Abstract
Identifying the events and molecular mechanisms that regulate oocyte growth has emerged as a key objective of research in human fertility, fuelled by evidence from human and animal studies indicating that disease and environmental factors can act on oocytes to affect the health of the resulting individual and by efforts to grow oocytes in vitro to enable fertility preservation of cancer survivors. Techniques that monitor the development of growing oocytes would be valuable tools to assess the progression of growth under different conditions. Most methods used to assess oocytes grown in vitro are indirect, however, relying on characteristics of the somatic compartment of the follicle, or compromise the oocyte, preventing its subsequent culture or fertilization. We investigated the utility of T-cell factor/lymphoid enhancer-binding factor (TCF/Lef)-LacZ transgene expression as a predictor of global transcriptional activity in oocytes and early embryos. Using a fluorescent β-galactosidase substrate combined with live-cell imaging, we show that TCF/Lef-LacZ transgene expression is detectable in growing oocytes, lost in fully grown oocytes and resumes in late two-cell embryos. Transgene expression is likely regulated by a Wnt-independent mechanism. Using chromatin analysis, LacZ expression and methods to monitor and inhibit transcription, we show that TCF/Lef-LacZ expression mirrors transcriptional activity in oocytes and preimplantation embryos. Oocytes and preimplantation embryos that undergo live-cell imaging for TCF/Lef-LacZ expression are able to continue development in vitro. TCF/Lef-LacZ reporter expression in living oocytes and early embryos is thus a sensitive and faithful marker of transcriptional activity that can be used to monitor and optimize conditions for oocyte growth.
Keywords: oocyte, transcription, assisted reproduction, transgene, in vitro growth
Introduction
Post-natal oocyte development comprises two stages—a prolonged period of growth, which requires about 4 months in humans, followed by a very brief process, termed meiotic maturation, which occurs coincident with ovulation and requires only about 36 h in humans. Although meiotic maturation has been studied extensively, oocyte growth has until recently received much less attention. Advances in medicine and technology, however, have sparked new and widespread interest in identifying key events and molecular mechanisms that regulate oocyte growth. On one hand, emerging evidence from human and animal studies now indicates that disease and environmental factors can act on oocytes in a manner that affects the health of the resulting individual and perhaps even succeeding generations (Wang and Moley, 2010; Luzzo et al., 2012; Ge et al., 2013; Lane et al., 2014). Although when and how their effects are transmitted to the oocyte are largely unknown, the prolonged duration of growth suggests that in many cases they likely target oocytes at this stage. On the other hand, the dramatic increase in the number of young cancer survivors owing to improved therapeutic treatments is generating reproductive-age adults burdened with a high risk of infertility as a result of therapy (Wallace, 2011; Donnez and Dolmans, 2013; De Vos et al., 2014). Fertility can be restored in some cases by transplantation of ovarian fragments that were removed prior to the therapy, but this option is not feasible in the case of cancers such as leukaemia that may have invaded the ovarian tissue (Demeestere et al., 2007; Bastings et al., 2013; Dolmans et al., 2013; Donnez et al., 2013). An alternative option is to grow oocytes in vitro; however, although several approaches have been developed using animal models, the success rate remains much too low for clinical translation (Desai et al., 2010; Smitz et al., 2010; Belli et al., 2012; Telfer and Zelinski, 2013; Shea et al., 2014).
During its growth, the oocyte undergoes a diverse set of processes, including an ∼200-fold increase in its volume (Wassarman, 1988), amplification of mitochondrial DNA (Mahrous et al., 2012), accumulation and storage of messenger RNAs (Flemr et al., 2010; Clarke, 2012), acquisition of genomic imprints (Lucifero et al., 2004; Anckaert et al., 2013), turnover of specific proteins (Chalupnikova et al., 2014) and a spatial reorganization of the chromatin, termed the non-surrounded nucleolus-to-surrounded nucleolus (NSN-SN) transition, at the end of growth (Wickramasinghe et al., 1991; Debey et al., 1993; Zuccotti et al., 1995; Christians et al., 1999; Tan et al., 2009). Underpinning and driving these changes is robust RNA synthesis, which is continuous in the growing oocyte until, when the oocyte reaches its full size, it declines to an undetectable level (Sánchez and Smitz, 2012). It is likely that some of these processes fail to occur normally under adverse health circumstances or environmental influences and during growth in vitro, resulting in arrested oocyte development or poor embryonic development after fertilization.
Techniques that could monitor these processes in individual oocytes would be valuable tools to assess the progression of oocyte growth. However, almost all current assays require oocytes to be fixed or physically disrupted and hence are unsuitable when the objective is to track oocyte development over time or to fertilize the in vitro-grown oocytes. Additionally, depending on the assay, it may be necessary to pool many oocytes to obtain sufficient material for analysis, so information about individuals cannot be obtained. Moreover, even though it is possible to measure the diameter of living oocytes, this is typically done following fixation or after removal from the follicle to ensure accuracy (Pesty et al., 2007; McLaughlin and Telfer, 2010) and in any case does not provide direct information regarding underlying molecular activity. In addition, the NSN-SN transition at the end of growth can be assayed in living oocytes using the cell-permeable fluorescent DNA stains, but these may damage DNA (Erba et al., 1988) and thus compromise subsequent development. Because of the technical difficulties associated with directly assaying the growing oocyte development, characteristics of the somatic portion of the follicle are often assayed instead. These typically include the size of the follicle, presence of an antrum and secretion of steroid hormones or growth factors (Adriaens et al., 2004; Telfer et al., 2008; Xu et al., 2009; McLaughlin and Telfer, 2010). Such surrogate measures are valuable but necessarily provide an indirect read-out of the quality of the oocyte. Thus, the arsenal of tools available to assess the progression of oocyte growth is surprisingly limited.
We previously generated and characterized a transgenic mouse line carrying the bacterial lacZ gene whose expression is controlled by a T-cell factor/lymphoid enhancer-binding factor (TCF/Lef) element that can be activated by nuclear β-catenin (Mohamed et al., 2004). Importantly, activity of the encoded β-galactosidase can be monitored in living cells. In a variety of somatic cell types, this reporter reflects activation of the Wnt signalling pathway (Mohamed et al., 2004, 2005; Ohyama et al., 2006; Lin et al., 2007; Abdul-Ghani et al., 2011; Paek et al., 2011; Usongo and Farookhi, 2012; Kuroda et al., 2013). We report here that the TCF/Lef-LacZ transgene is expressed in growing oocytes, likely by a Wnt-independent pathway in contrast to other cell types. When oocytes reach full size and become transcriptionally inactive, both the mRNA and the encoded protein become undetectable. Expression resumes at the two-cell stage after fertilization, coincident with the activation of embryonic transcription. This reporter thus provides a sensitive and easily assayed read-out of the transcriptional status of oocytes and thus of their developmental progression during growth. It should be useful for rapidly assaying the progression of oocyte growth under different conditions, both in vivo and in vitro, as well as for comparing the quality of individual oocytes within a population.
Methods
Experimental protocols were approved by the Animal Care Committee of the McGill University Health Centre following the regulations and policies of the Canadian Council on Animal Care (protocol 4735). Transgenic mice homozygous for the β-catenin/Tcf-responsive LacZ reporter gene were maintained at McGill and have been previously characterized (Mohamed et al., 2004). Five breeding pairs were maintained and replaced at yearly intervals. CD1 female mice were obtained from Charles River Laboratories (St-Constant, QC, Canada). Mice were euthanized by CO2 asphyxiation followed by cervical dislocation.
Collection and culture of granulosa–oocyte complexes (GOCs) and cumulus–oocyte complexes (COCs)
To obtain GOCs, ovaries were removed from two or three day-8 or day-12 TCF/Lef-LacZ female mice and disaggregated mechanically and enzymatically using 28-gauge needles in a 35-mm dish containing 2 ml of HEPES-buffered minimal essential media [MEM; pH 7.2, containing 28 ug/ml sodium pyruvate, 63 ug/ml penicillin G, 50 ug/ml streptomycin and 1 mg/ml bovine serum albumin (BSA)] (Sigma, Windsor, ON, Canada) supplemented with 0.01 mg/ml collagenase (Type V, Worthington Biochemical, Lakewood, NJ, USA) and 0.02 mg/ml DNase I (Sigma) at 37°C. To obtain denuded oocytes, oocytes were released from surrounding granulosa cells in calcium- and magnesium-free Hank's balanced salt solution (Gibco Life Technologies, Oakville, ON, Canada) supplemented with 0.05% (w/v) trypsin (Sigma). After collection, GOCs and oocytes were transferred to a 35-mm dish with 2 ml of fresh media using a mouth-operated pipette. For in vitro growth experiments, GOCs (100–140 μm in diameter) collected from day-12 mice were incubated in groups of 30 on type I collagen 3.0 micron inserts (VWR, Montreal, QC, Canada) in 24-well plates (VWR) containing 750 μl of pre-equilibrated serum-free bicarbonate-buffered MEM (MEM-NaHCO3) for 6 days in a humidified incubator at 37°C and 5% CO2 in air. One-third of the culture media was removed and replaced with pre-equilibrated fresh media on the third day of culture. To obtain COCs, ovaries were dissected from day-18 to day-21 TCF/Lef-LacZ female mice, and the large follicles were punctured using 28-gauge needles in a 35-mm dish containing 2 ml of HEPES-buffered media supplemented with 0.1 mg/ml dibutyryl cyclic adenosine monophosphate (dbcAMP, Sigma) to prevent meiotic maturation. In some experiments, oocytes were cultured in the presence of 10 mg/ul α-amanitin (Sigma, diluted from 1 mg/ml stock stored at −20°C).
Embryo collection and in vitro culture
Seven-week-old CD1 female mice were superovulated by intraperitoneal (i.p.) injection of 7.5 international units (IU) of equine chorionic gonadotropin (eCG, Sigma), followed by i.p. injection of 5 IU hCG (Sigma) 44–48 h later. Female mice were placed individually with adult TCF/Lef-LacZ males for mating overnight. One-cell embryos were collected from 2–3 females in the following morning (designated E0.5) in a 35-mm dish containing MEM-HEPES by puncturing the oviducts no later than 21 h post-hCG injection. Cumulus cells were disaggregated by incubating the embryo masses in 2 ml of MEM-HEPES supplemented with 0.01% hyaluronidase (Sigma) for 3–5 min. Embryos cleared of cumulus cells were transferred to fresh collection media. When required, two-cell-stage embryos were collected at E1.5 in MEM-HEPES by flushing the oviducts with 0.5 ml of MEM-HEPES using a 30-gauge needle. Flushed two-cell embryos were collected, washed three times and cultured in a 35-mm dish containing 40-μl drops of pre-equilibrated commercial potassium simplex optimized media (KSOM, Millipore, Toronto, ON, Canada) under 2 ml of in vitro fertilization-grade light mineral oil (Sigma) at 37°C and 5% CO2 in air. Seven-week-old CD1 females were superovulated as previously described (Clarke et al., 1992). In some experiments, embryos were cultured in the presence of 10 mg/ul α-amanitin (Sigma, diluted from 1 mg/ml stock stored at −20°C).
Fluorescein-di-galactoside (FDG) staining and fluorescent imaging
To detect LacZ activity, GOCs collected from TCF/Lef-LacZ females [live or after fixation for 5 min in 3.7% formaldehyde and permeabilization for 20 min in 0.5% Triton X-100 in phosphate-buffered saline (PBS)] were incubated for 1 hour at room temperature shielded from light in PBS containing 500 μM FDG (Marker Gene Technologies, Eugene, OR, USA). Embryos were incubated for 1 hour at 37°C and 5% CO2 in air in KSOM containing 500 μM FDG. FDG was diluted from 100 mM stock in dimethyl sulphoxide (DMSO) stored at −20°C. Cells intended for subsequent in vitro culture were transferred to pre-equilibrated MEM-NaHCO3 or KSOM prior to imaging; otherwise, cells were washed once in PBS and placed in 500 μl of PBS in one well of a four-well plate for visualization. Fluorescence was visualized using confocal microscopy (Zeiss CLSM 510 Meta) using an excitation wavelength of 488 nm and a 500–550 nm band pass emission filter. Cells were categorized as FDG+ (presence of any green fluorescence) or FDG– (no fluorescence detected). Cells for in vitro culture were maintained in a humidified incubator at 37°C and 5% CO2 in air.
WNT3A stimulation of oocytes
Oocytes were collected from day-12 TCF/Lef-LacZ females and cultured in MEM-NaHCO3 with or without 100 ng/ml recombinant mouse WNT3A (R&D Systems, Minneapolis, MN, USA) for 6 h at 37°C and 5% CO2 in air. Oocytes were subsequently processed for whole-mount immunofluorescence for total β-catenin.
FSH stimulation of granulosa cells
Granulosa cells were collected from day-21 CD1 female mice 24 h after i.p. injection of 5 IU eCG by puncturing large follicles with 28-gauge needles. Approximately 1× 106 cells were cultured in individual wells of a four-well plates containing 500 μl MEM-NaHCO3 supplemented with 5% foetal bovine serum (FBS) overnight at 37°C in 5% CO2 in air. Cells were then washed in serum-free MEM and cultured in MEM containing 100 ng/ml recombinant human follicle-stimulating hormone (FSH) (National Hormone & Peptide Program, USA) for 4 h. Cells were processed for whole-mount immunofluorescence to detect β-catenin.
Whole-mount indirect immunofluorescence
Cells were fixed and permeabilized in 2% para-formaldehyde containing 0.1% Triton X-100 in PBS for 15 min, transferred to blocking solution containing 3% BSA with 0.1% Triton X-100 in PBS, then incubated overnight at 4°C in anti-β-catenin antibody (Ab-37, VWR), which recognizes both phosphorylated and non-phosphorylated forms of the protein, diluted 1:400 in blocking solution. They were then washed for 2 × 5 min in blocking solution, incubated for 1 hour at room temperature with gentle agitation in CY3-conjugated goat anti-rabbit IgG antibody (Cedarlane, Peterborough, ON, Canada) diluted 1:400 in blocking solution containing 5 μM DRAQ5 and 1 μg/ml 4′,6-diamidino-2-phenylindole (DAPI), and then washed. Oocytes and embryos were mounted in 1 μl of PBS covered with mineral oil (Sigma) on a microscope slide. Granulosa cells were imaged in blocking solution. Fluorescence was visualized using confocal microscopy.
Immunohistochemistry
Ovaries were fixed overnight at 4°C in freshly prepared 4% para-formaldehyde in PBS and then embedded in paraffin (Demeestere et al., 2012). Sections were cut at 5 µm thickness and mounted on glass slides. Following deparaffinization, slides were incubated in blocking solution as mentioned earlier containing anti-β-galactosidase antibody (Life Technologies) overnight at 4°C with gentle agitation. Following washes, the slides were incubated with CY3-conjugated goat anti-rabbit IgG antibody as earlier, together with YOYO-1 (1:20 000, Life Technologies) to detect DNA. Slides were washed and covered with a glass cover slip. Fluorescence was visualized using confocal microscopy.
Detection of oocyte chromatin configuration
Oocytes were stained using 10 μg/ml Hoechst 33 358 (Sigma, 2.5 mg/ml stock stored at 20°C) in live cells or 5 μM DRAQ5 (New England Biolabs, Oakville, ON, Canada) diluted from 500 μM stock in 50% glycerol stored at −20°C after fixation and permeabilization. Stained cells were transferred to 500 μl PBS in one well of a four-well plate for visualization using UV light (Hoechst) or confocal microscopy (DRAQ5). Chromatin configuration was classified as non-surrounded nucleolus (NSN) or surrounded nucleolus (NS) as described (Bouniol-Baly et al., 1999).
5′-Ethynyl. uridine (EU) incorporation and detection
Cells were incubated in MEM-NaHCO3 containing 100 μM EU (Life Technologies) at 37°C and 5% CO2 in air for 2 h, fixed for 5 min in 3.7% formaldehyde in PBS (Fisher Scientific, Montreal, QC, Canada) freshly diluted from 37% formaldehyde, washed twice in 3% BSA/PBS and permeabilized in 0.5% Triton X-100 in PBS for 20 min. After washing, cells were incubated in the Click-iT cocktail reaction (Life Technologies) containing 4 mM CuSO4, 1 μM Alexa Fluor 594 azide, 1 μg/ml DAPI and 5 μM DRAQ5 for 30 min. After washing cells were incubated in 500 μM FDG in PBS for 30 min at room temperature shielded from light. Cells were mounted in 1 μl PBS covered with mineral oil on a microscope slide. Fluorescence was visualized using confocal microscopy. Cells with fluorescence present in the nucleus were classified as transcriptionally active.
Detection of LacZ mRNA
Pools of 40 growing oocytes (day-12), 40 fully grown oocytes (day-21), 30 one-cell embryos and 20 two-cell embryos were stored at −80°C until RNA extraction. Total RNA was extracted from each sample (Arcturus Picopure RNA, Life Technologies) according to manufacturer's instructions. DNA was removed by DNase I treatment (Sigma). cDNA was synthesized using SuperScript II Reverse Transcription kit (Life Technologies) and stored at −20°C until use. Two microlitres of cDNA was combined with 5 μl of 10× polymerase chain reaction (PCR) buffer, 1.5 μl of 50 mM MgCl2, 1 μl of 10 mM dNTPs, 1 μl of 10 μM forward and reverse primers (Sigma), 1 μl of Taq polymerase (Life Technologies) and 38.5 μl of sterile water in a total reaction volume of 50 μl. PCR was performed according to the following reaction cycle: 5 min at 95°C, 35 cycles of 95°C for 30 s, 53°C for 30 s, 72°C for 30 s and a final 10 min at 72°C. Amplification of Actb cDNA was used as a positive control and a no-template control (water instead of cDNA) as the negative control. Primers used for LacZ: forward 5′-ACTATCCCGACCGCCTTACT-3′ and reverse 5′-TAGCGGCTGATGTTGAACTG-3′. Primers used for Actb: forward 5′-GCTGTGCTATGTTGCTCTAG-3′ and reverse 5′-ATCGTACTCCTGCTTGCTGA-3′.
Statistical analysis
Statistical analyses were performed using GraphPad Prism (Version 6.0) software for Student's t-test (unpaired, equal variance) or two-way analysis of variance (ANOVA) with Tukey's honestly significant difference (HSD) test. Values presented in figures are the mean ± standard error of the mean. Probability values <0.05 (P < 0.05) were considered statistically significant. All experiments were repeated three times using independent replicates.
Results
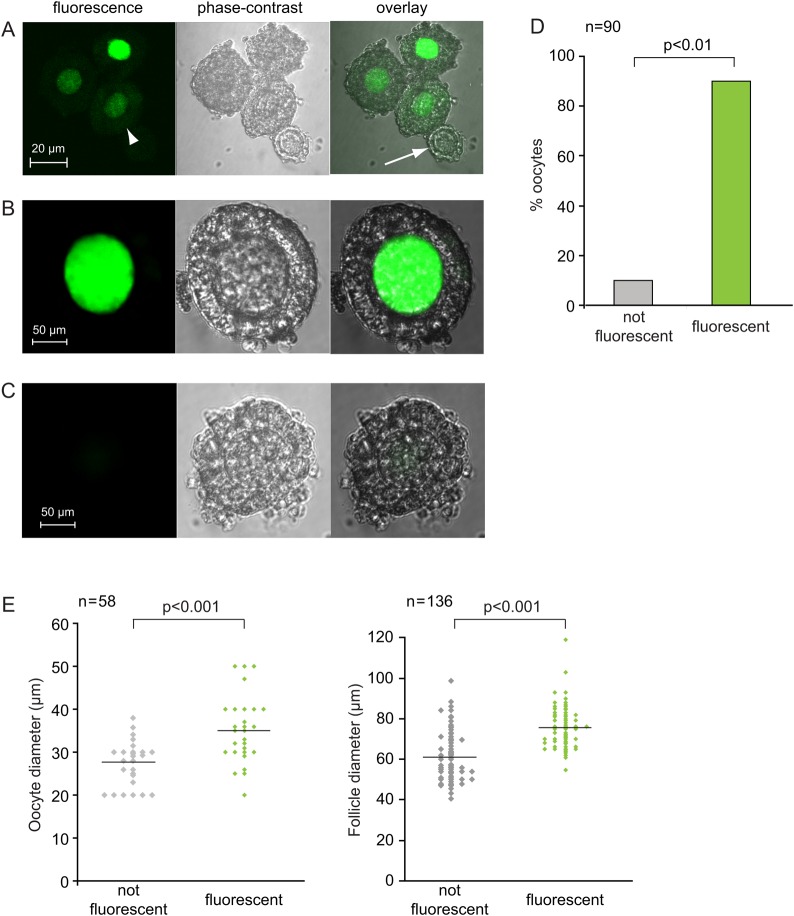
To determine whether the TCF/Lef-LacZ transgene was expressed in oocytes, we exploited the fact that a large group of oocytes and their follicles undergo growth synchronously during the first 3 weeks of post-natal life. We collected GOCs from 12-day-old pups, which contain a large population of secondary follicles. Such follicles contain mid-growth-stage oocytes surrounded by more than one layer of granulosa cells. When we stained the GOCs using FDG, a sensitive β-galactosidase substrate that can be taken up by living or fixed cells (Rakhmanova and MacDonald, 1998) and generates a fluorescent product, we observed that most of the oocytes in the GOCs were fluorescent (Fig. 1A, B and D). A small minority, however, were consistently non-fluorescent (Fig. 1A, arrow). Non-transgenic oocytes were never fluorescent (Fig. 1C). Weak fluorescence was occasionally observed in the granulosa cells of both non-transgenic and transgenic origins (Fig. 1A, arrowhead), but this was clearly distinguishable from the bright fluorescence of the transgenic oocytes. Fluorescence was never observed when FDG was omitted from the staining solution (not shown).
Figure 1.
Transgene-encoded β-galactosidase activity in oocytes. (A) Granulosa cell–oocyte complexes (GOCs) containing growing oocytes from TCF-Lef-LacZ females were stained using the fluorescent substrate, FDG. Bright fluorescence is visible in three oocytes but not in the fourth smaller oocyte (arrow). Very weak fluorescence is detectable in some granulosa cells (arrow head). (B) Higher-power view showing bright fluorescence in a growing oocyte. (C) Wild-type GOCs show no fluorescence. (D) Percentage of fluorescent and non-fluorescent growing oocytes. Data are summed from three independent replicates. Chi-square test. (E) Size of fluorescent and non-fluorescent growing oocytes (left) and their GOCs (right). Each point represents an individual oocyte or GOC. Two-sample t-test.
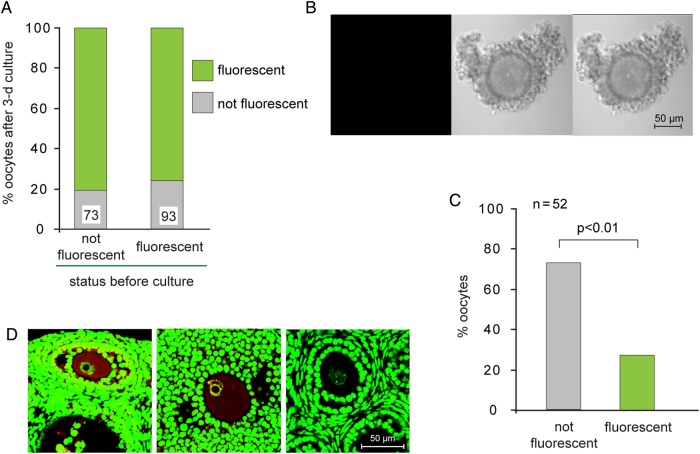
To determine why most oocytes contained transgene-encoded β-galactosidase whereas some did not, we collected GOCs at an earlier stage of growth from 8-day-old pups. We stained them as mentioned earlier and also measured the diameter of each follicle and its enclosed oocyte. We found that fluorescent oocytes and their GOCs were significantly larger than non-fluorescent oocytes and their follicles (Fig. 1E). This suggests that expression of the transgene was activated during oocyte growth. To test this idea, we collected GOCs from 8-day-old pups, stained them using FDG and separated the GOCs containing fluorescent or non-fluorescent oocytes. Both populations were cultured for 72 h and then stained again. We found that most oocytes that had been non-fluorescent at the time of collection became fluorescent during the culture period (Fig. 2A). These results strongly suggest that the transgene is not expressed at very early stages of growth and subsequently becomes active.
Figure 2.
Gain and loss of fluorescence during oocyte growth. (A) Granulosa cell–oocyte complexes (GOCs) containing growing oocytes were collected and stained using FDG. GOCs containing fluorescent and non-fluorescent oocytes were then incubated separately for 3 days and stained again to determine whether the oocyte was fluorescent. Data are summed from three experiments. The number of oocytes examined is shown at the base of each bar. (B) No fluorescence is detectable in fully grown oocytes. (C) Percentage of fluorescent and non-fluorescent fully grown oocytes. Data are summed from three experiments. Chi-square test. (D) Ovarian sections were stained using anti-β-galactosidase (red) and YOYO-1 (green). Left: Prominent β-galactosidase staining in growing oocyte of transgenic female. Middle: Extremely weak staining in fully grown oocyte of transgenic female. Right: Undetectable staining in growing oocyte of wild-type female. Note NSN chromatin configuration in left and right oocytes; SN configuration in middle oocyte.
Unexpectedly, we also observed that about one-quarter of the oocytes that had been fluorescent at the time of collection were no longer fluorescent after the culture period (Fig. 2A). To understand the basis for this, we collected and stained COCs containing fully grown oocytes from 18- to 21-day-old mice. Only about one-quarter of the oocytes were fluorescent, whereas the remaining three-quarters were non-fluorescent (Fig. 2B and C). Similarly, using ovarian tissue sections and immunofluorescence, we detected β-galactosidase protein in growing oocytes but not in larger oocytes (Fig. 2D). Taken together, these results show that β-galactosidase was absent at early stages of oocyte growth, accumulated during mid-growth and then was lost during late growth.
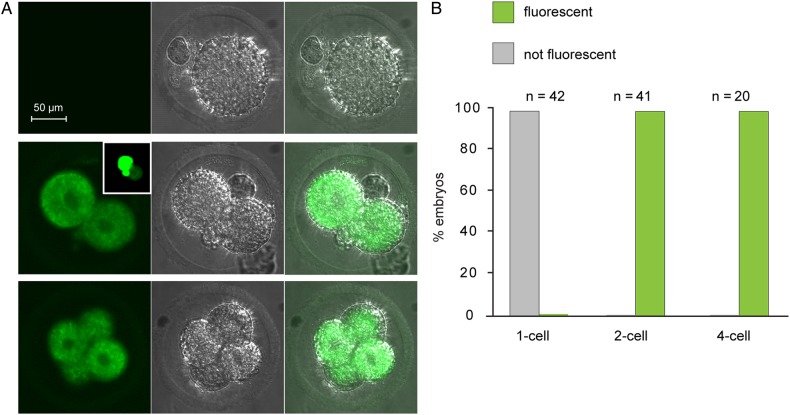
We then tested whether the β-galactosidase activity would re-appear following fertilization. We collected one-cell and two-cell embryos and stained them using FDG (Fig. 3A and B). No fluorescence was detectable in the one-cell embryos. In contrast, all embryos analysed at the late two-cell stage (46–48 h post-hCG injection) were fluorescent. In some embryos analysed at 46 h post-hCG, one blastomere displayed substantially brighter fluorescence than its sister, consistent with an asynchronous activation of expression in the sister blastomeres (Fig. 3B, inset). Moreover, the strong fluorescent signal persisted in four-cell embryos. Thus, β-galactosidase activity re-appeared at the time of the major activation of the embryonic genome.
Figure 3.
Transgene-encoded β-galactosidase activity in embryos. (A) One-cell (upper), two-cell (middle) and four-cell (lower) embryos are shown. For all panels, FDG is on the left, phase–contrast in the middle and overlay on the right. (B) Percentage of embryos at each stage that were fluorescent or not fluorescent. Data are summed from four experiments.
Because the TCF/Lef-LacZ transgene is expressed when the canonical Wnt-β-catenin signalling pathway is activated in a variety of cell types (see Introduction), we examined whether β-galactosidase activity in oocytes also reflected the activity of this pathway. In FSH-stimulated monolayer cultures from freshly isolated granulosa cells, nuclear β-catenin was easily detectable (Fig. 4A), as previously reported (Fan et al., 2010). In growing oocytes, however, although we detected immunoreactivity at the plasma membrane consistent with the known cytoplasmic localization of β-catenin, we never detected intra-nuclear reactivity (Fig. 4B). We did, however, consistently observe immunoreactivity at the nuclear envelope. These results show that nuclear β-catenin is undetectable in oocytes, even though they express the transgene as indicated by β-galactosidase activity.
Figure 4.
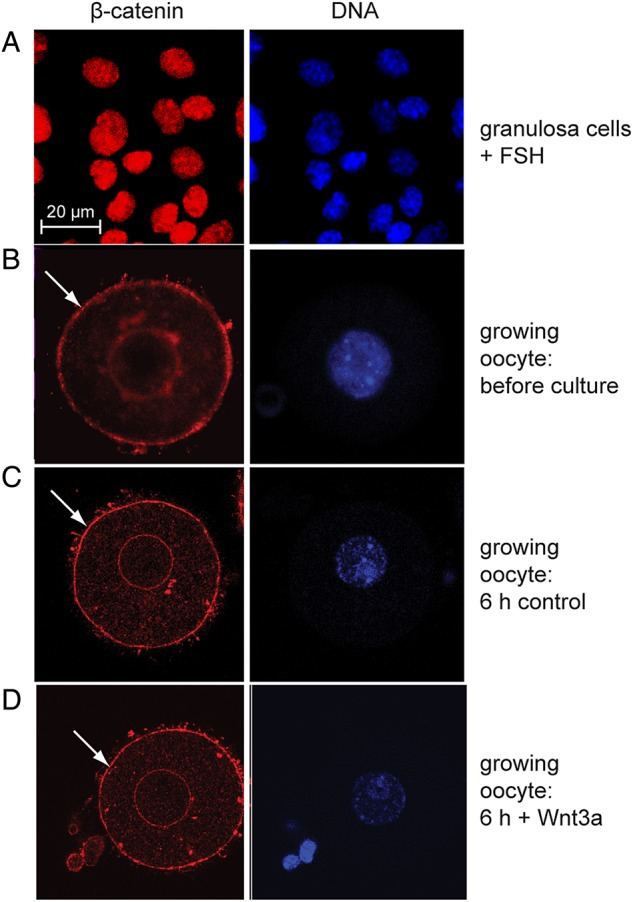
Intracellular localization of β-catenin. (A) Nuclear localization in granulosa cells treated with follicle-stimulating hormone. (B–D) Cytoplasmic localization of β-catenin in growing oocytes before culture and following culture in the absence or presence of WNT3A. Arrow shows staining near oocyte plasma membrane.
In a second approach, we tested whether β-catenin could localize to the oocyte nucleus in response to Wnt. Mid-growth-phase oocytes obtained from 12-day-old mice were cultured for 6 h in media with or without 100 ng/ml recombinant mouse WNT3A, an activator of canonical Wnt signalling. Oocytes cultured in the presence of WNT3A, like control oocytes, displayed only membrane-bound β-catenin (Fig. 4C and D), as well as the immunoreactivity at the nuclear envelope. These observations show that β-catenin is not localized to the nucleus in growing oocytes even when cultured in the presence of a canonical Wnt signal, WNT3A. These results strongly suggest that the growing oocytes express the TCF/Lef-LacZ transgene independently of β-catenin/TCF signalling.
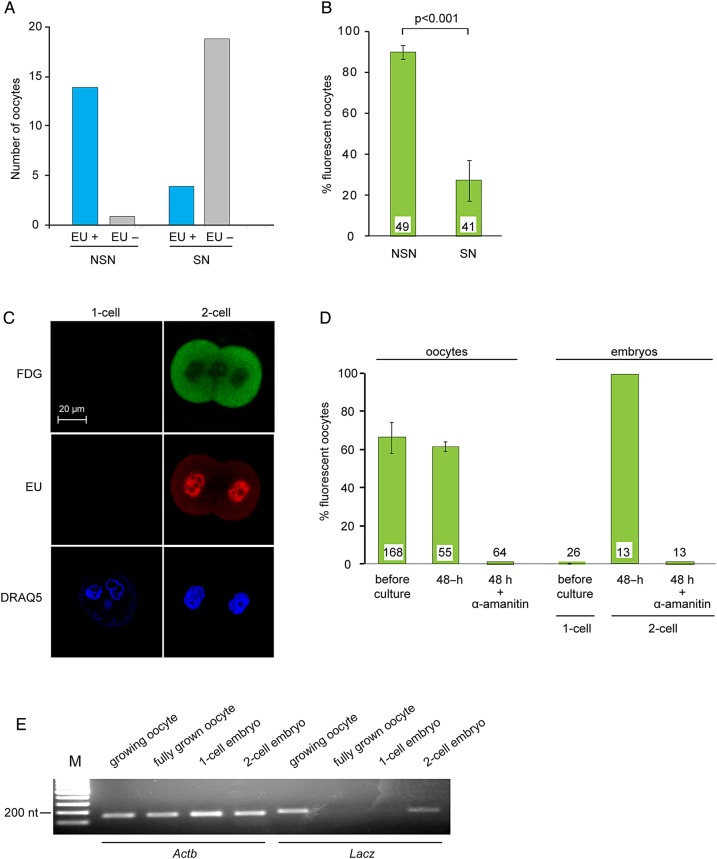
To understand the mechanisms regulating expression of β-galactosidase activity, we examined its relationship with known events of oocyte development. As growing oocytes near full size, their chromatin undergoes a change from a configuration termed NSN where the chromatin is decondensed and dispersed throughout the nucleus to one termed SN configurations where it is condensed and lines the perimeter of the nucleolus. These are easily distinguishable using a nuclear stain (Bouniol-Baly et al., 1999). Chromatin in the NSN configuration is transcriptionally active, while chromatin in the SN configuration has been associated with transcriptional arrest (Bouniol-Baly et al, 1999). Using EU incorporation to detect de novo RNA synthesis, we confirmed that NSN-type oocytes were transcriptionally active, whereas SN-type oocytes were not (Fig. 5A).
Figure 5.
Transcriptional regulation of β-galactosidase activity. (A) Oocytes within granulosa cell–oocyte complexes (GOCs) were incubated in the presence of EU to label newly synthesized RNA and then fixed and processed for EU detection and stained using DRAQ5 to assess chromatin configuration. Data are summed from four experiments. (B) Oocytes were stained using FDG to assess β-galactosidase activity and DRAQ5 to assess chromatin configuration. The experiment was performed three times. The number of oocytes examined is shown at the base of each bar. Two-sample t-test. (C) One-cell and two-cell embryos were incubated in the presence of EU and then processed to detect β-galactosidase activity (FDG) and RNA synthesis (EU). DNA was stained using DRAQ5. (D) Growing oocytes and one-cell embryos were stained using FDG. Other samples were then incubated in the absence or presence of α-amanitin for 48 h and then stained using FDG. The experiment was performed three times. The number of oocytes examined is shown at the base of each bar. (E) RNA was purified from oocytes and embryos at the indicated stages. RT-PCR was performed using primers corresponding to Actb and LacZ. The experiment was performed twice.
To determine whether β-galactosidase activity was correlated with chromatin configuration and thus transcriptional activity, we then collected GOCs from 12-day-old mice. Twenty were immediately stained with FDG and Hoechst, and in all cases, β-galactosidase activity was present and the chromatin was in the NSN configuration. The remaining GOCs were grown in vitro for 6 days and then stained as earlier. Slightly more than half retained the NSN configuration at the end of culture, and 90% of these were fluorescent (Fig. 5B). In contrast, most oocytes that progressed to the SN configuration in vitro were not fluorescent. Thus, the transition from NSN to SN chromatin configuration was associated with transcriptional arrest and with the loss of β-galactosidase activity. To confirm that β-galactosidase activity was linked to ongoing transcription, we then examined one-cell embryos, which are transcriptionally inactive, and two-cell embryos, which are active. As observed in oocytes, β-galactosidase activity paralleled nuclear incorporation of EU (Fig. 5C and D). Thus, β-galactosidase activity was closely linked to a state of active transcription in both oocytes and embryos.
To test whether the correlation between β-galactosidase activity and transcriptional activity reflected a causal relationship, GOCs and one-cell embryos were cultured in media containing α-amanitin, a highly specific inhibitor of RNA polymerase II (Lindell et al., 1970). Oocytes and embryos cultured in α-amanitin showed no detectable EU incorporation (data not shown). Moreover, when GOCs were cultured in the presence of α-amanitin for 48 h, β-galactosidase activity was no longer detectable, and one-cell embryos cultured in the presence of α-amanitin could divide to the two-cell stage, but no β-galactosidase activity was detectable (Fig. 5D). Hence, the generation of β-galactosidase activity depends on ongoing transcription in both oocytes and early embryos.
Because the stability of β-galactosidase protein in oocytes is unknown, we directly tested whether its activity faithfully reflected the presence of the encoding mRNA. We extracted RNA from growing oocytes, fully grown oocytes, one-cell and two-cell embryos of TCF/Lef-LacZ females. LacZ mRNA was detected in growing oocytes and two-cell embryos but not in fully grown oocytes or one-cell embryos (Fig. 5E). Thus, β-galactosidase activity was directly correlated with the presence of LacZ mRNA. This is consistent with the presence of the β-galactosidase protein in oocytes at early, but not late, stages of growth (Fig. 2D).
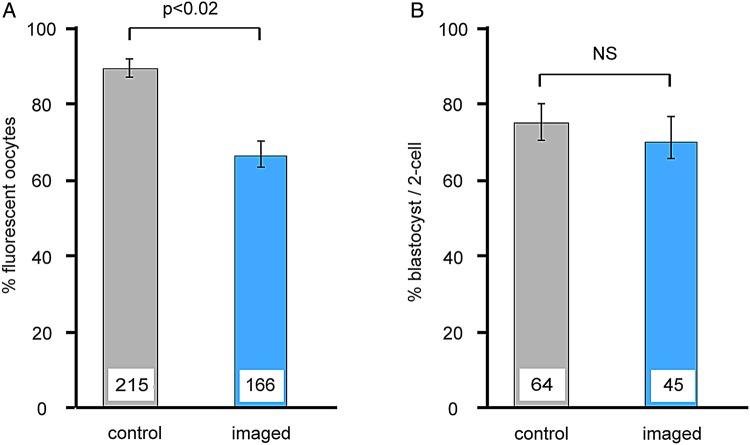
To determine whether FDG staining and imaging of live oocytes and embryos were compatible with subsequent development in vitro, we performed the procedure on GOCs and two-cell embryos and then incubated them. Following staining and imaging, GOCs were incubated for 6 days and two-cell embryos for 3 days, and then analysed. Figure 6 shows that most oocytes continued to develop following FDG imaging and became non-fluorescent indicating developmental progression, although the per cent survival was lower than in non-imaged oocytes. There was no difference between the ability of imaged and non-imaged embryos to reach the blastocyst stage. These results show that the majority of GOCs and embryos are able to continue to develop in vitro following FDG staining and imaging.
Figure 6.
Oocyte and embryo development after FDG staining and imaging. Freshly collected growing oocytes within granulosa cell–oocyte complexes (GOCs) or two-cell embryos were stained using FDG and imaged using confocal microscopy and then placed in culture for 6 days (oocytes) or 3 days (embryos). Staining and imaging were performed once for each GOC or embryo. Controls were subjected to the same culture conditions but were not stained and imaged. (A) The percentage of oocytes that remained healthy after culture as judged morphologically. (B) The percentage of embryos that reached the blastocyst stage after culture. The experiment was performed three times. The number of oocytes or embryos examined is shown at the base of each bar. Two-sample t-test.
Discussion
Previous transgenic lines have been described in which fluorescent proteins are expressed in oocytes. An OCT4-GFP fusion protein is expressed weakly in growing oocytes and more strongly in fully grown immature oocytes and metaphase II eggs (Yoshimizu et al., 1999). This marker can be useful for identifying and purifying oocytes, but its expression at metaphase II indicates that it does not reflect the transcriptional status of the germ cell. A line carrying a Dazl-Gfp transgene shows very weak fluorescence in fully grown immature oocytes, although testicular expression appears to be more robust (Nicholas et al., 2009). Most recently, a line carrying a Sohlh1-mCherry transgene has been reported, in which the reporter is expressed in oocytes of primordial and primary follicles, but not in more advanced follicles (Suzuki et al., 2013). Sohlh1-mCherry is thus a robust marker of non-growing and early growing oocytes, but its extinction at the secondary follicle stage precludes its use to monitor subsequent developmental progression. The β-galactosidase encoded by the TCF/Lef-LacZ transgene is detectable in oocytes and embryos that are transcriptionally active and is not detectable in oocytes and embryos that are transcriptionally inactive. Hence, it represents a marker that is detectable in live or fixed cells and that faithfully reflects the transcriptional activity of growing oocytes beyond the primary follicle stage and of embryos and thus of their developmental progression.
The mechanisms controlling expression of the TCF/Lef-LacZ transgene remain to be identified. Although its expression is regulated by Wnt signalling in a variety of somatic cell types and male germ cells (Yeh et al., 2011), our results suggest that its expression in oocytes and early embryos is Wnt independent. It seems probable that the transgene integrated into a relatively ‘open’ chromosomal region that contains genes that are active in oocytes and early embryos; however, we have not yet been able to identify the site of integration. We note also that there was some variability among oocytes in the intensity of the fluorescent signal. This could reflect variation among oocytes in the stage of growth (i.e. oocyte diameter) when the transgene becomes transcriptionally activated or in the processes that regulate mRNA and protein stability. The substantial depletion of the mRNA and protein in transcriptionally inactive fully grown oocytes suggests that both are short lived, at least in oocytes, and this characteristic enhances the value of the reporter as a marker of endogenous transcriptional activity.
Because the reporter gene described here permits the developmental progression of living oocytes to be assessed directly, rather than relying on surrogate markers of the somatic compartment of the follicle, it may significantly facilitate progress towards the goals of dissecting the effects of environmental and disease on oocyte development and of recapitulating female germ cell development in vitro. First, it can be used to easily track the developmental progression of oocytes, enabling different in vivo conditions or culture systems to be compared. Second, it will allow different oocytes within a group under culture to be compared. For example, by analogy with evidence that growing follicles send signals that inhibit neighbouring primordial follicles from initiating growth (Da Silva-Buttkus et al., 2009), factors secreted by growing oocytes might influence the growth of neighbouring oocytes, which would indicate whether oocytes should be grown individually or in groups. Third, it provides a simple and robust platform to rapidly assess the effect of drugs or other environmental agents on oocyte growth and development. Fourth, it provides a maker of normal oocyte development that should be useful in studies aimed at deriving functional germ cells from reprogrammed somatic cells (Dyce et al., 2011; Hayashi et al., 2012). In summary, this transgenic line should be useful for a wide range of studies that use in vitro systems either to generate fully grown mature oocytes that will give rise to healthy individuals or to uncover the molecular and mechanistic basis of oocyte growth and development.
Authors' roles
N.E. participated in experimental design, performed all experimental work and co-wrote the manuscript. R.F. participated in experimental design. H.J.C. participated in experimental design and co-wrote the manuscript.
Funding
Supported by the Natural Sciences and Engineering Research Council (H.J.C.) and by the Canadian Institutes of Health Research (H.J.C., R.F.). N.E. was supported by fellowships from the Réseau Québécois en Reproduction, the Centre for the Study of Reproduction at McGill and by the Faculty of Medicine at McGill. Support from the Fraser Fund of the Faculty of Medicine of McGill University is gratefully acknowledged.
Conflict of interest
The authors declare no conflicts of interest.
References
- Abdul-Ghani M, Dufort D, Stiles R, De Repentigny Y, Kothary R, Megeney LA. Wnt11 promotes cardiomyocyte development by caspase-mediated suppression of canonical Wnt signals. Mol Cell Biol 2011;31:163–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adriaens I, Cortvrindt R, Smitz J. Differential FSH exposure in preantral follicle culture has marked effects on folliculogenesis and oocyte developmental competence. Hum Reprod 2004;19:398–408. [DOI] [PubMed] [Google Scholar]
- Anckaert E, De Rycke M, Smitz J. Culture of oocytes and risk of imprinting defects. Hum Reprod Update 2013;19:52–66. [DOI] [PubMed] [Google Scholar]
- Bastings L, Beerendonk CC, Westphal JR, Massuger LF, Kaal SE, van Leeuwen FE, Braat DD, Peek R. Autotransplantation of cryopreserved ovarian tissue in cancer survivors and the risk of reintroducing malignancy: a systematic review. Hum Reprod Update 2013;19:483–506. [DOI] [PubMed] [Google Scholar]
- Belli M, Vigone G, Merico V, Redi CA, Zuccotti M, Garagna S. Towards a 3D culture of mouse ovarian follicles. Int J Dev Biol 2012;56:931–937. [DOI] [PubMed] [Google Scholar]
- Bouniol-Baly C, Hamraoui L, Guibert J, Beaujean N, Szollosi M, Debey P. Differential transcriptional activity associated to chromatin configuration in fully grown germinal vesicle mouse oocytes. Biol Reprod 1999;60:580–587. [DOI] [PubMed] [Google Scholar]
- Chalupnikova K, Solc P, Sulimenko V, Sedlacek R, Svoboda P. An oocyte-specific ELAVL2 isoform is a translational repressor ablated from meiotically competent antral oocytes. Cell Cycle 2014;13:1187–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christians E, Boiani M, Garagna S, Dessy C, Redi CA, Renard JP, Zuccotti M. Gene expression and chromatin organization during mouse oocyte growth. Dev Biol 1999;207:76–85. [DOI] [PubMed] [Google Scholar]
- Clarke H. Post-transcriptional control of gene expression during mouse oogenesis. In Kubiak JZ. (eds). Mouse Development. Springer Berlin Heidelberg, 2012, 1–21. [DOI] [PubMed] [Google Scholar]
- Clarke HJ, Oblin C, Bustin M. Developmental regulation of chromatin composition during mouse embryogenesis: somatic histone H1 is first detectable at the 4-cell stage. Development 1992;115:791–799. [DOI] [PubMed] [Google Scholar]
- Da Silva-Buttkus P, Marcelli G, Franks S, Stark J, Hardy K. Inferring biological mechanisms from spatial analysis: Prediction of a local inhibitor in the ovary. Proc Nat Acad Sci USA 2009;106:456–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debey P, Szollosi M, Szollosi D, Vautier D, Girousse A, Besombes D. Competent mouse oocytes isolated from antral follicles exhibit different chromatin organization and follow different maturation dynamics. Mol Reprod Dev 1993;36:59–74. [DOI] [PubMed] [Google Scholar]
- Demeestere I, Simon P, Emiliani S, Delbaere A, Englert Y. Fertility preservation: Successful transplantation of cryopreserved ovarian tissue in a young patient previously treated for Hodgkin's disease. Oncologist 2007;12:1437–1442. [DOI] [PubMed] [Google Scholar]
- Demeestere I, Streiff A, Suzuki J, Al-Khabouri S, Mahrous E, Tan SL, Clarke HJ. Follicle-stimulating hormone accelerates mouse oocyte development in vivo. Biol Reprod 2012;87:3, 1–11. [DOI] [PubMed] [Google Scholar]
- Desai N, Alex A, AbdelHafez F, Calabro A, Goldfarb J, Fleischman A, Falcone T. Three-dimensional in vitro follicle growth: overview of culture models, biomaterials, design parameters and future directions. Reprod Biol Endo 2010;8:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos M, Smitz J, Woodruff TK. Fertility preservation in women with cancer. Lancet 2014;384:1302–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolmans MM, Luyckx V, Donnez J, Andersen CY, Greve T. Risk of transferring malignant cells with transplanted frozen-thawed ovarian tissue. Fertil Steril 2013;99:1514–1522. [DOI] [PubMed] [Google Scholar]
- Donnez J, Dolmans MM. Fertility preservation in women. Nature Rev Endo 2013;9:735–749. [DOI] [PubMed] [Google Scholar]
- Donnez J, Dolmans MM, Pellicer A, Diaz-Garcia C, Sanchez Serrano M, Schmidt KT, Ernst E, Luyckx V, Andersen CY. Restoration of ovarian activity and pregnancy after transplantation of cryopreserved ovarian tissue: a review of 60 cases of reimplantation. Fertil Steril 2013;99:1503–1513. [DOI] [PubMed] [Google Scholar]
- Dyce PW, Liu J, Tayade C, Kidder GM, Betts DH, Li J. In vitro and in vivo germ line potential of stem cells derived from newborn mouse skin. PLoS One 2011;6:e20339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erba E, Ubezio P, Broggini M, Ponti M, D'Incalci M. DNA damage, cytotoxic effect and cell-cycle perturbation of Hoechst 33342 on L1210 cells in vitro. Cytometry 1988;9:1–6. [DOI] [PubMed] [Google Scholar]
- Fan H-Y, O'Connor A, Shitanaka M, Shimada M, Liu Z, Richards JS. β-catenin (CTNNB1) promotes preovulatory follicular development but represses LH-mediated ovulation and luteinization. Mol Endo 2010;24:1529–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemr M, Ma J, Schultz RM, Svoboda P. P-body loss is concomitant with formation of a messenger RNA storage domain in mouse oocytes. Biol Reprod 2010;82:1008–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge ZJ, Liang XW, Guo L, Liang QX, Luo SM, Wang YP, Wei YC, Han ZM, Schatten H, Sun QY. Maternal diabetes causes alterations of DNA methylation statuses of some imprinted genes in murine oocytes. Biol Reprod 2013;88:117. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Ogushi S, Kurimoto K, Shimamoto S, Ohta H, Saitou M. Offspring from oocytes derived from in vitro primordial germ cell-like cells in mice. Science 2012;338:971–975. [DOI] [PubMed] [Google Scholar]
- Kuroda K, Kuang S, Taketo MM, Rudnicki MA. Canonical Wnt signaling induces BMP-4 to specify slow myofibrogenesis of fetal myoblasts. Skel Musc 2013;3:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane M, Robker RL, Robertson SA. Parenting from before conception. Science 2014;345:756–760. [DOI] [PubMed] [Google Scholar]
- Lin L, Cui L, Zhou W, Dufort D, Zhang X, Cai CL, Bu L, Yang L, Martin J, Kemler R et al. . β-catenin directly regulates Islet1 expression in cardiovascular progenitors and is required for multiple aspects of cardiogenesis. Proc Natl Acad Sci USA 2007;104:9313–9318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindell TJ, Weinberg F, Morris PW, Roeder RG, Rutter WJ. Specific inhibition of nuclear RNA polymerase II by alpha-amanitin. Science 1970;170:447–449. [DOI] [PubMed] [Google Scholar]
- Lucifero D, Mann MR, Bartolomei MS, Trasler JM. Gene-specific timing and epigenetic memory in oocyte imprinting. Hum Mol Genet 2004;13:839–849. [DOI] [PubMed] [Google Scholar]
- Luzzo KM, Wang Q, Purcell SH, Chi M, Jimenez PT, Grindler N, Schedl T, Moley KH. High fat diet induced developmental defects in the mouse: oocyte meiotic aneuploidy and fetal growth retardation/brain defects. PLoS One 2012;7:e49217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahrous E, Yang Q, Clarke HJ. Regulation of mitochondrial DNA accumulation during oocyte growth and meiotic maturation in the mouse. Reproduction 2012;144:177–185. [DOI] [PubMed] [Google Scholar]
- McLaughlin M, Telfer EE. Oocyte development in bovine primordial follicles is promoted by activin and FSH within a two-step serum-free culture system. Reproduction 2010;139:971–978. [DOI] [PubMed] [Google Scholar]
- Mohamed OA, Clarke HJ, Dufort D. β-catenin signaling marks the prospective site of primitive streak formation in the mouse embryo. Dev Dyn 2004;231:416–424. [DOI] [PubMed] [Google Scholar]
- Mohamed OA, Jonnaert M, Labelle-Dumais C, Kuroda K, Clarke HJ, Dufort D. Uterine Wnt/β-catenin signaling is required for implantation. Proc Natl Acad Sci USA 2005;102:8579–8584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas CR, Xu EY, Banani SF, Hammer RE, Hamra FK, Reijo Pera RA. Characterization of a Dazl-GFP germ cell-specific reporter. Genesis 2009;47:74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama T, Mohamed OA, Taketo MM, Dufort D, Groves AK. Wnt signals mediate a fate decision between otic placode and epidermis. Development 2006;133:865–875. [DOI] [PubMed] [Google Scholar]
- Paek H, Hwang J-Y, Zukin RS, Hébert JM. β-catenin-dependent FGF signaling sustains cell survival in the anterior embryonic head by countering Smad4. Dev Cell 2011;20:689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesty A, Miyara F, Debey P, Lefevre B, Poirot C. Multiparameter assessment of mouse oogenesis during follicular growth in vitro. Mol Hum Reprod 2007;13:3–9. [DOI] [PubMed] [Google Scholar]
- Rakhmanova VA, MacDonald RC. A microplate fluorimetric assay for transfection of the β-galactosidase reporter gene. Anal Biochem 1998;257:234–237. [DOI] [PubMed] [Google Scholar]
- Sánchez F, Smitz J. Molecular control of oogenesis. Biochim Biophys Acta (BBA) 2012;1822:1896–1912. [DOI] [PubMed] [Google Scholar]
- Shea LD, Woodruff TK, Shikanov A. Bioengineering the ovarian follicle microenvironment. Ann Rev Biomed Engin 2014;16:29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smitz J, Dolmans MM, Donnez J, Fortune JE, Hovatta O, Jewgenow K, Picton HM, Plancha C, Shea LD, Stouffer RL et al. . Current achievements and future research directions in ovarian tissue culture, in vitro follicle development and transplantation: implications for fertility preservation. Hum Reprod Update 2010;16:395–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Dann CT, Rajkovic A. Generation of a germ cell-specific mouse transgenic CHERRY reporter, Sohlh1-mCherryFlag. Genesis 2013;51:50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan JH, Wang HL, Sun XS, Liu Y, Sui HS, Zhang J. Chromatin configurations in the germinal vesicle of mammalian oocytes. Mol Hum Reprod 2009;15:1–9. [DOI] [PubMed] [Google Scholar]
- Telfer EE, Zelinski MB. Ovarian follicle culture: advances and challenges for human and nonhuman primates. Fertil Steril 2013;99:1523–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telfer EE, McLaughlin M, Ding C, Thong KJ. A two-step serum-free culture system supports development of human oocytes from primordial follicles in the presence of activin. Hum Reprod 2008;23:1151–1158. [DOI] [PubMed] [Google Scholar]
- Usongo M, Farookhi R. Beta-catenin/Tcf-signaling appears to establish the murine ovarian surface epithelium (OSE) and remains active in selected postnatal OSE cells. BMC Dev Biol 2012;12:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace WH. Oncofertility and preservation of reproductive capacity in children and young adults. Cancer 2011;117:2301–2310. [DOI] [PubMed] [Google Scholar]
- Wang Q, Moley KH. Maternal diabetes and oocyte quality. Mitochondrion 2010;10:403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassarman PM. The mammalian ovum. In Knobil E. (eds). The Physiology of Reproduction. New York: Raven Press, 1988, 69–101. [Google Scholar]
- Wickramasinghe D, Ebert K, Albertini D. Meiotic competence acquisition is associated with the appearance of M-phase characteristics in growing mouse oocytes. Dev Biol 1991;143:162–172. [DOI] [PubMed] [Google Scholar]
- Xu M, Barrett SL, West-Farrell E, Kondapalli LA, Kiesewetter SE, Shea LD, Woodruff TK. In vitro grown human ovarian follicles from cancer patients support oocyte growth. Hum Reprod 2009;24:2531–2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh JR, Zhang X, Nagano MC. Wnt5a is a cell-extrinsic factor that supports self-renewal of mouse spermatogonial stem cells. J Cell Sci 2011;124:2357–2366. [DOI] [PubMed] [Google Scholar]
- Yoshimizu T, Sugiyama N, De Felice M, Yeom YI, Ohbo K, Masuko K, Obinata M, Abe K, Scholer HR, Matsui Y. Germline-specific expression of the Oct-4/green fluorescent protein (GFP) transgene in mice. Dev Gr Diff 1999;41:675–684. [DOI] [PubMed] [Google Scholar]
- Zuccotti M, Piccinelli A, Rossi P, Garagna S, Redi C. Chromatin organization during mouse oocyte growth. Mol Reprod Dev 1995;41:479–485. [DOI] [PubMed] [Google Scholar]