Abstract
Objective
Autism spectrum disorders (ASDs) were once considered lifelong disorders, but recent findings indicate that some children with ASDs no longer meet diagnostic criteria for any ASD and reach normal cognitive function. These children are considered to have achieved ‘optimal outcomes’ (OO). The present study aimed to retrospectively examine group differences in the intervention history of children and adolescents with OO and those with high-functioning autism (HFA)
Method
The current study examined intervention histories in 34 individuals with OO and 44 individuals with HFA (currently ages 8-21), who did not differ on age, sex, nonverbal IQ or family income. Intervention history was collected through detailed parent questionnaires.
Results
Children in the OO group had earlier parent concern, received earlier referrals to specialists, and earlier and more intensive intervention than those in the HFA group. Substantially more OO children received Applied Behavior Analysis (ABA) therapy than HFA children, although the intensity of ABA did not vary between groups. Children in the HFA group were more likely to have received medication, especially anti-psychotics and anti-depressants. There were no group differences in the percent of children receiving special diets or supplements.
Conclusion
These data suggest that OO individuals generally receive earlier, more intense interventions and more ABA, while HFA individuals receive more pharmacologic treatments. While the use of retrospective data is a clear limitation to the current study, the substantial differences in reported provision of early intervention, and ABA in particular, are highly suggestive and should be replicated in prospective studies.
Keywords: autism spectrum disorder, optimal outcomes, intervention history, medication
Autism Spectrum Disorders (ASDs) are generally considered lifelong disorders. However, some studies have described the phenomenon of “optimal outcome,” in which individuals lose their ASD diagnosis. In 1987, Lovaas first defined “recovery” in autism as achieving success in a regular classroom with average IQ scores, but did not determine whether autism symptoms had completely resolved.1 Mundy2 pointed out that these criteria do not by themselves constitute ‘recovery’, as some individuals with high-functioning autism (HFA) may reach this outcome while still showing significant autism symptoms. Subsequent studies have defined “optimal outcome” (OO) more stringently and proposed a definition requiring a well-documented history of ASD, no current criteria for ASD, and having both IQ and adaptive functioning within the average range.3 One of the most important questions about this group of ‘optimal outcome’ individuals is whether their intervention histories differ from those of individuals with high-functioning autism.
There are several comprehensive treatment approaches for autism, including Applied Behavior Analysis (ABA),1 Early Start Denver Model (ESDM),4 and Floor Time.5 In addition, many children receive ancillary therapies such as speech-language therapy (SLP), occupational therapy (OT) and physical therapy (PT).
ABA is generally considered the autism intervention supported by the most evidence.8 In the Lovaas study,1 47% of children with autism who received intensive ABA met his criteria for ‘recovery’, compared with only 2% in the less intensive control group1, and their gains were maintained.9 These studies have been criticized on methodological grounds (e.g., different pre- and post-intervention measures, non-random assignments, and inadequate control groups) and, as mentioned above, lack of outcome measures for core symptoms.10,11 Attempts at replication have yielded mixed results; while studies have been consistent in supporting intensive ABA, most outcomes have not been as positive as those reported byLovaas.12-14
Fewer studies of comprehensive treatment approaches other than ABA have been published. Dawson and colleagues4 examined the effectiveness of the ESDM, an intervention approach in which behavioral techniques are integrated into a developmental framework, and found that children who received the ESDM displayed significantly larger IQ and adaptive functioning gains compared with those who received standard community treatment. In addition, no controlled study to date for other early intervention approaches have reported outcomes of “recovery,” as was found for some children receiving ABA.
In addition to the Lovaas studies, Gabriels, Hill, Pierce, etal15 examined the intensity of behavioral intervention and subsequent gains in young children with autism, and found that, although not statistically different, the “high outcome” group received an average of 40.3 more treatment hours per month and an average of 1073 more total treatment hours than the “low outcome” group. Luiselli, Cannon, Ellis, and Sission16 also found that the number of months of treatment was related to improvements in language, cognitive, and socioemotional functioning. On the other hand, several other studies have found no meaningful relationship between number of intervention hours and cognitive and behavioral gains.16,17
There are several complicating factors in the relationship between treatment intensity and outcome. First, intervention is generally measured in terms of quantity rather than quality. It may be that both quantity and quality make independent contributions to outcome or that the effect of quantity is moderated by quality. Quality is particularly difficult to judge from retrospective reports. Second, children who make slower progress or are more impaired may receive more intensive treatment as a consequence, making interpretation of the relationship between progress and treatment intensity complex.3
While medical treatment is typically considered an adjunctive therapy, many children with autism do receive pharmacologic treatments (i.e., prescribed psychotropic medications).18 In general, medications are more useful in the treatment of ancillary symptoms such as irritability, aggression, hyperactivity, poor sleep, and poor attention, than in treating core symptoms.18-20
Some children with autism also receive complementary-alternative medicine (CAM) treatments, including dietary supplements, modified diets, neurofeedback; chelation and hyperbaric oxygen.18,21 Other than the use of melatonin for sleep, there is no clear evidence that any of these CAM treatments are efficacious for core or secondary symptoms of autism.18,21
Although prospective randomized controlled trials would be the most methodologically sound way to compare the number of children reaching ‘optimal outcome’ across treatments, such randomization would be fraught with practical and ethical issues; furthermore, the small percent of children likely to reach ‘optimal outcome’ would make the initial sample needed prohibitively large. We, therefore, used a sample of OO children and adolescents and collected retrospective reports of their therapies at different ages.
The current study utilizes the sample described in Fein et al.,22 which examined a group of OO children and adolescents aged 8 to 21 and compared them to children and adolescents with high-functioning autism (HFA) and typical development (TD). The authors found that the OO participants did not differ from the TD participants on summary measures of socialization, communication, face recognition, most language subscales. or any academic measure, including reading comprehension and written expression.21 A small number of the OO children continued to show some difficulty on components of executive function.22 To date, no one has obtained intervention histories for a group of OO children.
Thus, the aim of the present study is to retrospectively examine the intervention history of the OO and HFA individuals to determine if intervention differences may have contributed to their outcomes. The unique aspect of this study is having a group of OO children and adolescents who have clearly lost their autism diagnosis and are functioning essentially identically to their typically developing peers. Based on the previous literature, we hypothesized that a greater percent of the OO group than the HFA group would have received ABA and that the OO group would have received earlier and more intensive intervention than the HFA group. We did not have an a priori hypothesis about other interventions as these have not been linked to optimal outcome in other studies.
Methods
Participants
Participants were 25 individuals with a history of ASD and OO and 34 high-functioning individuals with a current ASD diagnosis (HFA). This subset of participants from the Fein et al.22 paper included those with complete intervention data. Participants ranged from 8 to 21 years. Groups did not differ on age (M(HFA)=14.0, M(OO)=12.7, t=1.70, p=.095), gender (HFA=31 males:3 females, OO=20:5, χ2(1, n=59)=1.54, p=.22), and nonverbal IQ (M(HFA)=110.1, M(OO)=109.8, t=0.075, p=.94), but were significantly different on verbal IQ(M(HFA)=104.4, M(OO)=114.4, t=2.76, p=.008). Participants tested at the University of Connecticut were primarily from Connecticut or Massachusetts. However, ten participants in the HFA group and thirteen participants in the OO group tested at the University of Connecticut were from other states (AZ, CO, DC, FL, ME, MI, MN, NH, NJ, NY, and UT) or from Canada. Because early intervention varies significantly by region, statistical analyses were conducted with the entire sample and then with the subset of the sample from Connecticut or Massachusetts (24 HFA and 12 OO participants), which have similar early intervention practices, in order to best equate opportunities based on location. Participants were mostly Caucasian, with 3 OO and 2 HFA individuals reporting other races or ethnicities. Families were generally of middle and high income, with 13 of 30 HFA families (43%) earning under $100,000 per year, and 17 earning over $100,000. For the OO group, 10 of 24 (42%) earned under $100,000 and 14 over $100,000. Dividing up the income groups in several different ways resulted in no significant Fisher's exact test or chi-square values. However, it should be noted that three families in the HFA group reported annual incomes of $30,000 or less, compared with none in the OO group, so it is possible that larger groups would have resulted in lower SES for the HFA group.
Recruitment was done through media outlets (newspaper stories, radio interviews), private practices, word of mouth between participant families, and clinic referrals. Participants were also referred from the principal investigators' private practices, the Psychological Services Clinic at the University of Connecticut, and from other ongoing studies at the University of Connecticut. The study was approved by Institutional Review Boards at the University of Connecticut, the Institute of Living of Hartford Hospital and Queens University. See Fein et al.22 for a flow chart of participant inclusion and exclusion.
Inclusion criteria
All participants had verbal, nonverbal, and full-scale IQ standard scores greater than 77 (within 1.5 SD of the average of 100). Additional OO criteria were:
Participants had an ASD diagnosis before the age of 5 by a physician or psychologist specializing in autism, in a written report, with documented early language delay (no words by 18 months or no phrases by 24 months). To confirm diagnosis, the report was edited to remove information about diagnosis, summary, and recommendations but leaving descriptions of behavior. One of the co-investigators (MB), an expert in diagnosis of ASD and Director of the University of Connecticut Psychological Services Clinic, reviewed these reports, blind to early diagnosis and current group membership. In addition to potential OO participants, she reviewed 24 “foil” reports for children with non-ASD diagnoses, such as global delay or language disorder. Four potential OO participants were rejected for insufficient early documentation, and were dropped from the study. All 24 foils were correctly rejected.
On phone screening, parents had to report that the participant had typically developing friends. During evaluation, participants could not meet criteria for any ASD on the Autism Diagnostic Observation Schedule (ADOS)23 administered by a research-reliable interviewer. In addition, the ADOS videotapes of all potential OO cases were reviewed by a clinician with more than 15 years of autism diagnostic experience (MB, IME, or DF) who confirmed that ADOS scores were below ASD thresholds and that, in their expert clinical judgment, an ASD was not present. Five potential OO participants were judged to have social impairments with an autistic quality and were excluded.
Communication and Socialization domains of the Vineland Adaptive Behavior Scales (Vineland)24 had to be greater than 77 (within 1.5SDs of the mean of 100).
Participants had to be fully included in regular education classrooms with no one-on-one assistance and no special education services to address autism deficits (e.g., no social skills training). However, participants could be receiving limited special education services or psychological support to address impairments not specific to ASDs, such as attention or academic difficulties.
To be included in the HFA group:
Following Collaborative Programs of Excellence in Autism guidelines,25 participants had to meet criteria for ASD on the ADOS (both Social and Communication domains and total score) and according to best estimate clinical judgment.
Exclusion criteria
Potential participants for any group were excluded if (1) at the time of telephone screening they exhibited major psychopathology (e.g., active psychotic disorder) that would impede full participation, (2) they had severe visual or hearing impairments, or (3) they had a seizure disorder, Fragile X, or head trauma with loss of consciousness. Two in the HFA group were excluded because of possible seizure disorder; none were excluded for other reasons.
Procedure
Potential participants who passed telephone screening were scheduled for an assessment. For participants under 18, parent consent and child assent were obtained prior to testing. For participants 18 and over, their informed consent was obtained. Intervention history was obtained through questionnaires completed by parents.
Measures
The Wechsler Abbreviated Scale of Intelligence (WASI)26 was used to assess verbal and nonverbal cognitive abilities. The Vineland Adaptive Behavior Scales (Vineland)24 assessed Communication and Socialization skills. Modules 3 or 4 of the Autism Diagnostic Observation Schedule (ADOS)23, a structured play and interview session, were used to assess symptoms of autism. The Autism Diagnostic Interview, Revised (ADI-R)27 and the lifetime version of the Social Communication Questionnaire (Lifetime-SCQ)28 were used to determine childhood symptom severity and age of first concerns.
Parents reported the specific type of intervention and hours per week for the following age periods: before 1.5 years; age 1.5-2; age 2-2.5; age 2.5-3; 1st year of preschool (age 3-4); and 2nd year of preschool (age 4-5). For each interval, parents were asked to indicate which of the following services the child received: ABA, Developmental Therapy (including FloorTime), Speech-Language Therapy, Occupational Therapy, Special Education Class/Special School, and Sensory Integration Therapy. The form also asked about current and previous medications, nutritional supplements, and special diets.
Results
Early Symptom Severity/Age of First Concerns
The SCQ-Lifetime was used to examine autism symptom severity in early childhood. Both groups scored above the autism cutoff; however, symptom severity was greater in the HFA group (M(HFA)=22.4, M(OO)=16.0, t=3.85, p<.001). On the ADI-R, parents in the OO group reported a somewhat earlier age of first concern about their child's development than parents in the HFA group (M(HFA)=22.0 months, M(OO)=16.7 months, t=2.00, p=.052). Still, both groups reported early concerns, as shown by mean ages before the second birthday. On the ADI-R, the interviewer's judgment of age when developmental abnormalities first manifested did not differ between the groups (M(HFA)=15.1 months, M(OO)=15.4 months, t=0.16, p=.87). This suggests that age of onset of symptoms did not differ between the groups, but that parents became concerned about five months later on average in the HFA group. The age at which children were reported to have been referred to a specialist (behavioral specialist, developmental pediatrician, geneticist, neurologist, psychiatrist, psychologist, speech/language pathologist) because of concerns about development was later in the HFA than OO group (M(HFA)=43.9 months, M(OO)=26.1 months, t=3.79, p=.001). The three low SES families of HFA children had an average age of first concern of 23 months, very similar to the HFA group as a whole.
Behavioral/Developmental Interventions
Significantly more participants in the OO group (83%) received Birth to Three services compared to participants in the HFA group (48%), χ2 (1, n=56)=6.73, p=.009, Cramer's V=0.35, a medium effect size. Similarly, significantly more participants in the OO group (92%) attended preschool than participants in the HFA group (56%), χ2 (1, n=58)=8.70, p=.003, Cramer's V=0.39.
As shown in Table 1, very few participants in either group received any intervention prior to 1.5 years. The percentage of children receiving intervention in each group increased continuously with age, except for a drop in the HFA group during the second year of preschool. Significantly more OO children received intervention between 2.5 and 3 years and between 4 and 5 years, with small-medium effect sizes, and with a trend at the 2-2.5 year age. A similar pattern held for the subset of the sample from CT and MA, although, because of the smaller sample size, the only significant difference was for the 4 to 5 age period.
Table 1. Percent of participants who received any type of intervention and mean number of weekly intervention hours, by age.
| Frequency of Participants Receiving Intervention | ||||
|---|---|---|---|---|
| Age | HFA Total N=28 | OO Total N=25 | Chi-Square p Value | Effect Size Cramer's V |
| Before 1.5 years | 4% N=1 | 12% N=3 | .25 | .16 |
| 1.5-2 years | 39% N=11 | 36% N=9 | .81 | .034 |
| 2-2.5 years | 43% N=12 | 68% N=17 | .066 | .25 |
| 2.5-3 years | 61% N=17 | 88% N=22 | .025 | .31 |
| 1st Year of Preschool (3-4 years) | 86% N=24 | 88% N=22 | .81 | .034 |
| 2nd Year of Preschool (4-5 years) | 68% N=19 | 92% N=23 | .031 | .30 |
| Mean Number of Weekly Intervention Hours | ||||
| Mean (SD)a Range | HFA Group Total N=34 | OO Group | p | Cohen's D |
| Before 1.5 Years | 4.0 (No SD) No Range N=1 | 5.3 (4.2) 2-10 N=3 | .81 | -0.44 |
| Between 1.5 and 2 years | 9.4 (15.3) 0.5-49 N=9 | 7.1 (6.4) 1-17 N=9 | .69 | 0.20 |
| Between 2 and 2.5 years | 4.1 (4.0) 0.5-13 N=8 | 14.8 (12.8) 1-42 N=16 | .006 | -1.13 |
| Between 2.5 and 3 years | 7.3 (9.5) 0.25-28 N=14 | 21.1 (16.9) 1-52 N=20 | .005 | -1.01 |
| 1st Year of Preschool (3-4 years) | 15.4 (15.1) 1-40.5 N=19 | 24.2 (15.5) 1-51 N=21 | .076 | -0.58 |
| 2nd Year of Preschool (4-5 years) | 18.9 (17.0) 0.5-47 N=17 | 25.8 (14.3) 1.5-60 N=22 | .17 | -0.44 |
Means, SDs, and ranges only include participants who received some intervention
The frequencies of the different types of intervention received for each age period are shown in Table 2. Data were not examined before 1.5 years of age because so few participants received intervention then.
Table 2. Types of therapy by age, entire sample.
| 1.5-2 years | HFA Total N=28 | OO Total N=25 | Chi-Square p Value | Effect Size Cramer's V |
|---|---|---|---|---|
| ABA | 4% | 16% | .12 | .21 |
| Speech | 29% | 28% | .96 | .006 |
| Occupational Therapy | 14% | 12% | .81 | .034 |
| Physical Therapy | 4% | 8% | .49 | .096 |
| Developmental Therapy | 0% | 16% | .028 | .30 |
| Sensory Integration Therapy | 4% | 8% | .49 | .096 |
| 2-2.5 years | HFA Total N=28 | OO Total N=25 | Chi-Square p Value | Effect Size Cramer's V |
| ABA | 4% | 40% | .001 | .45 |
| Speech | 32% | 52% | .14 | .20 |
| Occupational Therapy | 18% | 32% | .23 | .16 |
| Physical Therapy | 7% | 8% | .91 | .016 |
| Developmental Therapy | 0% | 20% | .013 | .34 |
| Special School | 0% | 8% | .13 | .21 |
| Sensory Integration Therapy | 4% | 12% | .25 | .16 |
| 2.5-3 years | HFA Total N=28 | OO Total N=25 | Chi-Square p Value | Effect Size Cramer's V |
| ABA | 7% | 56% | <.001 | .53 |
| Speech | 50% | 64% | .31 | .14 |
| Occupational Therapy | 29% | 48% | .15 | .20 |
| Physical Therapy | 14% | 8% | .47 | .099 |
| Developmental Therapy | 4% | 12% | .25 | .16 |
| Special School | 4% | 12% | .25 | .16 |
| Sensory Integration Therapy | 7% | 12% | .55 | .083 |
| 1st year of preschool (3-4 years) | HFA Total N=28 | OO Total N=25 | Chi-Square p Value | Effect Size Cramer's V |
| ABA | 32% | 60% | .042 | .28 |
| Speech | 71% | 56% | .24 | .16 |
| Occupational Therapy | 46% | 60% | .32 | .14 |
| Physical Therapy | 21% | 24% | .82 | .031 |
| Developmental Therapy | 4% | 4% | .94 | .011 |
| Special School | 18% | 40% | .07 | .25 |
| Sensory Integration Therapy | 7% | 4% | .62 | .068 |
| 2nd year of preschool (4-5 years) | HFA Total N=28 | OO Total N=25 | Chi-Square p Value | Effect Size Cramer's V |
| ABA | 25% | 72% | .001 | .47 |
| Speech | 50% | 68% | .18 | .18 |
| Occupational Therapy | 36% | 72% | .008 | .36 |
| Physical Therapy | 21% | 24% | .82 | .031 |
| Developmental Therapy | 4% | 20% | .06 | .26 |
| Special School | 14% | 36% | .07 | .25 |
| Sensory Integration Therapy | 0% | 4% | .29 | .15 |
Between 1.5 and 2 years, there was one significant difference, showing a higher frequency of developmental therapy in the OO than the HFA group (16% vs. 0%), with a small effect. About one third of participants in both groups were receiving SLP. Other interventions were infrequent in both groups.
Between 2 and 2.5 years, significantly more participants in the OO group (20%) were receiving developmental therapy, compared to the HFA group (0%). In addition, significantly more OO participants (40%) than HFA (4%) received ABA, a group difference with a medium effect size. The group difference in ABA was also present between 2.5 and 3 years, with a medium effect size (OO: 56%, HFA: 7%).
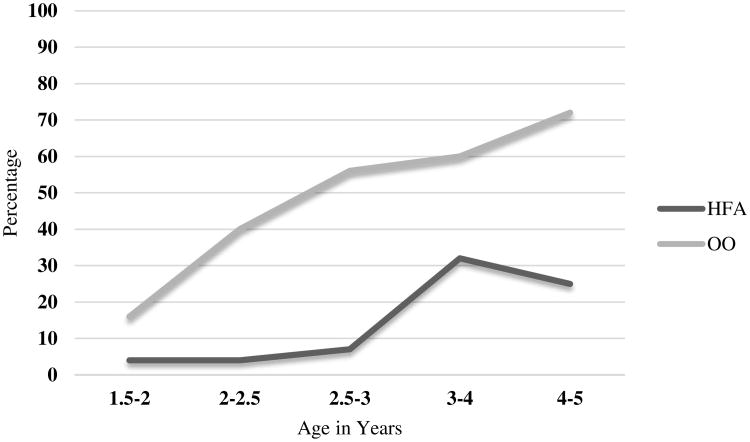
The pattern persisted during the first year of preschool (age 3 to 4), with significantly more children in the OO (60%) than the HFA (32%) group receiving ABA, with a small effect size, and during the second year of preschool (age 4 to 5), (OO group 72%; HFA group 25%) with a medium effect size. See Figure 1 for group differences in ABA frequency over time. In addition, there was a small effect of more participants in the OO group (72%) receiving OT than in the HFA group (36%) during the second year of preschool.
Figure 1. Percent of participants receiving ABA therapy, by age.
Similar results for frequency of intervention type across groups were found for the subset of children from CT and MA, although with fewer significant items, presumably because of the smaller sample size.
We also examined contrasts between groups for the number of hours of therapy weekly (see Table 1). The OO group received significantly more intervention hours than the HFA group in both six-month periods between the second and third birthdays. There were no group differences in the number of intervention hours prior to the age of two; however, very few children in either group received any intervention at that age. For the first and second year of preschool (age 3-5 years), the OO group received an average of eight more hours per week than the HFA group, a medium effect size, though this difference was not significant.
When examining the subset of the sample from Connecticut and Massachusetts, the groups were significantly different from the 2.5 to 3 year age period and the first year of preschool, with medium to large effect sizes, with the OO group receiving more intervention hours than the HFA group. While the groups were not significantly different on the 2 to 2.5 year age period or the second year of preschool, effect sizes were medium to large with the OO groups having higher mean number of hours than the HFA group.
Although the percent of children receiving ABA differed, there were no significant differences in the weekly number of hours of ABA for those who received it, at any age period, when examining either the total sample or the MA/CT subset of the sample. Not enough children in the HFA group received ABA therapy before the age of 3 to compare hours between groups. Between 2 and 2.5 years, 8 OO children (32%) received an average of 22.1 hours (SD=13.5), while between 2.5 and 3 years, 13 OO children (52%) received an average of 21.8 hours (SD=14.5). For the 3-4 age period, 7 HFA children (21%) received an average of 28.2 hours (SD=14.1), while 14 OO children (56%) received an average of 21.5 hours (SD=11.3), t=1.2, p=.25. At the 4-5 age period, 6 HFA children (18%) received an average of 31.2 hours (SD=8.8), while 14 OO children (56%) received an average of 24.0 hours (SD=10.5), t=1.5, p=.16. Results for the CT/MA subgroup were quite similar to those for the entire sample, with no significant group differences. Thus, for both HFA and OO groups, when children did receive ABA intervention, the average number of hour per week was generally between 20 and 30.
Total intervention hours and ABA hours, for the entire sample and the MA/CT subgroup, were reanalyzed using ANCOVAs, with age as a covariate, for two reasons. First, literature has shown systematic bias of parent report after different time periods,29 which was relevant given the wide age range of participants in the current study. Second, since children in the study were receiving early intervention across more than a decade of time, intervention practices might have changed. In general, the pattern of results remained the same, with age accounting for minimal variance. However, one exception was found: for mean ABA hours for the CT/MA sample for the 3-4 year age period, age accounted for 42% of the variance in the group means. Since no other time period for the CT/MA sample, and no age periods for the entire sample showed any other effects for current age, it is assumed that this is a chance result.
Pharmacologic and Complementary-Alternative Medicine (CAM) Treatments
Overall, the HFA group was more likely to be prescribed pharmacological treatments. Currently, 47% of the HFA group is on at least one medication, compared with only 20% of the OO group, a significant difference (see Table 3). A majority of the HFA participants were prescribed a pharmacological treatment at some point in their lives (64%), versus only 24% of the OO participants. The difference in medication frequency appears to be driven by the fact that the HFA participants were significantly more likely to be prescribed atypical antipsychotics and antidepressants.
Table 3. Pharmacologic and Complementary-Alternative Medicine (CAM) Treatments.
| OO N=25 | HFA N=36 | Chi-Square p Value | Effect Size Cramer's V | |
|---|---|---|---|---|
| Any Medication—Current | 20% | 47% | .029 | .28 |
| Any Medication—Past | 24% | 64% | .002 | .39 |
| CNS Stimulant—Current | 16% | 17% | .95 | .009 |
| CNS Stimulant—Ever | 24% | 36% | .32 | .13 |
| Antidepressant—Current | 4% | 25% | .029 | .28 |
| Antidepressant—Ever | 4% | 41% | <.001 | .47 |
| Atypical Antipsychotic—Current | 0% | 14% | .052 | .25 |
| Atypical Antipsychotic—Ever | 0% | 28% | .007 | .35 |
| Anticonvulsant—Current | 0% | 6% | .23 | .15 |
| Anticonvulsant—Ever | 0% | 6% | .23 | .15 |
| Norepinephrine Reuptake Inhibitor—Current | 0% | 8% | .14 | .19 |
| Norepinephrine Reuptake Inhibitor—Ever | 0% | 11% | .085 | .22 |
| Alpha-2 Adrenergic Agonist—Current | 4% | 8% | .50 | .09 |
| Alpha-2 Adrenergic Agonist—Ever | 4% | 8% | .50 | .09 |
| Benzodiazepine—Current | 0% | 6% | .23 | .15 |
| Benzodiazepine—Ever | 0% | 6% | .23 | .15 |
| Casein-Free, Gluten-Free (CFGF) Diet | 23% | 35% | .32 | .13 |
| Dietary Supplements | 40% | 36% | .76 | .04 |
The OO and HFA groups did not differ in the use of a casein-free, gluten-free (CFGF) diet or in the use of dietary supplements (see Table 3).
Discussion
Summary of results
Early symptoms/Age of concern: Overall severity was somewhat milder in the OO group; additional data in Fein et al (2013) suggest that these differences may have been in the social domain. Clinician judgment about the onset of developmental difficulties was not different between the groups, but parents of children in the HFA group became concerned about development an average of five months later, although still before the second birthday. The difference was much larger in referrals to specialists, with the OO group on average reportedly referred to a specialist at 26 months and the HFA group at 44 months, a difference of 18 months.
Total Intervention: Consistent with earlier age of parent concern and earlier referral to a specialist, the percent of OO children receiving any intervention was significantly higher than in the HFA group in the birth to three age period (83 vs. 48%) and also in the preschool period (92 vs. 56%). For more specific age spans, the differences were significant or close to significance for the 2-2.5, 2.5-3 and 4-5 year ages.
Intensity of Intervention: The number of hours of intervention per week differed between the OO and HFA groups, for those children receiving intervention at age 2-2.5 (average of 14.8 vs. 4.1 hours) and 2.5-3 (21 vs. 7.3 hours).
Specific therapies: More OO than HFA children received ABA at 2-2.5, 2.5-3, 3-4, and 4-5, with very striking differences (e.g., 40 vs. 4% at 2-2.5, 56 vs. 7% at 2.5-3). In addition, more OO children received developmental therapy at age 1.5-2 (16 vs. 0%) and at 2-2.5 (20 vs. 0%) and more OO children received OT at age 4-5 (72 vs. 36%).
Intensity of ABA: For those children receiving ABA, the number of hours per week was similar between groups, with 20 to 30 hours per week being the norm.
Other treatments: Children in the HFA group were more likely to have received medication, especially anti-psychotics and anti-depressants. There were no group differences in the percent of children receiving special diets or supplements.
Consistent with our hypothesis, the results of the present study suggest that children who go on to later achieve an optimal outcome are more likely to get intervention very early in life. The vast majority of the OO group (83%) got intervention before the age of 3 and most of the rest got intervention by preschool age, while only about half of the HFA children got intervention before kindergarten. Among those who did get intervention, the OO children received more hours. The biggest difference was in ABA, with very striking differences between groups, with large effect sizes between 2 and 3 years and between 4 and 5 years. The results suggest that most children in the HFA group are not getting ABA early in childhood; although the rates increase when they start school, they still never match the level of the OO group.
Contrary to our hypothesis, of children who did receive ABA, the children in the OO group did not receive more hours. This suggests that once ABA programs are implemented, the hours per week are relatively invariant. These findings are consistent with the study by Luiselli, Cannon, Ellis, and Sission16, suggesting that length of ABA intervention in months/years and an early start may be crucial.
Results also indicate that neither particular types of intervention nor the number of hours of early intervention is sufficient to predict outcome. Some children in the OO group had very limited early intervention, while some children in the HFA group received intensive early intervention. As treatment characteristics alone cannot predict outcome, other factors, such as child characteristics, need to be considered when studying prognosis. Results reported by Fein et al.22 indicated that despite severity of symptoms in all domains within the autistic range, the OO group appeared to show somewhat milder symptoms in the social domain, but not in communication or repetitive behavior, in early childhood.
The results of the present study indicate that children who retain their autism diagnosis are more likely to receive pharmacologic treatments, particularly antipsychotic and antidepressant medications. Although we do not have information about specifically when or why these medications were prescribed, it is likely that children in the HFA group had more mood and behavioral disturbances than those in the OO group. This is consistent with the findings in the literature that these disorders are frequently comorbid with autism.30 In addition, a quarter to a third of children and adolescents in both groups were given a CNS stimulant at some point in their lives, suggesting that attentional difficulties are clearly present and persistent in many children and adolescents with both HFA and OO.31
There were no differences between the groups in the use of special diets or dietary supplements. Therefore, consistent with previous literature,18,21 CAM treatments do not seem to effective in producing optimal outcomes from autism, although individual responders cannot be ruled out by our data.
Limitations and Future Directions
The most significant limitation of the present study is that the intervention data are based entirely on retrospective parent report. Particularly given the large age range of participants, parents were being asked to remember specific details about intervention years prior to the study. It is possible that parents recalled specific intervention details incorrectly. However, given that the groups were matched on age, recall bias due to age should be equally present across groups. It is possible that the children's different outcomes, although both groups were high functioning, influenced parent recall in a systematic way. Longitudinal studies that prospectively follow children from early childhood through latency age and adolescence would be definitive in measuring the impacts of early intervention.
An issue with the design of the study was that the children in the OO group had to be diagnosed with autism before the age of five, which was not required in the HFA group. Therefore, it is possible that the children in the HFA group were diagnosed later on average, leading to less early intervention. However, clinical judgment suggests that the developmental difficulties arose at the same time in both groups. In addition, based on parent report, overall autism symptom severity was somewhat greater in the HFA than OO group, although both groups had clear early histories of ASD, and many children in the OO group had severe early clinical pictures.22 Future studies should carefully match groups on age of diagnosis to eliminate this confound to intervention history. However, one would expect that more severe early picture (in the HFA group) would lead to earlier diagnosis, referral to specialists, and intervention, which suggests that group differences in age of symptom onset does not account for the later age of referral and intervention in the HFA group.
A confound in the results is that ABA treatments tend to be administered for many more hours than the other treatments parents reported. Therefore, since more of the OO group received ABA, they would be likely to receive more hours of treatment. In our data, it is not possible to disentangle whether the total intensity of treatment or ABA itself contributed more to the outcome.
Another major limitation is that quality of the interventions received by the children in the study could not be assessed. Even if parents accurately reported the hours and ages of intervention, it was not possible to evaluate the fidelity of the intervention or the provider's expertise. To attempt to address this, we examined the subset of the sample from Connecticut and Massachusetts since early intervention training and providers are more similar within and between these states than they may be elsewhere in the United States and in Canada. The findings with this subset were largely the same. Unfortunately, there is still considerable variability in quality of interventions between individual providers within any state, which could greatly impact a child's outcome. Therefore, randomized control trials looking at the efficacy and effectiveness of different types of early interventions, with assessment of quality, as well as different intensities of specific interventions, are necessary. As discussed by Reichow et al.,32 only one strong randomized control trial exists for early intervention in autism, indicating this is an important target for future research.
The sample was relatively homogenous, in geography, participants, ethnicity, and SES. This was both a strength and a weakness. Greater demographic similarity reduces the possibility of confounds due to these factors. That said, the results of the present study may not be generalizable to a more diverse sample. In addition, the makeup of the current sample argues against the results being accounted for by recruitment bias. Despite the fact that ABA is relatively more accessible in CT and MA than most other places, 70% of children in the HFA group came from CT and MA, compared with only 48% of children in the OO group.
Some limitations were also present in the method and design of the questionnaires filled out by parents in the present study. Because the information was obtained by questionnaire, there was a greater possibility of missing and incorrect data. In addition, the questionnaire did not ask about exact start and end dates of different treatment types, so total intervention hours from birth to kindergarten could not be computed and compared across groups. Finally, for the pharmacologic and CAM treatments, the parents were only asked about current and past treatments and treatments. Therefore, it was not possible to determine what children were given during the earliest years. Future studies may be best served by obtaining intervention data through structured interviews, rather than through questionnaires.
Although retrospective parent report has inherent weaknesses, the uniqueness of the current sample of OO children, matched on key characteristics to an HFA group, and the striking differences in reported provision of early intervention and ABA in particular, are worth considering as the basis for future, longitudinal studies. If confirmed, these results reinforce the importance of early, ongoing developmental surveillance and autism screening at appropriate ages, with prompt referral for diagnostic evaluation and intervention, to maximize the possibility of an optimal outcome.
Acknowledgments
This research was supported by the National Institutes of Mental Health grant R01MH076189
The authors are grateful to the adolescents and families who participated and the undergraduate research assistants who worked on the study
Footnotes
The authors have no conflicts of interest to declare
References
- 1.Lovaas OI. Behavioral treatment and normal educational and intellectual functioning in young autistic children. Journal of consulting and clinical psychology. 1987;55(1):3–9. doi: 10.1037//0022-006x.55.1.3. [DOI] [PubMed] [Google Scholar]
- 2.Mundy P. Normal versus high-functioning status in children with autism. American Journal on Mental Retardation. 1993;97(4):381–384. [Google Scholar]
- 3.Helt M, Kelley E, Kinsbourne M, et al. Can children with autism recover? If so, how? Neuropsychol Rev. 2008;18(4):339–366. doi: 10.1007/s11065-008-9075-9. [DOI] [PubMed] [Google Scholar]
- 4.Dawson G, Rogers S, Munson J, et al. Randomized, controlled trial of an intervention for toddlers with autism: the Early Start Denver Model. Pediatrics. 2010;125(1):e17–23. doi: 10.1542/peds.2009-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drew A, Baird G, Baron-Cohen S, et al. A pilot randomised control trial of a parent training intervention for pre-school children with autism. Preliminary findings and methodological challenges. European child & adolescent psychiatry. 2002;11(6):266–272. doi: 10.1007/s00787-002-0299-6. [DOI] [PubMed] [Google Scholar]
- 6.Aldred C, Green J, Adams C. A new social communication intervention for children with autism: pilot randomised controlled treatment study suggesting effectiveness. Journal of child psychology and psychiatry, and allied disciplines. 2004;45(8):1420–1430. doi: 10.1111/j.1469-7610.2004.00848.x. [DOI] [PubMed] [Google Scholar]
- 7.Green J, Charman T, McConachie H, et al. Parent-mediated communication-focused treatment in children with autism (PACT): a randomised controlled trial. Lancet. 2010;375(9732):2152–2160. doi: 10.1016/S0140-6736(10)60587-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rogers SJ, Vismara LA. Evidence-based comprehensive treatments for early autism. Journal of clinical child and adolescent psychology : the official journal for the Society of Clinical Child and Adolescent Psychology, American Psychological Association, Division 53. 2008;37(1):8–38. doi: 10.1080/15374410701817808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McEachin JJ, Smith T, Lovaas OI. Long-term outcome for children with autism who received early intensive behavioral treatment. Am J Ment Retard. 1993;97(4):359–372. discussion 373-391. [PubMed] [Google Scholar]
- 10.Gresham FM, MacMillan DL. Early Intervention Project: can its claims be substantiated and its effects replicated? J Autism Dev Disord. 1998;28(1):5–13. doi: 10.1023/a:1026002717402. [DOI] [PubMed] [Google Scholar]
- 11.Schopler E, Short A, Mesibov G. Relation of behavioral treatment to “normal functioning”: comment on Lovaas. J Consult Clin Psychol. 1989;57(1):162–164. doi: 10.1037//0022-006x.57.1.162. [DOI] [PubMed] [Google Scholar]
- 12.Cohen H, Amerine-Dickens M, Smith T. Early intensive behavioral treatment: replication of the UCLA model in a community setting. Journal of developmental and behavioral pediatrics : JDBP. 2006;27(2 Suppl):S145–155. doi: 10.1097/00004703-200604002-00013. [DOI] [PubMed] [Google Scholar]
- 13.Sallows GO, Graupner TD. Intensive behavioral treatment for children with autism: four-year outcome and predictors. American journal of mental retardation : AJMR. 2005;110(6):417–438. doi: 10.1352/0895-8017(2005)110[417:IBTFCW]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 14.Smith T, Groen AD, Wynn JW. Randomized trial of intensive early intervention for children with pervasive developmental disorder. American journal of mental retardation : AJMR. 2000;105(4):269–285. doi: 10.1352/0895-8017(2000)105<0269:RTOIEI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 15.Gabriels RL, Hill DE, Pierce RA, Rogers SJ, Wehner B. Predictors of treatment outcome in young children with autism: a retrospective study. Autism. 2001;5(4):407–429. doi: 10.1177/1362361301005004006. [DOI] [PubMed] [Google Scholar]
- 16.Luiselli JK, Cannon BM, Ellis JT, Sisson RW. Home-based behavioral intervention for young children with autism/pervasive developmental disorder. Autism. 2000;4:426–438. [Google Scholar]
- 17.Sheinkopf SJ, Siegel B. Home-based behavioral treatment of young children with autism. J Autism Dev Disord. 1998;28(1):15–23. doi: 10.1023/a:1026054701472. [DOI] [PubMed] [Google Scholar]
- 18.Huffman LC, Sutcliffe TL, Tanner IS, Feldman HM. Management of symptoms in children with autism spectrum disorders: a comprehensive review of pharmacologic and complementary-alternative medicine treatments. J Dev Behav Pediatr. 2011;32(1):56–68. doi: 10.1097/DBP.0b013e3182040acf. [DOI] [PubMed] [Google Scholar]
- 19.Siegel M, Beaulieu AA. Psychotropic medications in children with autism spectrum disorders: a systematic review and synthesis for evidence-based practice. Journal of autism and developmental disorders. 2012;42(8):1592–1605. doi: 10.1007/s10803-011-1399-2. [DOI] [PubMed] [Google Scholar]
- 20.Leskovec TJ, Rowles BM, Findling RL. Pharmacological treatment options for autism spectrum disorders in children and adolescents. Harvard review of psychiatry. 2008;16(2):97–112. doi: 10.1080/10673220802075852. [DOI] [PubMed] [Google Scholar]
- 21.Levy SE, Hyman SL. Complementary and alternative medicine treatments for children with autism spectrum disorders. Child Adolesc Psychiatr Clin N Am. 2008;17(4):803–820. ix. doi: 10.1016/j.chc.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fein D, Barton M, Eigsti IM, et al. Optimal outcome in individuals with a history of autism. Journal of child psychology and psychiatry. 2013;52(2):195–205. doi: 10.1111/jcpp.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lord C, Risi S, Lambrecht L, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30(3):205–223. [PubMed] [Google Scholar]
- 24.Sparrow SS, Balla DA, Cicchetti DV. Vineland Adaptive Behavior Scales (Interview Ed) Circle Pines, MN: American Guidance Service; 1985. [Google Scholar]
- 25.Luyster R, Richler J, Risi S, et al. Early regression in social communication in autism spectrum disorders: a CPEA Study. Dev Neuropsychol. 2005;27(3):311–336. doi: 10.1207/s15326942dn2703_2. [DOI] [PubMed] [Google Scholar]
- 26.Wechsler D. Wechsler abbreviated scale of intelligence (WASI) New York, NY: The Psychological Corporation; 1999. [Google Scholar]
- 27.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of autism and developmental disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 28.Berument SK, Rutter M, Lord C, Pickles A, Bailey A. Autism screening questionnaire: diagnostic validity. Br J Psychiatry. 1999;175:444–451. doi: 10.1192/bjp.175.5.444. [DOI] [PubMed] [Google Scholar]
- 29.Hus V, Taylor A, Lord C. Telescoping of caregiver report on the Autism Diagnostic Interview--Revised. Journal of child psychology and psychiatry, and allied disciplines. 2011;52(7):753–760. doi: 10.1111/j.1469-7610.2011.02398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanne SM, Abbacchi AM, Constantino JN. Multi-informant ratings of psychiatric symptom severity in children with autism spectrum disorders: the importance of environmental context. Journal of autism and developmental disorders. 2009;39(6):856–864. doi: 10.1007/s10803-009-0694-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frazier TW, Shattuck PT, Narendorf SC, Cooper BP, Wagner M, Spitznagel EL. Prevalence and correlates of psychotropic medication use in adolescents with an autism spectrum disorder with and without caregiver-reported attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2011;21(6):571–579. doi: 10.1089/cap.2011.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reichow B, Barton EE, Boyd BA, Hume K. Early intensive behavioral intervention (EIBI) for young children with autism spectrum disorders (ASD) Cochrane Database Syst Rev. 2012;10:CD009260. doi: 10.1002/14651858.CD009260.pub2. [DOI] [PubMed] [Google Scholar]