Abstract
Objective
To evaluate the expression of the genes COL1A1, COL1A2, COL3A1 and COL5A1 in the glenohumeral capsule of patients with traumatic anterior instability of the shoulder.
Methods
Samples from the glenohumeral capsule of 18 patients with traumatic anterior instability of the shoulder were evaluated. Male patients with a positive grip test and a Bankart lesion seen on magnetic resonance imaging were included. All the patients had suffered more than one episode of shoulder dislocation. Samples were collected from the injured glenohumeral capsule (anteroinferior region) and from the macroscopically unaffected region (anterosuperior region) of each patient. The expression of collagen genes was evaluated using the polymerase chain reaction after reverse transcription with quantitative analysis (qRT-PCR).
Results
The expression of COL1A1, COL1A2 and COL3A1 did not differ between the two regions of the shoulder capsule. However, it was observed that the expression of COL5A1 was significantly lower in the anteroinferior region than in the anterosuperior region (median ± interquartile range: 0.057 ± 0.052 vs. 0.155 ± 0.398; p = 0.028) of the glenohumeral capsule.
Conclusion
The affected region of the glenohumeral capsule in patients with shoulder instability presented reduced expression of COL5A1.
Keywords: Shoulder instability, Joint capsule, Gene expression, Extracellular matrix, Collagen
Resumo
Objetivo
Avaliar a expressão dos genes COL1A1, COL1A2, COL3A1 e COL5A1 na cápsula glenoumeral de pacientes com instabilidade anterior traumática do ombro.
Métodos
Foram avaliadas amostras de cápsula glenoumeral de 18 pacientes com instabilidade anterior traumática do ombro. Foram incluídos pacientes masculinos, com teste de apreensão positivo e lesão de Bankart no exame de ressonância magnética. Todos os pacientes sofreram mais de um episódio de luxação do ombro. Foram coletadas amostras da cápsula glenoumeral lesionada (região anteroinferior) e da região macroscopicamente não afetada (região anterossuperior) de cada paciente. A expressão dos genes de colágeno foi avaliada por reação em cadeia da polimerase após transcrição reversa com análise quantitativa (qRT-PCR).
Resultados
A expressão de COL1A1, COL1A2 e COL3A1 não diferiu entre as duas regiões da cápsula do ombro. No entanto, foi observado que a expressão de COL5A1 estava significantemente reduzida na região anteroinferior em relação à região anterossuperior (mediana ± intervalo interquartílico: 0,057 ± 0,052 vs 0,155 ± 0,398; p = 0,028) da cápsula glenoumeral.
Conclusão
A região afetada da cápsula glenoumeral de pacientes com instabilidade do ombro apresentou uma expressão reduzida de COL5A1.
Palavras-chave: Instabilidade do ombro, Cápsula articular, Expressão gênica, Matriz extracelular, Colágeno
Introduction
The great range of motion provided by the scapular belt allows the glenohumeral joint to be used as a stable fulcrum for placing the extremities of the upper limbs in a variety of spatial positions. However, one consequence of this great range of motion is that this joint has a propensity to become unstable.1
It is believed that the shoulder is the joint of the human body that most frequently suffers dislocation, with an incidence of 8.2–23.9 cases per 100,000 individuals per year.2, 3 Among these cases, 95% are caused by traumatic events and lesions of the anterior capsule are involved in 90% of these individuals.4, 5 Episodes of shoulder dislocation occur most frequently in young male individuals.6 Many of the individuals affected practice competitive sports.7 The recurrence rate for shoulder dislocation is high and reaches up to 100% among young athletes.8, 9
The anteroinferior (AI) region of the glenohumeral capsule is the location most affected in episodes of traumatic shoulder dislocation.10 After the first episode of anterior shoulder dislocation, it is common for patients to present shoulder instability.8, 9 Patients with shoulder instability generally present plastic deformation of the capsule, which may result in capsule laxity.10, 11 Previous studies have demonstrated that plastic deformation of the capsule is necessary even in the first dislocation.12, 13, 14 Currently, little is known about the structure of the capsule, especially among patients with shoulder instability. Better comprehension of the underlying biology is important for guiding patient management and for developing new therapeutic options that are complementary to surgery.
The capsule is composed of cellular and fibrous elements. The collagen content of the capsule progressively increases during embryonic development and at birth this tissue is generally fibrous.15 Types I, III and V fibrillar collagen are the commonest types in the shoulder capsule.16 Mutations in the genes that code for these collagens have been identified in most of the forms of Ehlers–Danlos syndrome (EDS) and imperfect osteogenesis,17, 18 which present frequent dislocations in several joints, including the shoulder joint. Thus, alterations to these genes may also play a role in shoulder instability.
The aim of the present study was to compare the messenger RNA (mRNA) expression of COL1A1, COL1A2, COL3A1 and COL5A1 between an injured region and another, uninjured region of the glenohumeral capsule in patients with traumatic anterior shoulder instability.
Materials and methods
Patients
Samples were collected from the glenohumeral capsule of 18 patients with traumatic anterior shoulder instability who underwent arthroscopic surgical treatment at Hospital São Paulo, Federal University of São Paulo (UNIFESP), between June 2011 and June 2013. During joint propaedeutics, any presence of associated lesions was noted. All the patients were seen to present capsule redundancy in the anteroinferior region. A free and informed consent statement was obtained from all the patients, as approved by UNIFESP's ethics committee (procedural number: 1085/11). The patients’ mean age at the time of the surgery was 30 years (range: 18–42). Their mean age at the time of the first episode of dislocation had been 25 years (range: 14–37). The mean time that elapsed between the first dislocation and sample collection was 5 years (range: 4 months–10 years).
Only patients with a positive grip test and a Bankart lesion shown on magnetic resonance imaging examination were included in the study. In addition, all the patients reported that they had had two or more episodes of shoulder dislocation. To reduce the heterogeneity of the sample, only male individuals were included. After the first episode of dislocation, the patients were treated with shoulder immobilization for at least two weeks. None of the patients had any history of previous surgery relating to shoulder injuries.
Patients with clinical signs of posterior and/or multidirectional instability and those with generalized hypermobility or hyperlaxity according to Beighton's scale were excluded from the study.19
Tissue samples
Tissue samples were collected from two regions of the capsule in each patient: one from the injured region (AI region) and the other from the corresponding macroscopically unaffected region (control), i.e. the anterosuperior (AS) region. All the samples were immediately immersed in RNAlater® solution (Qiagen, Germany) and were then stored at −20 °C until the time of RNA extraction.
Analysis of gene expression
The RNA extraction was performed using the RNeasy® mini-kit (Qiagen, Germany). The concentration and quality were determined using a NanoDrop® spectrophotometer (Kisker, Germany) and the integrity was ascertained by means of electrophoresis on 1% agarose gel. Synthesis of complementary DNA (cDNA) was performed using the high-capacity cDNA archive kit (Life Technologies, USA).
The expression of COL1A1, COL1A2, COL3A1 and COL5A1 was evaluated by means of the polymerase chain reaction after reverse transcription with quantitative analysis (qRT-PCR), using the 7500 Fast Real-Time PCR system (Life Technologies, USA). Os genes ACTB and GAPDH were selected as internal controls for normalizing the initial quantity of cDNA and correcting possible variations that might affect the efficiency of the qRT-PCR. All qRT-PCR runs were performed in triplicate for all the target genes (COL1A1: Hs00164004_mL; COL1A2: Hs00164099_mL; COL3A1: Hs00943809_mL; and COL5A1: Hs00609088_mL) and for the reference genes (β-actin, ACTB: 4352935e; and glyceraldehyde-3-phosphate dehydrogenase, GAPDH: Hs99999905_mL) through usage of commercially available primers and probes (Life Technologies, USA).
The gene expression was determined in accordance with the formula: ΔCt (cycle threshold) = Ct target gene (collagen) − geometric mean of the Ct of the reference genes.20 The quantification of the expression was adjusted for the amplification efficiency of each gene (COL1A1 = 92%; COL1A2 = 97%; COL3A1 = 103%; COL5A1 = 94%; ACTB = 91%; and GAPDH = 92%).
Statistical analysis
The Shapiro–Wilk test was performed to evaluate whether the data presented normal distribution. Since the majority of the variables analyzed did not present normal distribution, the nonparametric Wilcoxon test was used to compare gene expression between pairs of samples from the anteroinferior and anterosuperior regions. The effect size for the Wilcoxon test (r) was calculated in accordance with the formula r = Z/√N,21 in which r < 0.1 was considered to be a trivial effect, 0.1 ≤ r < 0.3 small, 0.3 ≤ r ≤ 0.5 moderate and r > 0.5 large.
Spearman's correlation was used to evaluate whether there was any correlation between collagen gene expression and the age at which the patient was affected, the age at the time of the surgery or the time that had elapsed between the first dislocation and sample collection.
p values <0.05 were considered statistically significant. Medians and interquartile intervals of the data obtained in this study are presented.
Results
The relative expression of the genes COL1A1, COL1A2, COL3A1 and COL5A1 presented wide variation among the individuals with traumatic anterior shoulder instability. Both increased and decreased collagen gene expression in the anteroinferior region, in relation to the anterosuperior region, was observed (Fig. 1). The expression of COL1A1, COL1A2 and COL3A1 did not differ significantly between the AI and AS regions (Table 1). However, the AI region presented reduced expression of COL5A1 in relation of the AS region of the glenohumeral capsule of patients with shoulder instability (p = 0.028; r = −0.3665; Table 1).
Fig. 1.
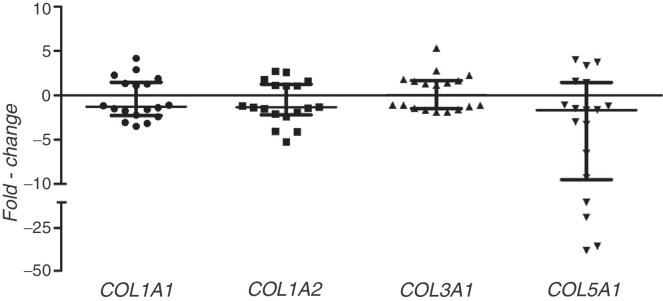
Alteration of collagen gene expression in the glenohumeral capsule of patients with shoulder instability. Expression values in the anteroinferior region in relation to the corresponding values in the anterosuperior region of the glenohumeral capsule (fold-change). Horizontal lines indicate the medians and interquartile intervals.
Table 1.
Collagen gene expression in the glenohumeral capsule of patients with shoulder instability.
| Gene | AI (median ± IQ) | AS (median ± IQ) | p value | Effect size (r) |
|---|---|---|---|---|
| COL1A1 | 1.068 ± 1.597 | 1.681 ± 1.801 | 0.372 | −0.1488 |
| COL1A2 | 0.555 ± 0.413 | 0.650 ± 0.455 | 0.145 | −0.2431 |
| COL3A1 | 0.716 ± 0.608 | 0.678 ± 0.662 | 0.879 | −0.0253 |
| COL5A1 | 0.057 ± 0.052 | 0.155 ± 0.398 | 0.028a | −0.3665 |
AI, samples from the anteroinferior region; AS, samples from the anterosuperior region; IQ, interquartile interval.
Statistically significant difference between the anteroinferior and anterosuperior regions, from Wilcoxon analysis, p < 0.05.
No correlation between the levels of collagen gene expression and the age at which the patient was affected, the age at the time of surgery or the time that had elapsed from the first dislocation to sample collection was observed in either of the regions studied (p > 0.05 for all the analyses).
Discussion
This study demonstrated that patients with anterior shoulder instability present decreased expression of the gene COL5A1 in the AI region of the glenohumeral capsule, in comparison with the AS region. Type V collagen accounts for 2–5% of the total amount of collagen in most tissues.22 This type of collagen intercalates with type I collagen, which is the predominant protein in the capsule, to form heterotypic fibrils.23 Although type V collagen is the fibrillar collagen present in the smallest quantities, it has a central role in regulating fibrillogenesis in the connective tissues.24 In EDS cases, decreased COL5A1 expression has been described in around 25–30% of the patients with mutations of this gene.25 In these patients, formation of abnormal collagen fibrils that are wide and irregular has been observed.26 In a classical animal model for EDS, decreased COL5A1 expression was associated with reductions in the numbers of fibrils and with presence of a very large and structurally aberrant subpopulation of fibrils with deficiencies of type V collagen.24 Deregulation of COL5A1 expression seems to have a fundamental role in structural alterations to the joint capsule, both in individuals with EDS and in animal models for this syndrome. Thus, we hypothesized that decreased COL5A1 expression in the AI region of the glenohumeral capsule in patients with shoulder instability could result in disorganized collagen fibrils, thereby contributing towards increased laxity of this tissue in the individuals evaluated.14, 27 On the other hand, decreased COL5A1 expression in the AI region in relation to the AS region might also be an intrinsic characteristic of the AI region that would contribute towards greater susceptibility of this region to injury, in the patients investigated.
The expression of the genes COL1A1, COL1A2 and COL3A1 did not differ between the AI and AS regions. However, the number of samples evaluated was a limiting factor in the present study. With the number of samples obtained, our analysis had a power of around 80% for detecting a variation with an effect size of 75% (large effect size), for a type I error of 5%. Large effect sizes were not detected in any of the analyses and therefore the observed power was low. Thus, our analyses had a high likelihood of not detecting a difference between the groups that in reality existed (false negatives). Further studies are needed in order to understand the role of COL1A1, COL1A2 and COL3A1 in the glenohumeral capsule of patients with shoulder instability.
In the present study, only male patients who had had more than one episode of shoulder dislocation were evaluated. The aim in making this selection was to homogenize our sample. In addition, paired samples with lesions and without lesions (controls) were obtained from the glenohumeral capsule of the same individual, so as to avoid the possibility of bias caused by biological variations between individuals. This is a strategy commonly used in molecular studies that seek to understand the processes of carcinogenesis.28 However, the mRNA levels of all the collagen genes studied were heterogenous between the patients with shoulder instability. This heterogeneity may have been due to different environmental exposures, such as variations in the number of episodes of dislocation and the time that elapsed between the dislocating events and the surgery. Moreover, like other acute soft-tissue injuries, shoulder instability is a multifactorial disease and therefore intrinsic factors, such as polymorphisms of collagen genes29, 30 or of genes coding for proteins involved in regulating their expression, may also contribute towards heterogeneity between patients and towards greater risk of the disease.
Because the only tissue samples studied were collected during the surgical treatment, it was not possible to assess the regulatory dynamics of gene expression. Nonetheless, it should be noted that this study, to the best of our knowledge, was the first to evaluate gene expression in the glenohumeral capsule of patients with shoulder instability. This study thus contributes towards comprehending this condition.
Conclusions
The injured region of the glenohumeral capsule of patients with shoulder instability presented decreased COL5A1 expression. Studying gene expression in the glenohumeral capsule of patients with shoulder instability is novel in the literature and opens up new research perspectives regarding this condition. Evaluation of gene expression may contribute towards greater comprehension of the biology of these lesions and thus towards development of new treatment strategies.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
We thank the National Council for Scientific and Technological Development (CNPq) and the Research Support Foundation of the State of São Paulo (FAPESP; grant no. 2011/10033-8) for the support provided for developing this work.
Footnotes
Please cite this article as: Belangero PS, Leal MF, de Castro Pochini A, Andreoli CV, Ejnisman B, Cohen M. Perfil de expressão de genes do colágeno na cápsula glenoumeral de pacientes com instabilidade traumática anterior do ombro. Rev Bras Ortop. 2014;49:642–646.
Work developed in the Discipline of Genetics and the Discipline of Exercise and Physical Activity Medicine of the Department of Orthopedics and Traumatology, Federal University of São Paulo, São Paulo, SP, Brazil.
References
- 1.Bulcholz R.W., Court-Brown C.M., Heckman J.D., Tornetta P., editors. Rockwood and Green's fractures in adults. 7th ed. Lippincott Williams & Wilkins; Philadelphia: 2009. [Google Scholar]
- 2.Owens B.D., Duffey M.L., Nelson B.J., DeBerardino T.M., Taylor D.C., Mountcastle S.B. The incidence and characteristics of shoulder instability at the United States Military Academy. Am J Sports Med. 2007;35(7):1168–1173. doi: 10.1177/0363546506295179. [DOI] [PubMed] [Google Scholar]
- 3.Tas M., Canbora M.K., Kose O., Egerci O.F., Gem M. Demographic and clinical characteristics of traumatic shoulder dislocations in an urban city of Turkey: a retrospective analysis of 208 cases. Acta Orthop Traumatol Turc. 2013;47(3):147–152. doi: 10.3944/aott.2013.3090. [DOI] [PubMed] [Google Scholar]
- 4.Kazar B., Relovszky E. Prognosis of primary dislocation of the shoulder. Acta Orthop Scand. 1969;40(2):216–224. doi: 10.3109/17453676908989501. [DOI] [PubMed] [Google Scholar]
- 5.Nordqvist A., Petersson C.J. Incidence and causes of shoulder girdle injuries in an urban population. J Shoulder Elbow Surg. 1995;4(2):107–112. doi: 10.1016/s1058-2746(05)80063-1. [DOI] [PubMed] [Google Scholar]
- 6.Zacchilli M.A., Owens B.D. Epidemiology of shoulder dislocations presenting to emergency departments in the United States. J Bone Joint Surg Am. 2010;92(3):542–549. doi: 10.2106/JBJS.I.00450. [DOI] [PubMed] [Google Scholar]
- 7.Buss D.D., Lynch G.P., Meyer C.P., Huber S.M., Freehill M.Q. Nonoperative management for in-season athletes with anterior shoulder instability. Am J Sports Med. 2004;32(6):1430–1433. doi: 10.1177/0363546503262069. [DOI] [PubMed] [Google Scholar]
- 8.Larrain M.V., Botto G.J., Montenegro H.J., Mauas D.M. Arthroscopic repair of acute traumatic anterior shoulder dislocation in young athletes. Arthroscopy. 2001;17(4):373–377. doi: 10.1053/jars.2001.23226. [DOI] [PubMed] [Google Scholar]
- 9.Te Slaa R.L., Wijffels M.P., Brand R., Marti R.K. The prognosis following acute primary glenohumeral dislocation. J Bone Joint Surg Br. 2004;86(1):58–64. [PubMed] [Google Scholar]
- 10.Wang V.M., Flatow E.L. Pathomechanics of acquired shoulder instability: a basic science perspective. J Shoulder Elbow Surg. 2005;14(1 Suppl. S):2S–11S. doi: 10.1016/j.jse.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Hovelius L., Eriksson K., Fredin H., Hagberg G., Hussenius A., Lind B. Recurrences after initial dislocation of the shoulder. Results of a prospective study of treatment. J Bone Joint Surg Am. 1983;65(3):343–349. [PubMed] [Google Scholar]
- 12.Rodeo S.A., Suzuki K., Yamauchi M., Bhargava M., Warren R.F. Analysis of collagen and elastic fibers in shoulder capsule in patients with shoulder instability. Am J Sports Med. 1998;26(5):634–643. doi: 10.1177/03635465980260050701. [DOI] [PubMed] [Google Scholar]
- 13.Speer K.P., Deng X., Borrero S., Torzilli P.A., Altchek D.A., Warren R.F. Biomechanical evaluation of a simulated Bankart lesion. J Bone Joint Surg Am. 1994;76(12):1819–1826. doi: 10.2106/00004623-199412000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Bigliani L.U., Pollock R.G., Soslowsky L.J., Flatow E.L., Pawluk R.J., Mow V.C. Tensile properties of the inferior glenohumeral ligament. J Orthop Res. 1992;10(2):187–197. doi: 10.1002/jor.1100100205. [DOI] [PubMed] [Google Scholar]
- 15.Aboul-Mahasen L.M., Sadek S.A. Developmental morphological and histological studies on structures of the human fetal shoulder joint. Cells Tissues Organs. 2002;170(1):1–20. doi: 10.1159/000047916. [DOI] [PubMed] [Google Scholar]
- 16.Kaltsas D.S. Comparative study of the properties of the shoulder joint capsule with those of other joint capsules. Clin Orthop Relat Res. 1983;(173):20–26. [PubMed] [Google Scholar]
- 17.Van Dijk F.S., Cobben J.M., Kariminejad A., Maugeri A., Nikkels P.G., van Rijn R.R. Osteogenesis imperfecta: a review with clinical examples. Mol Syndromol. 2011;2(1):1–20. doi: 10.1159/000332228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Callewaert B., Malfait F., Loeys B., De Paepe A. Ehlers–Danlos syndromes and Marfan syndrome. Best Pract Res Clin Rheumatol. 2008;22(1):165–189. doi: 10.1016/j.berh.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Beighton P., Solomon L., Soskolne C.L. Articular mobility in an African population. Ann Rheum Dis. 1973;32(5):413–418. doi: 10.1136/ard.32.5.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfaffl M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenthal R. Sage; Newbury Park: 1991. Meta-analytic procedures for social research. [Google Scholar]
- 22.Birk D.E. Type V collagen: heterotypic type I/V collagen interactions in the regulation of fibril assembly. Micron. 2001;32(3):223–237. doi: 10.1016/s0968-4328(00)00043-3. [DOI] [PubMed] [Google Scholar]
- 23.Chanut-Delalande H., Fichard A., Bernocco S., Garrone R., Hulmes D.J., Ruggiero F. Control of heterotypic fibril formation by collagen V is determined by chain stoichiometry. J Biol Chem. 2001;276(26):24352–24359. doi: 10.1074/jbc.m101182200. [DOI] [PubMed] [Google Scholar]
- 24.Wenstrup R.J., Florer J.B., Davidson J.M., Phillips C.L., Pfeiffer B.J., Menezes D.W. Murine model of the Ehlers–Danlos syndrome. col5a1 haploinsufficiency disrupts collagen fibril assembly at multiple stages. J Biol Chem. 2006;281(18):12888–12895. doi: 10.1074/jbc.M511528200. [DOI] [PubMed] [Google Scholar]
- 25.Mitchell A.L., Schwarze U., Jennings J.F., Byers P.H. Molecular mechanisms of classical Ehlers–Danlos syndrome (EDS) Hum Mutat. 2009;30(6):995–1002. doi: 10.1002/humu.21000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hausser I., Anton-Lamprecht I. Differential ultrastructural aberrations of collagen fibrils in Ehlers–Danlos syndrome types I–IV as a means of diagnostics and classification. Hum Genet. 1994;93(4):394–407. doi: 10.1007/BF00201664. [DOI] [PubMed] [Google Scholar]
- 27.Thomas S.C., Matsen F.A., 3rd. An approach to the repair of avulsion of the glenohumeral ligaments in the management of traumatic anterior glenohumeral instability. J Bone Joint Surg Am. 1989;71(4):506–513. [PubMed] [Google Scholar]
- 28.Leal M.F., Chung J., Calcagno D.Q., Assumpcao P.P., Demachki S., da Silva I.D. Differential proteomic analysis of noncardia gastric cancer from individuals of northern Brazil. PLoS ONE. 2012;7(7):e42255. doi: 10.1371/journal.pone.0042255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khoschnau S., Melhus H., Jacobson A., Rahme H., Bengtsson H., Ribom E. Type I collagen alpha1 Sp1 polymorphism and the risk of cruciate ligament ruptures or shoulder dislocations. Am J Sports Med. 2008;36(12):2432–2436. doi: 10.1177/0363546508320805. [DOI] [PubMed] [Google Scholar]
- 30.Collins M., Posthumus M., Schwellnus M.P. The COL1A1 gene and acute soft tissue ruptures. Br J Sports Med. 2010;44(14):1063–1064. doi: 10.1136/bjsm.2008.056184. [DOI] [PubMed] [Google Scholar]


