Abstract
Setting: Public sector hospitals and primary health clinics in the Mpumalanga Province of South Africa.
Objective: To determine whether failure to adhere to tuberculosis (TB) diagnostic guidelines (i.e., submit sputum for smear microscopy) contributed to the low bacteriological coverage reported for TB in 2008 in Mpumalanga Province.
Methods: We reviewed clinical records for new pulmonary TB cases at 30 of 118 randomly selected facilities that met the bacteriological coverage target of 80% and 30/87 facilities that did not. Data for hospital and clinic cases were abstracted into case report forms, captured electronically and compared with data from the electronic TB register (ETR). We assessed age, sex, human immunodeficiency virus (HIV) infection and facility type as potential confounders for recording of smear microscopy results.
Results: Age, sex and HIV infection did not influence recording of results. In hospitals, 61.8% of pulmonary TB cases had sputum smear results in their clinical records compared to 93.6% at clinics (P < 0.001). Of the 711 cases (30.3%) that did not have smear results in the ETR, 342 (48.1%) did have smear results in their clinical records.
Conclusion: Both poor clinical practice (especially in hospitals) and poor record keeping have contributed to the low bacteriological coverage reported. These shortcomings need to be addressed to improve patient care and programme management.
Keywords: smear recording, smear evaluation, microscopy, hospitals, primary care clinics
Abstract
Contexte : Hôpitaux publics et centres de santé primaire de la province de Mpumalanga en Afrique du Sud.
Objectif : Déterminer si le défaut de respecter les directives de diagnostic de la tuberculose (TB) (c'est-à-dire soumettre des crachats à la microscopie de frottis) a contribué à la faible couverture bactériologique rapportée en 2008.
Méthodes : Nous avons revu les dossiers cliniques des nouveaux cas de TB pulmonaire dans 30 de 118 structures sélectionnées au hasard qui ont atteint la couverture bacteriologique visée de 80% et 30 de 87 structures qui ne l'ont pas atteinte. Les données des cas suivis dans les hôpitaux et les dispensaires ont été résumées sur des formulaires, saisies en informatique et comparées aux données du registre électronique de la TB (ETR). Nous avons évalué l'âge, le sexe, l'infection au virus de l'immunodéficience humaine (VIH) et le type de structure en tant que facteurs de confusion de l'enregistrement des frottis.
Résultats : L'âge, le sexe et l'infection VIH n'ont pas influencé l'enregistrement des frottis. Dans les hôpitaux, 61,8% des cas de TB pulmonaire ont eu des résultats de frottis de crachats dans leurs dossiers cliniques comparés à 93,6% dans les dispensaires (P < 0,001). Sur les 711 cas (30,3%) qui n'avaient pas de résultats de frottis dans le ETR, 342 (48,1%) avaient des résultats de frottis dans leurs dossiers cliniques.
Conclusion : La faible couverture bactériologique constatée est due à la fois à des pratiques cliniques médiocres (surtout dans les hôpitaux) et à une maintenance insuffisante des dossiers. Ces deux problèmes doivent être résolus afin d'améliorer la prise en charge des patients et la gestion du programme.
Abstract
Marco de referencia: Los hospitales y los centros de atención primaria del sector público de salud de la provincia de Mpumalanga en Suráfrica.
Objetivo: Determinar si la falta de cumplimiento de las normas diagnósticas de la tuberculosis (TB) (por ejemplo, la presentación de muestras de esputo para baciloscopia) contribuye a la baja cobertura bacteriológica notificada en el 2008.
Métodos: Se analizaron las historias clínicas de los casos nuevos de TB pulmonar en 30 de los 118 establecimientos escogidos de manera aleatoria que cumplían con la meta de cobertura bacteriológica del 80% de los casos y en 30 de los 87 centros que no cumplían con esta meta. Los datos de los casos de los hospitales y los consultorios se consignaron en formularios de notificación, se captaron en un registro informático y se compararon con los datos del registro electrónico de la TB (ETR). Se evaluaron la edad, el sexo, la infección por el virus de la inmunodeficiencia humana (VIH) y el tipo de establecimiento como posible factores de confusión del registro de la baciloscopia.
Resultados: La edad, el sexo y la infección por el VIH no ejercieron ninguna influencia sobre el registro de la baciloscopia. En los hospitales, el 61,8% de los casos de TB pulmonar contaba con resultados de la baciloscopia del esputo en la historia clínica, en comparación con el 93,6% de los casos en los consultorios (P < 0,001). De los 711 casos que carecían de resultados de baciloscopia en el ETR (30,3%), 342 contaban con esta información en los expedientes clínicos (48,1%).
Conclusión: Se observaron prácticas clínicas inadecuadas (sobre todo en los hospitales) y deficiencias en los registros, que contribuyeron a la baja cobertura bacteriológica notificada. Es importante remediar estas insuficiencias con el fin de mejorar la atención que se presta a los pacientes y optimizar la gestión del programa contra la TB.
The examination of bacteriological specimens remains the mainstay of tuberculosis (TB) diagnosis. Access to quality assured sputum microscopy for case detection is a key component of the expanded DOTS strategy for effective TB control.1,2 Bacteriological coverage, defined as the proportion of pulmonary TB (PTB) cases with sputum smear test results (excluding children aged 0–7 years),3 is a measure both of the availability of laboratory services and of compliance with TB guidelines.
While guidelines require all presumptive pulmonary TB cases to submit at least two sputum samples obtained for smear microscopy,4,5 studies from Asia and Africa show that clinicians do not adhere to this recommendation, with an over-reliance on chest radiography and low utilisation of microscopy.6–8 Belief in the radiological diagnosis of TB persists, despite the fact that TB has various radiographic appearances and is mimicked by many other conditions, and several studies have shown that experts misdiagnose 26–43% of radiographs from confirmed cases.9
Routine data for 2008 showed that only 65% of new PTB cases in the Mpumalanga Province of South Africa were reported to have undergone smear tests, below the provincial target of 80% (source, Mpumalanga Department of Health). The reasons for the low bacteriological coverage were not known.
The aim of this study was to determine whether failure to adhere to TB diagnostic guidelines (i.e., submit sputum for evaluation) contributed to the low bacteriological coverage reported in the public health facilities. The contribution of poor record keeping, such as failure to document sputum testing results, was also assessed. Data obtained from primary health clinics were compared to those obtained from hospitals.
STUDY POPULATION AND METHODS
Setting
Mpumalanga is a largely rural province in South Africa with a population of 3.6 million and a TB case notification rate of 612 per 100 000 population in 2008. Of 22 298 TB cases reported, 89% were new TB cases and 92% had PTB. Unlike other provinces in South Africa, a high percentage of TB cases in Mpumalanga (~40%) were diagnosed and treated in hospitals, mainly on an out-patient basis. The public health sector diagnosed and treated over 95% of notified TB cases.
TB diagnosis and treatment was offered by 177 fixed public health clinics and 28 hospitals. In the study period, all presumptive TB cases were required to submit two sputum specimens for smear microscopy. The sputum specimens were transported to the local National Health Laboratory Services for microscopy. Nurses completed standardised clinical records for all patients diagnosed with TB, and recorded summary data into the paper-based facility TB registers. Self-carbonated copies of register pages were sent to the subdistrict/district level, where clerical staff captured the data into the electronic TB register (ETR), a national TB monitoring and evaluation tool. All summary TB reports, including bacteriological coverage, were drawn from the ETR. Data from three districts were collated to provide provincial reports.
Study population
Only public health facilities in Mpumalanga that reported TB patients in 2008 were included in the sampling frame. Mobile facilities were excluded for logistical reasons. The 205 facilities were sorted from best to worst bacteriological coverage, divided into two groups—118 ‘well performing’ facilities (bacteriological coverage ≥80%) and 87 ‘poorly performing’ facilities (bacteriological coverage <80%)—and 30 were randomly selected from each group. The sample included 42 primary health clinics and 18 hospitals.
Only new PTB patients at selected facilities were sampled. Patients aged <7 years, who were not diagnosed by sputum examination, were excluded, as were those who had ‘moved in’ or been ‘transferred in’, as diagnostic data for patients diagnosed elsewhere may have been incomplete.
Sample size
Open Source Epidemiological Statistics for Public Health, version 2.3 software (OpenEpi, http://www.openepi.com/Menu/OE_Menu.htm) was used to calculate the sample size for a proportion or descriptive study. We set the anticipated frequency (P) at 8%, the smallest estimated value expected for patients with no smears evaluated. The design effect was set at 2 to reflect clustering at facilities. Based on these assumptions, the sample size was calculated at n = 1370 at a 95% confidence level. We aimed to collect approximately double this number of cases to allow a comparison of hospitals and primary health clinics.
Methods
In this cross-sectional study, standardised clinical records were obtained for all new PTB cases registered in 2008 at the selected facilities. Clinical records that did not meet the inclusion criteria were discarded. A systematic sampling strategy was used and every fifth remaining record was reviewed by trained field researchers. Information from the clinical records and paper-based TB registers was abstracted into a case report form (CRF). Printed laboratory reports or written test results in the clinical record were taken as evidence of a recorded sputum result. Data were double-entered into an EpiData v3.1 database (The EpiData Association, Odense, Denmark) and verified.
Provincial ETR data for 2008 and CRF data were imported into a Microsoft SQL database (Microsoft Corp., Redmond, WA, USA). CRF data were matched on first name, family name and age or birth date to ETR data. Manual verification was undertaken for results that could not be matched electronically. Facility name, registration number and treatment start date or, where not available, registration date, were used to confirm all matches. Sputum records from the CRF and ETR were compared.
A comparative analysis of clinic and hospital data was undertaken using STATA 13 (StataCorp LP, College Station, TX, USA). Categorical data were summarised using proportions and compared using the χ2 test. Continuous data were summarised using means and standard deviations and compared using t-tests. We used a multivariable logistic regression analysis, accounting for clustering at facility level, to assess the effect of potential confounders (age, sex, human immunodeficiency virus [HIV] status and facility type) on smear recording.
Ethical considerations
The Health Research Ethics Committee of Stellenbosch University (Study number N10/09/287, 18 October 2010) and the Ethics Advisory Group of the International Union Against Tuberculosis and Lung Disease (Study number 57/10, 25 November 2010) approved the study. A waiver for informed consent was granted for the use of routine data. Permission to use routinely collected data was obtained from the Mpumalanga Department of Health.
RESULTS
Bacteriological coverage in clinical records
Sputum smear results were recorded for 2050 (78%) of the 2628 clinical records reviewed (Table 1). In the hospitals, 61.8% of clinical records had smear results recorded compared to 93.6% at the clinics (P < 0.001).
TABLE 1.
Comparison of PTB cases diagnosed at hospitals and primary health clinics

There were no significant sex differences between cases at the hospitals and clinics (Table 1). Cases at hospitals were slightly older than at the clinics (mean age 36.7 vs. 35.1 years, P = 0.002). The proportion of HIV-infected cases in the hospitals and clinics was similar (50.2% vs. 53.9%). A substantially higher proportion of cases were not tested for HIV at the hospitals than at the clinics (39.5% vs. 20.5%). HIV data were missing for 414 (15.8%) records overall: 274 (21.2%) from the hospitals and 140 (10.5%) from the clinics.
In the multivariable logistic regression analysis, age was not significantly associated with smear recording (odds ratio [OR] 1.0, 95% confidence interval [CI] 1.0–1.0, P = 0.313). There was no risk difference in smear recording between female and male cases (OR 1.2, 95%CI 0.9–1.5, P = 0.140). There was also no risk difference in smear recording between HIV-positive subjects (OR 0.7, 95%CI 0.5–1.1, P = 0.094) and those not tested for HIV (OR 0.6, 95%CI 0.4–1.0, P = 0.071) and HIV-negative cases. Cases diagnosed in clinics were more likely to have their smear results recorded than those diagnosed in hospital (OR 2.7, 95%CI 1.7–4.3, P < 0.001).
Recording comparison between clinical records and ETR
Amongst the 2628 clinical records reviewed at facilities, only 2346 (89.3%) were registered in the ETR (Figure). A higher proportion of cases were not registered at the hospitals than at the clinics (198/1292, 15.3% vs. 84/1336, 6.3%, P < 0.001).
FIGURE.
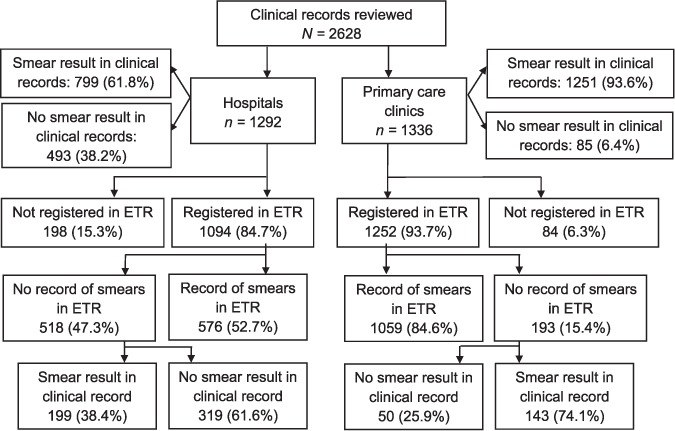
Smear recording in hospitals and primary health clinics. ETR = Electronic TB Register.
To assess the contribution of poor data capture, we compared clinical records with ETR data for the 2346 cases registered in the ETR (Table 2). In the hospitals, 199 cases (38.4%) with no smear results recorded in the ETR had smear results recorded in their clinical files compared to 143 (74.1%) at the clinics, while 98 cases at the hospitals (17.0%) and 23 cases (2.2%) at the clinics had smear results recorded in the ETR and not in their clinical records.
TABLE 2.
Correlation between smear results in clinical records and the ETR
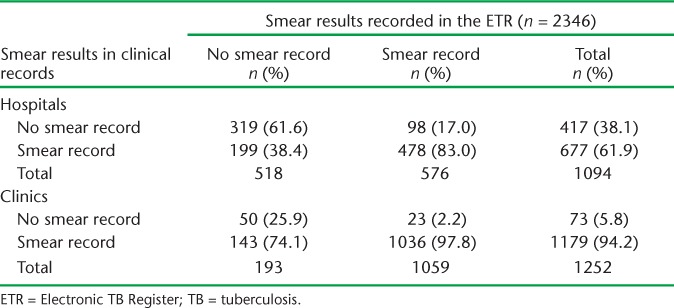
Inconsistencies between clinical records, the paper-based TB register and ETR
Sputum smears were recorded for 61.8% of clinical records in the hospitals compared to 93.6% in the clinics (P < 0.001). There was a decrease in smear recording from the clinical records to the paper-based TB registers, with 60.3% of smears recorded at hospitals and 86.8% at clinics. A further decrease occurred with capture into the ETR, with 52.7% of smears recorded in hospitals and 84.6% at the clinics.
These decreases do not reflect sequential losses between clinical records, the paper-based TB register and ETR, as some smear records are omitted and others are present, as shown above and in the comparison of clinical records and ETR data in Table 2.
DISCUSSION
The failure both to record a smear microscopy result in the clinical records and to document available results in ETR contributed to the low bacteriological coverage reported for new PTB cases in Mpumalanga Province.
We found that 22.0% of cases did not have smear results recorded in their clinical files. This was more common in hospitals (38.2%) than in clinics (6.4%). There may be several contributing factors, including clinician's failure to submit sputum for smear microscopy, the laboratory not receiving or processing the specimen, or results not being returned to the clinic or recorded in clinical files. We speculate that diagnostic practices in hospitals play a significant role, and that these may be influenced by the availability of chest X-ray facilities or by training. It is possible that clinicians in hospitals are less well trained in National TB Programme protocols than those in primary health care, as the latter tend to be the focus of TB control efforts. Irrespective of the reason, providing treatment without a smear result constitutes poor clinical practice; it contributes to the provision of empiric treatment and may result in patients being given incorrect treatment, wasting of resources and overburdening of treatment programmes.10 Other, equally important implications are that due to failure to submit sputum for evaluation, the most infectious TB cases cannot be identified, their response to treatment cannot be monitored bacteriologically and drug susceptibility cannot be evaluated. Urgent efforts are required to address these issues.
Clinicians in our setting sometimes attribute low bacteriological coverage to the inability of HIV-infected patients to produce sputum for smear microscopy. It was interesting to note that HIV status did not have a significant effect on the number of patients for whom smear results were recorded. The HIV analysis needs to be viewed with some caution in view of the 15.8% of cases with missing HIV data in the CRF. A higher proportion of cases in hospitals (39.5%) than in clinics (20.5%) did not undergo HIV testing, according to their clinical records. This has serious implications for the care of these patients, as many are likely to be HIV-infected and require antiretroviral treatment.
We also found under-reporting of new PTB cases in the ETR: 10.7% of the cases reviewed were not registered in the ETR. This was more common for cases at hospitals than at clinics. Under-reporting impacts the routine surveillance of TB control as well as for planning and management purposes, and needs to be addressed.
Among cases registered in the ETR, 711 (30.3%) did not have their smear results recorded in the ETR; however, 342 (48.1%) of these did have smear results in their clinical records. Under-reporting of smear results was more common in clinics than in hospitals: 74.1% of cases at clinics recorded with ‘no smear’ in the ETR had smear results in clinical records, compared to 38.4% in hospitals. The failure to systematically update registers may be responsible for the under-reporting, and regular supervision is required to address this problem.
The quality of ETR data is of concern, especially for hospital cases. Although the total number of smears recorded decreased between the clinical records and the facility register and then the ETR, this was not true sequential attrition that could be due, for example, to the failure to update the facility registers and the ETR. We found 98 (17.0%) cases at hospitals and 23 cases (2.2%) at clinics with smear results recorded in the ETR but with no smear results in their clinical records. As clinical records form the basis for capture into the facility paper-based TB register and then into the ETR, this may be due to incorrect recording in the facility TB register and/or data capture into the ETR. It is also possible that results are at times entered directly into the facility TB register without updating the clinical records. Several studies in South Africa have shown incorrect and incomplete recording and discrepancies between records,11–14 and efforts need to be made to improve the completeness of reporting and consistency between the different types of recording systems.
Limitations
The study had a number of limitations. First, in order to identify a mix of facilities with high and low bacteriological coverage, we stratified our sampling based on the bacteriological coverage reported in the ETR, which was found to be incomplete and inconsistent with clinical records. Second, as clinics and hospitals were not equally distributed in these two groups, the sample included may not be representative of all hospitals and clinics. Third, we did not verify that the clinical records obtained reflected all cases recorded in the TB register. It is possible that missing folders, for example for patients who had died, were not included and these are more likely to have been cases that were not appropriately evaluated. We may therefore have over-estimated bacteriological coverage. Finally, as the study is based on clinical records, we cannot determine whether the absence of a sputum result was due to sputum not being submitted, the laboratory not receiving or processing the specimen, or results not being returned to the clinic or filed. Additional studies are required to determine the influence of these factors.
Recommendations
We recommend that clinicians (both doctors and nurses) in hospitals be trained thoroughly on TB diagnostic algorithms and that regular peer or manager audits of clinical records are undertaken to ensure that training improves clinical practice. Regular register reviews to validate the correlation between data sources and improved supervision may help to improve the quality of the data.
CONCLUSION
Our findings suggest that both poor clinical practice (mostly in hospitals) and poor data capture (more commonly in clinics) contributed to the low bacteriological coverage reported in the ETR, and both need to be addressed. While the bacteriological coverage for the province was higher than routinely reported, the lack of availability of smear microscopy results for a high proportion of TB cases in hospitals has serious implications for patient care and needs to be addressed. Empirical treatment could contribute to over-burdening of TB services, and has particular relevance in an under-resourced province such as Mpumalanga.
Over the last 2 years the Xpert® MTB/RIF assay (Cepheid, Sunnyvale, CA, USA) has been introduced as a replacement for smear microscopy in South Africa, at substantial cost.15 The potential of this promising technology to improve TB control will not be realised unless sputum are correctly submitted for laboratory investigations.
Acknowledgments
This research was supported by a United States Agency for International Development (USAID) Cooperative Agreement (TREAT TB – Agreement No. GHN-A-00-800004-00). The contents are the responsibility of the authors and do not necessarily reflect the views of the USAID. The assistance of staff and managers from the Mpumalanga Department of Health is acknowledged.
Footnotes
Conflicts of interest: none declared.
References
- 1.World Health Organization. An Expanded DOTS Framework for Effective Tuberculosis Control. Geneva, Switzerland: WHO; 2002. WHO/CDS/TB/2002.297. [PubMed] [Google Scholar]
- 2.World Health Organization. Global tuberculosis report 2014. Geneva, Switzerland: WHO; 2014. WHO/HTM/TB/2014.08. [Google Scholar]
- 3.Centre for Disease Control (South Africa) ETR.Net Manual — The Electronic TB Register. Pretoria, South Africa: http://www.etrnet.info. Accessed February, 2015. [Google Scholar]
- 4.South Africa Department of Health. National Tuberculosis Management Guidelines. Pretoria, South Africa: Department of Health; 2009. [Google Scholar]
- 5.Hopewell P C, Pai M, Maher D, Upleker M, Raviglione M. International standards of tuberculosis care. Lancet Infect Dis. 2006;6:710–725. doi: 10.1016/S1473-3099(06)70628-4. [DOI] [PubMed] [Google Scholar]
- 6.Prasad R, Nautiyal R G, Mukherji P K, Jain A, Singh K, Ahuja R C. Diagnostic evaluation of pulmonary tuberculosis: what do doctors of modern medicine do in India? Int J Tuberc Lung Dis. 2003;71:52–57. [PubMed] [Google Scholar]
- 7.Shah S K, Sadiq H, Khalil M et al. Do private doctors follow national guidelines for managing pulmonary tuberculosis in Pakistan? East Mediterr Health J. 2003;9:776–788. [PubMed] [Google Scholar]
- 8.Suleiman B, Houssein A, Mehta F, Hinderaker S. Do doctors in north-western Somalia follow the national guidelines for tuberculosis management? East Mediterr Health J. 2003 Jul;9:789–795. [PubMed] [Google Scholar]
- 9.Koppaka R, Bock N. How reliable is chest radiography? In: Frieden T, editor. Toman's Tuberculosis. Case detection, treatment, and monitoring — questions and answers. 2nd ed. Geneva, Switzerland: World Health Organization; 2004. pp. 51–60. WHO/HTM/TB/2004.334. [Google Scholar]
- 10.Perkins M D, Cunningham J. Facing the crisis: improving the diagnosis of tuberculosis in the HIV era. J Infect Dis. 2007;196(Suppl 1):S15–S27. doi: 10.1086/518656. [DOI] [PubMed] [Google Scholar]
- 11.Dunbar R, Lawrence K, Verver S et al. Accuracy and completeness of recording of confirmed tuberculosis in two South African communities. Int J Tuberc Lung Dis. 2011;153:337–343. [PubMed] [Google Scholar]
- 12.Heunis C, Wouters E, Kigozi G et al. Accuracy of tuberculosis routine data and nurses' views of the TB-HIV information system in the Free State, South Africa. J Assoc Nurses AIDS Care. 2011;22:67–73. doi: 10.1016/j.jana.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 13.du Preez K D, Schaaf H S, Dunbar R et al. Incomplete registration and reporting of culture-confirmed childhood tuberculosis diagnosed in hospital. Public Health Action. 2011;1:19–24. doi: 10.5588/pha.11.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dilraj A, Bristow C C, Connolly C, Margot B, Dlamini S, Podewils L J. Validation of sputum smear results in the Electronic TB Register for the management of tuberculosis, South Africa. Int J Tuberc Lung Dis. 2013;17:1317–1321. doi: 10.5588/ijtld.12.0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyer-Rath G, Schnippel K, Long L et al. The impact and cost of scaling up GeneXpert MTB/RIF in South Africa. PLOS ONE. 2012;7:e36966. doi: 10.1371/journal.pone.0036966. [DOI] [PMC free article] [PubMed] [Google Scholar]


