Abstract
Objectives
We investigated whether HIV disease severity was associated with alterations in structural brain connectivity, and whether those alterations in turn were associated with cognitive deficits in youth with perinatally-acquired HIV (PHIV).
Design
PHIV youth (n=40) from the Pediatric HIV/AIDS Cohort Study (PHACS) (mean age: 16±2 yrs) were included to evaluate how current and past disease severity measures (recent/nadir CD4%; peak viral load) relate to white matter (WM) microstructure within PHIV youth. PHIV youth were compared to 314 controls from the Pediatric Imaging, Neurocognition, and Genetics (PING) study.
Methods
Diffusion tensor imaging and tractography were utilized to assess WM microstructure. Mediation analyses were conducted to examine whether microstructure alterations contributed to relationships between higher disease severity and specific cognitive domains in PHIV youth.
Results
Whole brain fractional anisotropy (FA) was reduced, but radial (RD) and mean (MD) diffusivity were increased, in PHIV compared to control youth. Within PHIV youth, more severe past HIV disease was associated with reduced FA of the right inferior fronto-occipital (IFO) and left uncinate tracts; elevated MD of the F minor; and increased streamlines comprising the left inferior longitudinal fasciculus (ILF). Associations of higher peak viral load with lower working memory performance were partly mediated by reductions in right IFO FA levels.
Conclusion
Our findings suggest that PHIV youth have higher risk of alterations in WM microstructure compared to typically developing youth, and certain alterations are related to past disease severity. Further, WM alterations potentially mediate associations between HIV disease and working memory.
Search terms: CD4 Cell Counts, Viral load, HIV, Diffusion tensor imaging (DTI), Diffusion tractography, cognition
Introduction
In the United States, many children with perinatally-acquired HIV (PHIV) have survived with combination antiretroviral treatment (cART). However, cognitive deficits have been observed among youth with PHIV compared to typically developing peers [1]. HIV affects central nervous system functioning, largely indirectly through damaging cytokines produced as a result of immune activation and inflammation [2, 3]. During replication, HIV destroys and leads to decreases in CD4+ immune T cells [4], a major indicator of disease severity. PHIV may produce unique effects on brain and cognition, considering HIV and associated immune changes likely impact early brain development.
Impaired cognitive performance [5-8] is associated with higher HIV RNA viral load (VL) and lower nadir CD4+ cell percentages [6, 7]. PHIV youth demonstrate deficits that are sensitive to HIV disease severity in working memory and processing speed [1]. In unaffected youth, white matter (WM) microstructure is important for plasticity underlying age-related increases in working memory capacity and processing speed [9, 10]. Thus, the present study investigated whether WM microstructure among youth with PHIV is altered compared to typically developing controls. We examined whether WM microstructure is affected by disease severity among PHIV youth, and if so, whether alterations in brain mediate the relationship between disease severity and poor cognitive performance.
WM is primarily made of organized myelinated axonal fiber bundles, which restrict water diffusion in a parallel direction to axons; increased maturation corresponds to increased restriction with age [11, 12]. Diffusion tensor imaging (DTI) measures this restriction of water diffusion [13]. Using tractography on DTI data, a three-dimensional estimate of WM tracts is generated [14], from which properties of water diffusion can be extracted. This technique's advantage includes not having to perform between-subject warping; making it a valuable tool for studies where disease may produce brain abnormalities [15]. One primary DTI variable is fractional anisotropy (FA) (i.e., magnitude of anisotropic diffusion). Increased FA levels likely reflect increases in myelination and/or axonal organization. However, if myelinated nerve sheaths are affected in a patient population, it is possible that neurobiological compensation could lead to myelin hyperplasia, and higher FA could be related to poorer cognition [16]. Beyond FA, other DTI parameters can be estimated and examined along each tract, such as diffusivity: 1) along principle axis (axial (AD)); 2) averaged across two minor axes (radial (RD)); 3) averaged across three axes (mean (MD)); and estimated number of streamlines comprising a specific tract (i.e., number of fiber bundles). Using tractography-based along-tract statistics, it is feasible to detect localized effects associated with PHIV [15].
DTI parameters have been shown to reflect HIV disease severity in the adult rodent [17], macaque [18, 19] and human brain [20-31]. Lower FA was observed in HIV-infected compared to non-infected adults [22, 24, 25], and even higher FA in some WM regions [22]. In the infected adult, HIV progression has been associated with lower FA, but higher AD, RD and MD [20]. Additionally, T-cell alterations were associated with DTI measures in SIV-infected non-human primates [18]. The effects of HIV on WM microstructure may possibly differ in youth with PHIV, compared to those who acquired HIV in adulthood, given that HIV exposure and cART occur when significant brain development is occurring. Sarma and colleagues [32] examined volume of WM in PHIV youth and identified WM atrophy that differed from those observed with adult-acquired HIV. To our knowledge, no study to date has utilized tractography to examine associations between disease severity markers and microstructural properties at specific points along WM tracts among PHIV youth.
The present study aims to address gaps in the literature by investigating WM microstructure: 1) in PHIV compared to typically developing youth; 2) as a function of disease severity markers; and 3) as a potential mediating factor between disease severity and cognitive performance within PHIV youth. Given overall lower FA values in HIV-infected adults [22, 24, 25], we hypothesized that PHIV youth would exhibit decreased FA compared to non-affected youth, and that greater disease severity among PHIV youth would relate to even lower FA values, which in turn, would contribute to lower cognitive performance.
Methods
Study population
PHIV
40 PHIV adolescents were selected from the Adolescent Master Protocol (AMP) study of the NIH Pediatric HIV/AIDS Cohort Study (PHACS) at a single site (Northwestern University, Chicago, IL). AMP is a prospective study designed to define the impact of perinatal HIV exposure, PHIV and cART on youth. Institutional review boards at Northwestern and Harvard approved the study. Parents, legal guardians or youth aged 18 years or older provided written informed consent for research participation; youth over 12 years provided assent.
Controls
A control group was generated using frequency-matching from the Pediatric Imaging, Neurocognition, and Genetics (PING) study (http://pingstudy.ucsd.edu/welcome.html) magnetic resonance imaging (MRI) database from five sites (MGH; Sackler Institute; UH; Yale; UCLA). Adolescents were frequency-matched by age, sex, and scanner type, totaling 314 PING youth.
Disease Markers and Cognitive Functioning in PHIV youth
Disease and cognitive functioning were examined among PHIV youth only. Information regarding clinical diagnoses and laboratory results, including CD4% and plasma HIV RNA concentration (viral load; VL), was obtained by medical chart abstraction at AMP study visits (see [8] for more detail). The lowest known CD4 percent (nadir CD4%) and highest known VL (“peak VL”) were collected. We considered three measures of disease severity: nadir CD4%, recent CD4%, and peak VL. Recent HIV RNA levels were not utilized in analyses due to lack of variability (only 6/40 (15%) had recent VLs above 400 copies/mL (range: <40-92,000)). Age at the time of acquisition of past disease markers was utilized as a covariate in analyses. Age at time of scan was highly correlated with age at assessment of current disease markers and cognitive testing, and was utilized as a second covariate in analyses. Biological sex was utilized as a covariate in all analyses. The mean interval between scanning and: (1) assessment of current disease markers was 1.8 months (SD±3.5 months); and (2) cognitive testing was 0.9 months (SD±6.9 months).
The Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV) (6-16 years), and the Wechsler Adult Intelligence Scale, Fourth Edition (WAIS-IV) for 17+ [33, 34] were used to evaluate cognitive performance in PHIV youth. We targeted: 1) Working memory (WMI); and 2) Processing speed (PSI) indexes.
Image Acquisition
Acquisition protocols were standardized across the PHACS and PING imaging sites (as described in [35]). At the PHACS Chicago location, whole brain diffusion weighted imaging data were acquired on a single 3-Tesla Siemens Tim Trio MRI scanner with 12-channel head coil. Diffusion weighted volumes (gradient encoding pulses applied in 30 directions, b0=1000 s/mm2, in-plane matrix of 96×96, axial slices with 2.5mm thickness and 90° angle, TE=86000ms, TR=172000ms) and one non-diffusion weighted volume (b=0s/mm2) were acquired. Similar protocols were used for PING 3T Siemens imaging sites with the exception of an 8-channel head coil.
Image Processing and Analyses
Between Group Analyses
Quality control and processing of PHIV and control youth were executed with the PING processing portal (http://pingstudy.ucsd.edu/investigators.html). Briefly, average whole brain FA was calculated using FMRIB Software Library (FSL) DTI toolbox, and an atlas-based method for labeling and characterizing tracts based at UCSD (described in detail in [35]. To investigate group differences in whole brain FA, AD, RD, and MD, ANOVA was performed in R software [36], adjusting for sex and age at scan. Post-hoc effect size estimates (Cohen's d) were calculated using each group's mean and standard deviation [37].
Within-PHIV Analyses
In order to investigate WM microstructure along major WM tracts in relation to disease severity and cognition, standard DTI preprocessing [38] and Trackvis [39] tractography were utilized followed by in-house scripts previously described [15]. Eddy current and motion distortions were corrected, a six parameter tensor model of diffusion was fit to the data to estimate voxelwise FA, AD, RD, and MD using a12-parameter affine to register to standardized space (MNI152 template). Next, whole-brain brute-force, atlas-based tractography was performed using the Diffusion Toolkit v0.6 (http://www.trackvis.org/dtk). This software implements Fiber Assignment by Continuous Tracking (FACT) algorithm [14] to generate deterministic streamlines by iteratively moving from voxel to voxel along the direction of maximal diffusion. The following constraints of (1) a whole-brain mask, (2) an FA threshold of 0.15 and (3) a tract-dependent turning angle threshold of 60° were used to reduce biologically implausible fibers. Successful tracts were defined as those with ≥1 streamline. Nine atlas-based WM tracts were identified using a multi-ROI approach established by [40] (Table 2). FA, AD, RD and MD at multiple points were parameterized along 9 major WM tracts using the along-tract mapping toolbox [15], MATLAB [41] and R software [36], as well as number of streamlines comprising each tract. Post-hoc effect size estimates using r2 were provided when appropriate to identify how well each model explained the proportion of total variation of outcomes.
Table 2.
Summary of significant associations between disease severity and DTI measures among youth with PHIV.
| White Matter Tract | Disease severity measure | DTI parameter | Hemisphere | r value | p value |
|---|---|---|---|---|---|
| Forceps minor (Fminor) | Nadir CD4% | AD | n/a | -0.39 | p<0.05 |
| Forceps minor (Fminor) | Nadir CD4% | MD | n/a | 0.54 | p<0.001 |
| Inferior fronto-occipital fasciculus (IFO) | Peak viral load | FA | right | -0.45 | p<0.01 |
| Inferior longitudinal fasciculus (ILF) | Peak viral load | Streamline count | left | 0.30 | p=0.05 |
| Uncinate fasciculus (UNC) | Peak viral load | FA | left | -0.56 | p<0.001 |
Associations between disease severity markers and FA, AD, MD and number of streamlines among youth with PHIV (adjusted p values following 1,000 permutations are reported). Pearson r represents directionality of association, for both the left and right hemispheres. There were no significant associations between Recent CD4% and DTI parameters. FA: fractional anisotropy; DTI: diffusion tensor imaging. AD: axial diffusivity; MD: mean diffusivity.
To examine within-subject relationships with FA/AD/RD/MD and disease severity or cognition, linear-mixed effect modeling was conducted in R [36], with DTI parameters along each tract as repeated measures. Analyses testing associations between streamline numbers and disease severity or cognition utilized linear modeling. To confirm previous findings of associations between disease severity and cognition [7] in the current sample, linear modeling was utilized using sex, age at peak VL, and age at cognitive testing as covariates. Significant associations were followed up with simple Pearson's correlation. Peak VL was not normally distributed (negatively skewed), and therefore was log transformed (base 10) prior to statistical analyses. Potential bivariate outliers were removed for secondary analyses to confirm statistical findings.
Mixed effect modeling was utilized to assess the association of biological disease markers with (i) DTI parameters (FA/AD/RD/MD) along each WM tract and (ii) streamline number of each tract, with sex, age at peak VL and age at scan as covariates. All linear mixed effect-modeling results (uncorrected p<0.05) were followed by permutations (n=1000) to obtain adjusted p-values, utilizing a threshold-free cluster enhancement algorithm [42]. Statistical trends were reported (corrected p<0.10).
To investigate the potential mediating role of WM microstructure alterations in associations observed between disease markers and cognition in PHIV youth, mediation analyses were conducted (mediation package in R [43]). Following analyses with brain and disease severity, mean values of FA/AD/RD/MD and streamline number were calculated from significant points along the tract and used in mediation analyses with disease severity and cognitive indices (WMI/PSI).
In order to reduce the likelihood of motion as a confounder in our analyses, motion during the scans was quantified in PHIV youth with FSL (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSLMotionOutliers) using frame-wise displacement [44]. Linear mixed-effects modeling was utilized to assess associations between motion and: (1) age at scan; (2) sex; and (3) markers of disease severity. As expected, there was a significant association between motion and age at scan with younger participants displaying increased motion, hence age at scan was used as a covariate in all analyses reported. There were no other significant associations with motion in PHIV youth.
Results
Study Population
40 PHIV youth and 314 Control youth from PING ranged in age from 11.6 to 20.7 years (Table 1). Within the PHIV youth, nadir CD4% and peak VL were not significantly correlated. For between group comparisons, two DTI scans could not be processed through the PING portal from the PHIV cohort, resulting in a sample of n=38 for PHIV versus Control between-group analyses. For within-group analyses with disease severity measures and cognition, all 40 PHIV youth were included.
Table 1.
Sample characteristics describing youth with PHIV and Control youth.
| PHIV (PHACS) | Control (PING) | ||
|---|---|---|---|
| General | Sample size | 40 | 314 |
| Age at time of scan | 16.7 ± 2.4 (11.6 - 20.7 years) |
16.1 ± 2.7 years (11.6 - 20.7 years) |
|
| Male:Female (%female) | 19:21 (52%) | 155:159 (50%) | |
|
|
|||
| Disease severity variables | Nadir CD4% | 16.0 ± 9.5 | |
| Age at nadir CD4% | 6.8 ± 5.4 years | ||
| Recent CD4% | 33.5 ± 11.5 | ||
| Log Peak viral load (copies/mL) | 5.4 ± 0.7 | ||
| Age at peak viral load | 4.0 ± 4.6 years | ||
| Recent viral load >400 copies/mL | 6 (15%) | ||
| CDC ‘C’ classification | 9 (22.5%) | ||
|
|
|||
| Cognition | Working Memory Index | 87.5 ± 16.8 | |
| Processing Speed Index | 95.3 ± 14.1 | ||
| Cognitive Proficiency Index | 90.2 ± 16.0 | ||
|
|
|||
Mean ± Standard Deviation or N (%), as appropriate. CDC= Centre for Disease Control; Cognitive (IQ) scores are normalized (mean=100±15). Note: Disease severity variables and Cognition were not assessed in Control participants.
Differences in whole brain DTI parameters in PHIV compared to control youth
Relative to typically developing control youth, PHIV youth exhibited significantly lower FA (by 8.3%; d=1.78), higher RD (by 10.9%; d=1.44), and higher MD (by 5.1%; d=1.13) averaged across the whole brain (all p<0.001), after adjustment for age at scan and sex. There were no statistical differences between groups in AD (d=.00).
Associations between disease markers and cognition among PHIV youth
In linear models adjusted for age at scan and sex, as well as age at peak VL, positive associations were found between peak VL and WMI (p<0.10; r2=.10) and PSI (p<0.05; r2=.17), with higher log peak VL associated with poorer performance on both cognitive domains. There were no significant associations between nadir CD4% (all r2=.03-.04) or recent CD4% (all r2=.009-.06) and cognitive indices.
Association of disease markers with DTI parameters among PHIV youth
A summary of findings can be found in Table 2
Fractional anisotropy (FA)
Significant negative associations were found between peak VL and FA along the right IFO. Specifically, peak VL was negatively associated with FA (r=-0.45, p<0.01; r2=.20), and this effect was observed only in the mid-to-anterior portion of the right IFO (Figure 1A). Additional significant associations were found between peak VL and FA along the left UNC (p<0.05), where peak VL was negatively correlated with FA (r=-0.56, p<0.001; r2=.31) only in the lateral-to-mid portions of the left UNC (Figure 1B). There were no significant associations between nadir CD4% or recent CD4% and FA.
Figure 1.
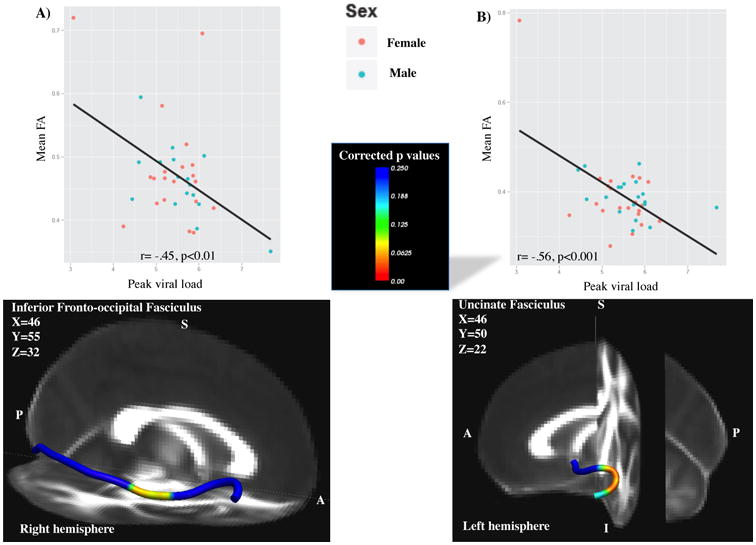
Higher peak VL was associated with lower FA levels in the A) right inferior fronto-occipital fasciculus (IFO); B) left uncinate fasciculus (UNC). Statistical representation of corrected p values overlaid onto 1mm FA standard image. A=anterior, I=inferior, P=posterior, R=right.
Axial diffusivity (AD)
Significant associations were found between nadir CD4% and AD along the forceps minor (Fminor) (p<0.10), where higher nadir CD4% was associated with lower AD (r=-0.39, p<0.05; r2=.15) only in the midline, crossing through the genu (Figure 2A). There were no significant associations between peak VL or recent CD4% and AD.
Figure 2.
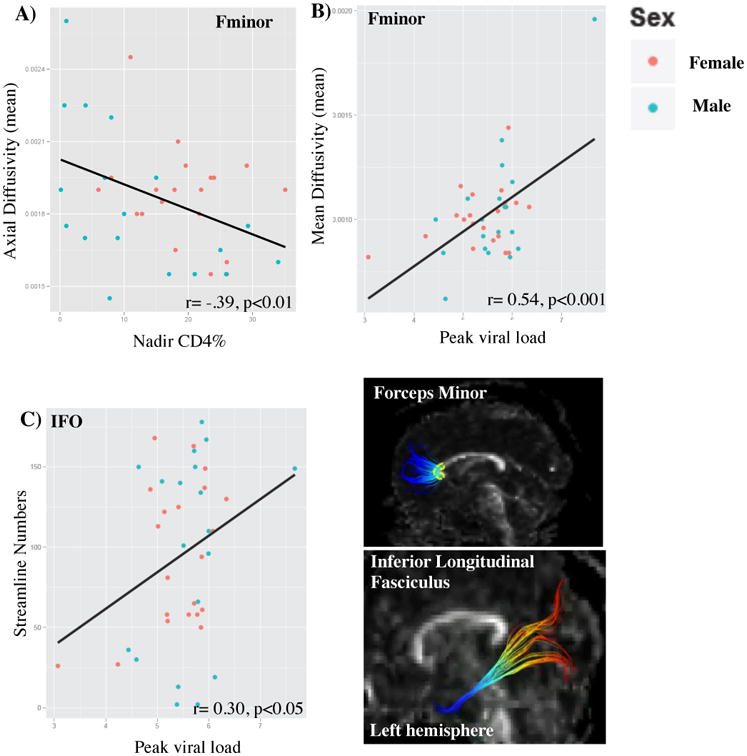
Associations between disease severity and AD, MD and streamline number. A) Greater disease severity was associated with higher AD values in the genu of the F minor (outlined in yellow). B) Greater disease severity was associated with higher MD values in the genu of the F minor. C) Greater disease severity was associated with overall more streamlines in the left ILF. AD=axial diffusivity; MD=mean diffusivity; ILF= inferior longitudinal fasciculus.
Radial diffusivity (RD)
There were no significant associations between peak VL, nadir CD4% (r2=.00) or recent CD4% (r2=.00) and RD.
Mean diffusivity (MD)
Significant associations were found between peak VL and MD along the Fminor (p<0.05), where a higher peak VL was associated with higher MD values (r=0.54, p<0.001; r2=.29) only in the midline, crossing through the genu (Figure 2B). After removal of a potential bivariate outlier (e.g., high MD and high Peak viral load), the association remained significant (r=0.32, p<0.05; r2=.10). There were no significant associations between nadir CD4% (r2=.00) or recent CD4% (r2=.00) and MD.
Streamlines
Significant positive associations were observed between peak VL and streamline number in the left ILF (r=0.30, p=0.05; r2=.09) (Figure 2C). There was a statistical trend for the association between nadir CD4% and streamline number in the left ILF (F(4,35)=2.48, p=0.06), where lower nadir CD4% was associated with more streamlines, but the follow-up correlation was not significant (r=-0.16; r2=.02).
Mediation analyses with disease severity, brain and cognition
Mediation analyses were conducted when cognition was significantly correlated with disease severity, disease severity was significantly correlated with DTI measures, and cognition was correlated with DTI measures at p<0.10. These conditions were met for the following: (1) peak VL, right IFO FA with WMI and PSI (p<0.10); (2) peak VL, left UNC FA with PSI (p<0.10); and (3) peak VL, Fminor streamline number with PSI (p<0.10). Out of these four 3-way associations, only one significant mediated effect was observed. Specifically, higher peak VL (mediated by reduced IFO FA) was associated with reduced WMI performance (total effect=61%), and the indirect effect of reduced FA accounted for 59% of the total effect of high peak VL.
Discussion
Significant differences in whole brain WM microstructure were observed in PHIV compared to typically developing youth (represented by overall lower FA and higher RD and MD). Within PHIV youth, advanced disease severity (e.g., lower nadir CD4% and higher peak VL) was associated with certain WM microstructure alterations in the expected direction, based on comparisons with typically developing youth in the present study. Specifically, higher peak VL was associated with lower FA (left UNC, right IFO), higher MD (F minor) and higher streamline counts (left ILF), whereas lower nadir CD4% was associated with higher AD (F minor). Additionally, association with working memory performance was partially mediated by reductions in FA along the right IFO. Higher disease activity may impact organization and/or myelination of underlying WM microstructure, suggesting a possible decrease in WM integrity with life-long HIV and cART exposure.
Alterations in whole brain DTI parameters observed in PHIV compared to control youth parallel those observed in HIV+ adults
PHIV youth exhibited whole brain reductions in FA, but higher RD and MD values compared to controls. The findings with FA and MD parallel those with adults, where reductions in FA [22, 24, 25] but increases in MD were observed in HIV+ compared to control adults [21, 26]. This suggests that axonal organization and/or myelination may be impacted by both perinatal and adulthood HIV. Further, markers of disease progression in HIV+ adults were associated with lower FA, but higher AD, RD and MD [20]. RD may be a proxy for myelination, with higher RD reflecting less myelination of tracts [20]. Higher MD values observed among children with developmental delays [45], may be a sensitive indicator of developmental problems. It is possible that higher MD, observed in PHIV youth and HIV+ adults [20], may represent neurodegeneration of WM: where a loss in neurons during neurodegeneration, corresponds to increased water molecules, possibly resulting in higher MD [46]. Supporting this hypothesis, smaller WM volumes have been observed among PHIV youth [32]. Adults with Hepatitis C virus (HCV) infection also exhibit lower FA and higher MD compared to healthy controls [47], suggesting that different types of chronic peripheral infections and inflammation may result in similar profiles of WM microstructure abnormalities.
Both peak VL and CD4 counts were associated with streamline number of the left ILF, where indices of increased disease severity were related to more streamlines (proxy for myelinated fiber bundles). The ILF connects occipital and temporal lobes; therefore, higher streamline number may be a compensatory mechanism to counteract the negative impact of HIV and/or cART exposure on the ILF. However, working memory deficits are observed in this and prior [1, 7] studies; thus, an increase in ILF width may not completely rescue cognitive deficits. Further, associations between both disease severity markers and FA, AD, RD and MD were observed in tracts that connect to the frontal lobes, suggesting that HIV may impact frontal tracts to a greater extent than posterior tracts. Lower nadir CD4 percentages were associated with higher AD of the F minor, again suggesting that the genu and frontal cortex may be particularly susceptible to PHIV. Previous studies have found similar alterations in WM microstructure in the corpus collosum of adult non-human primates and humans, where HIV+ participants exhibited low FA [24, 48] and high MD [18, 21, 25, 26, 49]. As WM in the frontal lobes are the last to fully mature [50], these regions could be more susceptible to HIV-related factors compared to posterior tracts. Interestingly, adult HIV is also associated with greater frontal compared to posterior brain alterations [20-24, 27].
VL reflects viral activity and replication, whereas CD4% reflects immune suppression; but, we observed associations of both measures with WM alterations. Higher peak VL related to lower FA along the right IFO and left UNC and higher MD along the F minor. The IFO and UNC connect the frontal and temporal lobes. Thus, peak VL, but not nadir CD4%, relates to FA alterations in tracts that are implicated in integration of auditory and visual cortices with the prefrontal cortex (IFO) and connections between temporal and frontal regions (UNC). The IFO connects the frontal, temporal and occipital lobes [51]. The UNC connects part of the limbic system (hippocampus, amygdala) with frontal regions, such as the orbitofrontal cortex. Reductions in FA along the right IFO and left UNC may reflect less axonal organization and/or myelination of these slowly maturing tracts. It is possible that perinatally acquired HIV produces a long-lasting increase in MD, and in the corpus callosum (midline F minor) as observed among PHIV youth.
Similar to Smith et al., [7], we found that peak VL was associated with reduced working memory and processing speed performance among PHIV youth. Lower FA in the right IFO and left UNC were associated with poorer processing speed, but lower FA in these tracts did not statistically mediate the relationship between higher disease severity and lower cognitive performance. However, lower FA in the right IFO mediated the relationship between higher disease severity and poorer working memory. Previous studies have demonstrated that working memory may be susceptible to HIV infection during brain development [1]. Associations of peak VL with FA along this tract may be evidence of cART effectiveness, along with corpus collosum alterations. This needs to be confirmed in a larger cohort of PHIV youth, as the present study had low power to detect brain-cognition associations given the relatively small sample size.
Limitations of the present study include that the control and patient samples were scanned at different sites. However, scanner type, acquisition protocol and pre-/post-processing methodologies were identical, and controls were frequency-matched by age and sex to PHIV youth, allowing for meaningful preliminary between group comparisons. It is important to note that this limitation only applies to between group comparisons with PHIV and control youth, and not the disease associations among PHIV youth, which are compelling on their own. Second, the cross-sectional nature of the present study limits our ability to assess the impact of HIV on brain development over time (e.g., change). Third, we assessed only a limited range of cognitive domains, and future studies should use a more comprehensive cognitive battery.
Conclusion
Overall, greater HIV-related disease severity early in life may impact organization and/or myelination of underlying WM microstructure, suggesting a possible decrease in WM integrity with life-long HIV. Alterations in brain development may contribute to cognitive deficits observed among PHIV youth. Understanding the impact of HIV disease severity on WM integrity further emphasizes the importance of medication adherence in order to control viral load early in life, as well as provide potentially useful clinical tools for evaluating cART efficacy during a dynamic period of brain development.
Supplementary Material
Supplemental Figure 1. Mean±SD. Alterations in whole brain DTI measures in PHIV compared to typically developing youth. A) Fractional anisotropy: Typical>PHIV; 2) Axial diffusivity: Typical=PHIV; 3) Radial diffusivity: PHIV>Typical; and 4) Mean diffusivity: PHIV>Typical.
Figure 3.
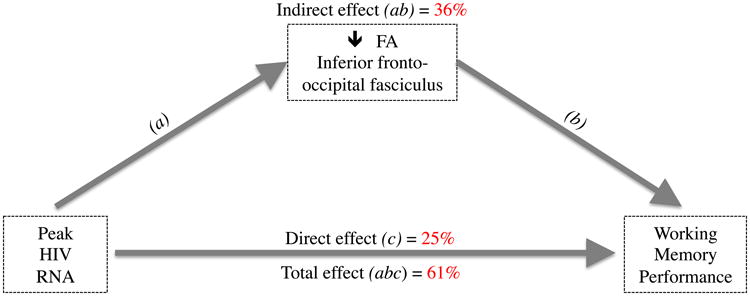
Mediation model for Peak HIV RNA, Right IFO FA and working memory. The association between higher Peak HIV RNA levels and poorer working memory performance was mediated, in part (36%), by lower FA in the right IFO. Indirect effect represents the mediating factor (FA), and direct effect represents the association between Peak HIV RNA and working memory, excluding FA.
Acknowledgments
We thank the children and families for their participation in the Pediatric HIV/AIDS Cohort Study (PHACS) and Pediatric Imaging, Neurocognition, and Genetics (PING) Study, and the individuals and institutions involved in the conduct of PHACS and PING. PING was funded as one of the signature projects through the American Recovery and Reinvestment Act of the National Institute of Drug Abuse (NIDA). PING and PHACS were supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development. PHACS was co-funded by NIDA, the National Institute of Allergy and Infectious Diseases, the Office of AIDS Research, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Deafness and Other Communication Disorders, the National Heart Lung and Blood Institute, the National Institute of Dental and Craniofacial Research, and the National Institute on Alcohol Abuse and Alcoholism, through cooperative agreements with the Harvard University School of Public Health (HD052102) (Principal Investigator: George 1 Seage; Project Director: Julie Alperen) and the Tulane University School of Medicine (HD052104) (Principal Investigator: Russell Van Dyke; Co-Principal Investigator: Kenneth Rich; Project Director: Patrick Davis). All co-authors made substantial contributions to the: 1) conception, design, acquisition, analysis or interpretation of the data; 2) drafting, interpretation or revision of content; and 3) final approval of the published version. The corresponding authors are accountable for all aspects of the work relating to accuracy and integrity of any part. Data management services were provided by Frontier Science and Technology Research Foundation (PI: Suzanne Siminski), and regulatory services and logistical support were provided by West at, Inc (PI: Julie Davidson). We thank Dr. John Colby for the original scripts to extract and analyze DTI parameters along tracts, Eric Kan for his assistance in modifying original scripts to process PHIV DTI data at Children's Hospital Los Angeles, and Dr. Russell Van Dyke at Tulane University School of Medicine for his valuable input in the study's design and interpretation.
Work was supported by the National Institute of Drug Abuse (NIDA) and by the National Institute of Child Health and Human Development (NICHD).
Footnotes
Conflict of Interest None of the authors have any conflicts of interest to report.
References
- 1.Koekkoek S, de Sonneville LM, Wolfs TF, Licht R, Geelen SP. Neurocognitive function profile in HIV-infected school-age children. Eur J Paediatr Neurol. 2008;12:290–297. doi: 10.1016/j.ejpn.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz M, Kipnis J. A conceptual revolution in the relationships between the brain and immunity. Brain Behav Immun. 2011;25:817–819. doi: 10.1016/j.bbi.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith R, Wilkins M. Perinatally acquired HIV infection: Long-term neuropsychological consequences and challenges ahead. Child Neuropsychol. 2014 doi: 10.1080/09297049.2014.898744. [DOI] [PubMed] [Google Scholar]
- 4.Koot M, Keet IP, Vos AH, de Goede RE, Roos MT, Coutinho RA, et al. Prognostic value of HIV-1 syncytium-inducing phenotype for rate of CD4+ cell depletion and progression to AIDS. Ann Intern Med. 1993;118:681–688. doi: 10.7326/0003-4819-118-9-199305010-00004. [DOI] [PubMed] [Google Scholar]
- 5.Martin SC, Wolters PL, Toledo-Tamula MA, Zeichner SL, Hazra R, Civitello L. Cognitive functioning in school-aged children with vertically acquired HIV infection being treated with highly active antiretroviral therapy (HAART) Dev Neuropsychol. 2006;30:633–657. doi: 10.1207/s15326942dn3002_1. [DOI] [PubMed] [Google Scholar]
- 6.Nachman S, Chernoff M, Williams P, Hodge J, Heston J, Gadow KD. Human immunodeficiency virus disease severity, psychiatric symptoms, and functional outcomes in perinatally infected youth. Arch Pediatr Adolesc Med. 2012;166:528–535. doi: 10.1001/archpediatrics.2011.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith R, Chernoff M, Williams PL, Malee KM, Sirois PA, Kammerer B, et al. Impact of HIV severity on cognitive and adaptive functioning during childhood and adolescence. Pediatr Infect Dis J. 2012;31:592–598. doi: 10.1097/INF.0b013e318253844b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wood SM, Shah SS, Steenhoff AP, Rutstein RM. The impact of AIDS diagnoses on long-term neurocognitive and psychiatric outcomes of surviving adolescents with perinatally acquired HIV. Aids. 2009;23:1859–1865. doi: 10.1097/QAD.0b013e32832d924f. [DOI] [PubMed] [Google Scholar]
- 9.Darki F, Klingberg T. The Role of Fronto-Parietal and Fronto-Striatal Networks in the Development of Working Memory: A Longitudinal Study. Cereb Cortex. 2014 doi: 10.1093/cercor/bht352. [DOI] [PubMed] [Google Scholar]
- 10.Erus G, Battapady H, Satterthwaite TD, Hakonarson H, Gur RE, Davatzikos C, et al. Imaging Patterns of Brain Development and their Relationship to Cognition. Cereb Cortex. 2014 doi: 10.1093/cercor/bht425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 12.Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- 13.Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed. 1995;8:333–344. doi: 10.1002/nbm.1940080707. [DOI] [PubMed] [Google Scholar]
- 14.Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45:265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 15.Colby JB, Soderberg L, Lebel C, Dinov ID, Thompson PM, Sowell ER. Along-tract statistics allow for enhanced tractography analysis. Neuroimage. 2012;59:3227–3242. doi: 10.1016/j.neuroimage.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Q, Sun J, Guo L, Zang Y, Feng Z, Huang X, et al. Increased fractional anisotropy in white matter of the right frontal region in children with attention-deficit/hyperactivity disorder: a diffusion tensor imaging study. Neuro Endocrinol Lett. 2010;31:747–753. [PubMed] [Google Scholar]
- 17.Dash PK, Gorantla S, Gendelman HE, Knibbe J, Casale GP, Makarov E, et al. Loss of neuronal integrity during progressive HIV-1 infection of humanized mice. J Neurosci. 2011;31:3148–3157. doi: 10.1523/JNEUROSCI.5473-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li C, Zhang X, Komery A, Li Y, Novembre FJ, Herndon JG. Longitudinal diffusion tensor imaging and perfusion MRI investigation in a macaque model of neuro-AIDS: a preliminary study. Neuroimage. 2011;58:286–292. doi: 10.1016/j.neuroimage.2011.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang X, Li C. Quantitative MRI Measures in SIV-Infected Macaque Brains. J Clin Cell Immunol. 2013;Suppl 7 doi: 10.4172/2155-9899.S7-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Y, An H, Zhu H, Stone T, Smith JK, Hall C, et al. White matter abnormalities revealed by diffusion tensor imaging in non-demented and demented HIV+ patients. Neuroimage. 2009;47:1154–1162. doi: 10.1016/j.neuroimage.2009.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfefferbaum A, Rosenbloom MJ, Adalsteinsson E, Sullivan EV. Diffusion tensor imaging with quantitative fibre tracking in HIV infection and alcoholism comorbidity: synergistic white matter damage. Brain. 2007;130:48–64. doi: 10.1093/brain/awl242. [DOI] [PubMed] [Google Scholar]
- 22.Pomara N, Crandall DT, Choi SJ, Johnson G, Lim KO. White matter abnormalities in HIV-1 infection: a diffusion tensor imaging study. Psychiatry Res. 2001;106:15–24. doi: 10.1016/s0925-4927(00)00082-2. [DOI] [PubMed] [Google Scholar]
- 23.Ragin AB, Wu Y, Storey P, Cohen BA, Edelman RR, Epstein LG. Diffusion tensor imaging of subcortical brain injury in patients infected with human immunodeficiency virus. J Neurovirol. 2005;11:292–298. doi: 10.1080/13550280590953799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thurnher MM, Castillo M, Stadler A, Rieger A, Schmid B, Sundgren PC. Diffusion-tensor MR imaging of the brain in human immunodeficiency virus-positive patients. AJNR. 2005;26:2275–2281. [PMC free article] [PubMed] [Google Scholar]
- 25.Wu Y, Storey P, Cohen BA, Epstein LG, Edelman RR, Ragin AB. Diffusion alterations in corpus callosum of patients with HIV. AJNR. 2006;27:656–660. [PMC free article] [PubMed] [Google Scholar]
- 26.Chang L, Wong V, Nakama H, Watters M, Ramones D, Miller EN, et al. Greater than age-related changes in brain diffusion of HIV patients after 1 year. J Neuroimmune Pharmacol. 2008;3:265–274. doi: 10.1007/s11481-008-9120-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gongvatana A, Schweinsburg BC, Taylor MJ, Theilmann RJ, Letendre SL, Alhassoon OM, et al. White matter tract injury and cognitive impairment in human immunodeficiency virus-infected individuals. J Neurovirol. 2009;15:187–195. doi: 10.1080/13550280902769756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mueller-Mang C, Law M, Mang T, Fruehwald-Pallamar J, Weber M, Thurnher MM. Diffusion tensor MR imaging (DTI) metrics in the cervical spinal cord in asymptomatic HIV-positive patients. Neuroradiology. 2011;53:585–592. doi: 10.1007/s00234-010-0782-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stubbe-Drger B, Deppe M, Mohammadi S, Keller SS, Kugel H, Gregor N, et al. Early microstructural white matter changes in patients with HIV: a diffusion tensor imaging study. BMC Neurol. 2012;12:23. doi: 10.1186/1471-2377-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wright PW, Heaps JM, Shimony JS, Thomas JB, Ances BM. The effects of HIV and combination antiretroviral therapy on white matter integrity. Aids. 2012;26:1501–1508. doi: 10.1097/QAD.0b013e3283550bec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu T, Zhong J, Hu R, Tivarus M, Ekholm S, Harezlak J, et al. Patterns of white matter injury in HIV infection after partial immune reconstitution: a DTI tract-based spatial statistics study. J Neurovirol. 2013;19:10–23. doi: 10.1007/s13365-012-0135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarma MK, Nagarajan R, Keller MA, Kumar R, Nielsen-Saines K, Michalik DE, et al. Regional brain gray and white matter changes in perinatally HIV-infected adolescents. Neuroimage Clin. 2014;4:29–34. doi: 10.1016/j.nicl.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wechsler D. Wechsler Intelligence Scale for Children - Fourth Edition. San Antonio, TX: Harcourt; 2003. [Google Scholar]
- 34.Wechsler D. Wechsler Adult Intelligence Scale-Fourth Edition. San Antonio, TX: Pearson; 2008. [Google Scholar]
- 35.Brown TT, Kuperman JM, Chung Y, Erhart M, McCabe C, Hagler DJ, Jr, et al. Neuroanatomical assessment of biological maturity. Curr Biol. 2012;22:1693–1698. doi: 10.1016/j.cub.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.R_Core_Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. [Google Scholar]
- 37.Becker L. Effect Size Calculators. Colorado Springs: University of Colorado; 1998. [Google Scholar]
- 38.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang R, Benner T, Sorensen A, Wedeen V. Diffusion Toolkit: A Software Package for Diffusion Imaging Data Processing and Tractography. Proc Intl Soc Mag Reson Med. 2007;15:3720. [Google Scholar]
- 40.Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36:630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.MATLAB. The MathWorks, Inc; Natick, MA: [Google Scholar]
- 42.Herting MM, Colby JB, Sowell ER, Nagel BJ. White matter connectivity and aerobic fitness in male adolescents. Dev Cogn Neurosci. 2014;7:65–75. doi: 10.1016/j.dcn.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tingley D, Yamamoto T, Keele L, Imai K. mediation: R Package for Causal Mediation Analysis. 2013 [Google Scholar]
- 44.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23 Suppl 1:S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 45.Filippi CG, Lin DD, Tsiouris AJ, Watts R, Packard AM, Heier LA, et al. Diffusion-tensor MR imaging in children with developmental delay: preliminary findings. Radiology. 2003;229:44–50. doi: 10.1148/radiol.2291020049. [DOI] [PubMed] [Google Scholar]
- 46.Cho H, Yang DW, Shon YM, Kim BS, Kim YI, Choi YB, et al. Abnormal integrity of corticocortical tracts in mild cognitive impairment: a diffusion tensor imaging study. J Korean Med Sci. 2008;23:477–483. doi: 10.3346/jkms.2008.23.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bladowska J, Zimny A, Knysz B, Malyszczak K, Koltowska A, Szewczyk P, et al. Evaluation of early cerebral metabolic, perfusion and microstructural changes in HCV-positive patients: a pilot study. J Hepatol. 2013;59:651–657. doi: 10.1016/j.jhep.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 48.Filippi CG, Ulug AM, Ryan E, Ferrando SJ, van Gorp W. Diffusion tensor imaging of patients with HIV and normal-appearing white matter on MR images of the brain. AJNR. 2001;22:277–283. [PMC free article] [PubMed] [Google Scholar]
- 49.Xuan A, Wang GB, Shi DP, Xu JL, Li YL. Initial study of magnetic resonance diffusion tensor imaging in brain white matter of early AIDS patients. Chin Med J (Engl) 2013;126:2720–2724. [PubMed] [Google Scholar]
- 50.Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;40:1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- 51.Kier EL, Staib LH, Davis LM, Bronen RA. MR imaging of the temporal stem: anatomic dissection tractography of the uncinate fasciculus, inferior occipitofrontal fasciculus, and Meyer's loop of the optic radiation. AJNR. 2004;25:677–691. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Mean±SD. Alterations in whole brain DTI measures in PHIV compared to typically developing youth. A) Fractional anisotropy: Typical>PHIV; 2) Axial diffusivity: Typical=PHIV; 3) Radial diffusivity: PHIV>Typical; and 4) Mean diffusivity: PHIV>Typical.


