Current genomics. 2010 Nov;11(7):519–27. doi: 10.2174/138920210793175921. Epub 2011/05/03. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.McDermott MF, Aksentijevich I, Galon J, McDermott EM, Ogunkolade BW, Centola M, et al. Germline mutations in the extracellular domains of the 55 kDa TNF receptor, TNFR1, define a family of dominantly inherited autoinflammatory syndromes. Cell. 1999 Apr 2;97(1):133–44. doi: 10.1016/s0092-8674(00)80721-7. Epub 1999/04/13. eng. [DOI] [PubMed] [Google Scholar]
- 122.Williamson LM, Hull D, Mehta R, Reeves WG, Robinson BH, Toghill PJ. Familial Hibernian fever. The Quarterly journal of medicine. 1982;51(204):469–80. Epub 1982/01/01. eng. [PubMed] [Google Scholar]
- 123.Stojanov S, McDermott MF. The tumour necrosis factor receptor-associated periodic syndrome: current concepts. Expert reviews in molecular medicine. 2005 Oct 10;7(22):1–18. doi: 10.1017/S1462399405009749. Epub 2005/10/12. eng. [DOI] [PubMed] [Google Scholar]
- 124.Jesus AA, Oliveira JB, Aksentijevich I, Fujihira E, Carneiro-Sampaio MM, Duarte AJ, et al. TNF receptor-associated periodic syndrome (TRAPS): description of a novel TNFRSF1A mutation and response to etanercept. European journal of pediatrics. 2008 Dec;167(12):1421–5. doi: 10.1007/s00431-008-0685-2. Epub 2008/04/15. eng. [DOI] [PubMed] [Google Scholar]
- 125.Haas SL, Lohse P, Schmitt WH, Hildenbrand R, Karaorman M, Singer MV, et al. Severe TNF receptor-associated periodic syndrome due to 2 TNFRSF1A mutations including a new F60V substitution. Gastroenterology. 2006 Jan;130(1):172–8. doi: 10.1053/j.gastro.2005.09.014. Epub 2006/01/13. eng. [DOI] [PubMed] [Google Scholar]
- 126.Moll M, Kuemmerle-Deschner JB. Inflammasome and cytokine blocking strategies in autoinflammatory disorders. Clinical immunology. 2013 Jun;147(3):242–75. doi: 10.1016/j.clim.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 127.Ben-Chetrit E, Levy M. Colchicine: 1998 update. Seminars in arthritis and rheumatism. 1998 Aug;28(1):48–59. doi: 10.1016/s0049-0172(98)80028-0. Epub 1998/09/03. eng. [DOI] [PubMed] [Google Scholar]
- 128.Moser C, Pohl G, Haslinger I, Knapp S, Rowczenio D, Russel T, et al. Successful treatment of familial Mediterranean fever with Anakinra and outcome after renal transplantation. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2009 Feb;24(2):676–8. doi: 10.1093/ndt/gfn646. Epub 2008/11/27. eng. [DOI] [PubMed] [Google Scholar]
- 129.Hashkes PJ, Spalding SJ, Giannini EH, Huang B, Johnson A, Park G, et al. Rilonacept for colchicine-resistant or -intolerant familial Mediterranean fever: a randomized trial. Annals of internal medicine. 2012 Oct 16;157(8):533–41. doi: 10.7326/0003-4819-157-8-201210160-00003. Epub 2012/10/17. eng. [DOI] [PubMed] [Google Scholar]
- 130.Bodar EJ, Kuijk LM, Drenth JP, van der Meer JW, Simon A, Frenkel J. On-demand anakinra treatment is effective in mevalonate kinase deficiency. Ann Rheum Dis. 2011 Dec;70(12):2155–8. doi: 10.1136/ard.2011.149922. Epub 2011/08/24. eng. [DOI] [PubMed] [Google Scholar]
- 131.Kostjukovits S, Kalliokoski L, Antila K, Korppi M. Treatment of hyperimmunoglobulinemia D syndrome with biologics in children: review of the literature and Finnish experience. Eur J Pediatr. 2015 Feb 27; doi: 10.1007/s00431-015-2505-9. Epub 2015/02/28. Eng. [DOI] [PubMed] [Google Scholar]
- 132.Caorsi R, Picco P, Buoncompagni A, Martini A, Gattorno M. Osteolytic lesion in PAPA syndrome responding to anti-interleukin 1 treatment. J Rheumatol. 2014 Nov;41(11):2333–4. doi: 10.3899/jrheum.140060. Epub 2014/11/05. eng. [DOI] [PubMed] [Google Scholar]
- 133.Bulua AC, Mogul DB, Aksentijevich I, Singh H, He DY, Muenz LR, et al. Efficacy of etanercept in the tumor necrosis factor receptor-associated periodic syndrome: a prospective, open-label, dose-escalation study. Arthritis and rheumatism. 2012 Mar;64(3):908–13. doi: 10.1002/art.33416. Epub 2011/10/19. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jacobelli S, Andre M, Alexandra JF, Dode C, Papo T. Failure of anti-TNF therapy in TNF Receptor 1-Associated Periodic Syndrome (TRAPS) Rheumatology (Oxford, England) 2007 Jul;46(7):1211–2. doi: 10.1093/rheumatology/kel298. Epub 2006/08/29. eng. [DOI] [PubMed] [Google Scholar]
- 135.Gattorno M, Pelagatti MA, Meini A, Obici L, Barcellona R, Federici S, et al. Persistent efficacy of anakinra in patients with tumor necrosis factor receptor-associated periodic syndrome. Arthritis and rheumatism. 2008 May;58(5):1516–20. doi: 10.1002/art.23475. Epub 2008/04/29. eng. [DOI] [PubMed] [Google Scholar]
- 136.Sacre K, Brihaye B, Lidove O, Papo T, Pocidalo MA, Cuisset L, et al. Dramatic improvement following interleukin 1beta blockade in tumor necrosis factor receptor-1-associated syndrome (TRAPS) resistant to anti-TNF-alpha therapy. The Journal of rheumatology. 2008 Feb;35(2):357–8. Epub 2008/02/09. eng. [PubMed] [Google Scholar]
- 137.Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011 Oct;63(10):3136–41. doi: 10.1002/art.30520. Epub 2011/07/30. eng. [DOI] [PubMed] [Google Scholar]
- 138.Arromdee E, Michet CJ, Crowson CS, O’Fallon WM, Gabriel SE. Epidemiology of gout: is the incidence rising? J Rheumatol. 2002 Nov;29(11):2403–6. Epub 2002/11/05. eng. [PubMed] [Google Scholar]
- 139.Wilkins E, Dieppe P, Maddison P, Evison G. Osteoarthritis and articular chondrocalcinosis in the elderly. Ann Rheum Dis. 1983 Jun;42(3):280–4. doi: 10.1136/ard.42.3.280. Epub 1983/06/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Liu-Bryan R, Scott P, Sydlaske A, Rose DM, Terkeltaub R. Innate immunity conferred by Toll-like receptors 2 and 4 and myeloid differentiation factor 88 expression is pivotal to monosodium urate monohydrate crystal-induced inflammation. Arthritis Rheum. 2005 Sep;52(9):2936–46. doi: 10.1002/art.21238. Epub 2005/09/06. eng. [DOI] [PubMed] [Google Scholar]
- 141.Joosten LA, Netea MG, Mylona E, Koenders MI, Malireddi RK, Oosting M, et al. Engagement of fatty acids with Toll-like receptor 2 drives interleukin-1beta production via the ASC/caspase 1 pathway in monosodium urate monohydrate crystal-induced gouty arthritis. Arthritis Rheum. 2010 Nov;62(11):3237–48. doi: 10.1002/art.27667. Epub 2010/07/28. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ng G, Sharma K, Ward SM, Desrosiers MD, Stephens LA, Schoel WM, et al. Receptor-independent, direct membrane binding leads to cell-surface lipid sorting and Syk kinase activation in dendritic cells. Immunity. 2008 Nov 14;29(5):807–18. doi: 10.1016/j.immuni.2008.09.013. Epub 2008/11/11. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006 Mar 9;440(7081):237–41. doi: 10.1038/nature04516. Epub 2006/01/13. eng. [DOI] [PubMed] [Google Scholar]
- 144.Martinon F. Signaling by ROS drives inflammasome activation. Eur J Immunol. 2010 Mar;40(3):616–9. doi: 10.1002/eji.200940168. Epub 2010/03/05. eng. [DOI] [PubMed] [Google Scholar]
- 145.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11(2):136–40. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 146.Dostert C, Guarda G, Romero JF, Menu P, Gross O, Tardivel A, et al. Malarial hemozoin is a Nalp3 inflammasome activating danger signal. PLoS One. 2009;4(8):e6510. doi: 10.1371/journal.pone.0006510. Epub 2009/08/05. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Choe JY, Jung HY, Park KY, Kim SK. Enhanced p62 expression through impaired proteasomal degradation is involved in caspase-1 activation in monosodium urate crystal-induced interleukin-1b expression. Rheumatology (Oxford, England) 2014 Jun;53(6):1043–53. doi: 10.1093/rheumatology/ket474. Epub 2014/03/04. eng. [DOI] [PubMed] [Google Scholar]
- 148.Joosten LAB, Netea MG, Fantuzzi G, Koenders MI, Helsen MMA, Sparrer H, et al. Inflammatory arthritis in caspase 1 gene–deficient mice: Contribution of proteinase 3 to caspase 1–independent production of bioactive interleukin-1β. Arthritis & Rheumatism. 2009;60(12):3651–62. doi: 10.1002/art.25006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Guma M, Ronacher L, Liu-Bryan R, Takai S, Karin M, Corr M. Caspase 1-independent activation of interleukin-1beta in neutrophil-predominant inflammation. Arthritis Rheum. 2009 Dec;60(12):3642–50. doi: 10.1002/art.24959. Epub 2009/12/02. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ea HK, Chobaz V, Nguyen C, Nasi S, van Lent P, Daudon M, et al. Pathogenic role of basic calcium phosphate crystals in destructive arthropathies. PLoS One. 2013;8(2):e57352. doi: 10.1371/journal.pone.0057352. Epub 2013/03/08. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rajamaki K, Lappalainen J, Oorni K, Valimaki E, Matikainen S, Kovanen PT, et al. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS One. 2010;5(7):e11765. doi: 10.1371/journal.pone.0011765. Epub 2010/07/30. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009 Jul 15;183(2):787–91. doi: 10.4049/jimmunol.0901363. Epub 2009/07/03. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kepp O, Galluzzi L, Kroemer G. Mitochondrial control of the NLRP3 inflammasome. Nat Immunol. 2011 Mar;12(3):199–200. doi: 10.1038/ni0311-199. Epub 2011/02/16. eng. [DOI] [PubMed] [Google Scholar]
- 154.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011 Jan 13;469(7329):221–5. doi: 10.1038/nature09663. Epub 2010/12/03. eng. [DOI] [PubMed] [Google Scholar]
- 155.Bauernfeind F, Bartok E, Rieger A, Franchi L, Nunez G, Hornung V. Cutting edge: reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. J Immunol. 2011 Jul 15;187(2):613–7. doi: 10.4049/jimmunol.1100613. Epub 2011/06/17. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011 May;31(5):986–1000. doi: 10.1161/ATVBAHA.110.207449. Epub 2011/04/22. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang Y, Viollet B, Terkeltaub R, Liu-Bryan R. AMP-activated protein kinase suppresses urate crystal-induced inflammation and transduces colchicine effects in macrophages. Ann Rheum Dis. 2014 Oct 31; doi: 10.1136/annrheumdis-2014-206074. Epub 2014/11/02. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.So A, De Smedt T, Revaz S, Tschopp J. A pilot study of IL-1 inhibition by anakinra in acute gout. Arthritis Res Ther. 2007;9(2):R28. doi: 10.1186/ar2143. Epub 2007/03/14. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ghosh P, Cho M, Rawat G, Simkin PA, Gardner GC. Treatment of acute gouty arthritis in complex hospitalized patients with anakinra. Arthritis Care Res (Hoboken) 2013 Aug;65(8):1381–4. doi: 10.1002/acr.21989. Epub 2013/05/08. eng. [DOI] [PubMed] [Google Scholar]
- 160.Ottaviani S, Molto A, Ea HK, Neveu S, Gill G, Brunier L, et al. Efficacy of anakinra in gouty arthritis: a retrospective study of 40 cases. Arthritis Res Ther. 2013;15(5):R123. doi: 10.1186/ar4303. Epub 2014/01/17. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chen K, Fields T, Mancuso CA, Bass AR, Vasanth L. Anakinra’s efficacy is variable in refractory gout: report of ten cases. Semin Arthritis Rheum. 2010 Dec;40(3):210–4. doi: 10.1016/j.semarthrit.2010.03.001. Epub 2010/05/25. eng. [DOI] [PubMed] [Google Scholar]
- 162.Terkeltaub RA, Schumacher HR, Carter JD, Baraf HS, Evans RR, Wang J, et al. Rilonacept in the treatment of acute gouty arthritis: a randomized, controlled clinical trial using indomethacin as the active comparator. Arthritis Res Ther. 2013;15(1):R25. doi: 10.1186/ar4159. Epub 2013/02/05. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Terkeltaub R, Sundy JS, Schumacher HR, Murphy F, Bookbinder S, Biedermann S, et al. The interleukin 1 inhibitor rilonacept in treatment of chronic gouty arthritis: results of a placebo-controlled, monosequence crossover, non-randomised, single-blind pilot study. Ann Rheum Dis. 2009 Oct;68(10):1613–7. doi: 10.1136/ard.2009.108936. Epub 2009/07/29. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mitha E, Schumacher HR, Fouche L, Luo SF, Weinstein SP, Yancopoulos GD, et al. Rilonacept for gout flare prevention during initiation of uric acid-lowering therapy: results from the PRESURGE-2 international, phase 3, randomized, placebo-controlled trial. Rheumatology (Oxford, England) 2013 Jul;52(7):1285–92. doi: 10.1093/rheumatology/ket114. Epub 2013/03/15. eng. [DOI] [PubMed] [Google Scholar]
- 165.Schumacher HR, Jr, Evans RR, Saag KG, Clower J, Jennings W, Weinstein SP, et al. Rilonacept (interleukin-1 trap) for prevention of gout flares during initiation of uric acid-lowering therapy: results from a phase III randomized, double-blind, placebo-controlled, confirmatory efficacy study. Arthritis Care Res (Hoboken) 2012 Oct;64(10):1462–70. doi: 10.1002/acr.21690. Epub 2012/05/03. eng. [DOI] [PubMed] [Google Scholar]
- 166.So A, De Meulemeester M, Pikhlak A, Yucel AE, Richard D, Murphy V, et al. Canakinumab for the treatment of acute flares in difficult-to-treat gouty arthritis: Results of a multicenter, phase II, dose-ranging study. Arthritis Rheum. 2010 Oct;62(10):3064–76. doi: 10.1002/art.27600. Epub 2010/06/10. eng. [DOI] [PubMed] [Google Scholar]
- 167.Schlesinger N, De Meulemeester M, Pikhlak A, Yucel AE, Richard D, Murphy V, et al. Canakinumab relieves symptoms of acute flares and improves health-related quality of life in patients with difficult-to-treat Gouty Arthritis by suppressing inflammation: results of a randomized, dose-ranging study. Arthritis Res Ther. 2011;13(2):R53. doi: 10.1186/ar3297. Epub 2011/03/29. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Schlesinger N, Mysler E, Lin HY, De Meulemeester M, Rovensky J, Arulmani U, et al. Canakinumab reduces the risk of acute gouty arthritis flares during initiation of allopurinol treatment: results of a double-blind, randomised study. Ann Rheum Dis. 2011 Jul;70(7):1264–71. doi: 10.1136/ard.2010.144063. Epub 2011/05/05. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Announ N, Palmer G, Guerne PA, Gabay C. Anakinra is a possible alternative in the treatment and prevention of acute attacks of pseudogout in end-stage renal failure. Joint Bone Spine. 2009 Jul;76(4):424–6. doi: 10.1016/j.jbspin.2009.01.001. Epub 2009/03/18. eng. [DOI] [PubMed] [Google Scholar]
- 170.Ottaviani S, Brunier L, Sibilia J, Maurier F, Ardizzone M, Wendling D, et al. Efficacy of anakinra in calcium pyrophosphate crystal-induced arthritis: a report of 16 cases and review of the literature. Joint Bone Spine. 2013 Mar;80(2):178–82. doi: 10.1016/j.jbspin.2012.07.018. Epub 2012/10/02. eng. [DOI] [PubMed] [Google Scholar]
- 171.Couderc M, Mathieu S, Glace B, Soubrier M. Efficacy of anakinra in articular chondrocalcinosis: report of three cases. Joint Bone Spine. 2012 May;79(3):330–1. doi: 10.1016/j.jbspin.2011.12.017. Epub 2012/02/22. eng. [DOI] [PubMed] [Google Scholar]
- 172.Zhang W, Doherty M, Pascual E, Barskova V, Guerne PA, Jansen TL, et al. EULAR recommendations for calcium pyrophosphate deposition. Part II: management. Ann Rheum Dis. 2011 Apr;70(4):571–5. doi: 10.1136/ard.2010.139360. Epub 2011/01/25. eng. [DOI] [PubMed] [Google Scholar]