Abstract
Osteochondromas are bone protuberances surrounded by a cartilage layer. They generally affect the extremities of the long bones in an immature skeleton and deform them. They usually occur singly, but a multiple form of presentation may be found. They have a very characteristic appearance and are easily diagnosed. However, an atypical site (in the axial skeleton) and/or malignant transformation of the lesion may sometimes make it difficult to identify osteochondromas immediately by means of radiographic examination. In these cases, imaging examinations that are more refined are necessary. Although osteochondromas do not directly affect these patients’ life expectancy, certain complications may occur, with varying degrees of severity.
Keywords: Osteochondroma/etiology, Osteochondroma/physiopathology, Osteochondroma/diagnosis, Bone neoplasms
Resumo
Osteocondromas são protuberâncias ósseas envolvidas por uma camada de cartilagem. Atingem, habitualmente, as extremidades dos ossos longos no esqueleto imaturo e os deformam. Em geral são únicos, mas a forma de apresentação múltipla pode ser encontrada. De aspecto bastante característico, são de fácil diagnóstico. Contudo, por vezes, a localização atípica (esqueleto axial) e/ou a malignização da lesão podem dificultar a sua pronta identificação por exames radiográficos. Nesses casos, exames de imagem mais apurados são necessários. Apesar de não afetarem diretamente a expectativa de vida do portador, algumas complicações, com variados graus de gravidade, podem ocorrer.
Palavras-chave: Osteocondroma/etiologia, Osteocondroma/fisiopatologia, Osteocondroma/diagnóstico, Neoplasias ósseas
Introduction
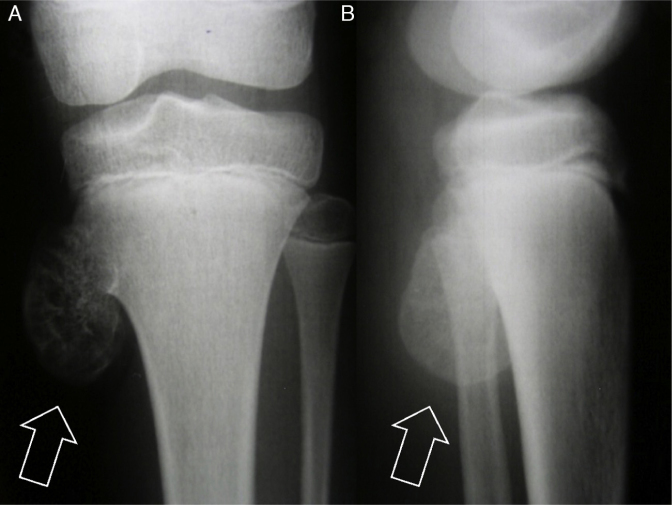
Debate continues as to whether osteochondroma is a developmental disorder (pseudotumoral lesion) or a neoplasm.1 Nonetheless, irrespective of whether it is a pseudotumoral lesion or a more common benign bone tumor,2 it is certainly an exostosis (external bone proliferation that deforms the bone).3 This bone protuberance is generally found in the immature skeleton of children and adolescents (Fig. 1).
Fig. 1.
Anteroposterior (AP) radiograph (A) and lateral radiograph (B) of the left knee. Note exostosis (osteochondroma – arrows) in the proximal region of the tibia in a skeletally immature patient.
According to the World Health Organization (WHO), osteochondromas are bone projections enveloped by a cartilage cover that arise on the external surface of the bone.1 Despite their predominant composition of bone, their growth takes place in the cartilaginous portion.4
They present two distinct clinical forms5: single lesions (solitary osteochondromas) and several lesions (multiple osteochondromas).
Solitary osteochondroma
This entity is also known as an osteochondromatous exostosis,1 osteocartilaginous exostosis4, 5 or simply exostosis.2
Multiple osteochondromas
Among the various synonyms used in the literature, the commonest ones are: hereditary multiple exostosis, multiple cartilaginous exostosis, hereditary osteochondromatosis and multiple hereditary osteochondromatosis.
Epidemiology
Solitary osteochondroma
This form constitutes 10% of all bone tumors and, among these, 35% (20–50%) of the benign tumors.1, 4, 5, 6, 7, 8 Single lesions are found in 85% of the individuals diagnosed with osteochondroma.5 The exostosis is commonly identified during childhood or adolescence.1, 4
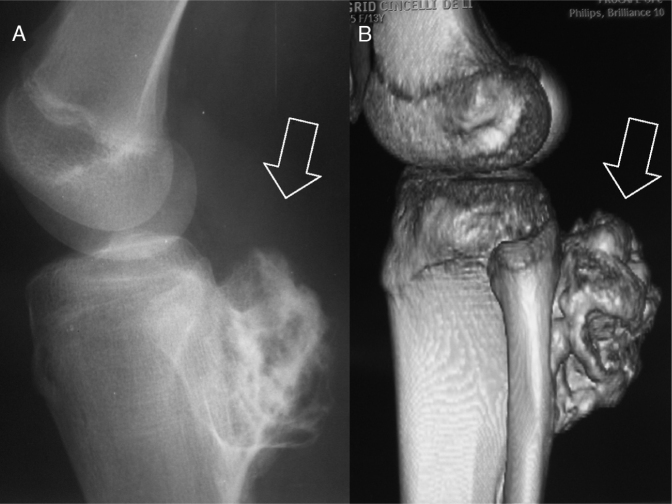
Osteochondromas more frequently affect the appendicular skeleton (upper and lower limbs).5 The long bones of the lower limbs are the bones most commonly affected.6, 9, 10, 11 The knee is the region most affected (40% of the cases) (Fig. 2).5, 6, 7, 12 After the knee, the proximal portions of the femur and the humerus are the sites preferentially affected. After osteochondromas appear in the long bones, they usually become located in the metaphysis and only rarely in the diaphysis.2 Flat bones like the scapula and hip may also be involved (Fig. 3).5
Fig. 2.
The long bones of the lower limbs (knee region) are most commonly affected. (A) Simple lateral radiograph. (B) Computed tomography with 3D reconstruction. Note lesion (arrows) in the proximal region of the tibia.
Fig. 3.
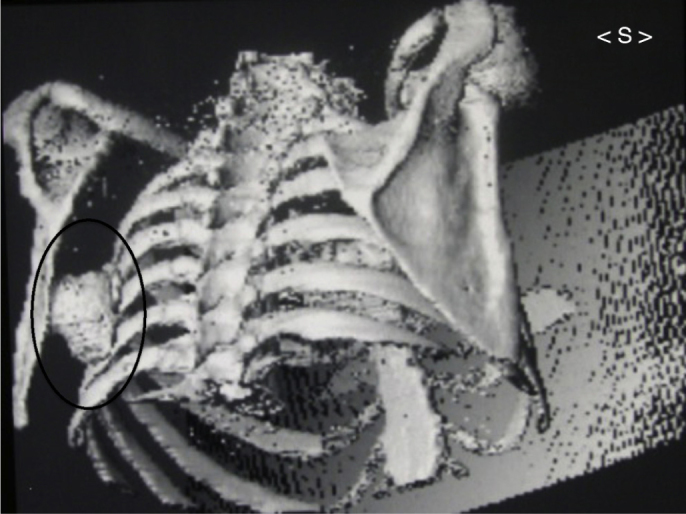
Image of 3D reconstruction from computed tomography of chest. Note single exostosis inside black oval figure in the region of the body of the left scapula, beside the ribs.
Despite the slight predominance of the male gender over the female gender that has been reported by some authors,4, 5, 7 it seems that there is no effective predilection according to sex.1
Multiple osteochondromas
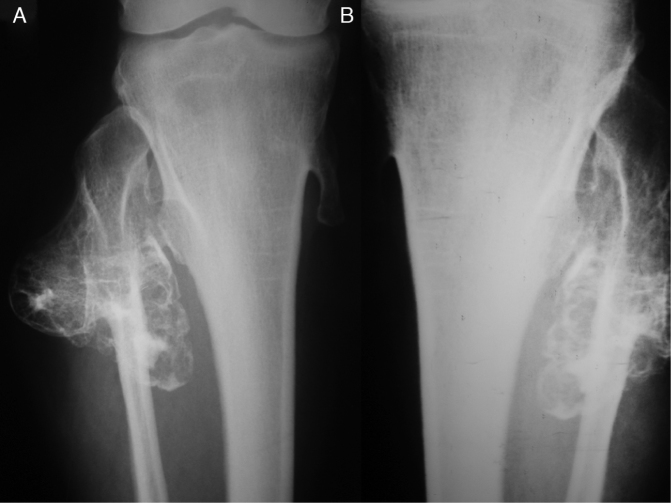
Some authors have reported that the incidence of multiple osteochondromas is 1:50,000 individuals.1, 13 Among patients with exostosis, 15% have multiple lesions.1 In this presentation, osteochondromas tend to be large and sessile, with a lobulated abundant cartilaginous cover.5 In the same way as seen with solitary osteochondromas, multiple osteochondromas have a predilection for the metaphysis of the long bones, and especially those of the lower limbs (Fig. 4).14
Fig. 4.
Hereditary multiple exostosis. (A and B) In the knees, radiographs showing multiple lesions in the proximal regions of the tibias and fibulas.
The ages of patients with multiple lesions are similar to those of others with single exostoses, and there is also no predilection according to sex.1
Etiology
The cause of osteochondromas remains unknown. Based on the similarity of the cartilaginous cover of the exostosis to the growth cartilage (growth plate) of the bone, several hypotheses have been put forward, all of them relating to alterations to the growth plate.1 Another fact that corroborates the possible correlation between the cartilage (of the osteochondroma and epiphyseal plate) is that when skeletal maturity is reached (after adolescence), the growth of the lesion usually also ceases.2 Thus, the lesion seems to result from separation of a fragment of growth cartilage (from the immature skeleton), which suffers herniation.2 Continuous growth of this loose piece of cartilage and its subsequent endochondral ossification forms a salience that projects from the bone surface, coated with a covering of cartilage.2 However, it is still unclear how this separation actually occurs.2
The variant with multiple lesions is a dominant autosomal alteration15, 16 that is transmitted by both sexes and is characterized by the presence of several osteochondromas.2 In this group, most of the individuals have a positive family history and/or mutation in one of the EXT genes.17, 18 These genes (EXT1, EXT2 and EXT3) are found in chromosomes 8, 11 and 19, respectively.19, 20, 21, 22
Clinical diagnosis
Solitary osteochondroma
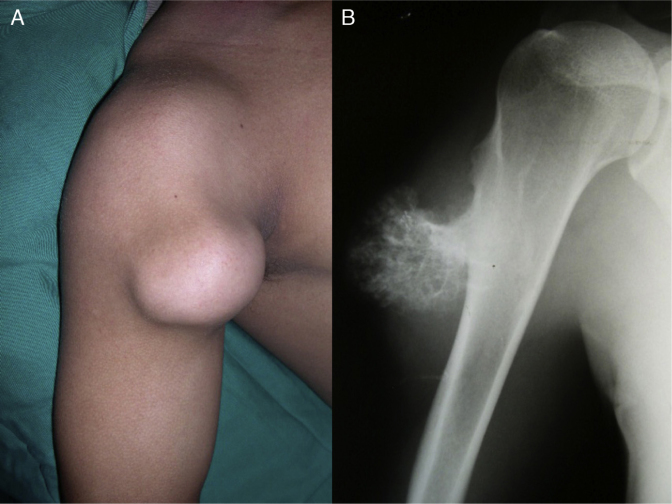
Among solitary osteochondromas, the vast majority are asymptomatic.7, 8, 15, 23 In fact, they are usually discovered by chance. After they have been detected, they present slowly increasing bulging and hardened consistency, but are painless (Fig. 5).1, 2
Fig. 5.
In the clinical examination (A), painless slowly growing bulging of hardened consistency is sometimes observed. (B) Radiograph of the proximal region of the right humerus of the same patient.
Symptomatic cases are often related to the size and location of the exostosis. In the immature skeleton, the osteochondroma grows slowly and progressively along with the bone involved, and it stops when skeletal maturity is reached.24
In a few cases, pain of greater intensity may be present, associated with complications of a mechanical origin1 that are promoted by the projection of hard tissue (bone) into the soft tissues.14 Whether due to simple contact, compression or friction, varying degrees of paresthesia, paresis, cracking, edema, redness or pallor can be observed, depending on the anatomical structure affected by the exostosis.
In osteochondromas of pedunculate type (see imaging diagnostics section), acute pain may occur due to fracturing of the base of the pedicle following local trauma.1, 4, 14, 25
Multiple osteochondromas
In the multiple form of this condition, low height, deformities of the bones affected and disproportion between the trunk and limbs can be observed.2, 5, 14, 17, 26, 27, 28 Severe involvement of some bones promotes shortening and osteoarticular deformity, with consequent limitation of joint range of motion.14 The main examples of this comprise deformity of the forearm (due to shortening of the ulna), inequality of the lengths of the lower limbs and angling (varus or valgus) of the knee (Fig. 6).13, 29, 30
Fig. 6.
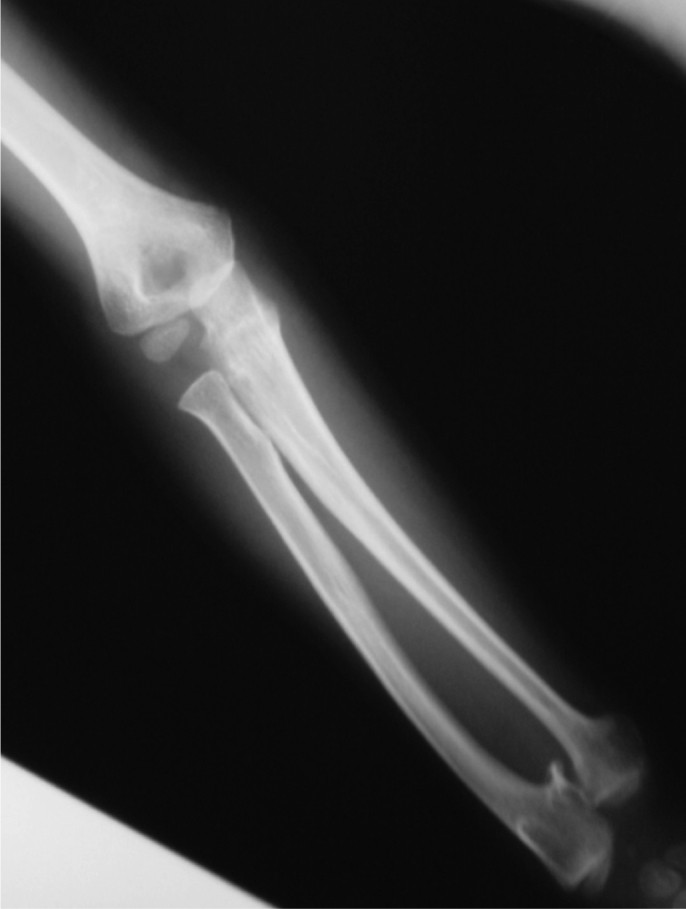
Radiograph of an individual with hereditary multiple exostosis. Note the deformity of the forearm (due to shortening of the ulna).
Malignant transformation
Rapidly increasing lesion size and local pain processes suggest that sarcomatous transformation is occurring in individuals with osteochondroma that was previously asymptomatic.1, 16, 28, 30, 31 Continuing growth of the lesion after skeletal maturity is reached should also awaken such suspicions. Other clinical findings that are occasionally reported include slight increases in soft tissues, elevation of temperature and local erythema.30
Imaging diagnostics
Simple radiographs
The radiographic appearance reflects the composite nature of the lesion, formed by cortical and medullary bone tissue,2 which projects outwards from the affected bone. It is precisely the continuity of the lesion with the surface of the host bone that is pathognomonic for osteochondroma.2 This continuity is easily observed in lesions that “inhabit” the long bones,2 in the standard radiographic views (two images in orthogonal planes). However, in planar bones (pelvis and scapula) and irregular bones (vertebrae), this relationship and consequently the diagnosis may not be evident on simple radiographs alone (Fig. 7).2
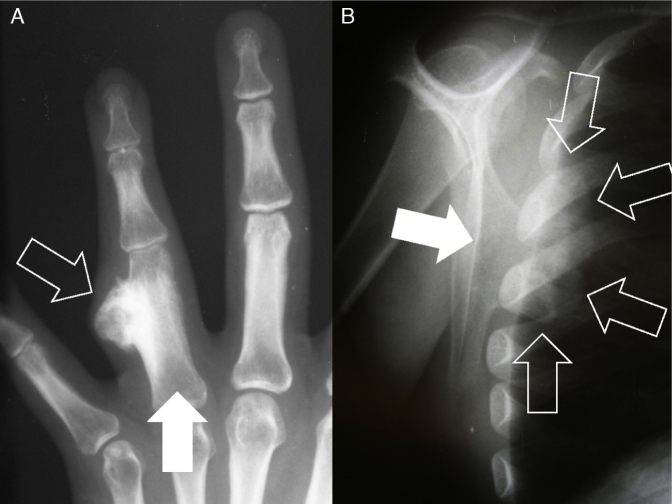
Fig. 7.
Radiographs showing projecting osteochondromas (open arrows) in different types of bone. (A) In the long bones (for example, the phalanx – filled arrow), the standard radiographic views (two images in orthogonal planes) are sufficient for the diagnosis. (B) However, in planar bones (for example, the scapula – filled arrow) and irregular bones, exostoses may not be so evident on simple radiographs alone.
The characteristic image consists of an external bone protuberance1, 4 and it may have a wide base (sessile) or a narrow base (pedicled or pedunculated) (Fig. 8). Because of the singular appearance of these lesions, it is possible in most cases, for example, to do away with biopsies for diagnosing them.
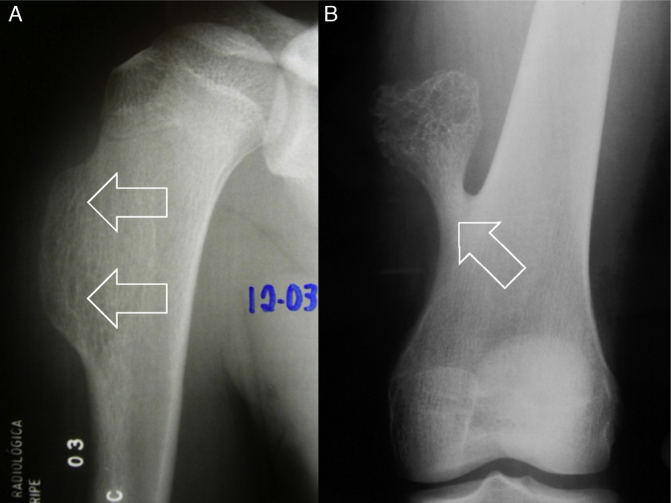
Fig. 8.
Different types of osteochondroma. Note that in examination (A), the lesion on the humerus is sessile (with wide base – arrows), while in (B), it is pedicled or pedunculated (narrow base [arrow], i.e. less in relation to its height).
The cartilaginous cover is often not visible in these examinations, because its density is similar to that of the surrounding soft tissues.15 However, cartilaginous calcifications may sometimes be observed.15, 23, 31 Irregular calcification is sometimes seen.1 However, on radiographs with excessive calcification of “flake” type,1 sarcomatous transformation of the osteochondroma should be suspected.
Computed tomography
This technique complements radiographs and shows details of the continuity of the cortical and spongy bone inside the lesion32, 33, 34, 35, 36, 37 and their relationship with the adjacent soft tissues (Fig. 9). Axial tomographic slices facilitate interpretation2 of the lesions located in anatomical sites of greater complexity,23 such as the spine and the belts of the upper and lower limb (Fig. 10).
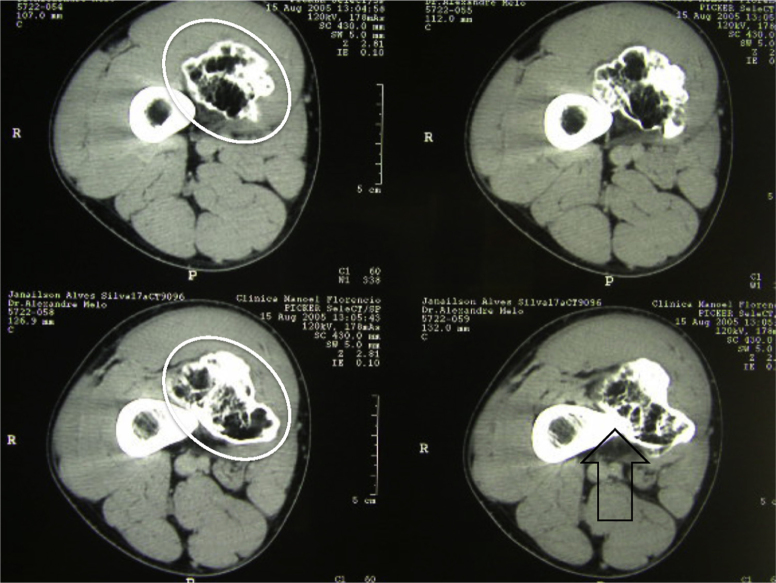
Fig. 9.
Axial computed tomography slices from the distal region of the thigh. Detail from exostosis in the medial region (white oval figure). Note continuity of the lesion with the cortical bone (open black arrow) and its relationship with the adjacent soft tissues.
Fig. 10.
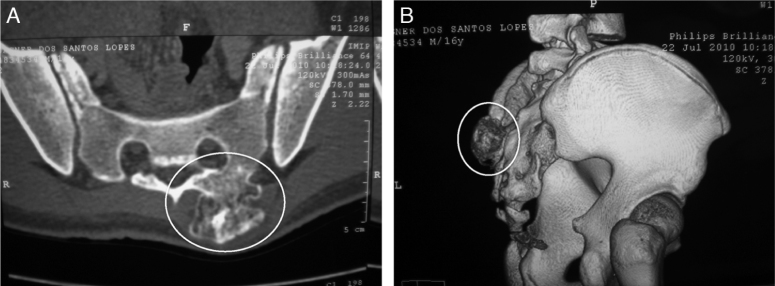
Computed tomography images facilitate locating the exostoses (white oval figures) at anatomical sites of greater complexity (such as the spine–sacral region). (A) Axial image. (B) 3D reconstruction.
Magnetic resonance
This is an examination that also demonstrates the cortical and medullary continuity between the osteochondroma and host bone.2 In the same way as seen in a normal piece of bone, the cortical bone of the exostosis presents low signal intensity (hyposignal) in all sequences, whereas the medullary component continues to have the appearance of the yellow medulla (Fig. 11A).2
Fig. 11.
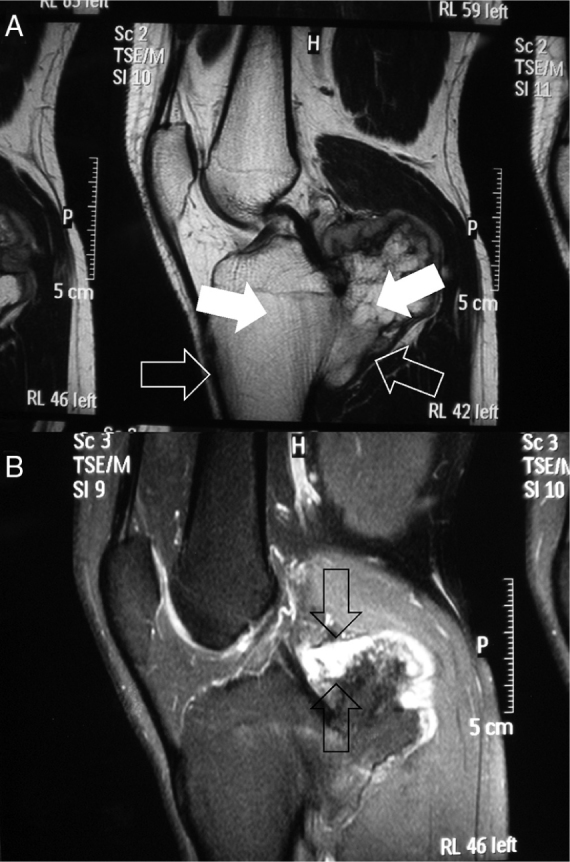
Magnetic resonance images. (A) T1-weighted sagittal image (note hyposignal of the cortical bone and the lesion [open arrows] and hypersignal of the bone medulla in both [filled arrows]). (B) T2-weighted sagittal image (note that the greatest thickness of the cartilaginous cover was around 1.5 cm [between arrows]).
This is accepted as the safest imaging method for evaluating structures adjacent to the osteochondroma and for observing and measuring the cartilage cover2, 30 that envelops the exostosis. The thickness of this layer is used as a criterion for differentiating suspected sarcomatous malignant transformation from cartilaginous tissue1, 30 (Fig. 11B). However, there is no consensus of opinions in this regard.30 Some authors1, 4, 38 have suggested that a thickness greater than 2 cm (in adults) may be indicative of malignant transformation, while others have accepted this possibility when it is greater than 1.5 cm.2 It has to be borne in mind that during childhood, this cartilage layer is naturally thicker than in the mature skeleton and may reach 3 cm. Calcified areas of the cover present low signal intensity in T1 and T2-weighted sequences.2 However, high concentrations of water in the non-calcified portion of this layer show an intermediate to low signal on T1-weighted images and a high signal on T2-weighted images.2
Bone scintigraphy
The cartilaginous tissue (cover) of the exostosis may or may not present high uptake of radiopharmaceuticals, both under conditions of normality and in situations of malignant transformation (secondary chondrosarcoma). For this reason, bone scintigraphy does not have great value in differentiating between benign and malignant cartilaginous lesions.39
Anatomopathological diagnosis
Macroscopic appearance
The lesion surface is lobulated and has an abundant cartilaginous cover (Fig. 12).5 These are lesions that vary in size considerably: from 1 to 10 cm.2 The cartilage cover may present dimensions of 1–3 cm in thickness in younger patients.6, 9, 12, 32, 33, 40, 41
Fig. 12.
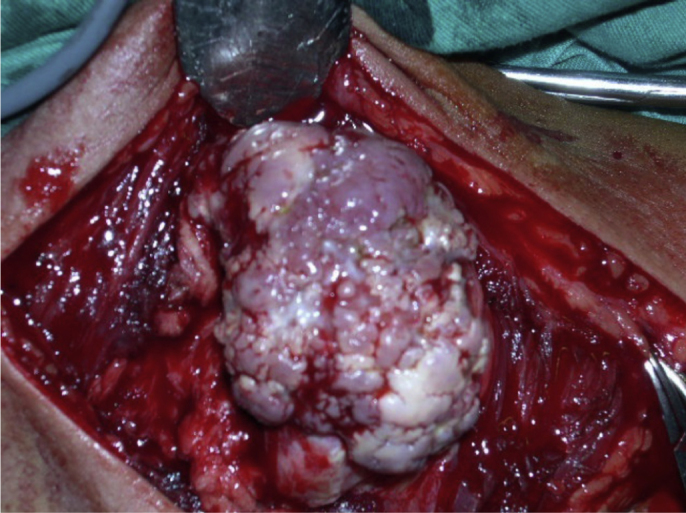
Intraoperative photograph of excision of an osteochondroma. Note its multilobulated surface and cartilage cover.
Microscopic appearance
Solitary and multiple osteochondromas are histologically similar.30 The lesion presents three layers1: perichondrium (most external), cartilage (intermediate) and bone (most internal).
Malignant transformation
Differentiation from normal cartilage is generally done in relation to secondary chondrosarcoma of low-grade malignity.30 Loss of cartilage architecture, mitotic activity, presence of cell atypia and necrosis are some of the findings that may indicate secondary malignant transformation.1
Treatment
Solitary osteochondroma
Presence of an exostosis is, in itself, insufficient reason for its surgical excision, especially in isolated cases.42 For individuals with single lesions, the management is expectant in the great majority of the cases, with successive return visits because of the chance (albeit small) of malignant transformation.
Surgical removal is indicated if the tumor causes pain or functional incapacity,4 either due to neurovascular compression or due to limitation of joint movement (Fig. 13). Another situation for surgical removal relates to fracturing of the base of the osteochondroma.25
Fig. 13.
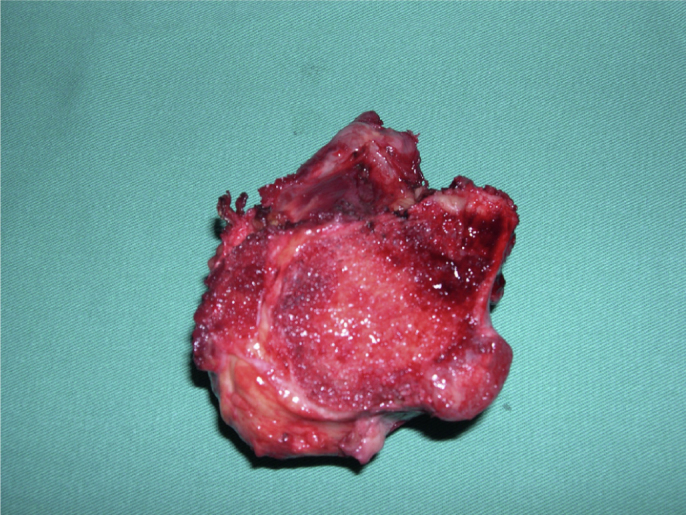
Surgical resection (specimen) was chosen for this exostosis that was causing vascular compression in the popliteal region.
Multiple osteochondromas
In these patients, the treatment is more complex. In the multiple forms of this pathological condition, osteochondromas are removed surgically for cosmetic reasons,43 in order to avoid progression of the bone deformities. In the forearm, for example, simple excision of the lesion (in the distal portion of the ulna) may impede local deformity.44
Malignant transformation
Sarcomatous transformation is generally treated by means of wide surgical resection, with preservation of the limb,30 while following rigorous oncological criteria.
Complications
Among the possible complications of these lesions are fractures (generally of pedunculated exostoses, at their base), vascular lesions (formation of pseudoaneurysm) and neurological complications (compression of peripheral nerves, which involves the spine or the periarticular regions), formation of a bursa (which affects the cartilaginous surface of the lesion, resulting from local friction) and malignant transformation.5, 14, 30, 45 This last complication, which is the most feared of all the complications, is very variable in frequency: in solitary osteochondroma cases, it occurs in less than 1%1, 16, 23, 45; while in patients with multiple lesions it may range from 1% to 30%1, 4, 5, 6, 9, 46, 47, 48 in different series. However, studies conducted more recently have suggested that the prevalence is lower: 3% to 5% in individuals with multiple osteochondromatosis.49, 50, 51, 52, 53, 54
Final remarks
Osteochondromas are benign lesions that do not affect life expectancy. However, the risk of malignant transformation (to secondary chondrosarcoma) should be taken onto consideration, especially in cases of multiple exostoses.
In symptomatic cases or those with atypical locations, other types of imaging examination should be requested, with a view to making a precise diagnosis. Furthermore, if there is clinical suspicion of malignant transformation and/or radiographic alterations in comparison with old examinations, magnetic resonance imaging is well indicated for detailed analysis on the thickness of the cartilaginous coating.
In situations in which excision of the osteochondroma is chosen, this is usually curative. Recurrence is seen in cases of incomplete removal.
The overall survival of patients with sarcomatous transformation is generally good. However, those with poorly differentiated lesions have a much worse prognosis.
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Please cite this article as: de Souza AMG, Bispo Júnior RZ. Osteocondroma: ignorar ou investigar?. Rev Bras Ortop. 2014;49:555–564.
References
- 1.Khurana J., Abdul-Karim F., Bovée J.V.M. Osteochondroma. In: Fletcher C.D., Unni K.K., Mertens F., editors. Pathology and genetics of tumours of the soft tissues and bones. IARC Press; Lyon: 2002. pp. 234–237. [Google Scholar]
- 2.Murphey M.D., Choi J.J., Kransdorf M.J., Flemming D.J., Gannon F.H. Imaging of osteochondroma: variants and complications with radiologic–pathologic correlation. Radiographics. 2000;20(5):1407–1434. doi: 10.1148/radiographics.20.5.g00se171407. [DOI] [PubMed] [Google Scholar]
- 3.Costeira O. Farmoquímica; Rio de Janeiro: 2001. Termos e expressões da prática médica. [Google Scholar]
- 4.Unni K.K. 5th ed. Thomas; Springfield: 1996. Osteochondroma. Dahlin's bone tumors: general aspects and data on 11,087 cases; pp. 11–23. [Google Scholar]
- 5.Dorfman H.D., Czerniak B. Mosby; St. Louis: 1998. Osteochondroma. Bone tumors; pp. 331–346. [Google Scholar]
- 6.Resnick D., Kyriakos M., Greenway G.D. Osteochondroma. In: Resnick D., editor. Diagnosis of bone and joint disorders. 3rd ed. Saunders; Philadelphia: 1995. pp. 3725–3746. [Google Scholar]
- 7.Giudici M.A., Moser R.P., Jr., Kransdorf M.J. Cartilaginous bone tumors. Radiol Clin North Am. 1993;31(2):237–259. [PubMed] [Google Scholar]
- 8.Scarborough M.T., Moreau G. Benign cartilage tumors. Orthop Clin North Am. 1996;27(3):583–589. [PubMed] [Google Scholar]
- 9.Mirra J.M. Lea & Febiger; Philadelphia: 1989. Benign cartilaginous exostoses: osteo-chondroma and osteochondromatosis. Bone tumors: clinical, radiologic, and pathologic correlations; pp. 1626–1659. [Google Scholar]
- 10.Milgram J.W. The origins of osteochondromas and enchondromas. A histopathologic study. Clin Orthop Relat Res. 1983;(174):264–284. [PubMed] [Google Scholar]
- 11.Keith A. Studies on the anatomical changes which accompany certain growth-disorders of the human body: I. The nature of the structural alterations in the disorder known as multiple exostoses. J Anat. 1920;54(Pt 2–3):101–115. [PMC free article] [PubMed] [Google Scholar]
- 12.Unni K.K. 5th ed. Thomas; Springfield: 1996. Chondrosarcoma (primary, secondary, dedifferentiated, and clear-cell). Dahlin's bone tumors: general aspects and data on 11,087 cases; pp. 71–108. [Google Scholar]
- 13.Schmale G.A., Conrad E.U., 3rd, Raskind W.H. The natural history of hereditary multiple exostoses. J Bone Joint Surg Am. 1994;76(7):986–992. doi: 10.2106/00004623-199407000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Stieber J.R., Dormans J.P. Manifestations of hereditary multiple exostoses. J Am Acad Orthop Surg. 2005;13(2):110–120. doi: 10.5435/00124635-200503000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Steiner G.C. Benign cartilage tumors. In: Taveras J.M., Ferrucci J.T., editors. Radiology: diagnosis – imaging – intervention. JB Lippincott; Philadelphia: 1992. pp. 1–3. [Google Scholar]
- 16.Harms S.E., Greenway G. Musculoskeletal tumors. In: Stark D.D., Bradley W.G., editors. Magnetic resonance imaging. 2nd ed. Mosby Year Book; St. Louis: 1992. pp. 2132–2133. [Google Scholar]
- 17.Legeai-Mallet L., Munnich A., Maroteaux P., Le Merrer M. Incomplete penetrance and expressivity skewing in hereditary multiple exostoses. Clin Genet. 1997;52(1):12–16. doi: 10.1111/j.1399-0004.1997.tb02508.x. [DOI] [PubMed] [Google Scholar]
- 18.Bovée J.V., Hogendoorn P.C. Multiple osteochondromas. In: Fletcher C.D., Unni K.K., Mertens F., editors. World Health Organization Classification of Tumours. Pathology and genetics of tumours of soft tissue and bone. IARC Press; Lyon: 2002. pp. 360–362. [Google Scholar]
- 19.Wu Y.Q., Heutink P., de Vries B.B., Sandkuijl L.A., van den Ouweland A.M., Niermeijer M.F. Assignment of a second locus for multiple exostoses to the pericentromeric region of chromosome 11. Hum Mol Genet. 1994;3(1):167–171. doi: 10.1093/hmg/3.1.167. [DOI] [PubMed] [Google Scholar]
- 20.Lüdecke H.J., Johnson C., Wagner M.J., Wells D.E., Turleau C., Tommerup N. Molecular definition of the shortest region of deletion overlap in the Langer-Giedion syndrome. Am J Hum Genet. 1991;49(6):1197–1206. [PMC free article] [PubMed] [Google Scholar]
- 21.Parrish J.E., Wagner M.J., Hecht J.T., Scott C.I., Jr., Wells D.E. Molecular analysis of overlapping chromosomal deletions in patients with Langer-Giedion syndrome. Genomics. 1991;11(1):54–61. doi: 10.1016/0888-7543(91)90101-j. [DOI] [PubMed] [Google Scholar]
- 22.Hecht J.T., Hogue D., Strong L.C., Hansen M.F., Blanton S.H., Wagner M. Hereditary multiple exostosis and chondrosarcoma: linkage to chromosome II and loss of heterozygosity for EXT-linked markers on chromosomes II and 8. Am J Hum Genet. 1995;56(5):1125–1131. [PMC free article] [PubMed] [Google Scholar]
- 23.Resnick D., Kyriakos M., Greenway G.D. Tumors and tumor-like lesions of bone: imaging and pathology of specific lesions. In: Resnick D., Niwayama G., editors. Diagnosis of bone and joint disorders. 2nd ed. Saunders; Philadelphia: 1988. pp. 3648–3720. [Google Scholar]
- 24.Margolis M., McLennan M.K. Radiology rounds. Osteochondroma. Can Fam Physician. 1995;41(216):220–222. [PMC free article] [PubMed] [Google Scholar]
- 25.Tanigawa N., Kariya S., Kojima H., Komemushi A., Fujii H., Sawada S. Lower limb ischaemia caused by fractured osteochondroma of the femur. Br J Radiol. 2007;80(952):e78–e80. doi: 10.1259/bjr/44678280. [DOI] [PubMed] [Google Scholar]
- 26.Wicklund C.L., Pauli R.M., Johnston D., Hecht J.T. Natural history study of hereditary multiple exostoses. Am J Med Genet. 1995;55(1):43–46. doi: 10.1002/ajmg.1320550113. [DOI] [PubMed] [Google Scholar]
- 27.McCormick C., Duncan G., Tufaro F. New perspectives on the molecular basis of hereditary bone tumours. Mol Med Today. 1999;5(11):481–486. doi: 10.1016/s1357-4310(99)01593-2. [DOI] [PubMed] [Google Scholar]
- 28.Hennekam R.C. Hereditary multiple exostoses. J Med Genet. 1991;28(4):262–266. doi: 10.1136/jmg.28.4.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shapiro F., Simon S., Glimcher M.J. Hereditary multiple exostoses. Anthropometric, roentgenographic, and clinical aspects. J Bone Joint Surg Am. 1979;61(6):815–824. [PubMed] [Google Scholar]
- 30.Shah Z.K., Peh W.C., Wong Y., Shek T.W., Davies A.M. Sarcomatous transformation in diaphyseal aclasis. Australas Radiol. 2007;51(2):110–119. doi: 10.1111/j.1440-1673.2007.01679.x. [DOI] [PubMed] [Google Scholar]
- 31.Greenspan A. Tumors of cartilage origin. Orthop Clin North Am. 1989;20(3):347–366. [PubMed] [Google Scholar]
- 32.Kenney P.J., Gilula L.A., Murphy W.A. The use of computed tomography to distinguish osteochondroma and chondrosarcoma. Radiology. 1981;139(1):129–137. doi: 10.1148/radiology.139.1.6937887. [DOI] [PubMed] [Google Scholar]
- 33.Lange R.H., Lange T.A., Rao B.K. Correlative radiographic, scintigraphic, and histological evaluation of exostoses. J Bone Joint Surg Am. 1984;66(9):1454–1459. [PubMed] [Google Scholar]
- 34.Hudson T.M., Springfield D.S., Spanier S.S., Enneking W.F., Hamlin D.J. Benign exostoses and exostotic chondrosarcomas: evaluation of cartilage thickness by CT. Radiology. 1984;152(3):595–599. doi: 10.1148/radiology.152.3.6611561. [DOI] [PubMed] [Google Scholar]
- 35.Kobayashi H., Kotoura Y., Hosono M., Fujimoto R., Tsuboyama T., Itoh H. 3D-spiral CT of multiple exostoses. Comput Med Imaging Graph. 1995;19(5):419–422. doi: 10.1016/0895-6111(95)00025-9. [DOI] [PubMed] [Google Scholar]
- 36.Lee P.C., Chen W.J., Tu Y.K., Chen L.H. Solitary osteochondroma of the lumbar spine with cord compression: a case report. Changgeng Yi Xue Za Zhi. 1998;21(2):227–231. [PubMed] [Google Scholar]
- 37.Moriwaka F., Hozen H., Nakane K., Sasaki H., Tashiro K., Abe H. Myelopathy due to osteochondroma: MR and CT studies. J Comput Assist Tomogr. 1990;14(1):128–130. doi: 10.1097/00004728-199001000-00025. [DOI] [PubMed] [Google Scholar]
- 38.Lee J.K., Yao L., Wirth C.R. MR imaging of solitary osteochondromas: report of eight cases. AJR Am J Roentgenol. 1987;149(3):557–560. doi: 10.2214/ajr.149.3.557. [DOI] [PubMed] [Google Scholar]
- 39.Lee F.Y., Yu J., Chang S.S., Fawwaz R., Parisien M.V. Diagnostic value and limitations of fluorine-18 fluorodeoxyglucose positron emission tomography for cartilaginous tumors of bone. J Bone Joint Surg Am. 2004;86(12):2677–2685. doi: 10.2106/00004623-200412000-00014. [DOI] [PubMed] [Google Scholar]
- 40.Malghem J., Vande Berg B., Noël H., Maldague B. Benign osteochondromas and exostotic chondrosarcomas: evaluation of cartilage cap thickness by ultrasound. Skeletal Radiol. 1992;21(1):33–37. doi: 10.1007/BF00243091. [DOI] [PubMed] [Google Scholar]
- 41.Garrison R.C., Unni K.K., McLeod R.A., Pritchard D.J., Dahlin D.C. Chondrosarcoma arising in osteochondroma. Cancer. 1982;49(9):1890–1897. doi: 10.1002/1097-0142(19820501)49:9<1890::aid-cncr2820490923>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 42.Bispo Júnior R.Z., de Souza A.M.G., Mello Júnior C.F. Osteocondroma. In: Bispo Júnior R.Z., Mello Júnior C.F., editors. Ortopedia Básica. Cap 6. Revinter; Rio de Janeiro: 2014. pp. 63–69. [Google Scholar]
- 43.Bovée J.V. Multiple osteochondromas. Orphanet J Rare Dis. 2008;3:3. doi: 10.1186/1750-1172-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Akita S., Murase T., Yonenobu K., Shimada K., Masada K., Yoshikawa H. Long-term results of surgery for forearm deformities in patients with multiple cartilaginous exostoses. J Bone Joint Surg Am. 2007;89(9):1993–1999. doi: 10.2106/JBJS.F.01336. [DOI] [PubMed] [Google Scholar]
- 45.Severo A., Calieron L.G., Kuhn A. Compressäo do nervo fibular comum por osteocondroma: relato de caso. Rev Bras Ortop. 2001;36(9):356–358. [Google Scholar]
- 46.Meissner S.A., Vieth V., August C., Winkelmann W. Radiology–pathology conference: osteosarcoma in a cartilaginous exostosis of the femur. Clin Imaging. 2006;30(3):206–209. doi: 10.1016/j.clinimag.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 47.Pierz K.A., Stieber J.R., Kusumi K., Dormans J.P. Hereditary multiple exostoses: one center's experience and review of etiology. Clin Orthop Relat Res. 2002;(401):49–59. doi: 10.1097/00003086-200208000-00008. [DOI] [PubMed] [Google Scholar]
- 48.Lee K.C., Davies A.M., Cassar-Pullicino V.N. Imaging the complications of osteochondromas. Clin Radiol. 2002;57(1):18–28. doi: 10.1053/crad.2001.0719. [DOI] [PubMed] [Google Scholar]
- 49.Fischgrund J.S., Cantor J.B., Samberg L.C. Malignant degeneration of a vertebral osteochondroma with epidural tumor extension: a report of the case and review of the literature. J Spinal Disord. 1994;7(1):86–90. doi: 10.1097/00002517-199407010-00013. [DOI] [PubMed] [Google Scholar]
- 50.Young C.L., Sim F.H., Unni K.K., McLeod R.A. Chondrosarcoma of bone in children. Cancer. 1990;66(7):1641–1648. doi: 10.1002/1097-0142(19901001)66:7<1641::aid-cncr2820660732>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 51.Norman A., Sissons H.A. Radiographic hallmarks of peripheral chondrosarcoma. Radiology. 1984;151(3):589–596. doi: 10.1148/radiology.151.3.6718712. [DOI] [PubMed] [Google Scholar]
- 52.Willms R., Hartwig C.H., Böhm P., Sell S. Malignant transformation of a multiple cartilaginous exostosis – a case report. Int Orthop. 1997;21(2):133–136. doi: 10.1007/s002640050136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bell R.S. Musculoskeletal images. Malignant transformation in familial osteochondromatosis? Can J Surg. 1999;42(1):8. [PMC free article] [PubMed] [Google Scholar]
- 54.Ostlere S.J., Gold R.H., Mirra J.M., Perlman R.D. Case report 658: chondrosarcoma of the proximal phalanx of right fourth finger secondary to multiple hereditary exostoses (MHE) Skeletal Radiol. 1991;20(2):145–148. doi: 10.1007/BF00193831. [DOI] [PubMed] [Google Scholar]