Abstract
Prostaglandin F2α (PGF2α) plays a critical role in the initiation and process of parturition. Since human labor has been described as an inflammatory event, we investigated the role of PGF2α in the inflammatory process using cultured human uterine smooth muscle cells (HUSMCs) isolated from term pregnant women as a model. Using a multiplex assay, HUSMCs treated with PGF2α changed their output of a number of cytokines and chemokines, with a distinct response pattern that differed between HUSMCs isolated from the upper and lower segment region of the uterus. Confirmatory enzyme-linked immunosorbent assays (ELISAs) showed that PGF2α stimulated increased output of interleukin (IL) 1β, IL6, IL8 (CXCL8) and monocyte chemotactic protein-1 (MCP1, also known as chemokine (c-c motif) ligand 2, CCL2) by HUSMCs isolated from both upper and lower uterine segments. In contrast, PGF2α inhibited tumor necrosis factor α (TNFα) release by HUMSCs from the lower uterine segment while the output of TNFα was undetectable in the upper segment. Small interfering (si) RNA mediated knockdown of the PGF2α receptor prevented the changes in cytokine and chemokine output by the HUSMCs. Since the PGF2α receptor (PTGFR) couples via the Gq protein and subsequently activates the phospholipase C (PLC) and protein kinase C (PKC) signaling pathways, we examined the role of these pathways in PGF2α modulation of the cytokines. Inhibition of PLC and PKC reversed the effects of PGF2α. PGF2α activated multiple signaling pathways including extracellular signal-regulated kinases (ERK) 1/2, phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K), P38, calcineurin/nuclear factor of activated T-cells (NFAT) and NF-κB signaling. Inhibition of ERK reversed PGF2α-induced IL1β, IL6 and CCL2 output, while inhibition of PI3K blocked the effect of PGF2α on IL6, CXCL8 and CCL2 output and inhibition of NF-κB reversed PGF2α-induced IL1β and CCL2 output. NFAT was involved in PGF2α modulation of CCL2 and TNFα output. In conclusion, our results support a role of PGF2α in creating an inflammatory environment during the late stage of human pregnancy.
Keywords: PGF2α, inflammation, myometrium, pregnancy, labor
Introduction
Prostaglandins (PGs), a family of hormones produced in all tissues of the body, modulate various functions via endocrine, paracrine and autocrine mechanisms. In female reproductive systems, PGs are involved in many events including ovulation, blastocyst transport, implantation, pregnancy maintenance, luteolysis and parturition. In most mammalian species, PGs produced by gestational tissues play a central role in the initiation and progression of labor being involved in all aspects of parturition including ripening of the cervix, membrane rupture and induction of uterine contraction (Lundin-Schiller and Mitchell, 1990; Olson et al., 1995).
Accumulating evidence demonstrates that human labor is an inflammatory process, characterized by increased leukocyte infiltration into uterine tissues and increased expression and release of numerous cytokines and chemokines, including interleukin 6 (IL 6), IL1, interleukin 8 (also known as chemokine (c-x-c motif) ligand 8, CXCL8) and CCL2 (Goldenberg et al., 2000; Osman et al., 2003). The increased cytokines and chemokines further promote the recruitment of leukocytes into uterine tissues in a feed-forward fashion thereby creating an ‘inflammatory microenvironment’ (Kobayashi, 2008; White et al., 2013). Within the uterus, such inflammatory cascades result in the up-regulation of uterine activation proteins (UAPs), thereby leading to the onset of parturition (Hertelendy et al., 1993; Young et al., 2002).
It is well known that PGs can serve as pro-inflammatory mediators due to their high expression in inflamed tissues and ability to induce inflammatory symptoms. One such example, prostaglandin F2α (PGF2α), is produced by gestational tissues with high levels observed at parturition (Olson et al., 1995; Fortier et al., 2008; Maddipati et al., 2014), and has a recognized physiological role in stimulating myometrial contractions. However, increased PGF2α concentrations observed in the maternal circulation occur early on in, or precede, the labor process (Kinoshita et al., 1977), suggesting that PGF2α is involved in additional parturition events besides uterine contraction. We previously reported that PGF2α may contribute to transformation of the relatively quiescent uterus of gestation, to the powerful contractile uterus of parturition, by up-regulating expression of the UAPs (Xu et al., 2013). Moreover, PGF2α can also serve as an inflammatory mediator, for example, in the female reproductive system, PGF2α induces CCL2 in the ovary (Luo et al., 2011) and CXCL8 in endometrial adenocarcinoma cells (Pollard and Mitchell, 1996). The PGF2α signal is mediated by the PGF2α receptor (PTGFR), which couples to the G protein Gq to activate multiple signaling pathways which include phospholipase C/protein kinase C (PLC/PKC), mitogen activated protein kinase (MAPKs), phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) and calcineurin/nuclear factor of activated T-cells (NFAT) signaling pathways (Sales et al., 2009; Goupil et al., 2010; Kondo et al., 2012).
Human uterine smooth muscle cells (HUSMCs) isolated from the pregnant uterus synthesize and secrete chemokines and cytokines such as IL1β, IL6, CCL2 and CXCL8 (Hua et al., 2012; Shynlova et al., 2013a). In addition, HUMSCs demonstrate increased expression of UAPs such as connexin 43(CX43) and cyclo-oxygenase-2 (COX-2, also known as PTGS-2) in response to treatment with PGF2α (Xu et al., 2013). Thus, we hypothesize that PGF2α regulates chemokine and cytokine output in the myometrium during pregnancy, thereby amplifying the inflammatory responses within uterus.
Finally, in the human uterus, the concept of a functional regionalization has been proposed. This suggests that the upper segment (US) displays a relaxed state during gestation to accommodate the growing fetus but at labor contracts to expel the baby, while the lower segment (LS) maintains a state conducive to passage of the fetal head during labor (Luckas and Wray, 2000). Our previous study demonstrated that PGF2α-induced changes in UAP abundance differed in HUSMCs isolated from the upper and lower uterine segments, indicating potential differentiation of roles between US and LS in pregnancy and parturition (Xu et al., 2013). However, some studies show no difference in contractility between US and LS myometrium. The expression pattern of some UAPs such as PTGFR in US and LS during labor is similar (Hay et al., 2010) whereas some other UAPs, for instance, the PGE2 receptors, PTGER2 and PTGER3, display different expression levels in US and LS (Grigsby et al., 2006). Thus, investigating the role of PGF2α in the regulation of cytokine and chemokine output comparing US and LS myometrial cells will expand our knowledge about functional regionalization in the human uterus.
The objectives of the present study are to (i) assess if PGF2α participates in the regulation of cytokine and chemokine output in the uterus during pregnancy and (ii) define the signaling pathways involved in the PGF2α mediated regulation of chemokine and cytokine output. Our study will provide insight into the mechanisms of human parturition and indicate new strategies for development of tocolytics.
Materials and Methods
Isolation and culture of HUSMCs
This study was approved by the specialty committee on ethics of biomedicine research, Second Military Medical University, Shanghai, China as well as the Conjoint Health Research Ethics Board, University of Calgary. Written informed consent was obtained from all the patients involved in this study.
Biopsies of LS human myometrium (n = 11) were obtained from pregnant women undergoing elective cesarean section at term (the average gestational age was 38 weeks, with a range of 37–42 weeks) in Changhai hospital, Shanghai. Among the patients who were recruited in this study, cesarean section was performed due to breech presentation, previous cesarean section, cephalopelvic disproportion or maternal request. Women who had evidence of underlying disease, such as hypertension, diabetes, pre-eclampsia, intrauterine growth restriction, were not included in this study. Biopsies were excised from the middle portion of upper edge of the incision line in the lower uterine segment. HUSMCs from LS were isolated by enzymatic dispersion as described previously (Xu et al., 2011). Briefly, myometrial pieces were incubated with phenol-red-free Dulbecco's modified Eagles medium (DMEM) containing 1 mg/ml collagenase type II (Invitrogen, Grand Island, NY), and 1 mg/ml deoxyribonuclease I (Invitrogen) at 37°C for 45 min. Following filtration by 100 μm cell strainer (Corning), the cell suspension was centrifuged at 600g for 10 min, and the cell pellet resuspended in DMEM containing 10% fetal calf serum (FCS), penicillin (100 U/ml) and streptomycin (100 mg/ml). The cells were then plated into 25-cm2 flasks and kept at 37°C in 5% CO2-95% air humidified atmosphere until confluent (∼2 weeks) and all experiments were performed with these cells at passage 2. For the treatment experiments, when the cell density is up to 90% confluence, 0.05% trypsin was used to disperse cells and placed in 6-well plates with DMEM containing 10% FCS. After the cells had grown to ∼80% confluence, the media was changed to DMEM without FCS. Subsequently, cells were treated with various concentrations of PGF2α (Sigma-Aldrich, US) in the presence or absence of kinase inhibitors, including PLC inhibitor (U73122), PKC inhibitor (chelerythrine), ERK inhibitor (PD98059), PI3K inhibitor (LY294002), Calcineurin inhibitor (CsA), the blocker of calcineurin and NFAT interaction (Inca-6), NFAT-AP1 complex inhibitor (RA), P38 inhibitor (SB202190) or NFκB inhibitor (PDTC), and incubated for 24 h. The vehicle control was treated with same volume of solvent (ethanol, ≤0.1% v/v). Concentration of the above inhibitors was determined according to the literature (Pollard and Mitchell, 1996) and our previous studies (You et al., 2012; Xu et al., 2015). All the above inhibitors were purchased from Sigma-Aldrich.
Seven paired biopsies from both US and LS uterine segments were collected from pregnant women undergoing elective cesarean sections at term. The upper segment biopsies were all taken from the side opposite the placenta on the anterior or posterior aspect of the upper segment. Palpation and visualization of the uterus determined where the upper segment began, and the decidual layer was dissected away before a small piece of myometrium was grasped with fine forceps and dissected with Iris scissors. Biopsies of LS and US were dispersed and cultured as described above. Cells were cultured to passage 7 and placed in 6-well plates with DMEM containing 10% FCS and 1 × antimycotic (100 units/ml Penicillin g sodium, 100 μg/ml streptomycin sulfate, 0.25 μg/ml amphotericin B) at 37°C with 5% CO2. Following growth to ∼80% confluence, cells were serum deprived overnight then treated with various concentration of PGF2α (10−8–10−5 M) and incubated for 24 h. After incubation, supernatant and cells were collected. The in vitro characteristics of the cells from upper (US-HUSMCs) and lower (LS-HUSMCs) segments were maintained to at least 10 passages as described previously (Mosher et al., 2013).
RNA interference
For knockdown of PTGFR, sequence-specific small interfering RNA (siRNA) targeting human PTGFR (sense 5′-GGUGUAUUGGAGUCACAAAtt-3′; antisense 5′-UUUGUGACUCCAAUACACCgc-3′) was purchased from Santa Cruz, US (sc-44987). The following nonsense siRNA (sense 5′-GAAUCUGGGAUGUUAACCAtt-3′; antisense 5′-UGGUUAACAUCCCAGAUUCtg-3′) was also provided by Santa Cruz and used as the negative control. Cultured HUSMCs were transfected with PTGFR siRNA or control siRNA using Lipofectamine™ RNAi MAX (Invitrogen) for 6 h, followed by 18 h of incubation with DMEM only. The cells were treated with increasing concentrations of PGF2α (10−8–10−5M) for 24 h.
Multiplexed fluorescent bead-based immunoassays
The multiplex immunoassays built on magnetic beads were custom-designed and obtained from Eve® Technologies (Calgary, Canada). In total, 42 cytokines, chemokines and growth factors were evaluated in the cell supernatants from primary HUSMCs in absence and presence of 10−6 M PGF2α. The multiplex assay was carried out by the manufacturer Eve® Technologies (Calgary, Canada).
Enzyme-linked immunosorbent assay
The concentrations of IL6, CCL2, CXCL8, IL1β and tumor necrosis factor α (TNFα) in culture media of myometrial cells were determined with specific enzyme-linked immunoassays (R&D Systems, Inc., Minneapolis, MN, USA) according to the manufacturer's instructions.
Western blotting analysis
HUSMCs were harvested in the presence of M-Per lysis buffer (Pierce Biotechnology, US) and the protein extracted following the manufacturer's protocol. Seventy μg protein was separated by SDS (10% w/v)-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. Subsequently, membranes were incubated with specific antibodies including PTGFR, p65, phospho-p65(ser-529), ERK1/2, phospho-ERK1/2, NFATC1, p38, phospho-p38, PI3K and phospho-PI3K(Tyr508) overnight at 4°C. After incubation with a secondary horseradish peroxidase-conjugated antibody, membranes were visualized using enhanced chemiluminescence (Santa Cruz). The intensities of light-emitting bands were detected and quantified using Sygene Bio Image system (Synoptics Ltd, UK). The levels of phospho-PI3K, phospho-p65, phospho-ERK1/2 and phospho-p38 were normalized to the unphosphorylated type of these proteins, while the level of NFATC1 was normalized to β-actin. The information of all antibodies including manufacture, catalog number and dilution is shown in Supplementary Table SI.
Statistics
The results for all protein determinations are presented as the mean ± SEM. Data were tested by SPSS software and found to be normally distributed. Data were then analyzed by two-way ANOVA followed by the Least Significant Difference (LSD) multiple comparison method. Significance was achieved at P ≤ 0.05.
Results
PGF2α regulates output of chemokines and cytokines in US and LS HUSMCs
We first examined the cytokine and chemokine output, in response to PGF2α treatment, comparing paired US and LS HUSMCs, using a Multiplex assay. One micromolar PGF2α modulated the secretion of a number of chemokines and cytokines during a 24-h incubation. PGF2α robustly stimulated granulocyte-macrophage colony stimulating factor (GM-CSF), IL6, interferon (IFN) α, CXCL8 and CCL2 output in both US and LS cells (Supplementary Fig. S1). However, the response pattern of some cytokines differed between US and LS, for example, PGF2α induced fibroblast growth factor (FGF) 2, IL12 and IL1β in LS-HUSMCs, but inhibited FGF2 and IL12 output, and had no effect on IL1β output by US-HUSMCs. In general, we observed that PGF2α induced more pro-inflammatory cytokines in the LS-HUSMCs compared with the US-HUSMCs.
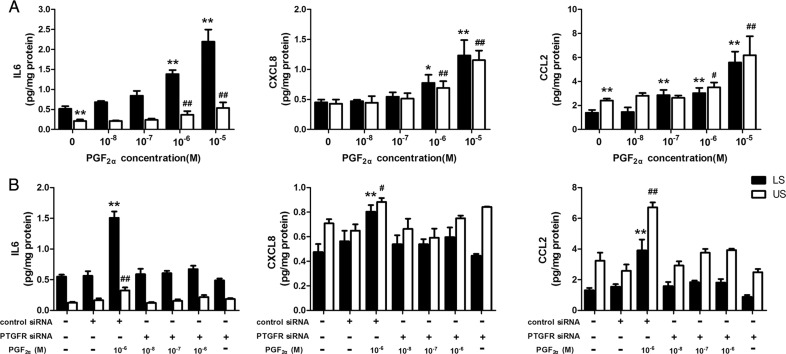
To confirm the effect of PGF2α on cytokines, we used ELISAs, to measure several pro-inflammatory cytokines, whose secretion was stimulated by PGF2α in both the LS and US cells in the above experiment. As shown in Fig. 1A, PGF2α (10−8–10−6 M) treatment for 24 h increased the levels of CCL2, CXCL8 and IL6 in culture media of both LS and US cells in a dose-dependent manner.
Figure 1.
Role of prostaglandin F2α (PGF2α) and its receptor (PTGFR) in mediating cytokine and chemokine output in paired upper segment (US) and lower segment (LS) myometrial cells. (A) Human uterine smooth muscle cells (HUSMCs) isolated from paired US and LS were treated with increasing concentrations of PGF2α (10−8–10−5 M) for 24 h. Following incubation, the cell culture media supernatants were collected for enzyme-linked immunosorbent assay (ELISA) to determine concentration of interleukin-6 (IL-6), CXCL8 and monocyte chemoattractant protein (CCL-2). (B) Sequence-specific small interfering RNA (siRNA) targeting PTGFR to confirm the role of PTGFR in regulating cytokine and chemokine output. HUSMCs were transfected with specific PTGFR siRNA and treated with PGF2α as indicated. Following a 24-h incubation, cell media supernatants were collected for ELISA to determine IL-6, CXCL8 and CCL-2. Values are presented as mean ± SEM. n = 7 (from seven patients). **P < 0.01 compared with vehicle control in HUSMCs of LS. #P < 0.05, ##P < 0.01 versus vehicle control in HUSMCs of US.
The effects of PGF2α are mediated by PTGFR
To investigate whether the modulation of cytokine output by PGF2α occurred as a direct effect via its own receptor, we used sequence-specific siRNA targeting PTGFR to knockdown the levels of PTGFR in the cells. The efficiency of interference with for PTGFR siRNA reached about 72% (Supplementary Fig. S2). As shown in Fig. 1B, knockdown of PTGFR reversed the PGF2α induced up-regulation of CXCL8, CCL2 and IL6 output in both US and LS cells.
The intracellular signaling pathways involved in PGF2α regulation of cytokine and chemokine output in the LS HUSMCs
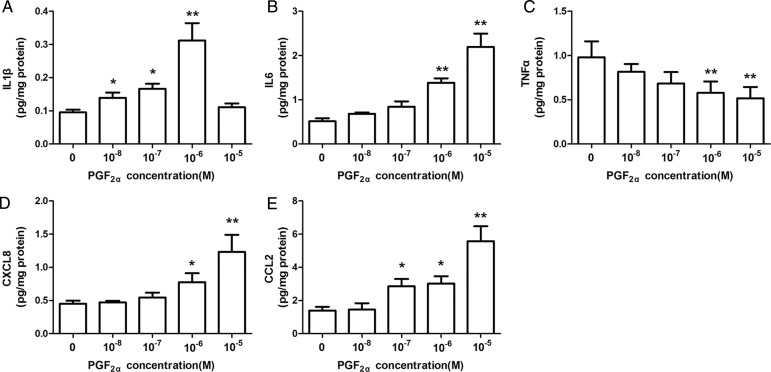
We then investigated the signaling pathways involved in PGF2α regulation of cytokine and chemokine output in human pregnant myometrium. Considering that the amount of US biopsies was limited, the following study was performed with the LS myometrial cells. Multiplex results showed that PGF2α stimulated IL1β output but inhibited TNFα output in LS cells. These results were confirmed using ELISA. As shown in Fig. 2, PGF2α inhibited TNFα output (Fig. 2C) but stimulated IL1β output (Fig. 2A) by the LS cells in a dose-dependent manner, and as expected, PGF2α stimulated IL6, CXCL8 and CCL2 output (Fig. 2B, D and E).
Figure 2.
Prostaglandin F2α (PGF2α) modulates interleukins 1β, 6 and 8 (IL-1β, IL-6, CXCL8) tumor necrosis factor α (TNFα), and monocyte chemoattractant protein (CCL-2) output by lower segment (LS) myometrial cells. Isolated LS human uterine smooth muscle cells (HUSMCs) were treated with PGF2α (10−8–10−5 M) for 24 h. Enzyme-linked immunosorbent assays (ELISA) were used to determine concentrations of (A) IL-1β, (B) IL-6, (C) TNFα, (D) CXCL8 and (E) CCL-2. Values are presented as mean ± SEM. n = 7 (from seven patients). *P < 0.05, **P < 0.01 compared with vehicle control.
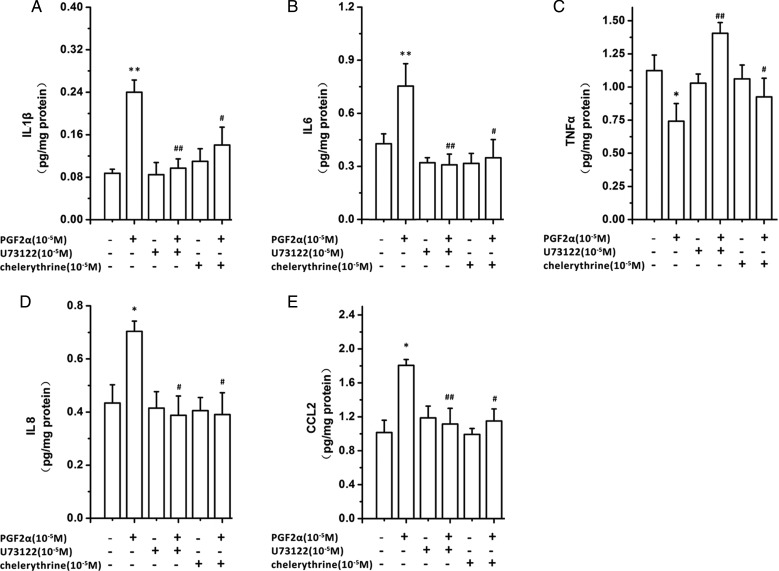
PGF2α activation of the PTGFR, a G protein coupled receptor, leads to Gq coupling and subsequent activation of downstream signaling pathways which include phospholipase C β (PLCβ), and protein kinase C (PKC) (Goupil et al., 2010; Kondo et al., 2012). To determine the role of these downstream signaling pathways in the PGF2α-induced modulation of chemokine and cytokine output, we utilized PLC and PKC inhibitors. Inhibition of either PLC with U73122 (10−5 M) or PKC with chelerythrine (10−5 M) blocked the PGF2α-induced IL1β, IL6, CXCL8, and CCL2 output as well as PGF2α inhibition of TNF-α output (Fig. 3).
Figure 3.
Inhibition of phospholipase C (PLC) and protein kinase C (PKC) modulates the effect of prostaglandin F2α (PGF2α) induced cytokine and chemokine output. (A) Human uterine smooth muscle cells (HUSMCs) from lower segment (LS) were cultured and incubated for 24 h with the PLC inhibitor (U73122) or the PKC inhibitor (chelerythrine), in presence or absence of PGF2α (10−6 M). The supernatants were collected for enzyme-linked immunosorbent assays (ELISA) to determine concentration of; (A) IL-1β, (B) IL-6, (C) TNFα, (D) CXCL8 and (E) CCL-2. Values are presented as mean ± SEM. n = 4 (from four patients). *P < 0.05, **P < 0.01 compared with vehicle control. #P < 0.05, ##P < 0.01 compared with PGF2α10−6 M.
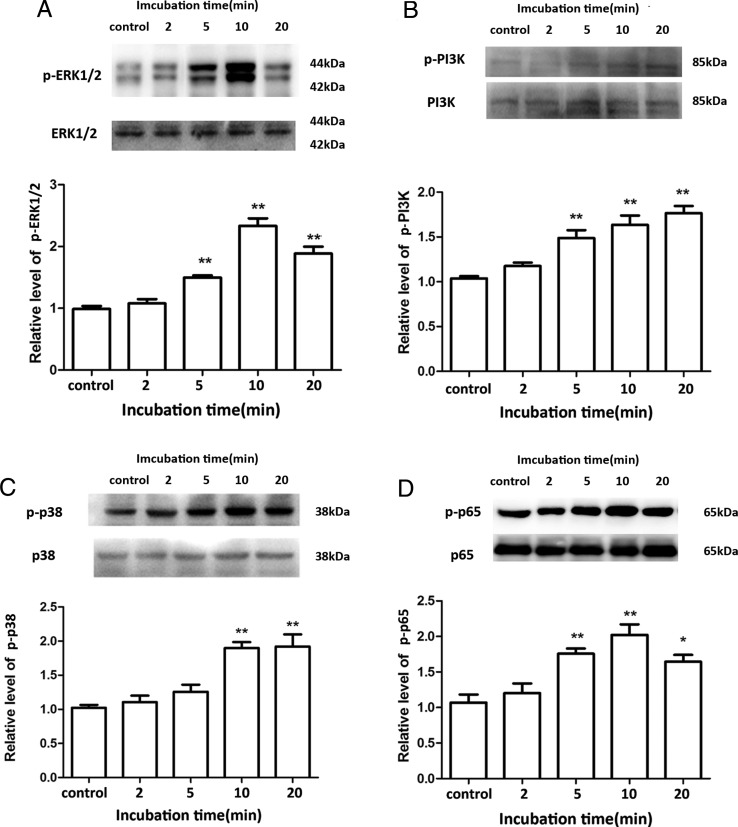
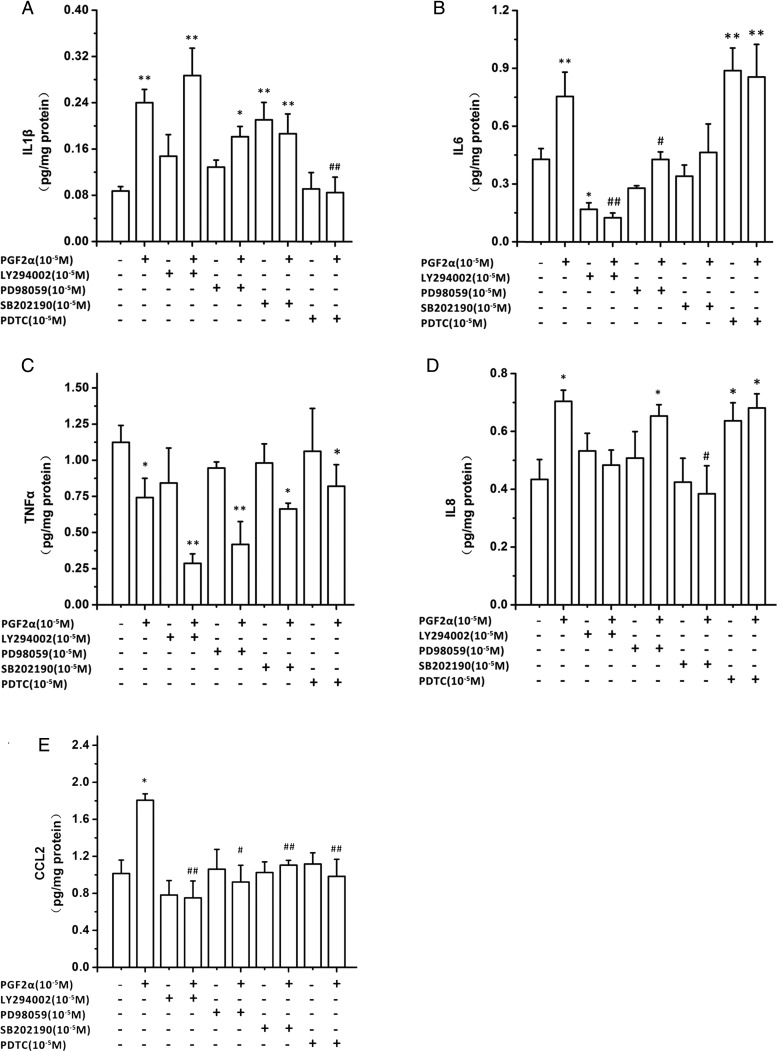
PGF2α has been shown to activate PI3K, ERK1/2 and P38 signaling pathways (Goupil et al., 2010; Kondo et al., 2012). We therefore confirmed these effects in LS HUSMCs. As shown in Fig. 4A–C, PGF2α (10−6 M) increased the levels of phospho-ERK1/2, phospho-PI3K and phospho-p38 in a time-dependent manner. Inhibition of ERK with PD98059 (10−5 M) blocked PGF2α-induced IL1β, IL6 and CCL2, but not CXCL8 output (Fig. 5A–E). Inhibition of PI3K with LY294002 (10−5 M) blocked PGF2α-induced IL6, CXCL8 and CCL2, but not IL1β output. Neither PD98059 nor LY294002 affected PGF2α inhibition of TNFα output. The inhibitor of P38, SB 202190 (10−5 M) reversed the increased output of IL6, CXCL8 and CCL2 by PGF2α. However, treatment of cells with LY294002 alone inhibited IL6 output, while SB202190 treatment caused an increase in IL1β output.
Figure 4.
Prostaglandin F2α (PGF2α) activates phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K), extracellular signal receptor kinase (ERK1/2), P38 and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling pathways. Human uterine smooth muscle cells (HUSMCs) from lower segment (LS) were treated with PGF2α (10−6 M) for the indicated time. The levels of (A) phospho-PI3K, (B) phospho- ERK1/2, (C) phospho-P38 and (D) phospho-p65 were determined by western blotting. Representative blots are presented at the top of corresponding diagram. Values are presented as mean ± SEM. n = 4 (from four patients). *P < 0.05, **P < 0.01 compared with vehicle control.
Figure 5.
The role of phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K), extracellular signal receptor kinase (ERK1/2), P38 and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling pathways in prostaglandin F2α (PGF2α) modulation of cytokine outputs. Human uterine smooth muscle cells (HUSMCs) from lower segment (LS) were treated with PI3K inhibitor (LY294002), and ERK inhibitor (PD98059), P38 inhibitor (SB202190) and NF-κB inhibitor (PDTC) in the presence or absence of PGF2α (10−6 M). Supernatants were collected and analyzed by enzyme-linked immunosorbent assays (ELISA) to determine concentration of (A) interleukin (IL)-1β, (B) IL-6, (C) tumor necrosis factor α (TNFα), (D) interleukin 8 (CXCL8) and (E) monocyte chemoattractant protein (CCL-2). Values are presented as mean ± SEM. n = 4 (from four patients). *P < 0.05, **P < 0.01 compared with vehicle control. #P < 0.05, ##P < 0.01 compared with PGF2α10−6 M.
NF-κB, the archetypal inflammatory transcription factor, is known to drive chemokine and cytokine production. PGF2α (10−6 M) increased the levels of phospho-p65 in time-dependent manner (Fig. 4D). The NF-κB inhibitor, PDTC (10−5 M), reversed the PGF2α-induced IL1β and CCL2 output, but not that of IL6, TNFα or CXCL8 (Fig. 5A–E). Notably, PDTC itself significantly stimulated IL6 (P < 0.01 versus vehicle) and CXCL8 output (P < 0.05 versus vehicle).
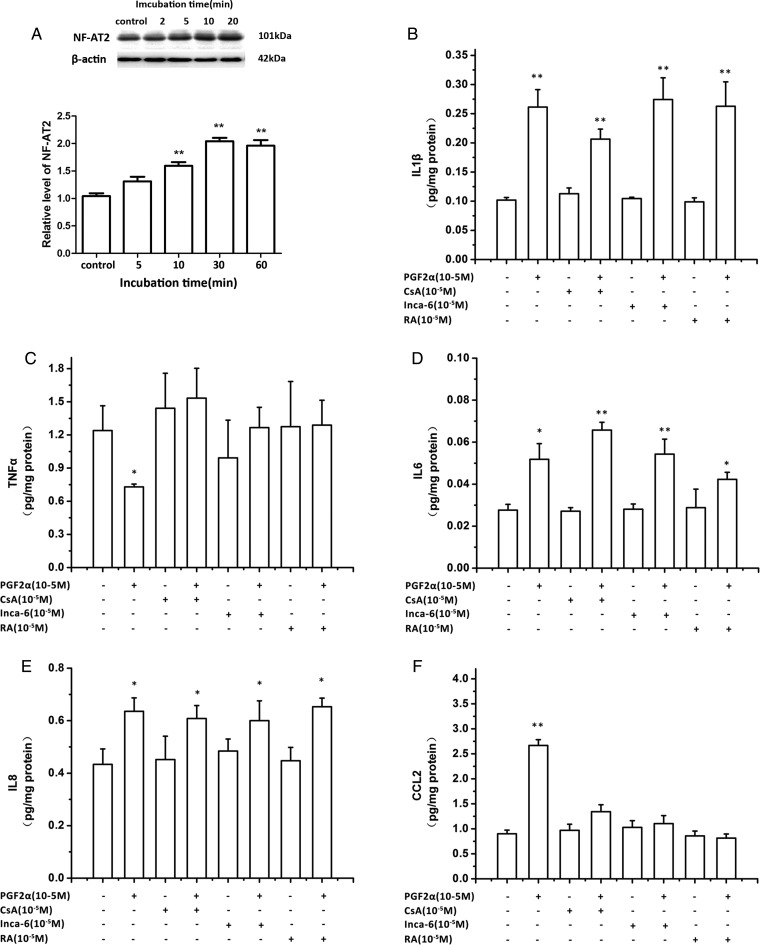
Stimulation of the Gq/PLC signaling pathway leads to Ca2+ release from intracellular calcium stores and subsequently activation of the calcineurin/NFAT pathway. Five members of the NFAT family of transcription factors have been isolated: NFATC2 (NF-AT1/p), NFATC1 (NF-AT2), NFATC4 (NF-AT3), NFATC3 (NF-AT4/x) and NFAT5 (TonEBP) (Rao et al., 1997). Normally, Ca2+ induces activation of calcineurin which leads to NFAT dephosphorylation. In human myometrium, NFATC1 has been shown to be activated by Ca2+ signaling (Pont et al., 2012). As shown in Fig. 6A, PGF2α (10−6 M) time-dependently increased the level of NFATC1. A series of inhibitors were then applied to explore the role of the calcineurin/NFAT pathway in PGF2α regulation of cytokine and chemokine outputs. With the administration of calcineurin inhibitor, CsA, the robust stimulation of CCL2 output by PGF2α was reversed (Fig. 6B). A similar trend was confirmed by the application of Inca-6, a blocker of calcineurin and NFAT interaction, and RA, an inhibitor of NFAT-AP1 complex. PGF2α-induced suppression of TNFα output was also blocked by CsA, Inca-6 and RA.
Figure 6.
The role of calcineurin/nuclear factor of activated T-cells (NFATC1) pathway in prostaglandin F2α (PGF2α) modulation of cytokine output. (A) Human uterine smooth muscle cells (HUSMCs) from lower segment (LS) were treated with PGF2α (10−6 M) for the time indicated. The level of NFATC1 was determined by western blotting. (B–F) HUSMCs were treated with calcineurin inhibitor (CsA), the blocker of calcineurin and NFAT interaction (InCA-6), NFAT-AP1 complex inhibitor (RA) in presence or absence of PGF2α (10−6 M). The supernatants were collected for enzyme-linked immunosorbent assays (ELISA) to determine concentration of; (B) IL-1β, (C) IL-6, (D) TNFα, (E) CXCL8 and (F) CCL-2. Values are presented as mean ± SEM. n = 4 (from four patients). *P < 0.05, **P < 0.01 compared with vehicle control.
Discussion
Human parturition is an inflammatory event characterized by increased communication between uterine chemotactic signals and leukocytes in late gestation, which peaks at parturition and leads to leukocyte invasion of the uterus at every delivery (Zourbas et al., 2001; Tornblom et al., 2005; Golightly et al., 2011; Singh et al., 2011; Gomez-Lopez et al., 2013). Even though there is an absence of infection, pro-inflammatory cytokines and chemokines are increased in both of preterm and term birth (Romero et al., 1990; Osmers et al., 1995; Young et al., 2002; Esplin et al., 2005). The invading leukocytes promote a positive feed-forward cycle by secreting pro-inflammatory cytokines and chemokines such as IL1β, IL6, CXCL8 and CCL2 that drive PG synthesis (Golightly et al., 2011). Previous studies mostly consider that an increase in PG concentration induced by cytokines triggers uterine contractility (Young et al., 2002; Keelan et al., 2003). However, PGF2α is more than just a potent stimulator of myometrial contraction. PGF2α has an important signaling role during parturition, aiding in the transformation of the uterus of gestation to the uterus of delivery near the end of pregnancy. In this study, we extend our knowledge regarding the involvement of PGF2α in parturition by clearly showing it as one of the mediators that promote the establishment of a pro-inflammatory intrauterine environment by stimulation of pro-inflammatory cytokine and chemokine production in myometrium, leading to the initiation of labor. In this sense, the circle becomes complete; pro-inflammatory cytokines promote synthesis of PGF2α and its receptor, PTGFR, and PGF2α promotes cytokine and chemokine synthesis via its receptor.
A number of studies have demonstrated that the rise of IL1β and IL6 in gestational tissues, amniotic fluid and maternal blood prior to labor indicates a role in parturition (Romero et al., 1990; Osman et al., 2003; Shynlova et al., 2013b; Maddipati et al., 2014). IL6 and IL1β can stimulate PGE2 and PGF2α production in gestational tissues such as myometrium, amnion and decidual cells (Mitchell et al., 1991; Keelan et al., 2003). In the uterus, IL6 and IL1β also induce labor onset by up-regulating expression of UAPs in myometrium (Young et al., 1997; Fang et al., 2000; Rauk et al., 2001). Our study demonstrated that PGF2α administration triggered an up-regulation of IL6 and IL1β output in myometrial cells. Taken together, it may suggest that a positive interaction between PGF2α and the pro-inflammatory cytokines IL1β and IL6 might occur within uterus, which amplifies inflammation and uterine activation as parturition is initiated.
Both CXCL8 and CCL2 are responsible for the recruitment of monocytes, memory T cells and dendritic cells to sites of inflammation (Xu et al., 1996; Kobayashi, 2008; White et al., 2013). CXCL8 is not only secreted from placental and decidual tissues (Saito et al., 1994; el Maradny et al., 1996; Denison et al., 1998) but higher expression is detected in myometrium at labor (Osmers et al., 1995). CCL2 is also expressed in human myometrium and greatly increased after onset of labor (Esplin et al., 2005). Our data indicate that PGF2α significantly enhances CXCL8 and CCL2 production in HUSMCs which may suggest that PGF2α is involved in the process of uterine activation at end of gestation.
In the present study, we found that, unlike IL1β and IL6, TNFα output was suppressed by PGF2α in HUSMCs. It is hard to know the significance of PGF2α inhibition of TNFα. Previous studies have investigated the expression of TNFα in relation to labor in a variety of gestational tissues with inconsistent results (Winkler et al., 2001; Tattersall et al., 2008; Thomakos et al., 2010; Alexander et al., 2012). TNFα expression has been shown to be increased in amnion, chorion, and isolated choriodecidua with labor (Thomakos et al., 2010). Some studies have reported that TNFα protein concentrations were low in decidua, fetal membranes, and myometrium, and that they did not change during the onset of labor (Winkler et al., 2001; Tattersall et al., 2008; You et al., 2014). Given that TNFα can be induced by other pro-inflammatory cytokines such as IL1β and IL6, our findings that PGF2α suppressed TNFα production in myometrium might partly explain why the expression of TNFα is not changed during labor even though the level of IL1β and IL6 is higher.
PTGFR, a member of the G-protein coupled receptor superfamily, principally couples to Gq protein leading to the activation of PLCβ/Ca2+/PKC signaling pathways. Our data indicate that PGF2α modulation of chemokine and cytokine production is dependent on the PLC/PKC signaling pathway. It is known that Ca2+ triggers calcineurin/NFAT signaling. PGF2α up-regulates CXCL8 production via calcineurin/NFAT signaling pathway in endometrial cancer (Sales et al., 2009). In the present study, we show that calcineurin/NFAT signaling pathway is involved in PGF2α stimulation of CCL2 and suppression of TNFα, but not CXCL8 production, suggesting that different signaling pathways are responsible for PGF2α modulation of cytokines in different cells. Moreover, our data showed that PGF2α regulation of IL1β and CCL2 is through NF-κB activation while its regulation of IL6 production is dependent on ERK, PI3K and P38 signaling pathways and CXCL8 secretion is through P38 signaling. Interestingly, PGF2α regulation of CCL2 output occurs via multiple signaling molecules including ERK, PI3K, P38 and NF-κB. Taken together, we suggest that divergent signaling pathways mediate PGF2α modulation of chemokines and cytokines in myometrium (Fig. 7).
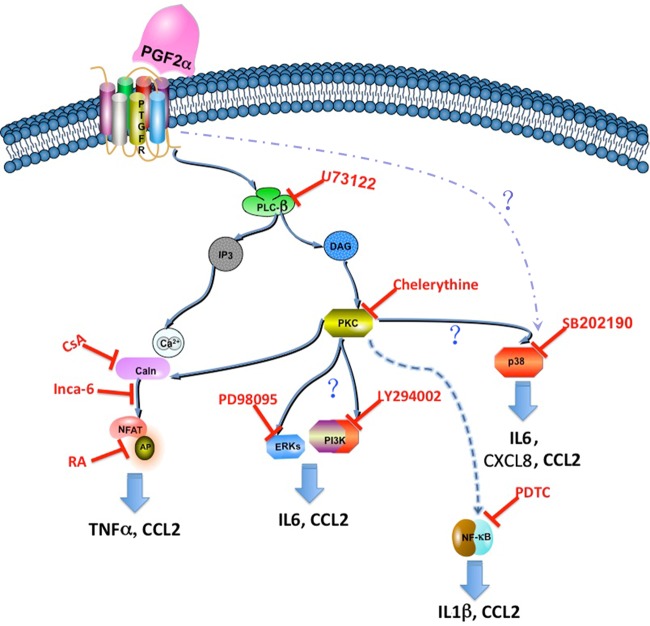
Figure 7.
Scheme illustrating the signaling pathway involved in the production of chemokines and cytokines induced by prostaglandin F2α (PGF2α). Prostaglandin F2α receptor (PTGFR) primary couples to Gq protein. PTGFR can activate phospholipase β (PLCβ), which catalyzes the hydrolysis of membrane phosphoinositol lipids and leads to the release of inositol-1, 4, 5-triphosphate (IP3) and diacylglycerol (DAG), which subsequently activate protein kinase C (PKC) and trigger the release of Ca2+ from endoplasmic reticulum (ER). PKC then activates phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K), extracellular signal receptor kinase (ERK), P38 and nuclear factor-kappa light-chain-enhancer of activated B cells (NFκB) signaling pathways. Ca2+ activates calcineurin/nuclear factor of activated T-cells (NFAT) pathway and eventually activates AP-1 signaling.
Notably, the present study has shown that several kinase inhibitors themselves have corresponding effects on cytokine output, such as P38 inhibitor stimulated IL1β output while PDTC increased IL6 and CXCL8 output. Currently, it is unknown why these reagents increased secretion of the above cytokines. However, some studies have demonstrated the interaction between P38 and NF-κB signaling. Kanaji et al. (2012) have shown that P38 inhibitor enhances the level of phospho-p65 in endothelial cells, while PDTC has also been shown to induce P38 activation in vascular smooth muscle cells (Moon et al., 2004). Interestingly, in the present study, we found that NF-κB activation leads to an increase in IL1β output while P38 signaling mediates PGF2α stimulation of IL6 and CXCL8 output. Nevertheless, whether PDTC enhancing IL6 and CXCL8 output is through P38 signaling and P38 inhibitor increasing IL1β output is associated with NF-κB activation remains to be further elucidated.
Our previous study demonstrated that PGF2α-induced changes in UAP abundance differ between US and LS HUMSCs (Xu et al., 2013). Our findings that the response pattern of some cytokines to PGF2α was different between LS and US cells confirm our earlier observation that the responsiveness to PGF2α can vary between US and LS cells, and indicate potential differential roles of PGF2α in US and LS during pregnancy and parturition. Although the mechanisms underlying different responsiveness to PGF2α in US and LS are unknown, different PTGFR receptor densities in the two regions, different intracellular pathways, or the possibility that PGF2α stimulates other mediators with varying effects in each region might attribute to the discrepancy of PGF2α effects in US and LS.
In conclusion, our study systematically demonstrated that PGF2α modulates chemokine and cytokine output in human pregnant myometrium no matter upper and lower segment. Multiple signaling pathways are involved in PGF2α regulation of chemokine and cytokine output in myometrium. Our findings corroborate the hypothesis that the initiation and amplification of non-infectious inflammation is a positive feedback reaction. This complex interrelationship of PGs, cytokines and chemokines, activates the uterus and completes its transfer to a contractile state.
Accessing research data
Data can be accessed via email by contacting nixin@smmu.edu.cn and david.olson@ualberta.ca
Supplementary data
Supplementary data are available at http://molehr.oxfordjournals.org/.
Authors' roles
The authors were responsible for the following aspects of the study. C.X. and W.L.: study design, cell culture and treatment, acquisition, analysis and interpretation of data from ELISA, manuscript draft preparation; X.Y.: cell culture and treatment, acquisition, analysis and interpretation of data from western blotting; K.L. and K.P.: cell culture, siRNA transfection and ELISA; X.F., D.M.S., S.L.W, Q.S. and H.G. patient recruitment, tissue isolation, and clinical data collection. D.M.O. and X.N.: study design and coordination, data interpretation. X.N. and D.M.S.: critical revision of manuscript. All of the authors critically revised the manuscript and approved the final version.
Funding
This work was supported by Canadian Institutes of Health Research (CIHR, MOP 119513) (D.M.O.), the CIHR-NSFC Joint Initiative (CCI 9220) (D.M.O., X.N.), the Science and Technology Commission of Shanghai Municipals (13430722900) (X.N.), the Alberta Innovates Health Solutions (AIHS) Interdisciplinary Preterm Birth and Healthy Outcomes Team (PreHOT, ITG 201100532) (D.M.O., S.L.W., D.M.S.), March of Dimes (D.M.O., RCCHUSJ21), AIHS Summer Studentship (K.P.), and the Global Alliance for the Prevention of Prematurity and Stillbirth (GAPPS) Preventing Preterm Birth Initiative, an initiative of Seattle Children's Hospital (D.M.O. and X.N.).
Conflict of interest
None declared.
Supplementary Material
Acknowledgements
The authors wish to thank the nursing and medical staff of the delivery suite and the patients in Changhai Hospital and Foothills Hospital Calgary, for their participation and cooperation.
References
- Alexander HA, Sooranna SR, Myatt L, Johnson MR. Myometrial tumor necrosis factor-alpha receptors increase with gestation and labour and modulate gene expression through mitogen-activated kinase and nuclear factor-kappaB. Reprod Sci 2012;19:43–54. [DOI] [PubMed] [Google Scholar]
- Denison FC, Kelly RW, Calder AA, Riley SC. Cytokine secretion by human fetal membranes, decidua and placenta at term. Hum Reprod 1998;13:3560–3565. [DOI] [PubMed] [Google Scholar]
- el Maradny E, Kanayama N, Maehara K, Kobayashi T, Terao T. Expression of interleukin-8 receptors in the gestational tissues before and after initiation of labour: immunohistochemical study. Acta Obstet Gynecol Scand 1996;75:790–796. [DOI] [PubMed] [Google Scholar]
- Esplin MS, Peltier MR, Hamblin S, Smith S, Fausett MB, Dildy GA, Branch DW, Silver RM, Adashi EY. Monocyte chemotactic protein-1 expression is increased in human gestational tissues during term and preterm labour. Placenta 2005;26:661–671. [DOI] [PubMed] [Google Scholar]
- Fang X, Wong S, Mitchell BF. Effects of LPS and IL-6 on oxytocin receptor in non-pregnant and pregnant rat uterus. Am J Reprod Immunol 2000;44:65–72. [DOI] [PubMed] [Google Scholar]
- Fortier MA, Krishnaswamy K, Danyod G, Boucher-Kovalik S, Chapdalaine P. A postgenomic integrated view of prostaglandins in reproduction: implications for other body systems. J Physiol Pharmacol 2008;59(Suppl 1):65–89. [PubMed] [Google Scholar]
- Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med 2000;342:1500–1507. [DOI] [PubMed] [Google Scholar]
- Golightly E, Jabbour HN, Norman JE. Endocrine immune interactions in human parturition. Mol Cell Endocrinol 2011;335:52–59. [DOI] [PubMed] [Google Scholar]
- Gomez-Lopez N, Tanaka S, Zaeem Z, Metz GA, Olson DM. Maternal circulating leukocytes display early chemotactic responsiveness during late gestation. BMC Pregnancy Childbirth 2013;13(Suppl 1):S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goupil E, Tassy D, Bourguet C, Quiniou C, Wisehart V, Petrin D, Le Gouill C, Devost D, Zingg HH, Bouvier M et al. . A novel biased allosteric compound inhibitor of parturition selectively impedes the prostaglandin F2alpha-mediated Rho/ROCK signaling pathway. J Biol Chem 2010;285:25624–25636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigsby PL, Sooranna SR, Adu-Amankwa B, Pitzer B, Brockman DE, Johnson MR, Myatt L. Regional expression of prostaglandin E2 and F2alpha receptors in human myometrium, amnion, and choriodecidua with advancing gestation and labour. Biol Reprod 2006;75:297–305. [DOI] [PubMed] [Google Scholar]
- Hay A, Wood S, Olson D, Slater DM. Labour is associated with decreased expression of the PGF2alpha receptor (PTGFR) and a novel PTGFR splice variant in human myometrium but not decidua. Mol Hum Reprod 2010;16:752–760. [DOI] [PubMed] [Google Scholar]
- Hertelendy F, Romero R, Molnar M, Todd H, Baldassare JJ. Cytokine-initiated signal transduction in human myometrial cells. Am J Reprod Immunol 1993;30:49–57. [DOI] [PubMed] [Google Scholar]
- Hua R, Pease JE, Sooranna SR, Viney JM, Nelson SM, Myatt L, Bennett PR, Johnson MR. Stretch and inflammatory cytokines drive myometrial chemokine expression via NF-kappaB activation. Endocrinology 2012;153:481–491. [DOI] [PubMed] [Google Scholar]
- Kanaji N, Nelson A, Allen-Gipson DS, Sato T, Nakanishi M, Wang X, Li Y, Basma H, Michalski J, Farid M et al. . The p38 mitogen-activated protein kinases modulate endothelial cell survival and tissue repair. Inflamm Res 2012;61:233–244. [DOI] [PubMed] [Google Scholar]
- Keelan JA, Blumenstein M, Helliwell RJ, Sato TA, Marvin KW, Mitchell MD. Cytokines, prostaglandins and parturition—a review. Placenta 2003;24(Suppl A):S33–S46. [DOI] [PubMed] [Google Scholar]
- Kinoshita K, Satoh K, Sakamoto S. Prostaglandin F2alpha and E1 in plasma and amniotic fluid during human pregnancy and labour. Endocrinol Jpn 1977;24:155–162. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y. The role of chemokines in neutrophil biology. Front Biosci 2008;13:2400–2407. [DOI] [PubMed] [Google Scholar]
- Kondo A, Otsuka T, Kato K, Natsume H, Kuroyanagi G, Mizutani J, Ito Y, Matsushima-Nishiwaki R, Kozawa O, Tokuda H. AMP-activated protein kinase inhibitor decreases prostaglandin F2alpha-stimulated interleukin-6 synthesis through p38 MAP kinase in osteoblasts. Int J Mol Med 2012;30:1487–1492. [DOI] [PubMed] [Google Scholar]
- Luckas MJ, Wray S. A comparison of the contractile properties of human myometrium obtained from the upper and lower uterine segments. BJOG 2000;107:1309–1311. [DOI] [PubMed] [Google Scholar]
- Lundin-Schiller S, Mitchell MD. The role of prostaglandins in human parturition. Prostaglandins Leukot Essent Fatty Acids 1990;39:1–10. [DOI] [PubMed] [Google Scholar]
- Luo W, Diaz FJ, Wiltbank MC. Induction of mRNA for chemokines and chemokine receptors by prostaglandin F2alpha is dependent upon stage of the porcine corpus luteum and intraluteal progesterone. Endocrinology 2011;152:2797–2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddipati KR, Romero R, Chaiworapongsa T, Zhou SL, Xu Z, Tarca AL, Kusanovic JP, Munoz H, Honn KV. Eicosanomic profiling reveals dominance of the epoxygenase pathway in human amniotic fluid at term in spontaneous labour. FASEB J 2014;28:4835–4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell MD, Dudley DJ, Edwin SS, Schiller SL. Interleukin-6 stimulates prostaglandin production by human amnion and decidual cells. Eur J Pharmacol 1991;192:189–191. [DOI] [PubMed] [Google Scholar]
- Moon SK, Jung SY, Kim CH. Transcription factor Sp1 mediates p38MAPK-dependent activation of the p21WAF1 gene promoter in vascular smooth muscle cells by pyrrolidine dithiocarbamate. Biochem Biophys Res Commun 2004;316:605–611. [DOI] [PubMed] [Google Scholar]
- Mosher AA, Rainey KJ, Bolstad SS, Lye SJ, Mitchell BF, Olson DM, Wood SL, Slater DM. Development and validation of primary human myometrial cell culture models to study pregnancy and labour. BMC Pregnancy Childbirth 2013;13(Suppl 1):S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson DM, Mijovic JE, Sadowsky DW. Control of human parturition. Semin Perinatol 1995;19:52–63. [DOI] [PubMed] [Google Scholar]
- Osman I, Young A, Ledingham MA, Thomson AJ, Jordan F, Greer IA, Norman JE. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol Hum Reprod 2003;9:41–45. [DOI] [PubMed] [Google Scholar]
- Osmers RG, Blaser J, Kuhn W, Tschesche H. Interleukin-8 synthesis and the onset of labour. Obstet Gynecol 1995;86:223–229. [DOI] [PubMed] [Google Scholar]
- Pollard JK, Mitchell MD. Intrauterine infection and the effects of inflammatory mediators on prostaglandin production by myometrial cells from pregnant women. Am J Obstet Gynecol 1996;174:682–686. [DOI] [PubMed] [Google Scholar]
- Pont JN, McArdle CA, Lopez Bernal A. Oxytocin-stimulated NFAT transcriptional activation in human myometrial cells. Mol Endocrinol 2012;26:1743–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol 1997;15:707–747. [DOI] [PubMed] [Google Scholar]
- Rauk PN, Friebe-Hoffmann U, Winebrenner LD, Chiao JP. Interleukin-6 up-regulates the oxytocin receptor in cultured uterine smooth muscle cells. Am J Reprod Immunol 2001;45:148–153. [DOI] [PubMed] [Google Scholar]
- Romero R, Avila C, Santhanam U, Sehgal PB. Amniotic fluid interleukin 6 in preterm labour. Association with infection. J Clin Invest 1990;85:1392–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito S, Kasahara T, Sakakura S, Umekage H, Harada N, Ichijo M. Detection and localization of interleukin-8 mRNA and protein in human placenta and decidual tissues. J Reprod Immunol 1994;27:161–172. [DOI] [PubMed] [Google Scholar]
- Sales KJ, Maldonado-Perez D, Grant V, Catalano RD, Wilson MR, Brown P, Williams AR, Anderson RA, Thompson EA, Jabbour HN. Prostaglandin F(2alpha)-F-prostanoid receptor regulates CXCL8 expression in endometrial adenocarcinoma cells via the calcium-calcineurin-NFAT pathway. Biochim Biophys Acta 2009;1793:1917–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shynlova O, Lee YH, Srikhajon K, Lye SJ. Physiologic uterine inflammation and labour onset: integration of endocrine and mechanical signals. Reprod Sci 2013a;20:154–167. [DOI] [PubMed] [Google Scholar]
- Shynlova O, Nedd-Roderique T, Li Y, Dorogin A, Lye SJ. Myometrial immune cells contribute to term parturition, preterm labour and post-partum involution in mice. J Cell Mol Med 2013b;17:90–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Chaudhry P, Asselin E. Bridging endometrial receptivity and implantation: network of hormones, cytokines, and growth factors. J Endocrinol 2011;210:5–14. [DOI] [PubMed] [Google Scholar]
- Tattersall M, Engineer N, Khanjani S, Sooranna SR, Roberts VH, Grigsby PL, Liang Z, Myatt L, Johnson MR. Pro-labour myometrial gene expression: are preterm labour and term labour the same? Reproduction 2008;135:569–579. [DOI] [PubMed] [Google Scholar]
- Thomakos N, Daskalakis G, Papapanagiotou A, Papantoniou N, Mesogitis S, Antsaklis A. Amniotic fluid interleukin-6 and tumor necrosis factor-alpha at mid-trimester genetic amniocentesis: relationship to intra-amniotic microbial invasion and preterm delivery. Eur J Obstet Gynecol Reprod Biol 2010;148:147–151. [DOI] [PubMed] [Google Scholar]
- Tornblom SA, Klimaviciute A, Bystrom B, Chromek M, Brauner A, Ekman-Ordeberg G. Non-infected preterm parturition is related to increased concentrations of IL-6, IL-8 and MCP-1 in human cervix. Reprod Biol Endocrinol 2005;3:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White GE, Iqbal AJ, Greaves DR. CC chemokine receptors and chronic inflammation—therapeutic opportunities and pharmacological challenges. Pharmacol Rev 2013;65:47–89. [DOI] [PubMed] [Google Scholar]
- Winkler M, Kemp B, Fischer DC, Maul H, Hlubek M, Rath W. Tissue concentrations of cytokines in the lower uterine segment during preterm parturition. J Perinat Med 2001;29:519–527. [DOI] [PubMed] [Google Scholar]
- Xu LL, Warren MK, Rose WL, Gong W, Wang JM. Human recombinant monocyte chemotactic protein and other C-C chemokines bind and induce directional migration of dendritic cells in vitro. J Leukoc Biol 1996;60:365–371. [DOI] [PubMed] [Google Scholar]
- Xu C, Gao L, You X, Dai L, Li Y, Gu H, Slater DM, Olson DM, Ni X. CRH acts on CRH-R1 and -R2 to differentially modulate the expression of large-conductance calcium-activated potassium channels in human pregnant myometrium. Endocrinology 2011;152:4406–4417. [DOI] [PubMed] [Google Scholar]
- Xu C, Long A, Fang X, Wood SL, Slater DM, Ni X, Olson DM. Effects of PGF2alpha on the expression of uterine activation proteins in pregnant human myometrial cells from upper and lower segment. J Clin Endocrinol Metab 2013;98:2975–2983. [DOI] [PubMed] [Google Scholar]
- Xu C, You X, Liu W, Sun Q, Ding X, Huang Y, Ni X. Prostaglandin F2alpha regulates the expression of uterine activation proteins via multiple signalling pathways. Reproduction 2015;149:139–146. [DOI] [PubMed] [Google Scholar]
- You X, Gao L, Liu J, Xu C, Liu C, Li Y, Hui N, Gu H, Ni X. CRH activation of different signaling pathways results in differential calcium signaling in human pregnant myometrium before and during labour. J Clin Endocrinol Metab 2012;97:E1851–E1861. [DOI] [PubMed] [Google Scholar]
- You X, Liu J, Xu C, Liu W, Zhu X, Li Y, Sun Q, Gu H, Ni X. Corticotropin-releasing hormone (CRH) promotes inflammation in human pregnant myometrium: the evidence of CRH initiating parturition? J Clin Endocrinol Metab 2014;99:E199–E208. [DOI] [PubMed] [Google Scholar]
- Young LJ, Muns S, Wang Z, Insel TR. Changes in oxytocin receptor mRNA in rat brain during pregnancy and the effects of estrogen and interleukin-6. J Neuroendocrinol 1997;9:859–865. [DOI] [PubMed] [Google Scholar]
- Young A, Thomson AJ, Ledingham M, Jordan F, Greer IA, Norman JE. Immunolocalization of proinflammatory cytokines in myometrium, cervix, and fetal membranes during human parturition at term. Biol Reprod 2002;66:445–449. [DOI] [PubMed] [Google Scholar]
- Zourbas S, Dubanchet S, Martal J, Chaouat G. Localization of pro-inflammatory (IL-12, IL-15) and anti-inflammatory (IL-11, IL-13) cytokines at the foetomaternal interface during murine pregnancy. Clin Exp Immunol 2001;126:519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.