Abstract
Rationale and Goal
Glutamine, the most abundant amino acid in plasma, has attracted considerable interest for its cardioprotective properties. The primary effect of glutamine in the heart is commonly believed to be mediated via its anaplerotic metabolism to citric acid cycle (CAC) intermediates; however, there is little direct evidence to support this concept. Another potential candidate is the hexosamine biosynthetic pathway (HBP), which has recently been shown to modulate cardiomyocyte function and metabolism. Therefore, the goal of this study was to evaluate the contribution of anaplerosis and the HBP to the acute metabolic effects of glutamine in the heart.
Methods
Normoxic ex vivo working rat hearts were perfused with 13C-labeled substrates to assess relevant metabolic fluxes perfused with a physiological mixture of carbohydrates and a fatty acid (control) or under conditions of restricted pyruvate anaplerosis.
Results
Addition of a physiological concentration of glutamine (0.5 mM) had no effect on contractile function of hearts perfused under the control condition, but improved that of hearts perfused under restricted pyruvate anaplerosis. Changes in CAC intermediate concentrations as well as 13C-enrichment from [U-13C]glutamine did not support a major role of glutamine anaplerosis under any conditions. Under the control condition, however, glutamine significantly increased the contribution of exogenous oleate to β-oxidation, 1.6-fold, and triglyceride formation, 2.8-fold. Glutamine had no effect on malonyl-CoA or AMPK kinase activity levels; however, it resulted in a higher plasma membrane level of the fatty acid transporter CD36. These metabolic effects of glutamine were reversed by azaserine, which inhibits glucose entry into the HPB.
Conclusion
Our results reveal a metabolic role of physiological concentration of glutamine in the healthy working heart beyond anaplerosis. This role appears to involve the HBP and regulation of fatty acid entry and metabolism via CD36.
Keywords: Glutamine, energy metabolism, isolated working heart, anaplerosis, CD36
1. Introduction
Glutamine is the most abundant amino acid in the plasma and although considered a “non essential“ amino acid, it nevertheless regulates several cell specific processes, including growth and gene expression (see for review, refs [1,2]). In the heart, which is the specific focus of this study, it has also been linked to cardioprotection [3–6]. However, the molecular mechanisms underlying the effects of glutamine on the heart are not well understood. It is commonly believed that the primary metabolic pathway for glutamine metabolism involves its conversion to glutamate and subsequently to the citric acid cycle (CAC) intermediate α-ketogluratate. This represents a mechanism for potentially replenishing the CAC intermediate pool, which might be partially depleted in response to stress or acute increases in energetic demand and thereby ensuring optimal CAC flux. This pathway is termed anaplerosis and in the intestine, kidney or proliferating cells (for reviews see refs [7,8]), the metabolism of endogenous amino acids, such as glutamate or glutamine, via anaplerosis is well supported [7]; however, there is little data to support this pathway in the heart (For review, see ref [9]). In fact we have previously reported negligible labelling of α-ketoglutarate (<5%) when hearts were perfused with [U-13C5]glutamate under both normoxia or low-flow ischemia [10]. Consequently, to the best of our knowledge, there is no definitive data demonstrating that the heart is able to use glutamine as an anaplerotic substrate.
Another major pathway by which glutamine may exert its effects on the heart is via the hexosamine biosynthetic pathway (HBP). Over the past decade, the HBP has been extensively studied in the context of diabetic complications [11,12], and more recently, of cardiac ischemia [13]. Glutamine is the co-substrate along with fructose-6-phosphate for the first- and rate-limiting enzyme of the HBP, namely glutamine fructose 6-phosphate amidotransferase (GFAT) and is therefore essential for HBP activity. Glucose metabolism via the HBP pathway in part regulates protein modification by O-linked β-N-acetylglucosamine (O-GlcNAc), which has been shown to regulate a wide range of cellular functions including, cell survival, signal transduction, transcriptional activity and protein stability (for recent reviews, see [13,14]). Our understanding of the role and regulation of the HBP in the heart is limited; however, we found that the addition of glucosamine, which directly stimulates the HBP and enhances protein O-GlcNAcylation, induced a shift in energy substrate selection from carbohydrates (CHOs) to long chain fatty acids (LCFAs) via CD36 recruitment to the plasma membrane [15,16]. However, glucosamine is non-physiologically relevant substrate that enters the HBP bypassing the regulatory GFAT reaction. Consequently, while the findings with glucosamine raised the possibility that the HBP could play a role in regulating cardiac substrate metabolism, it remains to be determined as to whether glutamine might have similar effects under physiologically-relevant condition.
Therefore, the goal of this study was to determine whether, in an established ex vivo working rat heart model, the acute effects of glutamine on cardiac metabolism are mediated by anaplerosis or the HBP. Of note, despite the fact that glutamine is the most abundant amino acid in plasma, the majority of perfusion studies, including our own, do not routinely add glutamine to the perfusion medium. Conversely, those studies that have examined the cardiac effects of glutamine in perfused hearts used buffer that lacked many of the substrates that are physiologically relevant fuels for the in vivo heart and/or used supraphysiological concentrations of glutamine. Here, we found that physiological concentrations of glutamine had marginal effects on anaplerotic pathways, but significantly enhanced the contribution of exogenous LCFA to β-oxidation and triglyceride (TG) formation. This is the first demonstration that the primary mechanism underlying the acute metabolic effects of physiological concentration of glutamine on the heart is not anaplerosis. Additional data suggest the involvement of the HBP and increased CD36 recruitment to the plasma membrane.
2. Material and Methods
2.1. Chemicals
The sources of chemicals, biological products, and 13C-labeled substrates as well as the procedure for the dialysis of BSA fatty acid-free (BSA fraction V, Intergen) have been described previously [17–19].
2.2. Animals and heart perfusion experiments
All animal experiments were approved by the local animal care committee in compliance with the guidelines of the Canadian Council on Animal Care guidelines. Male Wistar rats (14-week-old; Charles River) were provided with food and water ad libitum. A previous publication [20] describes the procedure for heart isolation and its ex vivo perfusion in the working mode with continuous monitoring of functional parameters as well as equations to calculate myocardial oxygen consumption (µmol.min−1.gww−1), rate pressure product (mmHg.beats.min−1) as well as cardiac power and cardiac efficiency (mW.µmol O2−1.min−1.gww−1). Rat hearts were perfused under normoxia at physiological pre- and afterloads pressure with a semi-recirculating modified Krebs-Henseleit buffer containing various substrates and hormones according to five different protocols. For one set of perfusions, the buffer contained physiological concentrations of glucose (5.5 mM), lactate (1.0 mM), pyruvate (0.2 mM), oleate (0.4 mM; bound to 3% albumin), carnitine (50 μM), insulin (8 nM) and epinephrine (5 nM) to provide unlimited energy substrate supply either in the absence (control; n=19) or presence of 0.5 mM glutamine (n=19) with or without 20 µM azaserine (n=10). In the other set of perfusion, hearts were perfused in the absence of pyruvate and insulin to restrict pyruvate anaplerosis in the absence (n=15) or presence of 0.5 mM glutamine (n=11). For any given perfusion, one of the unlabeled substrates was replaced by its corresponding labeled substrate, namely [U-13C18]oleate (25–35% initial molar percent enrichment (MPE)) or [U-13C6]glucose (25–35% initial MPE). In order to further explore the anaplerotic effect of glutamine, perfusion (n=2) has been conducted with [U-13C5]glutamine (initial MPE >99%). Throughout perfusion, influent and effluent perfusates were collected to document lactate dehydrogenase release rates (every 5 min), oxygen and carbon dioxide partial pressures (at 10 and 20 min), and lactate and pyruvate efflux rates (at 30 min). Subsequent to each perfusion period, the hearts were freeze-clamped with metal tongs chilled in liquid nitrogen and weighed. All samples were stored at −80°C until further analysis.
2.2.1. Various measurements
Our previously published study [20] provides (i) definitions of the 13C terminology and detailed descriptions for measurements of CAC intermediates and relevant metabolites by gas chromatography-mass spectrometry (GCMS; Hewlett-Packard 6890N gas chromatograph coupled to a 5973N mass spectrometer), as well as (iii) equations for the calculation of: 1) flux ratios relevant to substrate selection for citrate synthesis from 13C-enrichment of the acetyl (carbons 4+5: ACCIT) and OAA (carbons 1+2+3+6; OAACIT) moiety of citrate, and 2) efflux rates of unlabeled lactate and pyruvate reflecting glycolysis from exogenous glucose (for perfusion with [U-13C6]glucose). The 13C-enrichement of oleate and total tissue fatty acids in heart tissue triglycerides (TG) were analyzed by GCMS as their methyl ester derivatives as previously described [16,17]. Tissue 13C-enrichment and concentration of glutamate, glutamine and aspartate were assessed using a modified method [21]. In brief, this involves tissue (50 mg) extraction with methanol (70%), ultrasonication (2 × 20 sec) followed by acid hydrolysis (70°C, 15 min), centrifugation (20,000 g; 10 min), supernatant evaporation and solubilization in 25 µL of pyridine (45°C, 90 min) followed by derivatization with 75 µL N-methyl-N-tert-butyldimethylsilyltrifluoroacetamide (MTBSTFA; 90°C; 4h). Samples (1 µL) were injected onto the GCMS operated under conditions similar to those described previously for CAC intermediates [22] except for the temperature program: 150°C for 0.5 min; increased at 7°C.min−1 until 210°C, kept for 3 min, increased at 7°C.min−1 until 310°C, kept for 6 min, increased at 10°C.min−1 until 320°C, and kept for 2 min. The ions sets monitored were at m/z = 431–436, 432–437 and 418–422 for glutamine, glutamate and aspartate, respectively. For quantification, tissue samples were spiked with the corresponding labeled internal standards, L-[13C5,15N2]glutamine, L-[13C5,15N]glutamic acid and L-[13C4,15N]aspartic acid, for which the following ions were monitored: 438, 438 and 423, respectively. CoA derivatives were measured as previously described by high performance liquid chromatography [23].
2.2.2. Immunoblot analysis
The abundance and electrophoretic mobility of CD36 was studied at the protein level after membrane isolation by immunoblotting as described previously (for detail regarding the protein preparation please see: [16]). Proteins were resolved on 7.5% acrylamide SDS-PAGE and then transferred onto PVDF membranes (Millipore, Billerica, MA, USA) for 2 h at 100 V and 5°C. Membranes were probed with horseradish peroxidase (HRP)-conjugated-CD36 antibody (Covance, USA) and visualized, as described previously [16]. Cadherin (Invitrogen, Burlington, ON, Canada), a transmembrane protein, served as loading control, P-ACC and ACC, AMPK and P-AMPK were purchased from Cell Signaling Technology (Danvers, MA, USA) [24].
2.3. Statistical analysis
Data are expressed as mean±SE. Statistical significance was reached at P<0.05 using a one or two-way ANOVA, followed by the Bonferroni selected-comparisons post-test.
3. Results
3.1. Functional effects of glutamine in the ex vivo working rat heart
The impact of adding a physiological concentration of glutamine (0.5 mM) was examined in hearts perfused under two conditions. First, hearts were perfused with a mixture of CHOs and LCFA (control) supplied at concentrations mimicking the in vivo milieu. Second, hearts were perfused in the absence of pyruvate and insulin, in order to restrict pyruvate anaplerosis through its carboxylation (restricted pyruvate anaplerosis) and thereby set conditions that would favor glutamine anaplerosis [9].
In Table 1 we show that under the control condition, the addition of 0.5mM glutamine had no major effects on cardiac function except for a 20% increase in -dP/dt (P<0.05). In contrast, under condition of restricted pyruvate anaplerosis, cardiac function was depressed compared to control conditions, as evidenced by a lower heart rate, rate pressure product and cardiac output; the addition of glutamine normalized these parameters. These data demonstrate that the effect of glutamine on cardiac function is dependent on substrate availability and has minimal effect on contractile function in hearts provided with physiologically relevant substrates.
Table 1.
Effect of 0.5 mM glutamine on functional parameters of rat hearts perfused ex vivo in working mode under conditions of unlimited substrate supply or restricted pyruvate anaplerosis
| Conditions | Unlimited substrate supply | Restricted pyruvate anaplerosis | ||
|---|---|---|---|---|
| Addition/ Parameters |
None | Glutamine | None | Glutamine |
| Heart rate (beat.min−1) |
306±6 | 302±8 | 266±9*** | 290±8 |
| max-Pressure (mmHg) |
132±3 | 141±3 | 134±3 | 133±4 |
| min-Pressure (mmHg) |
−3,2±1.3 | −6,1±15 | −2,2±1.2 | −2,1±0.9 |
| LDVP (mmHg) |
134±4 | 147±5 | 134±4 | 135±5 |
| +dP/dt (mmHg.s−1) |
5710±258 | 6024±205 | 6129±213 | 5928±216 |
| −dP/dt (mmHg.s−1) |
3758±241 | 4416±195* | 3855±175 | 3917±251 |
| Rate pressure product (mmHg.beats−1.min−1) |
38728±1476 | 40780±1223 | 34430±1584* | 38105±1532 |
| Coronary flow (ml.min−1) |
22.1±0.9 | 21.3±0.9 | 18.2±0.7** | 20.8±0.2 |
| Cardiac output (ml.min−1) |
58.1±2.3 | 57.7±2.2 | 43.4±3.0*** | 51.4±3.1 |
| Stroke volume (ml.beat−1) |
0.208±0.011 | 0.209±0.009 | 0.161±0.008** | 0.163±0.011**$$ |
| Cardiac power (mWatts) |
16.0±0.9 | 16.9±0.8 | 17.30±0.6 | 15.80±0.6 |
| MVO2 (µmol.min−1) |
22.5±1.8 | 25.4±2.6 | 22.7±0.8 | 24.4±1.8 |
Values are mean ± SEM of those assessed between 25-30 min of perfusion for 10-19 hearts.
P<0.05,
P<0.01,
P<0.001 vs. control,
P<0.01 vs. glutamine.
LDVP = left ventricular developed pressure.
3.2. Metabolic effects of glutamine in the ex vivo working heart: Role of anaplerosis
The cardiac effects of glutamine are typically attributed to it metabolism via anaplerotic pathways; therefore, we assessed the effects of glutamine on cardiac anaplerosis, by determining tissue levels and 13C-enrichment of CAC intermediates and related metabolites (Tables 2 and 3) in working hearts perfused under conditions of unlimited substrate supply (control) and under restricted pyruvate anaplerosis. There was no significant difference in glutamine concentrations between control and the restricted pyruvate anaplerosis groups, and the addition of exogenous glutamine significantly increased tissue glutamine levels by almost 2-fold in both groups. This is similar to previous reports in the perfused rat heart [25] and suggest that there is a rapid turnover of glutamine. It is noteworthy that, in contrast to glutamine, the tissue level of glutamate, its primary metabolite, as well as aspartate, was relatively unaffected by the addition of glutamine and similar between in the two perfusion groups. As anticipated perfusion under restricted pyruvate anaplerosis resulted in significantly lower tissue levels of pyruvate, citrate and malate; whereas, acetyl-CoA or CoA levels were not different. Although glutamine addition did increase significantly tissue level of succinyl-CoA by 2 to 4 nmol/g heart tissue, this intermediate represents only 1% of the total CAC intermediate pool. Interestingly, however, irrespective of substrate supply, the addition of glutamine did not increase total tissue level of CAC intermediates; in fact, in hearts perfused under the control condition, the addition of glutamine paradoxically resulted in a decrease in total CAC intermediate pool by 15% (P<0.05). This is contrary to what would be anticipated if there was a substantial metabolic flux of glutamine via anaplerotic pathways.
Table 2.
Effect of glutamine addition on tissue levels of CAC intermediates and related metabolites in working hearts perfused under conditions of unlimited substrate supply or restricted pyruvate anaplerosis.
| Conditions | Unlimited substrate supply | Restricted pyruvate anaplerosis | ||
|---|---|---|---|---|
| Addition/Metabolites | None | Glutamine | None | Glutamine |
| Glutamine | 1665±112 | 3480±287*** | 2019±195 | 3020±250++ |
| Glutamate | 2855±208 | 3057±166 | 3447±80** | 3016±67 |
| Aspartate | 587±13 | 516±19 | 588±37 | 587±34 |
| Lactate | 820±59 | 676±36 | 596±7 | 690±38 |
| Pyruvate | 137±16 | 147±6 | 63±3*** | 61±3$$$ |
| Acetyl-CoA | 1.84±0.14 | 2.41±0.31 | 1.56±0.22 | 2.35±0.38 |
| CoA | 63.6±3.2 | 56.0±4.2 | 63.0±3.5 | 66.6±8.8 |
| Citrate | 260±10 | 218±6* | 195±9*** | 177±18* |
| α-ketoglutarate | 31±3 | 43±3 | 30±3 | 36±5 |
| Succinyl-CoA | 6.97±0.36 | 9.28±0.70* | 4.21±0.38 | 9.40±0.81+++ |
| Succinate | 94±4 | 75±7 | 85±9 | 89±8 |
| Fumarate | 21±1 | 18±1 | 19±1 | 18±1 |
| Malate | 128±5 | 102±3*** | 104±4*** | 101±3 |
| Total | 537±17 | 465±11* | 439±14** | 430±30** |
Values, expressed in nmol per gram wet weight, are mean ± SEM of 6 – 9 perfused hearts.
P<0.05,
P<0.01,
P<0.001 vs. control,
P<0.05,
P<0.001 vs. glutamine
P<0.01,
P<0.001 vs. restricted pyruvate anaplerosis.
Table 3.
Molar percent enrichment (MPE) of glutamine, glutamate and CAC intermediates in isolated working heart perfused with [U-13C]glutamine under control condition.
| Metabolites /MPE |
M+1 | M+2 | M+3 | M+4 | M+5 |
|---|---|---|---|---|---|
| Glutamine | n.d. | n.d. | 0.6 | 3.6 | 47.5 |
| Glutamate | 0.5 | 0.2 | 0.4 | 0.1 | 2.6 |
| α-Ketoglutarate | 0.1 | 0.3 | 0.5 | 0.6 | 1.3 |
Values are means of 2 heart perfusion experiments. MPE values for other CAC intermediates were <1.1%.
To better assess the potential anaplerotic metabolism of glutamine, hearts were perfused with [U-13C5]glutamine (MPE=100%) and the MPE of tissue glutamine, glutamate and CAC intermediates determined. As expected, tissue glutamine was exclusively enriched in M+5 isotopomers; however, while tissue glutamine MPE was 47.5% that of glutamate and of α-ketoglutarate were only 2.6% and 1.3%, respectively, and other CAC intermediates were close to the detection limit (Table 3). Conversely, hearts perfused under all conditions with [U-13C6]glucose (MPE: 25–35%; Figure 1) or [U-13C18]oleate (MPE=25–35%; data not shown) resulted in substantial 13C-labeling of citrate, α-ketoglutarate and glutamate; whereas that of tissue glutamine was close to detection level. Of note, the isotopic dilution between α-ketoglutarate and glutamate was marginal thereby supporting the notion of a rapid equilibrium between these two metabolites and negligible entry of unlabeled substrate at this level.
Figure 1. 13C-labeling of tissue citrate, α-ketoglutarate, glutamate and glutamine from [U-13C6]glucose in working rat heart perfused ex vivo under conditions of unlimited substrate supply or restricted pyruvate ansplerosis.
Data are means ± SEM of 5-11 heart perfusion experiments conducted under conditions of unlimited (A, B) or restricted energy substrate supply (C, D) in the absence (A, C) or presence (B, D) of 0.5 glutamine. Freeze-clamped hearts were processed for the analysis of 13Clabeling of metabolites by GCMS.
Taken together, these results provide little or no evidence to support metabolism of glutamine via anaplerosis in the normoxic healthy working heart regardless of substrate availability. These data also suggest while there appears to be rapid turnover of glutamine in the heart, there appears to be little direct crosstalk between exogenous glutamine and the predominant intracellular pool of glutamate.
3.3. Metabolic effects of glutamine in the working heart: Energy substrate selection and storage
Our previous studies have shown that glucosamine, which enters the HBP downstream of the regulatory GFAT reaction and enhances protein O-GlcNAcylation, resulted in increased exogenous LCFA oxidation which appears to be mediated by increased plasma membrane levels of the LCFA transporter CD36 [15–16]. We postulated therefore, that glutamine, which is required for GFAT activity and is essential for glucose metabolism via the HBP, might have a similar effect. As shown in Fig. 2, addition of glutamine to hearts perfused under the control condition (Fig. 2A–F; left panel) significantly increased the contribution of exogenous oleate to both β-oxidation (~50%; Fig. 2A)) and TG formation (>3-fold; Fig. 2B), which is consistent with the previously reported effects of glucosamine. In contrast, glutamine had no significant effect on (i) glycolysis, as reflected by the contribution of exogenous glucose to pyruvate formation (Fig. 2C) or lactate and pyruvate production rates (Fig. 2D), or on (ii) the exogenous glucose contribution to oxidation (via pyruvate decarboxylation to acetyl-CoA): Fig. 2E); however, it decreased significantly pyruvate metabolism via anaplerosis (via pyruvate carboxylation to oxaloacetate) by 3-fold. (Fig. 2F).
Figure 2. Effect of glutamine on substrate fluxes relevant to energy metabolism assessed in working rat heart perfused ex vivo under conditions of unlimited substrate supply or restricted pyruvate anaplerosis.
The Experimental Procedures section provides details on the determination of flux ratios, which reflect the contributions of exogenous fatty acid (oleate) to acetyl-CoA (oxidation; A) and triglycerides (storage; B) as well as glucose to pyruvate (C) and acetyl-CoA (oxidation; E) and/or OAA (anaplerosis; F) formation, which are expressed relative to citrate synthesis (CS), and finally, glycolysis (D). Data are means ± SEM of 5-11 heart perfusion experiments conducted under conditions of unlimited energy susbtrate supply (control; white bars) or restricted pyruvate anaplerosis (hatched bars) in the absence or presence (grey bars) of 0.5 mM glutamine (Gln). Statistics: *P<0.05, ***P<0.001 vs. control.
The situation differed, however, in hearts perfused under condition of restricted pyruvate anaplerosis (Fig. 2A–F; right panel). Firstly, compared to their control heart counterparts perfused without glutamine, these hearts displayed a significantly increased contribution of exogenous (i) oleate to β-oxidation, albeit not to TG formation, and (ii) glucose to pyruvate, albeit not to acetyl-A (oxidation). These flux ratios were not significantly modified by glutamine addition.
In summary, our results substantiate for the first time that similar to glucosamine, physiological concentration of glutamine can modulate LCFA metabolism in the healthy normoxic working heart perfused under conditions of unlimited substrate supply.
3.4. Mechanisms underlying the metabolic effects of glutamine on LCFA metabolism
Since our initial experiments indicated that glutamine was not metabolized by anaplerotic pathways, we examined the role of HBP by perfusing hearts under control condition with and without azaserine. Azaserine is a structural analogue of glutamine that competes with glutamine in binding to its metabolizing enzymes, including GFAT, which controls glucose entry into the HBP. We have previously reported that in the perfused heart, azaserine is effective in attenuating glutamine metabolism via its inhibition of GFAT [5]. In these perfusions, we assessed all functional and metabolic parameters reported in Tables 1 and 2 as well as Figure 2. For simplicity, Table 4 reports only functional and metabolic parameters that were found to be significantly affected by the addition of azaserine when compared to hearts perfused under the control condition in the absence or presence of glutamine. In brief, we found that in hearts perfused under control condition with glutamine, the addition of azaserine resulted in a significant decrease in cardiac function of ~20%; however, function was stable throughout the perfusion period. At the metabolic level, azaserine reversed the effects of glutamine on both exogenous oleate β-oxidation and TG formation, but had no effect on the pyruvate anaplerotic flux ratio, or any other parameters measured.
Table 4.
Effect of azaserine on the functional and metabolic effects of glutamine in working hearts perfused under control condition.
| Conditions | Effects (% control) | |
|---|---|---|
| Addition/Measured parameters | GLN | GLN + AZA |
| A) Functional parameters (n=10–19) | ||
| Heart rate | − 1% | − 14%* |
| RPP | + 5% | − 10%* |
| Stroke volume | − 1% | − 22%*$ |
| B) Metabolic flux parameters (n=6–9) | ||
| Oleate to acetyl-CoA via β-oxidation (%) | + 53%* | + 18% |
| Oleate to triglycerides (%) | + 186%** | + 42%*$ |
Data are means ± SEM of 10 – 19 heart perfusion experiments. They are expressed relative to values obtained in hearts perfused under control conditions in the absence of glutamine (gln), which are reported in Tables 1 and 2, as well as Figure 2. This Table reports only functional and metabolic parameters that were significantly modified by the addition of azaserine (AZA).
P<0.05,
P<0.01 vs. control,
P<0.05,
P<0.01 vs. Glutamine (GLN).
To examine in more detail the potential mechanism by which glutamine increases both exogenous LCFA oxidation and esterification to TGs, we first assessed the levels of malonyl-CoA, a recognized potent mediator of LCFA β-oxidation via carnitine palmitoyl transferase I [24] as well as the ratio of phosphorylated-to-total protein level for acetyl-CoA carboxylase and AMPK, which reflect the activity of enzymes involved in malonyl-CoA synthesis and regulation, respectively. AMPK is also known to play a crucial role in the translocation of the LCFA transporter CD36 from endogenous stores to the plasma membrane, which is an essential step in enhancing exogenous LCFA uptake [26]. As shown in Fig 3, glutamine had no effect on any of these parameters; however, azaserine increased malonyl-CoA tissue levels, which may be a consequence of the activation of AMPK and subsequent inhibition of acetyl-CoA carboxylase.
Figure 3. Effect of glutamine on mechanisms regulating β-oxidation in ex vivo working hearts.
Tissue levels of malonyl-CoA, assessed by HPLC (A). Representative immunoblots and densitometry of phosphorylated-to total acetyl-CoA carboxylase (P-ACC/ACC; B) and AMP kinase (P-AMPK/AMPK; C). Data are means ± SEM of 4–5 heart perfused under control (Crl) conditions in the absence or presence of 0.5 mM glutamine (Gln) without or with 20 µM azaserine (Aza). Statistics: *P<0.05, vs. control.
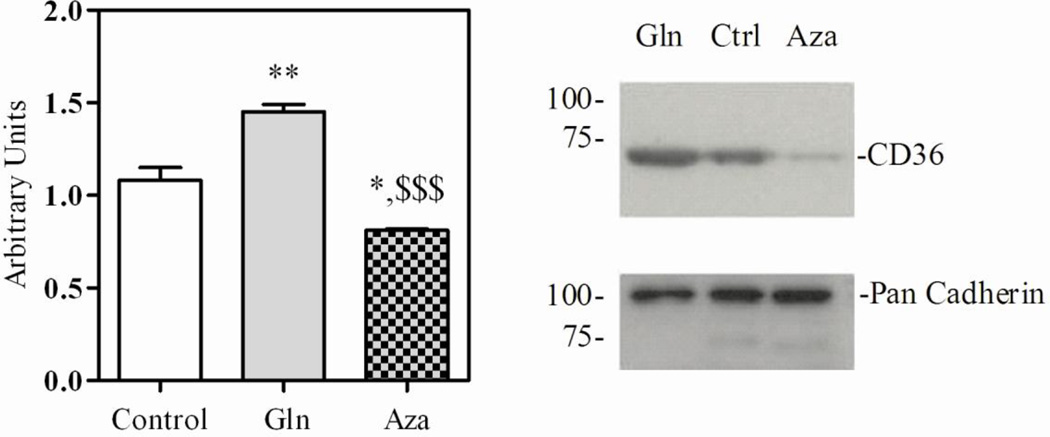
In the light of our previous finding that glucosamine enhanced LCFA oxidation associated with an increase in membrane CD36 levels CD36 [15,16], we examined whether glutamine may have a similar effect. This was assessed using immunoblot analysis of plasma membranes prepared from ex vivo working hearts perfused under control condition in the absence of glutamine and in the presence of glutamine with or without azaserine. Figure 4 demonstrates a greater recruitment of CD36 to the plasma membrane in the presence of glutamine (1.08±0.07 vs. 1.45±0.04, P<0.01), an effect that is completely reversed by addition of azaserine (0.81±0.01, P<0.001 vs. glutamine). With glucosamine treatment, we showed that this was associated with an increase O-GlcNAc modification of total proteins and of CD36 [15–16]. However, in this study, using previously described immunoprecipitation and immunoblotting techniques [15], while we were able to confirm that CD36 is O-GlcNAcylated, we were unable to reliably demonstrate any effect of glutamine on the level of CD36 O-GlcNAcylation (data not shown).
Figure 4. CD36 immunoreactivity.
Representative CD36 immunoblots and densitometry of membranes isolated from hearts perfused under control conditions in the absence or presence of 0.5 mM glutamine (Gln) with or without azaserine (Aza). Data are means ± SEM of 3–4 heart perfusion experiments. Statistics: *P<0.05, **P<0.01 vs. control, $$P<0.01 vs. glutamine.
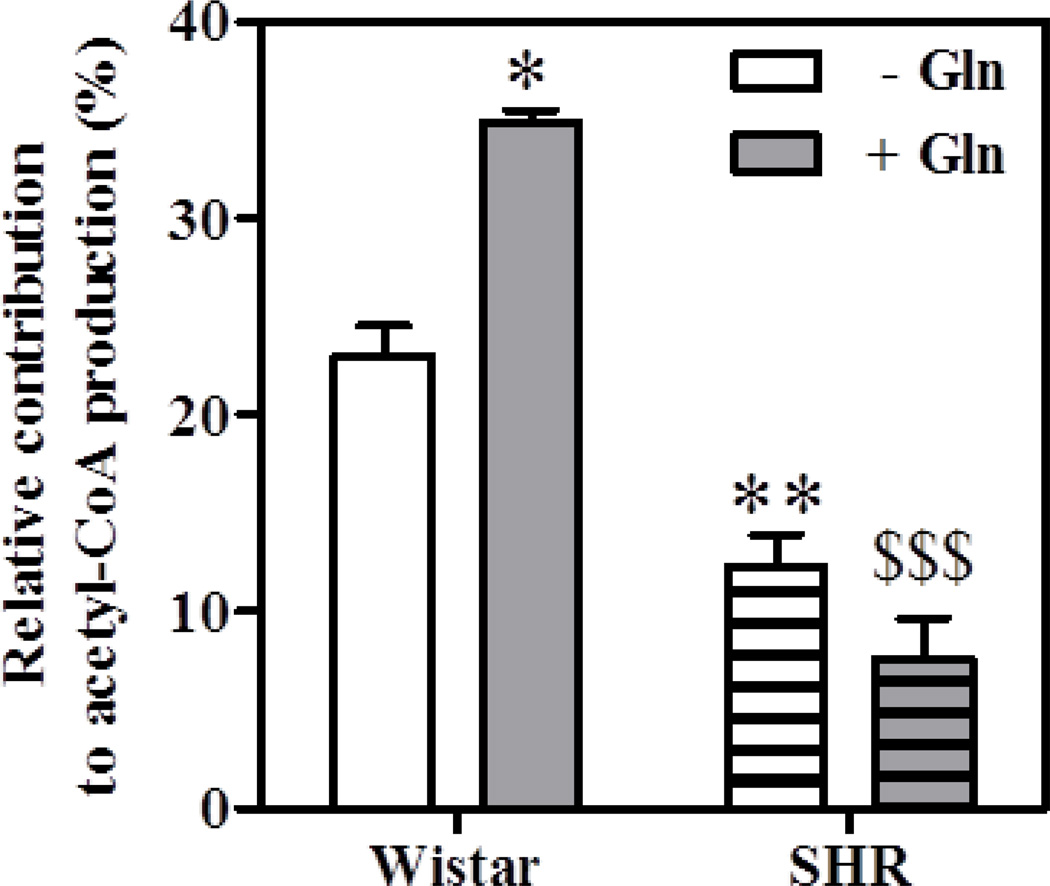
Therefore, in order to provide additional support for the involvement of CD36, we compared the contribution of exogenous oleate to β-oxidation in hearts from Wistar rats to that of spontaneously hypertensive rats (SHR) that carry a CD36 gene defect resulting in decrease post-translational modification by N-glycosyation. Concurring with the previously reported effect of glucosamine, glutamine increased oleate oxidation in hearts from Wistar rats, but had no effect on β-oxidation in SHR hearts (Figure 5).
Figure 5. Effect of glutamine on exogenous oleate β-oxidation in ex vivo working hearts from Wistar and spontaneously hypertensive (SHR) rats.
Flux values were calculated as described in Figure 2. Data are means ± SEM of 3–4 heart perfusion experiments conducted under control condition in the absence or presence of 0.5 mM glutamine. Statistics: *p<0.05 and **P<0.01 vs. Wistar without glutamine (−Gln), $$$P<0.01 vs. Wistar plus glutamine (+ Gln).
Taken altogether, these results support the notion that the effects of physiological concentration of glutamine on exogenous LCFA metabolism involve CD36 and the HBP but not the malonyl-CoA or AMPK pathway. However, while collectively these data with glutamine supports our earlier results with glucosamine, the mechanism by which glutamine increases plasma membrane levels of CD36 remains to be ascertained.
4. Discussion
This study demonstrates for the first time that in the healthy perfused heart a physiological concentration (0.5 mM) of glutamine has a marginal anaplerotic potential, regardless of substrate availability. Interestingly, however, we found that in hearts perfused with physiological concentration of CHO and a LCFA, glutamine increased exogenous LCFA contribution to β-oxidation and TG formation as well as plasma membrane CD36 recruitment. These metabolic effects of glutamine were reversed by azaserine, a structural analogue of glutamine that competes with glutamine in binding to its metabolizing enzymes, including GFAT, which regulates HBP flux. The effects of glutamine are similar to those previously reported with glucosamine, which directly enters the HBP flux downstream of GFAT. Therefore, results from this study support the notion that that the predominant acute metabolic effects of glutamine in the normoxic healthy heart are not mediated by anaplerosis but mostly likely via the HBP. This suggests that the acute modulation of the HBP by glutamine represents a previously unrecognized mechanism regulating cardiac energy substrate metabolism.
4.1. Glutamine has a marginal anaplerotic potential in the ex vivo working heart
Anaplerosis has frequently been assumed to underlie glutamine’s cardioprotection reported in ex vivo heart models of hypoxia or ischemia [3–6] perfused with glucose as the sole substrate. However, the anaplerotic role of glutamine was not supported by existing evidence [27]. To the best of our knowledge, this is the first study to assess and quantify the anaplerotic potential of glutamine in hearts perfused under normoxia with a mixture of substrates, CHOs and a LCFA, at concentrations mimicking the in situ milieu. Hearts were also perfused in the absence of pyruvate and insulin to restrict pyruvate carboxylation, a recognized anaplerotic pathway of the normoxic heart [9]. The latter condition is similar to providing glucose and a fatty acid as the only exogenous substrates, which is commonly used in studies of the perfused heart.
As we have discussed in detail elsewhere, the quantification of anaplerosis in the intact heart can be challenging due to the complexity of the metabolic networks involved (see for review: Des Rosiers et al., [9]). Furthermore, anaplerotic substrates can label CAC intermediates without net anaplerotic flux as a result of label recycling due to metabolism via the CAC, as well as by exchange reactions between CAC intermediates and other metabolites such as aspartate and glutamate. For this reason, we quantified tissue levels and 13C-enrichment of CAC intermediates and related metabolites in hearts perfused with [U-13C]labeled glucose, oleate and glutamine.
Under our conditions, the addition of glutamine to the perfusate almost doubled its tissue level, in contrast to that of glutamate and aspartate, which remained stable. These results extend the observations of Rennie et al. [28], who reported stable concentrations of different metabolites, but not glutamine, in the post-ischemic heart perfused ex vivo. Together, they emphasize the crucial role of exogenous glutamine in maintaining its myocardial tissue level. In this regard, our finding of a ~16-fold lower enrichment of glutamine than glutamate (0.5 vs. 8%, respectively) in hearts perfused with [U-13C]glucose, support with the notion that glutamine synthesis from glutamate is relatively unimportant in the heart under normal conditions [29–30].
Despite the aforementioned increase in tissue glutamine level, anaplerosis from glutamine appears to be marginal as evidenced by the quantification of CAC intermediate tissue levels, as well as their 13C-labeling from [U-13C5]glutamine. In fact, paradoxically, addition of glutamine seemed to decrease total tissue CAC intermediates levels, particularly citrate and malate, which would suggest an inhibitory effect on another anaplerotic reaction, specifically pyruvate carboxylation. This effect of glutamine was not modulated by azaserine, nor was it observed in hearts perfused under conditions of restricted pyruvate anaplerosis, thereby suggesting that it is not mediated by the HBP but modulated by pyruvate availability, respectively. Glutamine addition did not reduce pyruvate level as assessed in whole heart homogenates; but we cannot exclude the possibility that pyruvate availability was restricted in a mitochondrial subpool accessible to pyruvate carboxylase [31–32]. In contrast, under both conditions, glutamine increased the tissue level of succinyl-CoA, a site of anaplerotic entry from the catabolism of branched amino acids valine and isoleucine; however, it is unclear at this time how glutamine could influence this particular anaplerotic pathway.
4.2. Glutamine modulates LCFA metabolism in the ex vivo working heart
In contrast to its marginal effects on anaplerosis, the addition of glutamine markedly affected metabolic fluxes relevant to energy substrate metabolism; specifically, it increased the contribution of exogenous oleate to both β-oxidation (from 25 to 40%) and TG formation (3-fold) under the control condition. Since the contribution of exogenous glucose to acetyl-CoA via oxidation was unchanged (20%) that of other sources of acetyl-CoA was decreased from 56% to 40%. Most likely these sources are exogenous unlabeled lactate and pyruvate, which were added to the perfusion buffer at 1 and 0.2 mM, respectively. In support of this interpretation, we previously reported that addition of glucosamine to perfused rat hearts resulted in similar changes in oxidation of exogenous palmitate (increased), glucose (unchanged) and lactate plus pyruvate (decreased) [15].
Several other effects of glutamine reported in this study are also consistent with our earlier studies using glucosamine [15–16]. Firstly, the effects of glutamine on LCFA metabolism could not be explained by changes in the tissue level or activity of metabolites and enzymes known to be involved in the regulation of the β-oxidation pathway, namely malonyl-CoA, acetyl-CoA carboxylase or AMPK [24]. Secondly, glutamine enhanced the recruitment of the LCFA transporter CD36 to the plasma membrane and was not able to increase exogenous LCFA β-oxidation in the SHR, which harbors a mutant CD36 gene [15,16]. Finally, the effects of glutamine on LCFA β-oxidation and esterification to TG as well as on membrane CD36 recruitment were reversed by the addition of azaserine, thereby suggesting the involvement of the HBP in mediating the effects of glutamine. Indeed, azaserine has been used to attenuate HBP flux via its inhibition of GFAT. However, it should be noted that azaserine is not specific for GFAT and it was found to increase tissue levels of malonyl-CoA and phosphorylated-to-total AMPK. Therefore, we cannot entirely rule out the possibility that some of the effects of azaserine could be independent of the HBP.
4.3 Study limitations and future directions
It should be noted that we did not directly assess fatty acid uptake in these studies; however, the fact that glutamine increased both exogenous oleate oxidation and TG synthesis indicates that the uptake of this LCFA must also have increased. Also, we did not assess CD36 levels in endosomal stores, from which CD36 had to be mobilized to be recruited at the plasma membrane [26]; however, it appears unlikely that its level differed between hearts at the beginning of the perfusion. Most likely, the presence of glutamine during heart perfusion promoted CD36 recruitment at the plasma membrane. Our previous studies with glucosamine supported the importance of CD36 O-GlcNAcylation in this process [15–16]. In this study, the fact that azaserine reverses glutamine effects on LCFA metabolism as well as on CD36 membrane levels strongly supports a role for the HBP in this process, albeit we were unable to provide evidence for increased membrane CD36 O-GlcNAcylation with glutamine. However, since glucosamine bypasses GFAT, the regulatory and rate limiting step of the HBP, its potential for increasing protein O-GlcNAc levels is likely much greater than glutamine. It is also possible that other proteins involved in CD36 trafficking rather than CD36 itself are targets for O-GlcNAcylation. Interestingly, in this regard, activation of FoxO1, a target of O-GlcNAcylation [33], has been reported to increase membrane CD36 recruitment, FA oxidation and TG synthesis in muscle cells [34], effects that resembled those reported herein for glutamine. Clearly, there is a complex interplay of signaling pathways regulating CD36 trafficking, which remain to be better understood. Additional studies appear warranted to clarify the role of HBP in regulating CD36 trafficking since this may represent one of on a few pathways that may differentially modulate CD36 (increased) vs. GLUT4 (decreased) recruitment [26].
It is also important to emphasize how little is known about the regulation of the HBP flux in the heart. This is due in part to the fact that the HBP flux has never been directly quantified. It is generally assumed to represent about 2 to 4% of the glucose that is transported into the cell [14]. Based on the glycolytic rate assessed in working hearts perfused with exogenous [13C6]glucose (~1 µmol/min/gww), the HBP flux would be estimated to be at most 0.02-0.04 µmol/min/gww. This value concurs with the absolute flux from glutamine to glutamate (~0.06 µmol.min−1.gww−1 in this study) estimated from: (i) the relative contribution of glutamine to glutamate formation (~5%: from the MPE M+5 ratio of tissue glutamate-to-glutamine: 2.5/47.5%) and (ii) assuming that the remaining 95% of glutamate formation occurs from α-ketoglutarate, which in the rat heart is estimated to be equal to the CAC flux rate (i.e., ~1.2 µmol.min−1.gww−1) [29].
Clearly in the heart, much remains to be learned about glutamine metabolism under various physiological and pathological conditions, as well as how this metabolism impacts on cell signaling pathways such as that involving protein O-GlcNAcylation [13,14], but also protein kinase A and mTOR signaling [35]. Importantly many other factors appear to modulate cardiac glutamine metabolism including the thyroid status [36], glucocorticoids [37] and cMyc expression [38]. Nevertheless, it is interesting to note that enzymes involved in glucose metabolism, glutamine synthesis and the HBP all display similar circadian rhythms, peaking in the heart during the active/awake phase, thereby suggesting a coordinated regulation of all these pathways [39].
In conclusion, results from this study demonstrate that the addition of physiological concentration of glutamine to working hearts perfused ex vivo modulates energy substrate metabolism by both HBP-independent and -dependent pathways. Specifically, while glutamine has a marginal potential for anaplerosis, it nevertheless inhibits anaplerotic pyruvate carboxylation possibily by decreasing pyruvate availability. However, the predominant metabolic effect of glutamine in the normoxic heart is to increase exogenous LCFA oxidation and storage, an effect that appears to involve the HBP and enhanced CD36 recruitment to the plasma membrane. Therefore these findings help to further substantiate conclusions from our earlier studies [15,16] that glucose metabolism via the HBP represents a new mechanism for the acute regulation of cardiac energy metabolism.
Highlights.
Physiological concentration of glutamine has a marginal anaplerotic potential in the healthy heart
The primary effect of glutamine in the heart is to increase exogenous fatty acid oxidation and storage
Glutamine modulates fatty acid metabolism via CD36 and the hexosamine biosynthetic pathway (HBP)
The HBP represents a new mechanism for the acute regulation of cardiac energy metabolism.
Acknowledgments
We thank Caroline Daneault and Isabelle Robillard-Frayne for technical assistance as well as France Thériault for secretarial assistance.
Funding
This study was supported by the Canadian Institutes of Health Research (CIHR Grants # 9575 to C.D.R.) and by “Fondation Bettencourt Schueller”, HSFC, FRSQ (B.L.) and the National Heart, Lung, and Blood Institute (HL101192 to J.C.C.).
ABBREVIATIONS
- AMPK
AMP-activated protein kinase
- CAC
citric acid cycle
- CHO
carbohydrate
- GCMS
Gas chromatography-mass spectrometry
- GFAT
glutamine:fructose 6-phosphate amidotransferase
- HBP
hexosamine biosynthetic pathway
- LCFA
long-chain fatty acid
- MPE
molar percent enrichment
- TG
triglyceride
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
The present work was presented in part at meetings of (i) the Society for Heart and Vascular Metabolism in Kananaskis, Alberta, Canada, in 2010 and in Brussels, Belgium, in 2011, (ii) the Experimental Biology meeting in Washington, DC, USA, in 2011, and (iii) the European Society of Cardiology in Paris, France, in 2011.
References
- 1.Wu G. Amino acids: metabolism, functions, and nutrition. Amino Acids. 2009;37:1–17. doi: 10.1007/s00726-009-0269-0. [DOI] [PubMed] [Google Scholar]
- 2.Curi R, Newsholme P, Procopio J, Lagranha C, Gorjao R, Pithon-Curi TC. Glutamine, gene expression, and cell function. Front Biosci. 2007;12:344–357. doi: 10.2741/2068. [DOI] [PubMed] [Google Scholar]
- 3.Khogali SE, Harper AA, Lyall JA, Rennie MJ. Effects of L-glutamine on post-ischaemic cardiac function: protection and rescue. J Mol Cell Cardiol. 1998;30:819–827. doi: 10.1006/jmcc.1998.0647. [DOI] [PubMed] [Google Scholar]
- 4.Khogali SE, Pringle SD, Weryk BV, Rennie MJ. Is glutamine beneficial in ischemic heart disease? Nutrition. 2002;18:123–126. doi: 10.1016/s0899-9007(01)00768-7. [DOI] [PubMed] [Google Scholar]
- 5.Liu J, Marchase RB, Chatham JC. Glutamine-induced protection of isolated rat heart from ischemia/reperfusion injury is mediated via the hexosamine biosynthesis pathway and increased protein O-GlcNAc levels. J Mol Cell Cardiol. 2007;42:177–185. doi: 10.1016/j.yjmcc.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wischmeyer PE, Van den Hoek TL, Li C, Shao Z, Ren H, Riehm J, et al. Glutamine preserves cardiomyocyte viability and enhances recovery of contractile function after ischemia-reperfusion injury. JPEN J Parenter Enteral Nutr. 2003;27:116–122. doi: 10.1177/0148607103027002116. [DOI] [PubMed] [Google Scholar]
- 7.Brunengraber H, Roe CR. Anaplerotic molecules: current and future. J Inherit Metab Dis. 2006;29:327–331. doi: 10.1007/s10545-006-0320-1. [DOI] [PubMed] [Google Scholar]
- 8.Owen OE, Kalhan SC, Hanson RW. The key role of anaplerosis and cataplerosis for citric acid cycle function. J Biol Chem. 2002;277:30409–30412. doi: 10.1074/jbc.R200006200. [DOI] [PubMed] [Google Scholar]
- 9.Des Rosiers C, Labarthe F, Lloyd SG, Chatham JC. Cardiac anaplerosis in health and disease: food for thought. Cardiovasc Res. 2011;90:210–219. doi: 10.1093/cvr/cvr055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Comte B, Vincent G, Bouchard B, Benderdour M, Des Rosiers C. Reverse flux through cardiac NADP(+)-isocitrate dehydrogenase under normoxia and ischemia. Am J Physiol Heart Circ Physiol. 2002;283:H1505–H1514. doi: 10.1152/ajpheart.00287.2002. [DOI] [PubMed] [Google Scholar]
- 11.Akimoto Y, Hart GW, Hirano H, Kawakami H. O-GlcNAc modification of nucleocytoplasmic proteins and diabetes. Med Mol Morphol. 2005;38:84–91. doi: 10.1007/s00795-004-0264-1. [DOI] [PubMed] [Google Scholar]
- 12.Marsh SA, Dell’Italia LJ, Chatham JC. Activation of the hexosamine biosynthesis pathway and protein O-GlcNAcylation modulate hypertrophic and cell signaling pathways in cardiomyocytes from diabetic mice. Amino Acids. 2011;40:819–828. doi: 10.1007/s00726-010-0699-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chatham JC, Not LG, Fulop N, Marchase RB. Hexosamine biosynthesis and protein O-glycosylation: the first line of defense against stress, ischemia, and trauma. Shock. 2008;29:431–440. doi: 10.1097/shk.0b013e3181598bad. [DOI] [PubMed] [Google Scholar]
- 14.Fulop N, Marchase RB, Chatham JC. Role of protein O-linked N-acetyl-glucosamine in mediating cell function and survival in the cardiovascular system. Cardiovasc Res. 2007;73:288–297. doi: 10.1016/j.cardiores.2006.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laczy B, Fulop N, Onay-Besikci A, Des Rosiers C, Chatham JC. Acute regulation of cardiac metabolism by the hexosamine biosynthesis pathway and protein O-GlcNAcylation. PLoS One. 2011;6:e18417. doi: 10.1371/journal.pone.0018417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lauzier B, Merlen C, Vaillant F, McDuff J, Bouchard B, Beguin PC, et al. Post-translational modifications, a key process in CD36 function: lessons from the spontaneously hypertensive rat heart. J Mol Cell Cardiol. 2011;51:99–108. doi: 10.1016/j.yjmcc.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Gelinas R, Thompson-Legault J, Bouchard B, Daneault C, Mansour A, Gillis MA, et al. Prolonged QT interval and lipid alterations beyond beta-oxidation in very long-chain acyl-CoA dehydrogenase null mouse hearts. Am J Physiol Heart Circ Physiol. 2011;301:H813–H823. doi: 10.1152/ajpheart.01275.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khairallah RJ, Khairallah M, Gelinas R, Bouchard B, Young ME, Allen BG, et al. Cyclic GMP signaling in cardiomyocytes modulates fatty acid trafficking and prevents triglyceride accumulation. J Mol Cell Cardiol. 2008;45:230–239. doi: 10.1016/j.yjmcc.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lauzier B, Vaillant F, Gelinas R, Bouchard B, Brownsey R, Thorin E, et al. Ivabradine reduces heart rate while preserving metabolic fluxes and energy status of healthy normoxic working hearts. Am J Physiol Heart Circ Physiol. 2011;300:H845–H852. doi: 10.1152/ajpheart.01034.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vincent G, Bouchard B, Khairallah M, Des Rosiers C. Differential modulation of citrate synthesis and release by fatty acids in perfused working rat hearts. Am J Physiol Heart Circ Physiol. 2004;286:H257–H266. doi: 10.1152/ajpheart.00717.2003. [DOI] [PubMed] [Google Scholar]
- 21.Jiye A, Trygg J, Gullberg J, Johansson A, Jonsson P, Antti H, et al. Extraction and GC/MS analysis of the human blood plasma metabolome. Anal Chem. 2005;77:8086–8094. doi: 10.1021/ac051211v. [DOI] [PubMed] [Google Scholar]
- 22.Comte B, Vincent G, Bouchard B, Jette M, Cordeau S, Rosiers CD. A 13C mass isotopomer study of anaplerotic pyruvate carboxylation in perfused rat hearts. J Biol Chem. 1997;272:26125–26131. doi: 10.1074/jbc.272.42.26125. [DOI] [PubMed] [Google Scholar]
- 23.Dyck JR, Barr AJ, Barr RL, Kolattukudy PE, Lopaschuk GD. Characterization of cardiac malonyl-CoA decarboxylase and its putative role in regulating fatty acid oxidation. Am J Physiol. 1998;275(6 Pt 2):H2122–H2129. doi: 10.1152/ajpheart.1998.275.6.H2122. [DOI] [PubMed] [Google Scholar]
- 24.Cuthbert KD, Dyck JR. Malonyl-CoA decarboxylase is a major regulator of myocardial fatty acid oxidation. Curr Hypertens Rep. 2005;7:407–411. doi: 10.1007/s11906-005-0034-z. [DOI] [PubMed] [Google Scholar]
- 25.Chatham JC, Forder JR. Glutamate and glutamine exchange in the isolated perfused heart. FASEB J. 2001;15:A1142. [Google Scholar]
- 26.Steinbusch LKM, Schwenk RW, Ouwens DM, Diamant M, Glatz JFC, Luiken JJFP. Subcellular trafficking of the substrate transporters GLUT4 and CD36 in cardiomyocytes. Cell. Mol. Life Sci. 2011;68:2525–2538. doi: 10.1007/s00018-011-0690-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen DM, Guthrie PH, Gao X, Sakai R, Taegtmeyer H. Glutamine cycling in isolated working rat heart. Am J Physiol Endocrinol Metab. 2003;285:E1312–E1316. doi: 10.1152/ajpendo.00539.2002. [DOI] [PubMed] [Google Scholar]
- 28.Rennie MJ, Bowtell JL, Bruce M, Khogali SE. Interaction between glutamine availability and metabolism of glycogen, tricarboxylic acid cycle intermediates and glutathione. J Nutr. 2001;131(9 Suppl):2488S–24890S. doi: 10.1093/jn/131.9.2488S. discussion 96S-7S. [DOI] [PubMed] [Google Scholar]
- 29.Ziegler A, Zaugg CE, Buser PT, Seelig J, Kunnecke B. Non-invasive measurements of myocardial carbon metabolism using in vivo 13C NMR spectroscopy. NMR Biomed. 2002;15:222–234. doi: 10.1002/nbm.764. [DOI] [PubMed] [Google Scholar]
- 30.He Y, Hakvoort TB, Kohler SE, Vermeulen JL, de Waart DR, de Theije C, et al. Glutamine synthetase in muscle is required for glutamine production during fasting and extrahepatic ammonia detoxification. J Biol Chem. 2010;285:9516–9524. doi: 10.1074/jbc.M109.092429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Des Rosiers C, Lloyd S, Comte B, Chatham JC. A critical perspective of the use of (13)C-isotopomer analysis by GCMS and NMR as applied to cardiac metabolism. Metab Eng. 2004;6:44–58. doi: 10.1016/j.ymben.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 32.Nelson D, Rumsey WL, Erecinska M. Glutamine catabolism by heart muscle. Properties of phosphate-activated glutaminase. Biochem J. 1992;282(Pt 2):559–564. doi: 10.1042/bj2820559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Housley MP, Rodgers JT, Udeshi ND, Kelly TJ, Shabanowitz J, Hunt DF, Puigserver P, Hart GW. O-GlcNAc regulates FoxO activation in response to glucose. J Biol Chem. 2008;283:16283–16292. doi: 10.1074/jbc.M802240200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bastie CC, Nahlé Z, McLoughlin T, Esser K, Zhang W, Unterman T, Abumrad NA. FoxO1 stimulates fatty acid uptake and oxidation in muscle cells through CD36-dependent and -independent mechanisms. J Biol Chem. 2005;280:14222–14229. doi: 10.1074/jbc.M413625200. [DOI] [PubMed] [Google Scholar]
- 35.Xia Y, Wen HY, Young ME, Guthrie PH, Taegtmeyer H, Kellems RE. Mammalian target of rapamycin and protein kinase A signaling mediate the cardiac transcriptional response to glutamine. J Biol Chem. 2003;278:13143–13150. doi: 10.1074/jbc.M208500200. [DOI] [PubMed] [Google Scholar]
- 36.Hyyti OM, Ning XH, Buroker NE, Ge M, Portman MA. Thyroid hormone controls myocardial substrate metabolism through nuclear receptor-mediated and rapid posttranscriptional mechanisms. Am J Physiol Endocrinol Metab. 2006;290:E372–E379. doi: 10.1152/ajpendo.00288.2005. [DOI] [PubMed] [Google Scholar]
- 37.Yao Z, DuBois DC, Almon RR, Jusko WJ. Modeling circadian rhythms of glucocorticoid receptor and glutamine synthetase expression in rat skeletal muscle. Pharm Res. 2006;23:670–679. doi: 10.1007/s11095-005-9608-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahuja P, Zhao P, Angelis E, Ruan H, Korge P, Olson A, et al. Myc controls transcriptional regulation of cardiac metabolism and mitochondrial biogenesis in response to pathological stress in mice. J Clin Invest. 2010;120:1494–1505. doi: 10.1172/JCI38331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Durgan DJ, Pat BM, Laczy B, Bradley JA, Tsai JY, Grenett MH, et al. O-GlcNAcylation, novel post-translational modification linking myocardial metabolism and cardiomyocyte circadian clock. J Biol Chem. 2011;286:44606–44619. doi: 10.1074/jbc.M111.278903. [DOI] [PMC free article] [PubMed] [Google Scholar]