Abstract
Protecting human participants requires consideration and minimization of the burdens imposed by research. Effective conceptualizations of research burden should include appraisals of indirect burdens depending on research duration, intensity, and invasiveness. Introducing the concept of perceived research burden, we developed, tested, and validated a psychometric instrument for measuring burden, using vignettes of research studies presented to research volunteers and family members. We found high internal consistency of the Perceived Research Burden Assessment (PeRBA), across research scenarios (Cronbach’s alpha .87 – .96). We demonstrated convergent validity by correlating research burden with likelihood for enrolling in a research study. Because perceived research burden was largely unrelated to perceived social support, we interpreted PeRBA as demonstrating discriminant validity.
Keywords: human subjects, research burden, recruitment, measurement
At a fundamental level, the protection of human subjects participating in research requires investigators, regulatory bodies, and oversight agencies to consider and minimize the degree of burden that research poses to participants. Efforts to address the burden of clinical research have historically focused on the direct risks associated with research interventions or data collection procedures, yet there is a strong need to examine the broader construct of the burden imposed by the overall process of research participation. The current paper is based on the premise that a full conceptualization of research burden encompasses not only perceptions of a study’s directs risks, but also includes appraisals of the indirect burden (or, degree of inconvenience) that likely varies depending upon the study duration, intensity, and the invasiveness of study procedures; and may be modulated by a number of other factors, particularly perceptions of benefit. While a basic moral imperative undergirds the need to minimize the burden of the research process, there is also a compelling pragmatic basis for identifying ways to minimize research participant burden. Lessons from the field of survey research suggest that patients may be less likely to participate, or be enrolled by their family members, in research that is perceived to be unduly burdensome (Groves, Cialdini, & Couper, 1992). Research into this possibility is limited by a lack of standardized measures of research participant burden. Therefore, the objective of this paper is to describe a pilot study of the development and initial psychometric testing of a new measure of perceived research participant burden.
Conceptual Underpinnings: From Respondent Burden to Perceived Research Burden
With roots in the field of survey research, the concept of respondent burden has traditionally been defined as a multidimensional construct encompassing: the length of an interview, the amount of effort required of the respondent, the amount of stress on the respondent, and the frequency with which the respondent is interviewed (Bradburn, 1978; Frankel & Sharp, 1983). Concerns regarding respondent burden were initially couched in terms of the potential effect of respondent fatigue on data integrity (Sharp & Frankel, 1983). Although early work on the construct incorporated both objective and subjective aspects of interview participation, subsequent empirical studies have adopted a narrow and indirect approach to measuring respondent burden, operationalizing it as the time required to complete a survey or interview (Gibson, Koepsell, Diehr, & Hale, 1999; Kim, Dahlberg, & Hagell, 2006; McCarty, Killworth, & Rennell, 2007). Departing from this tradition, the notion of respondent burden has recently been carried over to the research ethics literature. Ulrich and colleagues applied the construct to illustrate research participant protection concerns arising from a case presentation of a seriously ill individual who is approached for participation in multiple clinical research studies, each with varying degrees of risk and associated burden (Ulrich, Wallen, Feister, & Grady, 2005). They redefined respondent burden as a subjective phenomenon involving the perception by the subject of psychological, physical, and/or economic hardships associated with participation in the research process. In a similar vein, Lingler and colleagues (2006) have delineated the need for investigators and regulatory bodies to extend their conceptualizations of research-related burden beyond the risks of direct harm to subjects to include the burden that is incurred by the family caregivers who facilitate the involvement of frail, older subjects in clinical research (Lingler, Parker, DeKosky, & Schulz, 2006).
The research described in this paper adopts a broader, global conceptualization of research-related burden that encompasses each of the features described by Ulrich while at the same time acknowledging that, in the case of patient-oriented research, perceptions of burden may also be held by the family members of research subjects (Demi & Warren, 1995; Lingler et al., 2006; Neumark, Stommel, Given, C. & Given, B. 2001). The need to consider such third party perceptions of burden may be especially impactful for researchers seeking to recruit individuals who are typically accompanied to the research setting by a family caregiver.
Because the phrase “respondent burden” has a specific definition and rich tradition of usage in the field of survey research, we use the terminology of perceived participant burden. Features of this phrasing include the emphasis on burden’s subjective nature, as well as the connotation that participation in the research process is dynamic, versus the more passive and unidimensional role that may be implied by the term “respondent.”
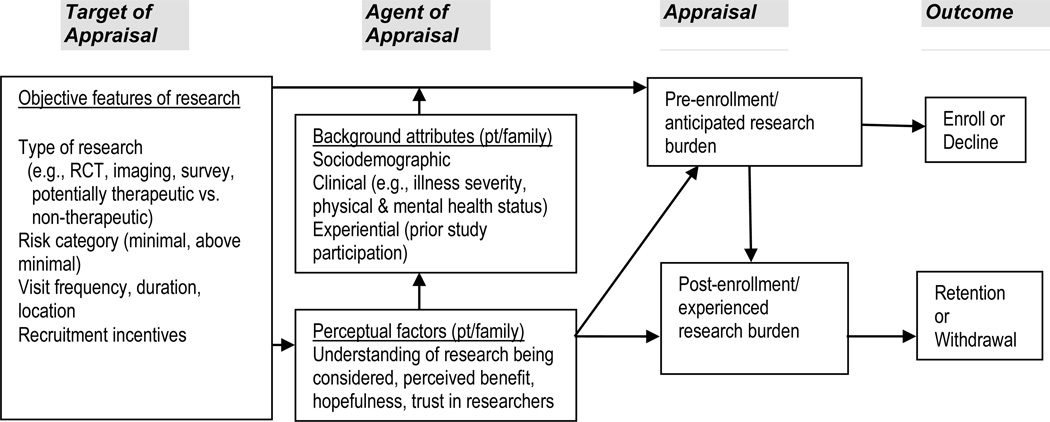
As depicted in Figure 1, the conceptualization of burden promoted here further expands upon previous theoretical work (e.g., Ulrich et al., 2012) by distinguishing pre enrollment perceptions of participant burden from post enrollment perceptions. We define pre enrollment appraisals of burden as a potential participant’s expectations of the degree of burden to be incurred should one opt to participate in a particular research study. Post enrollment appraisals of research burden are defined as the degree of burden that is experienced during actual study participation. Both anticipated and experienced burden are subjective in nature and are posited to vary depending upon the characteristics of a given research study as well as an array of patient and, in some cases, caregiver or family member factors. Distinguishing and measuring pre and post enrollment perceptions of burden may be particularly useful for examining how appraisals of research burden affect research participation outcomes. Our framework conceptualizes pre enrollment appraisals of burden as potentially influencing study enrollment outcomes while post enrollment appraisals of burden are hypothesized to influence retention outcomes (Figure 1). Our model also addresses the possibility that both patient and family members’ perceptions of research burden are potential factors in decisions to enroll or remain in research studies.
Figure 1. Conceptual Model of Role of Perceived Participant Burden in Recruitment and Retention.
Note: RCT = randomized controlled trial; pt = patient; “Family” refers to relatives or close friends who may share in decision-making.
A standardized measure of perceived research burden would enable researchers to test the relationships posited in this model as well as to systematically assess perceptions of burden among potential and actual research participants. The purpose of this pilot study, therefore, was to develop and describe the psychometric properties of patient and family member versions of a Perceived Research Burden Assessment (PeRBA).
Method
Phase I of this investigation focused on item generation and survey development. Phase II focused on piloting the survey and conducting psychometric testing, using a sample of decisionally intact participants and caregivers recruited from an Alzheimer Disease Research Center (ADRC). The research was approved by the University of Pittsburgh Institutional Review Board.
Item Generation and Survey Development
We assembled an initial item pool based upon input from an urban Community Advisory Council (Support: State Grant # SAP 4100027294; PI: DeKosky) and an extensive review of the literature. Given the importance of ensuring cultural competence when developing interview-based assessment tools, and the possibility that studies in our literature review may under represent persons from racial and ethnic minority groups, we sought input from an existing Community Advisory Council that had initially been formed to enhance the recruitment of African Americans into research on cognitive aging and Alzheimer’s disease. The Council was comprised of multiple stakeholders, including older African Americans with and without cognitive impairment who have and have not participated in research, as well as health and social service professionals from Pittsburgh’s African American community. Initial talk of assessing participant burden arose from a general discussion about various research studies and potential barriers to recruitment. Field notes and meeting minutes were scrutinized during the process of PeRBA item generation. As plans for the development of PeRBA evolved, additional feedback was sought from this group.
We simultaneously conducted a literature review, pairing the terms “burden” and “barriers” with key words “human experimentation,” “research methodology,” and “research subjects” in an electronic database search of English-language publications from Medline (January 1979–December 2009). We screened 244 articles and excluded those focused on: administrative burden of research, burden of research decision-making, or barriers to activities other than research participation. Our in-depth review of the remaining 34 articles confirmed the absence of a published tool for measuring perceived participant burden, and clustering of similar content revealed 6 considerations: the time required by study participation, the level of invasiveness, the accessibility of the research site; as well as the economic, physical, and psychological burden associated with participation.
Twenty two potential scale items were generated to reflect the above content areas. Once the scale items were generated, face validity and readability were established by initial review of the instrument with methodology experts in geriatric psychiatry at the University of Pittsburgh. Based on feedback from this group, the scale was revised; 5 items were dropped and the remaining items were reworded to ensure clarity, simplicity, and neutrality (e.g., avoidance of “loaded” questions). The revised 17 item tool, along with a 22 item family member version, was evaluated by a local multidisciplinary panel of investigators from Nursing, General Internal Medicine, Psychiatry, Public Health, and Dentistry, including the director of a large clinical research registry. The family member version contains additional items designed to capture aspects of research burden that are unique to those individuals who may be actively facilitating a patient’s involvement in research. An example of such an item is, “I feel I that I may have to persuade or coax my loved one to cooperate with certain aspects of the research study.”
The resulting assessment, coined “PeRBA,” consists of 17 items in the patient version and 22 items in the family member version, with pre-enrollment and post-enrollment phrasing options for both versions (See Appendix). These items are measured on a 5-point Likert scale ranging from 1 (strongly disagree) to 5 (strongly agree). As shown in the Appendix, our final conceptualization of research burden groups the content areas of time, accessibility and economic considerations into the broader conceptual domain of logistical burden. Psychological and physical burden comprise the remaining two conceptual domains in our final conceptual scheme. This final conceptualization was the result of ongoing discussions among the research team as the study progressed. During this process, it was noted that only one item targeted the domain of physical burden. To address this limitation, four new items were later added to each version of PeRBA to provide additional information about the perceived physical burdens of research participation.
Survey Administration
Our convenience sample included decisionally intact research volunteers and family members of those consecutively presenting for a regularly scheduled annual visit at the University of Pittsburgh Alzheimer Disease Research Center (ADRC). All research volunteers met the ADRC enrollment criteria (Lopez et al., 2000). To be eligible for this pilot study, patient participants also had to: 1) carry an ADRC consensus-based diagnosis of normal cognition, mild cognitive impairment (Pertersen et al., 2001; Lopez et al., 2003) or Probable Alzheimer’s disease (McKhann et al., 1984), 2) have a Mini-Mental Status Exam (Folstein, M., Folstein, F., & McHugh, 1975) score of at least 16, 3) be community-dwelling, 4) live within a 50-mile radius of the University of Pittsburgh; and 5) be willing to participate and, for those with a cognitive disorder, have a primary family member who is willing to participate. Decisional capacity was verified after entry into our study as described below. Research volunteers and family members were recruited as pairs and their interviews were conducted separately in private rooms at the ADRC. As per ADRC protocol, participants with normal cognition did not have family member co-participants.
All participants provided written informed consent to the study. Proxy consent was not employed. Rather, those who did not express adequate comprehension of the purpose, risks, and benefits of the study were excluded from participation as a basic understanding of the concept of perceived research burden was necessary to complete the study procedures.
Each participant independently completed up to 3 consecutively administered PeRBA surveys, rating their perception of the burden associated with 3 hypothetical clinical research recruitment scenarios for studies at differing levels of risk. The presentation of each vignette and its respective PeRBA administration took less than 10 minutes. Cognitively normal participants were included in the group completing the patient version of PeRBA to ensure a sample that reflects the full range of individuals who are likely to consider participation in research on dementia as the field moves toward interventions targeting older adults before the onset of cognitive impairment. The hypothetical recruitment scenarios were adapted from a series was developed specifically for use in the context of dementia research and has been in use for over a decade (Sachs et al., 1994). Our minor modifications to the scenarios reflect current research trends (See appendix). In order to ensure that participants understood the content of each vignette well enough to assess the burden associated with the hypothetical study, we administered the 10-item University of San Diego Brief Assessment of Capacity to Consent to Research (UBACC; Jeste et al., 2007) to both research volunteer and family member participants after each vignette was presented, and only proceeded with the PeRBA when participants scored above the recommended cut off on this screen for decisional capacity.
Measures of Convergent and Discriminant Validity
Convergent validity
Convergent validity was assessed by comparing PeRBA scores across research scenarios in which perceived burden is expected to differ, and by examining the correlation between PeRBA scores and ratings of participants’ self-reported likelihood of enrolling themselves or their family members in a research study like the one depicted in each vignette. Ratings were ascertained using a 3 point Likert scale ranging from “not very likely” to “highly likely” to enroll in a research study like the one described in the vignette. This measure was administered immediately following the presentation of each vignette.
Discriminant validity
We used perceived satisfaction with social support as an indicator of PeRBA’s discriminant validity. Satisfaction with social support was selected because it represents a construct that is not expected to be highly correlated with perceived research burden. Note that social support is not a variable in our conceptual framework. The choice of this variable is also supported by a large review of empirical studies on clinical research participation which failed to relate social support to receptiveness to research (Cox & McGarry, 2003). Perceived satisfaction with social support was evaluated using the satisfaction with social support item from the Interpersonal Support Evaluation List (ISEL; Cohen & Hoberman, 1983).
Additional measures
Sociodemographic and clinical characteristics of each participant were abstracted from the ADRC record with participants’ explicit consent.
Data Analysis
Descriptive statistics were used to characterize the sample population of research volunteers control participants and patient and caregiver participants (Table 1). Mean, standard deviation, and range of scores for each administration of PeRBA were generated. Characteristics of the research volunteers with and without cognitive impairment were compared before combining for further analysis. Family member responses were analyzed separately.
Table 1.
Sample Characteristics (n = 134)
| Research Volunteers |
Family Member (n = 52) |
||
|---|---|---|---|
| Normal cognition (n = 30) |
Patient (n = 52) |
||
| Mean age in years (SD) | 75.7 (10.3) | 74.3 (9.8) | 69.9 (11.5) |
| Sex, n (%)* | |||
| Male | 9 (30.0) | 28 (53.8) | 18 (22.0) |
| Female | 21 (70.0) | 24 (46.2) | 34 (78.0) |
| Race, n (%)* | |||
| White | 21 (70.0) | 48 (92.3) | 48 (92.3) |
| Black | 9 (30.0) | 4 (7.7) | 4 (7.7) |
| Mean years of education (SD) | 15.9 (3.1) | 15.4 (3.1) | 14.9 (2.6) |
| Relation to patient, n (%) | |||
| Spouse or partner | -- | -- | 43 (82.7) |
| Child | -- | -- | 6 (11.5) |
| Other | -- | -- | 3 (5.8) |
Note:
P value < .05
Internal consistency reliability was examined for both the patient and family member versions of PeRBA using Cronbach’s alpha for three increasingly burdensome research scenarios. This generated a total of six values for Cronbach’s alpha (See Table 3). We set an a priori standard for acceptable reliability of PeRBA at alpha values of .70 or higher (Nunnally & Bernstein, 1994).
Table 3.
Preliminary Reliability and Validity of PeRBA to Measure Anticipated Research Burden (n = 134)
| Scenario/Analysis |
Internal Consistency: Cronbach’s alpha |
Correlation with related construct: likelihood of participating (rho) |
Correlation with unrelated construct: social support (r) |
|||
|---|---|---|---|---|---|---|
| P | F | P | F | P | F | |
| Scenario A: Venipuncture to develop a diagnostic test | .95 | .96 | −.565*** | −.454** | −.289* | −.131 |
| Scenario B: Early phase investigational drug trial | .87 | .94 | −.451*** | −.643*** | −.167 | −.257 |
| Scenario C: Investigation of neurosurgical procedure | .88 | .92 | −.456*** | −.470** | −.204 | −.112 |
Note:
P indicates patient version of PeRBA scale, F indicates family member version of PeRBA scale
*P value < .05; **P value < .01; ***P value < .001
rho = Spearman’s Rho correlation; r = Pearson’s r correlation
Convergent and discriminant validity of the PeRBA instrument were assessed by examining scores on the patient and caregiver versions of PeRBA using the variables described above. We performed paired t-tests to compare participants’ PeRBA ratings across the 3 research scenarios. Normality checks were conducted to determine which correlational tests were best suited to the data. We used Spearnan’s Rho to test the hypothesis that individuals scoring higher on PeRBA (high perceived research burden) would be less likely to agree to participate in a study such as that described in the hypothetical research scenario. To establish discriminant validity, we hypothesized that scores on PeRBA would be weakly or modestly correlated with responses indicating levels of social support (Pearson’s r <.3; Cohen, 1988). A power analysis was performed, indicating that computing these correlation coefficients using a two tailed test and an alpha of .05 would require a sample size of 82 to achieve 80% power and to detect an effect size as low as .3.
Results
Eighty-two research volunteer and 52 family member participants were recruited through the local ADRC and, as shown in Table 1, the characteristics of the sample reflect the generally higher levels of education that are common in this population. It should be noted that the family member sample size is smaller than the research volunteer sample as those volunteers who were cognitive normal did not have family members accompanying them to annual ADRC visits. An updated power analysis showed that the family member sample size of 52 provides 80% power to detect an effect as low as .37 using two-tailed correlation tests.
Few significant differences were noted between individuals with cognitive impairment and cognitively normal research volunteers. Age and education did not differ between groups, however, there was a higher proportion of men in the patient group and a higher proportion of African Americans in the normal cognition group. Family members, as compared to research volunteers, were on average five years younger (Table 1).
Table 2 shows the mean values of total PeRBA scores for both versions of the tool, by scenario. As category of risk in the scenarios increased, mean total ratings of perceived research burden also significantly increased, as expected (Table 2). Internal consistency was high for both versions of PeRBA (patient and caregiver), with Cronbach’s alpha rated between .87 and .96 across all three research scenarios (Table 3). Table 4 displays the mean values for each item and inter-item correlations for both patient and family member versions of PeRBA, across all three research scenarios.
Table 2.
PeRBA scores in three scenarios (n=134)
| PeRBA-P (n = 82) |
PeRBA-F (n = 52) |
|
|---|---|---|
| Scenario A: Venipuncture to develop a diagnostic test, M (s.d.) | 30.7 (9.5) | 43.9 (12.7) |
| Scenario B: Early phase investigational drug trial, M (s.d.) | 38.7 (8.4) | 53.9 (13.2) |
| Scenario C: Investigation of neurosurgical procedure, M (s.d.) | 41.8 (9.8) | 58.2 (13.2) |
| P value for paired t test within each group | ||
| A-B: comparison between scores for scenario A & B | P (A-B) < 0.001 | P (A-B) < 0.001 |
| A-C: comparison between scores for scenario A & C | P (A-C) < 0.001 | P (A-C) < 0.001 |
| B-C: comparison between scores for scenario B & C | P (B-C) = 0.001 | P (B-C) < 0.001 |
Note: P indicates patient version of PeRBA scale, F indicates family member version of PeRBA scale.
Table 4.
Item characteristics
| PeRBA-P (17 items) |
PeRBA-F (21 items) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Scenario A | Scenario B | Scenario C | Scenario A | Scenario B | Scenario C | |||||||
| Mean (SD) |
Corrected item-total correlation |
Mean (SD) |
Corrected item-total correlation |
Mean (SD) |
Corrected item-total correlation |
Mean (SD) |
Corrected item-total correlation |
Mean (SD) |
Corrected item-total correlation |
Mean (SD) |
Corrected item-total correlation |
|
| Item 1 | 1.70 (.744) |
.779 | 2.58 (1.117) |
.512 | 2.86 (1.202) |
.620 | 1.87 (.817) |
.756 | 3.02 (1.108) |
.718 | 3.04 (1.106) |
.739 |
| Item 2 | 1.79 (.695) |
.835 | 2.59 (.998) |
.544 | 2.79 (1.074) |
.674 | 1.87 (.715) |
.880 | 2.84 (1.028) |
.682 | 3.00 (1.195) |
.783 |
| Item 3 | 1.72 (.637) |
.797 | 2.30 (.982) |
.643 | 2.47 (1.126) |
.641 | 1.85 (.697) |
.845 | 2.24 (.969) |
.674 | 2.46 (1.129) |
.687 |
| Item 4 | 1.76 (.686) |
.800 | 2.00 (.687) |
.678 | 2.10 (.790) |
.546 | 1.85 (.638) |
.764 | 2.12 (.807) |
.701 | 2.40 (.926) |
.640 |
| Item 5 | 1.83 (.737) |
.641 | 1.95 (.685) |
.284 | 2.07 (.718) |
.507 | 1.87 (.687) |
.790 | 2.41 (.911) |
.677 | 2.52 (.931) |
.613 |
| Item 6 | 1.83 (.717) |
.719 | 2.05 (.797) |
.629 | 2.17 (.805) |
.527 | 1.92 (.682) |
.790 | 2.10 (.467) |
.617 | 2.16 (.548) |
.499 |
| Item 7 | 1.85 (.804) |
.591 | 1.99 (.754) |
.387 | 2.00 (.787) |
.376 | 1.94 (.698) |
.840 | 2.12 (.564) |
.585 | 2.22 (.616) |
.449 |
| Item 8 | 1.92 (.770) |
.635 | 2.18 (.839) |
.409 | 2.25 (.931) |
.404 | 1.94 (.669) |
.752 | 2.14 (.707) |
.691 | 2.24 (.687) |
.597 |
| Item 9 | 2.07 (.915) |
.650 | 2.40 (.982) |
.462 | 2.38 (.941) |
.426 | 1.98 (.754) |
.645 | 2.08 (.702) |
.598 | 2.20 (.782) |
.516 |
| Item 10 | 1.89 (.785) |
.608 | 2.08 (.846) |
.354 | 2.13 (.804) |
.363 | 2.04 (.791) |
.534 | 2.20 (.866) |
.585 | 2.26 (.828) |
.515 |
| Item 11 | 1.73 (.696) |
.741 | 2.22 (.961) |
.689 | 2.58 (1.148) |
.569 | 2.23 (.983) |
.652 | 2.43 (.957) |
.430 | 2.60 (1.069) |
.437 |
| Item 12 | 1.72 (.721) |
.728 | 2.97 (1.178) |
.381 | 3.22 (1.189) |
.439 | 2.23 (.942) |
.671 | 2.57 (.979) |
.519 | 2.50 (1.074) |
.590 |
| Item 13 | .187 (.827) |
.783 | 3.04 (1.252) |
.523 | 3.22 (1.178) |
.570 | 2.13 (.886) |
.477 | 2.24 (.925) |
.468 | 2.22 (.910) |
.435 |
| Item 14 | 1.79 (.773) |
.718 | 2.85 (1.151) |
.383 | 3.17 (1.210) |
.610 | 2.13 (.742) |
.590 | 2.67 (.966) |
.730 | 3.18 (1.063) |
.602 |
| Item 15 | 1.72 (.680) |
.772 | 2.04 (.873) |
.676 | 2.24 (.896) |
.584 | 1.94 (.639) |
.813 | 3.06 (1.049) |
.505 | 3.52 (1.074) |
.398 |
| Item 16 | 1.77 (.760) |
.767 | 1.92 (.741) |
.610 | 2.06 (.748) |
.635 | 2.13 (.929) |
.623 | 2.49 (.982) |
.702 | 2.92 (1.122) |
.552 |
| Item 17 | 1.77 (.590) |
.761 | 2.00 (.799) |
.527 | 2.07 (.775) |
.464 | 2.13 (.971) |
.736 | 2.47 (.938) |
.729 | 2.88 (1.118) |
.598 |
| Item 18 | N/A | N/A | N/A | N/A | N/A | N/A | 2.08 (.837) |
.800 | 3.00 (1.041) |
.756 | 3.58 (1.071) |
.630 |
| Item 19 | N/A | N/A | N/A | N/A | N/A | N/A | 2.04 (.907) |
.749 | 2.96 (1.060) |
.726 | 3.46 (1.182) |
.613 |
| Item 20 | N/A | N/A | N/A | N/A | N/A | N/A | 1.96 (.713) |
.720 | 2.35 (.991) |
.704 | 2.56 (1.091) |
.656 |
| Item 21 | N/A | N/A | N/A | N/A | N/A | N/A | 1.88 (.676) |
.651 | 2.24 (.969) |
.483 | 2.38 (1.086) |
.484 |
| Item 22 | N/A | N/A | N/A | N/A | N/A | N/A | 1.90 (.664) |
.833 | 2.16 (.825) |
.624 | 2.24 (.797) |
.409 |
Convergent validity analyses of both the patient and family member versions of the tool showed that PeRBA ratings were highly negatively associated with ratings of likelihood of participating in a “study like this”. This suggests that the more burdensome a research study is perceived to be, as measured by PeRBA, the less likely an individual is to enroll either himself or a family member as a participant. This finding was consistent across three increasingly burdensome research scenarios (Table 3). Discriminant validity analyses strongly suggested that research burden was perceived independently of satisfaction with social support, indicating that PeRBA measures a distinctly appraised concept (Table 3). Perceived research burden and social support were modestly correlated (r = –.289, p< .05) in only the low risk research scenario.
Discussion
We established reliability and face, convergent and discriminant validity of PeRBA, the first instrument to measure perceived research burden among key stakeholders in research, potential research participants and their family members. Perceived research burden was inversely associated with likelihood of enrolling in a similar research study and emerged as an independent construct, generally unassociated with perceived satisfaction with social support. As expected, perceived research burden among research volunteers and their family members increased as risk categorization of the hypothetical studies progressed from minimal to greater than minimal.
Limitations
The hypothetical nature of the research scenarios utilized in the development of PeRBA may have biased participants toward lower reports of burden and is a limitation of the current report. Small sample sizes limited the breadth of the psychometric analyses, and larger, more confirmatory analyses are needed to further examine PeRBA item responses. Limited involvement of underrepresented populations is an additional concern. There is a need for specific consideration of research burden in other clinical and ethnic populations from which research participants are recruited.
Best Practices
This tool offers a sound means of assessing perceived research burden in the context of Alzheimer’s disease and holds considerable promise for research focused on other conditions. Across disease contexts, research recruitment and retention are of critical importance in ensuring the applicability and reliability of clinical research findings for translation into clinical practice. Perceived participant burden is a likely, but up until now immeasurable, factor that may contribute to enrollment, retention, and attrition in research studies. The ability to assess this factor would be useful in several ways. For example, during pilot work, investigators could prospectively gauge perceived research burden in order to refine protocols and/or adjust recruitment goals. An instrument such as PeRBA could also be used to track or monitor the impact of perceived burden on recruitment and retention during the conduct of clinical research studies. The availability of both patient and family member versions of the scale will facilitate assessments of the relative impact of patient vs. family member perceptions of burden and to identify factors that may attenuate such perceptions.
The current investigation was grounded in the assumption that objective features of a research study, like the frequency and intensity of visits, and the categorization of its risk level, are highly correlated with perceived burden among potential participants. In addition to the objective features of a given study, a number of background attributes and perceptual factors are likely to contribute to an individual’s appraisal of the burden associated with research participation. For example, expectations of a benefit to self or others may temper perceptions of the burden associated with research participation (Argarwal et al., 2007; Brody, Annett, Schere, Perryman, & Cofrin, 2005; Brown & Topcu, 2003; Jenkins & Fallowfield, 2000; Marcantonio et al., 2008). The literature also indicates that intra and interpersonal characteristics such as degree of hope and level of trust in researchers and/or doctors are important to consider (Jenkins & Fallowfield, 2000; Mainous, Smith, Geesey, & Tilley, 2006), especially in the context of research involving seriously ill, disenfranchised or vulnerable populations (Demi & Warren, 1995; Tait, Voepel-Lewis, & Malviya (2003). Finally, sociodemographic factors such as ethnicity (Areán, Alvidrez, Nery, Estes, & Linkins, 2003; Corbie-Smith, Thomas, Williams, & Moody-Ayers, 1999; Fouad et al, 2000; Freimuth et al., 2001) and age (Neumark et al., 2001; Trauth, Musa, Siminoff, Jewell, & Ricci, 2000; Williams, Shuster, Clay, & Burgio, 2006) have been linked to willingness to participate in research, and may therefore be associated with perceived research burden. Best practices for addressing research participant burden should take into account the likely complex inter-relationships of these variables. Stated another way, characteristics of an investigator’s sample may be equally as influential as features of the research study in predicting how burdensome the study will appear to potential participants.
Research Agenda
It should be noted that as the study reported here progressed, four new items were added to each version of PeRBA to provide additional information about the perceived physical burdens of research participation. Because only a subgroup of our participants received the longer version of PeRBA, the analyses reported above used the 17-item patient version and 21-item family member version. Psychometric evaluation of the full version of PeRBA is needed, as is testing of the post-enrollment version of the tool. Future research using PeRBA may also incorporate factor analytic approaches to ascertain the underlying properties of the measure or item response theory to examine item-level characteristics. Although the PeRBA was designed to address perceived burden along key dimensions (physical, psychological, and logistical), it is yet to be established whether this perceived research burden is indeed multidimensional, or if there is a more unitary concept of burden that encompasses all three dimensions.
Educational Implications
Recruitment and retention issues plague clinical research (Reynolds, 2011). The current study highlights the need to advise research trainees and investigators at all levels that although reasons for refusing participation in research vary, anticipated or perceived research burden may also be an important contributor to low rates of participant accrual. Addressing this issue requires not only additional data on factors affecting recruitment, including perceived burden, but also educating investigators and research staff on the importance of considering participant perspectives as they design and implement research studies. Investigators could use PeRBA results to enhance their understanding of and empathy toward their primary research populations, which may in turn lead to greater satisfaction on the part of the research participants and ultimately greater success in completing clinical trials.
With this in mind, the University of Pittsburgh Clinical Translational Science Institute recently featured PeRBA in a Responsible Conduct of Research Training session. The program was well received with most attendees (n = 18) rating themselves as more confident in knowledge of participant burden, having gained practical tools or skills, and more confident to advance their research progress (mean scores of 4.69, 4.33, and 4.47, respectively on a scale of 0–6) following the presentation on PeRBA. In addition, one attendee followed up with the PeRBA presenter to request copies of the instrument for use in another research lab on campus.
Conclusion
PeRBA is a new tool for assessing perceptions of research burden among potential research participants and their family members. Initial psychometric evaluation of PeRBA reveals good internal consistency, evidence of face validity, and acceptable convergent and discriminant validity. A realistic assessment of perceived burden, obtained in advance of conducting research studies, could improve recruitment and retention in study populations. Using PeRBA will likely yield better estimates of the feasibility of recruiting participants from populations of interest to clinical investigators. By using PeRBA among potential research participants, investigators will be able to refine their understanding of research study burden independent of generalized concepts of social support and other predictors of likelihood to participate.
Supplementary Material
Acknowledgements
This research was supported by a University of Pittsburgh ADRC seed monies grant and NIH grants P50 AG05133, UL1 RR024153, and UL1 TR000005. The authors thank MaryAnn Oakley, MA, Trevor Nissel, BSN, and Catherine Toth, BSN for their assistance with the conduct of this research project.
Contributor Information
Jennifer H Lingler, University of Pittsburgh.
Karen Schmidt, Email: kschmidt@pitt.edu, University of Pittsburgh.
Amanda Gentry, Email: amg168@pitt.edu, University of Pittsburgh.
Lu Hu, Email: luh16@pitt.edu, University of Pittsburgh.
Lauren Terhorst, Email: terhorstl@ccbh.com, University of Pittsburgh Medical Center.
References
- Areán PA, Alvidrez J, Nery R, Estes C, Linkins K. Recruitment and retention of older minorities in mental health services research. The Gerontologist. 2003;43(1):36–44. doi: 10.1093/geront/43.1.36. [DOI] [PubMed] [Google Scholar]
- Agarwal SK, Estrada S, Foster WG, Wall LL, Brown D, Revis ES, et al. What motivates women to take part in clinical and basic science endometriosis research? Bioethics. 2007;21(5):263–269. doi: 10.1111/j.1467-8519.2007.00552.x. [DOI] [PubMed] [Google Scholar]
- Bradburn N. Health Survey Research Methods: Second Biennial Conference. Washington, DC: U.S. Government Printing Office; 1978. Respondent burden. (Williamsburg, VA) [Google Scholar]
- Brody JL, Annett RD, Scherer DG, Perryman ML, Cofrin KMW. Comparisons of adolescent and parent willingness to participate in minimal and aboveminimal risk pediatric asthma research protocols. Journal of Adolescent Health. 2005;37(3):229–235. doi: 10.1016/j.jadohealth.2004.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DR, Topcu M. Willingness to participate in clinical treatment research among older African Americans and Whites. The Gerontologist. 2003;43(1):62–72. doi: 10.1093/geront/43.1.62. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Erlbaum; 1998. p. 1988. [Google Scholar]
- Cohen S, Hoberman HM. Positive events and social supports as buffers of life change stress. Journal of Applied Social Psychology. 1983;13:99–125. [Google Scholar]
- Corbie-Smith G, Thomas SB, Williams MV, Moody-Ayers S. Attitudes and beliefs of African Americans toward participation in medical research. Journal of General Internal Medicine. 1999;14(9):537–546. doi: 10.1046/j.1525-1497.1999.07048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox K, McGarry J. Why patients don’t take part in cancer clinical trials: An overview of the literature. European Journal of Cancer Care. 2003;12(2):114–122. doi: 10.1046/j.1365-2354.2003.00396.x. [DOI] [PubMed] [Google Scholar]
- Demi AS, Warren NA. Issues in conducting research with vulnerable families. Western Journal of Nursing Research. 1995;17(2):188–202. doi: 10.1177/019394599501700206. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fouad MN, Partridge E, Green BL, Kohler C, Wynn T, Nagy S, et al. Minority recruitment in clinical trials: A conference at Tuskegee, researchers and the community. Annals of Epidemiology. 2000;10(Suppl. 8):S35–S40. doi: 10.1016/s1047-2797(00)00199-x. [DOI] [PubMed] [Google Scholar]
- Frankel J, Sharp LM. Measurement of respondent burden: Study design and early findings. Washinton, D.C.: Bureau of Social Science Research; 1980. [Google Scholar]
- Freimuth VS, Quinn SC, Thomas SB, Cole G, Zook E, Duncan T. African Americans’ views on research and the Tuskegee Syphilis Study. Social Science and Medicine. 2001;52(5):797–808. doi: 10.1016/s0277-9536(00)00178-7. [DOI] [PubMed] [Google Scholar]
- Gibson P, Koepsell T, Diehr P, Hale C. Increasing response rates for mailed surveys of Medicaid clients and other low-income populations. American Journal of Epidemiology. 1999;149:1057–1062. doi: 10.1093/oxfordjournals.aje.a009751. [DOI] [PubMed] [Google Scholar]
- Groves RM, Cialdini RB, Couper MP. Understanding the decision to participate in a survey, Public Opinion Quarterly. 1992;56(4):475–495. [Google Scholar]
- Jenkins V, Fallowfield L. Reasons for accepting or declining to participate in randomized clinical trials for cancer therapy. British Journal of Cancer. 2000;82(11):1783–1788. doi: 10.1054/bjoc.2000.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeste DV, Palmer BW, Appelbaum PS, Golshan S, Glorioso D, Dunn LB, Kraemer HC. University of California, San Diego Brief Assessment of Capacity to Consent (UBACC) Archives of General Psychiatry. 2007;64(8):966–974. doi: 10.1001/archpsyc.64.8.966. [DOI] [PubMed] [Google Scholar]
- Kim MY, Dahlberg A, Hagell P. Respondent burden and patient-perceived validity of the PDQ-39. Acta Neurologica Scandinavica. 2006;113(2):132–137. doi: 10.1111/j.1600-0404.2005.00549.x. [DOI] [PubMed] [Google Scholar]
- Lingler JH, Martire LM, Hunsaker AE, Greene MG, Dew MA, Schulz R. Feasibility of a patient-driven approach to recruiting older adults, caregivers, and clinicians for provider-patient communication research. Journal of the American Academy of Nurse Practictioners. 2009;21:377–383. doi: 10.1111/j.1745-7599.2009.00427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingler JH, Parker LS, DeKosky ST, Schulz R. Caregivers as subjects of clinical drug trials: A review of human subjects protection practices in published studies of Alzheimer disease pharmacotherapies. IRB: Ethics & Human Research. 2006;28(3):11–18. [PubMed] [Google Scholar]
- Lopez OL, Becker JT, Klunk WE, Saxton J, Hamilton R, Sweet RA, et al. Research evaluation and diagnosis of possible Alzheimer’s disease over the last 2 decades: I. Neurology. 2000;55:1854–1862. doi: 10.1212/wnl.55.12.1854. [DOI] [PubMed] [Google Scholar]
- Lopez OL, Jagust WJ, DeKosky ST, Becker JT, Fitzpatrick A, Dulberg C, et al. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study: Part 1. Archives of Neurology. 2003;60(10):1385–1389. doi: 10.1001/archneur.60.10.1385. [DOI] [PubMed] [Google Scholar]
- Marcantonio ER, Aneja J, Jones RN, Alsop DC, Fong TG, Crosby GJ, et al. Maximizing clinical research participation in vulnerable older persons: Identification of barriers and motivators. Journal of the American Geriatrics Society. 2008;56(8):1522–1527. doi: 10.1111/j.1532-5415.2008.01829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainous AG, III, Smith DW, Geesey ME, Tilley BC. Development of a measure to assess patient trust in medical researchers. Annals of Family Medicine. 2006;4(3):247–252. doi: 10.1370/afm.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty C, Killworth PD, Rennell J. Impact of methods for reducing respondent burden on personal network structural measures. Social Networks. 2007;29:300–315. [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Neumark DE, Stommel M, Given CW, Given BA. Research design and subject characteristics predicting nonparticipation in a panel survey of older families with cancer. Nursing Research. 2001;50(6):363–368. doi: 10.1097/00006199-200111000-00006. [DOI] [PubMed] [Google Scholar]
- Nunnally JC, Bernstein IH. Psychometric Theory. New York: McGraw Hill; 1994. [Google Scholar]; Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, et al. Current concepts in mild cognitive impairment. Archives of Neurology. 2001;58(12):1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- Peteresen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, et al. Current concepts in mild cognitive impairment. Archives of Neurology. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- Reynolds T. Clinical trials: can technology solve the problem of low recruitment? British Medical Journal. 2011;342:d3662. doi: 10.1136/bmj.d3662. [DOI] [PubMed] [Google Scholar]
- Sachs GA, Stocking CB, Stern R, Cox DM, Hougham G, Sachs RS. Ethical aspects of dementia research: Informed consent and proxy consent. Clinical Research. 1994;42(3):403–412. [PubMed] [Google Scholar]
- Sharp LM, Frankel J. Respondent burden: A test of some common assumptions. Public Opinion Quarterly. 1983;47:36–53. [Google Scholar]
- Tait AR, Voepel-Lewis T, Malviya S. Participation of children in clinical research: Factors that influence a parent’s decision to consent. Anesthesiology. 2003;99(4):819–825. doi: 10.1097/00000542-200310000-00012. [DOI] [PubMed] [Google Scholar]
- Trauth JM, Musa D, Siminoff L, Jewell IK, Ricci E. Public attitudes regarding willingness to participate in medical research studies. Journal of Health and Social Policy. 2000;12(2):23–43. doi: 10.1300/J045v12n02_02. [DOI] [PubMed] [Google Scholar]
- Ulrich CM, Knafl KA, Ratcliffe SJ, Richmond TS, Grady C, Miller-Davis C, Wallen GR. Developing a model of the benefits and burdens of research participation in cancer clinical trials. AJOB Primary Research. 2012;3(2):10–23. doi: 10.1080/21507716.2011.653472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich CM, Wallen GR, Feister A, Grady C. Respondent burden in clinical research: When are we asking too much of subjects? IRB: Ethics and Human Research. 2005;27(4):17–20. [PubMed] [Google Scholar]
- Williams CJ, Shuster JL, Jr, Clay OJ, Burgio KL. Interest in research participation among hospice patients, caregivers, and ambulatory senior citizens: Practical barriers or ethical constraints? Journal of Palliative Medicine. 2006;9(4):968–974. doi: 10.1089/jpm.2006.9.968. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.