Abstract
Background
Children with sickle cell disease (SCD) are susceptible to recurrent infections, which are often life threatening and necessitate frequent vaccinations. Given the altered baseline immunity and proinflammatory state associated with SCD, we sought to determine the relative safety and efficacy of vaccination in transgenic SCD mice.
Methods
Eight week-old SCD mice were vaccinated with ovalbumin (OVA) and aluminum hydroxide weekly for three weeks by the intraperitoneal (IP) or intramuscular (IM) route. One week after the third vaccination, serum cytokines/chemokines, immunoglobulins, and bronchoalveolar lavage (BAL) fluid cytokines were measured.
Results
Only SCD mice were prone to mortality associated with vaccination as 40% of the animals died after the IP vaccinations and 50% died after the IM vaccinations. Serum IgG2b and IgM were significantly lower in SCD than C57Bl/6 mice after vaccination, but OVA-specific IgE was significantly higher. Serum interleukin 1 alpha (IL-1α), IL-2, IL-5, macrophage inflammatory protein 1 alpha (MIP-1α), and granulocyte macrophage-colony stimulating factor (GM-CSF) were significantly lower in SCD mice than C57Bl/6 mice after vaccination, whereas BAL fluid IL-1β and IL-6 were elevated.
Conclusions
Mice with SCD appear to have a dysregulated immune response to vaccination. Thus, the relative safety and immunogenicity of vaccination should be studied in greater detail in the context of SCD.
INTRODUCTION
Children suffering from sickle cell disease (SCD) are prone to frequent and severe infections that can lead to premature death if prompt antibiotic treatment is not administered. One of the most common infections in children with SCD is caused by Streptococcus pneumoniae, which often manifests as pneumonia and can lead to septicemia if the bacterium becomes invasive. The incidence of invasive S. pneumoniae infection in individuals with SCD is between 30–600 fold higher (depending on age) than what is observed in the general population (1). As a consequence, children with SCD typically adhere to strict vaccination schedules which often include more frequent booster shots than children without SCD. The introduction of pneumococcal vaccines has reduced the incidence of mortality associated with S. pneumoniae infection in children with SCD by 80–90% (2, 3); however, infection in vaccinees has nevertheless been reported in this population (4). Vaccination against both S. pneumoniae and Influenza A virus appear to result in low antigen-specific IgG and IgM antibody titers (5, 6), the latter of which is likely a function of a reduced number of IgM producing B-cells (7, 8). Furthermore, a recent study has shown an association between chronic transfusion of children with SCD and a lack of a protective post-vaccination antibody response to influenza A (9). Taken together, these findings bring into question the relative immunogenicity of vaccination in children with SCD when compared to control subjects and indicate that hypo-responsiveness to vaccine antigens may not be uncommon.
The phase one safety evaluations of vaccines are usually tested in the general population but are not tested in individuals with uncommon diseases such as SCD. The recently developed intranasal influenza vaccine (FluMist, MedImmune, Gaithersburg, MD) is one such example and, consequently, administration of this vaccine to SCD patients is not recommended by the CDC. Even when a vaccine is routinely administered as part of the standard vaccination schedule, such as is the case with the trivalent inactivated influenza (TIV) vaccine, controversy may arise pertaining to its safety in people with uncommon diseases. Indeed, recent retrospective studies using the vaccine safety datalink project have indicated that the TIV vaccine is not associated with hospitalization in children or adults with SCD (10, 11); however, a previous report by this group had shown that people with SCD had more frequent fever or pain episodes resulting in an inpatient visit within two weeks of influenza vaccination than control subjects (12). To our knowledge, no published prospective studies have been conducted in humans or mice to definitively determine if vaccination is associated with adverse effects in SCD.
Very little work has been conducted in transgenic SCD mice to study the effects of experimental treatment on basic outcomes that cannot be tested in humans. One of the few papers to do so demonstrated that NKT-cells are an important source of pulmonary dysfunction at baseline in NY1DD SCD mice (13). Another report used intraperitoneal (IP) injection of lipopolysaccharide (LPS) into the “Berkeley” (Berk) transgenic SCD mouse strain to determine the effects of systemic challenge with an inflammatory agent on markers of disease (14). Many of these mice died shortly after injection and the survivors exhibited negative respiratory outcomes and had increased inflammatory markers. In another study, experimental asthma was induced in SCD mice by subcutaneous (SC) implantation of ovalbumin (OVA), followed by OVA aerosol challenge (15). Mortality of SCD mice was associated with SC implantation of OVA, and marked increases in IgE was observed. A follow-up study by the same group also demonstrated increases in bronchoalveolar lavage (BAL) cytokines (including IL-1β and IL-6) after the induction of asthma in SCD mice (16). Taken together, these findings indicate that SCD results in exaggerated inflammatory responses in reaction to antigenic stimuli.
There appears to be a dichotomy in SCD between hypo-responsiveness to some antigens and an overzealous inflammatory response to others. Our original goal was to study asthma in a murine model of SCD using our previously published IP OVA/Alum vaccination/sensitization protocol (17). However, upon experiencing approximately 50% mortality with sensitization alone in multiple experiments, we shifted focus to understand how transgenic SCD mice respond to vaccination. Herein, we describe changes in systemic and pulmonary cytokines, serum antibodies, and antigen-specific IgE responses in SCD mice surviving vaccination as compared with wild-type and hemizygous mice. Implications for infection and vaccination in children with SCD are discussed.
RESULTS
Vaccination-Induced Mortality
IP vaccination of SCD mice with the protein antigen OVA and adjuvant alum resulted in significant mortality (40%) when compared to either C57Bl/6 or hemizygous (no deaths in either group; p<0.05; Figure 1). This effect occurred with increasing frequency at each additional IP injection. In order to validate that the route of inoculation was not the major factor of SCD mouse mortality, additional mice were inoculated with OVA/alum via the IM route. Again, only SCD mice died after the injections (50%), which was significantly different from C57Bl/6 and hemizygous mice. Interestingly, the kinetics of mortality were different between the two routes of inoculation, with IP resulting in the greatest mortality after the third vaccination and IM only resulting in mortality after one vaccination.
Figure 1.
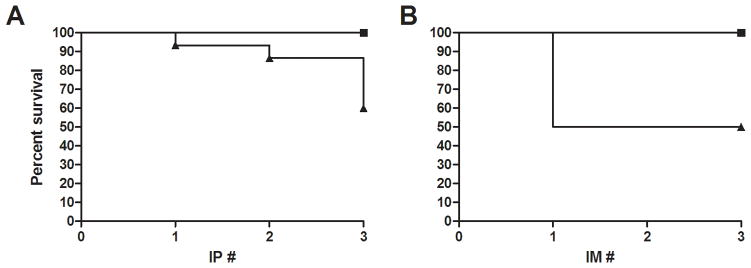
Post-vaccination Kaplan-Meier survival curves. (A) Percent survival of C57Bl/6 (n=11), hemizygous (n=11), or SCD mice (n=15) after each IP vaccination. (B) Percent survival of C57Bl/6 (n=6), hemizygous (n=7), or SCD mice (n=6) after each IM vaccination. Curves for SCD mice for both routes of inoculation were significantly different from C57Bl/6 and hemizyous mice (p<0.05). Squares = C57Bl6 and hemizygous mice; triangle = SCD.
Antibody Responses
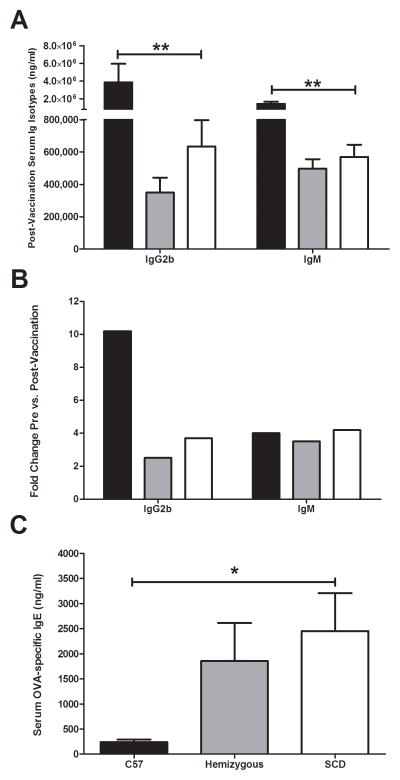
Concentrations of post-vaccination serum immunoglobulin class and sub-classes were measured one week after the third vaccination using a Luminex assay. When compared to C57Bl/6 mice, SCD mice exhibited significantly lower serum concentrations of IgG2b (Figure 2A). Serum concentrations of IgM were also significantly reduced in SCD mice. No differences were observed in any post-vaccination Ig class/sub-class between SCD and hemizygous mice. When comparing pre- versus post- vaccination serum antibody levels, dramatically higher fold-change increases were observed for IgG2b in C57Bl/6 mice than in SCD mice, indicating that the lower level of this Ig sub-class post-vaccination is attributable to an inability of SCD mice to produce it in response to vaccination (Figure 2B). No difference in the fold-change of IgM concentrations in the pre- versus post-vaccination samples was observed among the groups (Figure 2B), which is likely attributed to the previously reported reduction of IgM at baseline in SCD mice (8). When compared with C57Bl/6 mice, SCD mice exhibited dramatically increased OVA-specific IgE titers after vaccination (Figure 2C), indicating that SCD mice may be more prone to allergic sensitization than C57Bl/6 mice. No difference was observed in OVA-specific IgE titers when SCD and hemizygous mice were compared.
Figure 2.
Serum antibody responses to vaccination. (A) Significantly different serum immunoglobulin concentrations post-vaccination as measured by Luminex assay (IgG2b: C57Bl/6 = 3.88×106 ng/ml (SEM, 2,072,000), hemizygous = 350,000 ng/ml (SEM, 92,000), SCD = 635,000 ng/ml (SEM, 162,000); p<0.01) (IgM: C57Bl/6 = 1.44×106 ng/ml (SEM, 244,000), hemizygous = 497,000 ng/ml (SEM, 59,000), SCD = 570,000 ng/ml (SEM, 75,000); p<0.001). (B) Pre- versus postvaccination fold-change in significantly different serum immunoglobulins postvaccination. (C) Serum concentrations of OVA-specific IgE antibodies as measured by ELISA (C57Bl/6 = 242 ng/ml (SEM, 48), hemizygous = 1854 ng/ml (SEM, 758), SCD = 2452 ng/ml (SEM, 754); p<0.05). Bars represent mean levels +/− standard error of the mean. Significant differences were determined by one-way analysis of variance and pairwise comparisons were conducted using the Dunnett’s post-hoc test with SCD serving as the control group. Black bars = C57Bl/6; grey bars = hemizygous; white bars = SCD. *p<0.05, **p<0.01.
Cytokine/Chemokine Responses
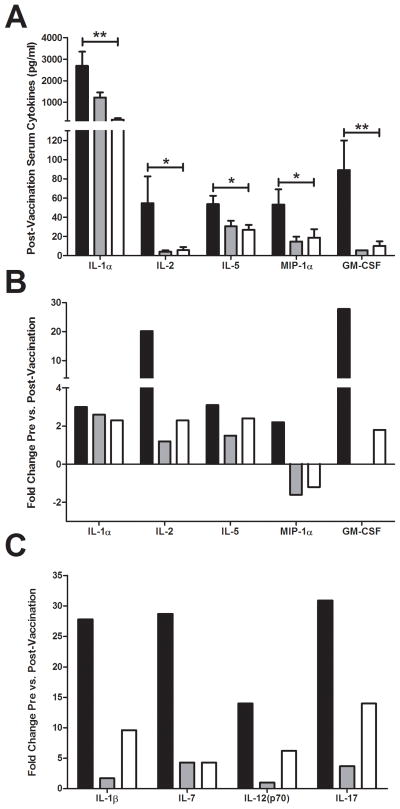
Concentrations of post-vaccination serum cytokines and chemokines were measured one week after the third vaccination using a Luminex assay. When compared to C57Bl/6 mice, SCD mice had lower serum concentrations of the cytokines IL-1α, IL-2, and IL-5 (Figure 3A). Reduced concentrations of serum chemokines MIP-1α and GM-CSF were also found. No significant differences in the aforementioned cytokines/chemokines were measured between SCD and hemizygous mice. When comparing pre- versus post-vaccination cytokine levels, fold-change differences in IL-1α were not noticeably different among the groups (Figure 3B), which is likely attributed to the previously reported reduction of this cytokine at baseline in SCD mice (8). Dramatically higher pre- versus post-vaccination fold-change increases were observed for both IL-2 and GM-CSF in C57Bl/6 mice than SCD mice, indicating that the lower levels of these cytokines post-vaccination is attributed to an inability of SCD mice to produce them in response to vaccination. Moreover, when pre- versus post-vaccination titers were compared, SCD mice exhibited reduced levels of MIP-1α. This indicates that SCD has a severely dysregulated MIP-1α response after vaccination.
Figure 3.
Serum cytokine and chemokine responses to vaccination. (A) Significantly different postvaccination cytokine/chemokine serum concentrations as measured by Luminex assay (IL-1α: C57Bl/6 = 2692 pg/ml (SEM, 659), hemizygous = 1224 pg/ml (SEM, 236), SCD = 195 pg/ml (SEM, 64); p<0.001) (IL-2: C57Bl/6 = 55 pg/ml (SEM, 28), hemizygous = 4.2 pg/ml (SEM, 1.4), SCD = 5.8 pg/ml (SEM, 3.2); p<0.05) (IL-5: C57Bl/6 = 54 (SEM, 8.7), hemizygous = 31 pg/ml (SEM, 5.8), SCD = 27 pg/ml (SEM, 5.2); p<0.05) (MIP-1α: C57Bl/6 = 53 pg/ml (SEM, 16), hemizygous = 15 pg/ml (SEM, 4.9), SCD = 19 pg/ml (SEM, 8.8); p<0.05) (GM-CSF: C57Bl/6 = 89 pg/ml (SEM, 31), hemizygous = 5.5 pg/ml (SEM, NA), SCD = 10 pg/ml (SEM, 4.7); p<0.01). (B) Pre- versus post-vaccination fold-change in significantly different post-vaccination serum cytokines/chemokines. (C) Fold-change in serum cytokines that were only significantly different pre- versus post-vaccination in C57Bl/6 mice. Bars represent mean levels +/− standard error of the mean. Significant differences were determined by one-way analysis of variance and pairwise comparisons were conducted using the Dunnett’s post-hoc test with SCD serving as the control group. Black bars = C57Bl/6; grey bars = hemizygous; white bars = SCD. *p<0.05, **p<0.01.
Several serum cytokines were significantly increased in response to vaccination in C57Bl/6 mice, but not in SCD or hemizygous mice (Figure 3C). These cytokines include IL-1β, IL-7, IL-12(p70), and IL-17. These cytokines exhibited more variability within groups, thereby contributing to a lack of significance when post-vaccination concentrations were compared. However, SCD mice (and hemizygous) appear to have a reduced ability to produce these cytokines in response to vaccination. Taken together, SCD mice that survive inoculation appear to be hypo-responsive to vaccination, as demonstrated by their lack of cytokine production in serum post-vaccination.
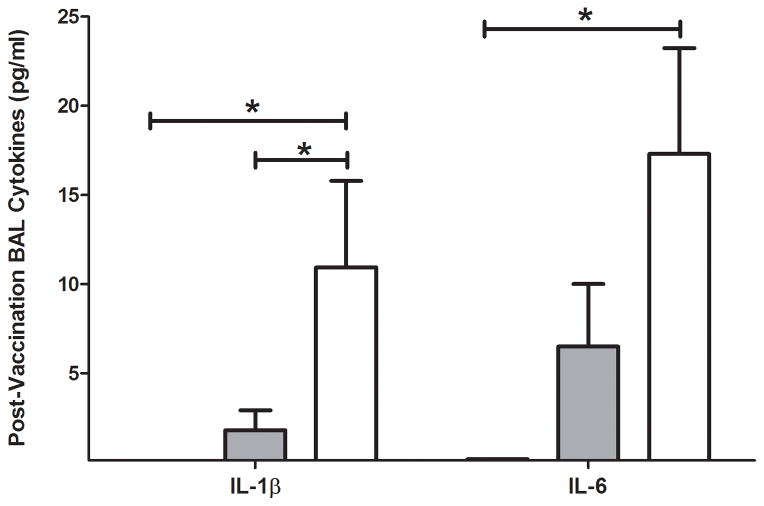
In contrast to the hypo-responsiveness exhibited by SCD mice in the production of serum cytokines after vaccination, cytokines in BAL fluid appeared to be higher in SCD mice (Figure 4). BAL fluid IL-1β was not measurable in C57Bl/6 mice post-vaccination (MDL = 0.20 pg/ml) and was significantly higher in SCD mice when compared with either hemizygous mice or C57Bl/6 mice. Similarly, IL-6 was elevated in the BAL fluid of SCD mice when compared with C57Bl/6 mice (not measurable, MDL = 0.28 pg/ml) after vaccination. No differences in post-vaccination BAL fluid IL-6 were observed between hemizygous mice and SCD mice.
Figure 4.
BAL fluid cytokines measured in response to vaccination. No cytokines were detected above the MDL before vaccination (IL-1β: C57Bl/6 < 0.20 pg/ml, hemizygous = 1.8 pg/ml (SEM, 1.1), SCD = 11 pg/ml (SEM, 4.9); p<0.05) (IL-6: C57Bl/6 < 0.28 pg/ml, hemizygous = 6.5 pg/ml (SEM, 3.5), SCD = 17 pg/l (SEM, 5.9); p<0.05). Bars represent mean levels +/− standard error of the mean. Significant differences were determined by one-way analysis of variance and pairwise comparisons were conducted utilizing the Dunnett’s post-hoc test with SCD serving as the control group. Black bars = C57Bl/6; grey bars = hemizygous; white bars = SCD. *p<0.05
DISCUSSION
Relative safety and immunogenicity are important features to consider when administering vaccines, yet these two basic parameters are not well-understood in children with uncommon diseases such as SCD. Death of SCD mice after injection of inflammatory agents is a phenomenon that has been known for some time and is presumably associated with heightened baseline inflammation. A previous study showed that IP inoculation with the proinflammatory molecule LPS resulted in approximately 60% mortality of SCD mice, whereas sham inoculation with saline had no effect (14). Protein antigens may also produce mortality in SCD mice, as has been shown by SC implantation of OVA (15). Our findings of approximately 40% mortality in SCD mice after IP OVA/alum injection and 50% mortality after IM OVA/alum injection correlate with these previous reports. Thus, our model of vaccination has clinical implications pertaining to possible adverse events associated with vaccination of children with SCD.
The gold standard of immunogenicity for most vaccines is elicitation of the production of antigen-specific serum antibodies. As previously mentioned, SCD mice that survived vaccination had significantly higher OVA-specific IgE antibody serum concentrations than C57Bl/6 mice, indicating that these animals may be especially prone to allergic sensitization. Conversely, these same mice were hypo-responsive in terms of IgG2b and IgM production after vaccination. All groups of mice increased their IgM titers 4-fold in response to vaccination, which indicates that differences in post-vaccination IgM concentrations are due to low baseline production of this immunoglobulin. However, SCD mice only induced a 4-fold increase in IgG2b after vaccination, whereas C57Bl/6 mice increased serum concentrations more than 10-fold. The inability of SCD mice to induce wild-type levels of IgG2b antibodies has potential clinical implications as murine IgG2b is similar to human IgG3 (and to some degree IgG1) (18), with the ability to fix complement and bind to protein antigens. It is crucially important for vaccines to induce these functions for maximum efficacy. These findingsare also in line with the observation that children living with SCD have diminished antibody responses to the hepatitis B vaccine (19). Taken together, these findings have translational implications that should be investigated further in children living with SCD.
The ability of lymphocytes to become activated and proliferate in response to stimuli is essential for vaccine immunogenicity. Cytokines such as IL-2, IL-7, and IL-12 are responsible for allowing lymphocytes to mature/differentiate, proliferate, and generate immunological memory. The reduced post-vaccination serum concentrations and apparent inability of these cytokines to be sufficiently stimulated after vaccination indicate that lymphocytes in SCD mice surviving vaccination may have sub-par effector and memory functions. Furthermore, chemokines MIP-1α and GM-CSF, which are important for stimulating (20) and mobilizing (21) antigen presenting cells (APCs) in response to antigen, are not produced at wildtype levels by SCD mice following vaccination. In fact, MIP-1α appears to be down-regulated in response to vaccination in SCD mice. This has serious implications as MIP-1α is important for mobilization of DC precursors into the blood (22). MIP-1α is also a ligand for CCR5, which has been shown to be important for protection from pathogens such as influenza (23). Furthermore, it has been reported that there is a higher proportion of SCD patients in Brazil that also have the CCR5D32 mutant allele when compared to controls (24). Extrapolation of our results from the mouse model then indicates that these individuals may mount suboptimal immune responses to vaccination which may then leave them more susceptible to infection. SCD mice are also hypo-responsive to IL-17 production post-vaccination and this cytokine is important for clearance of mucosal bacterial pathogens. Indeed, protection of mice from S. pneumoniae colonization appears to be IL-17-dependent (25), which suggests that lack of IL-17 production in SCD mice may contribute to their increased susceptibility to pneumococcal infection (26).
Mouse models of acute lung injury are associated with increased levels of TNF-α, IL-1β, and IL-6 in BAL fluid. This milieu has been shown to increase the production of secretory IgA in the lungs to help protect from infection when the natural barrier defenses are weakened (27). SCD mice that survived vaccination had significantly elevated IL-1β and IL-6 in their BAL fluid one week after the third vaccination when compared with C57Bl/6 mice, indicating that lung injury associated with vaccination had occurred. Given the long-elapsed time between inoculation and measurement of these cytokines, it would not be surprising if they were in fact higher in concentration in the lung airways at earlier time points. It would also not be surprising if TNF-α is elevated early on as well. A study of acute lung injury in SCD mice also showed increased IL-6 in BAL fluid within hours of LPS injection (14), which corroborates our findings. The finding of increased IL-1β in BAL fluid of SCD mice that survived vaccination also has clinical implications, as this cytokine has been associated with ischemic reperfusion injury in human SCD (28), which is also associated with acute chest syndrome. Thus, therapeutics targeting IL-6 and IL-1β may help to reduce pulmonary-related morbidity and mortality in people living with SCD.
Vaccination-related death of SCD mice raises the question by what mechanism these animals are dying. This study does not directly address this question, as all outcomes were measured one week after the third vaccination only in animals that tolerated the OVA/alum inoculation. However, we were able to determine that the SCD mice that tolerated IP vaccination had similarly high levels of antigen-specific serum IgE as the hemizygous controls, which were much higher than those found in C57Bl/6 mice. This raises the possibility that the sensitive SCD mice may have had even higher IgE levels and died of shock from a type I hypersensitivity reaction. Anecdotal support of this hypothesis comes from the observation that all SCD mice that died after vaccination were found dead within hours of OVA/alum injection. Furthermore, all but one SCD mouse died after the second or third IP injection, indicating that the mice had to be first sensitized to OVA/alum before they became susceptible to death from vaccination. Interestingly, susceptible SCD mice that were inoculated by the IM route all died after the first vaccination, indicating that these mice did not have to be sensitized to antigen to be susceptible to death associated with this route of vaccination. It therefore seems likely that these animals died from an exaggerated inflammatory response, possibly driven by the presence of the adjuvant alum. While we do not know the cause of vaccination-related death of SCD mice, it is likely a combination of multiple etiologies which may include both hypersensitivity reactions and exaggerated inflammatory responses. Further work is needed in order to more definitively answer this question.
Many studies have shown that both humans and mice with SCD live in an immunologically altered state. The study presented herein demonstrates that SCD mice are prone to death associated with vaccination, which is likely linked to the altered baseline immunity observed in these animals. Consequently, animals that survive inoculation appear to have dysregulated systemic immune responses to vaccination. Enhanced pulmonary production of IL-1β and IL-6 suggests acute lung injury may develop as a consequence of antigenic challenge. Our data suggest that the relative safety and immunogenicity of vaccination should be studied in greater detail in the context of SCD. Although it is clear that humans with SCD do not suffer mortality as a consequence of vaccination, there may be subclinical deleterious consequences that warrant further investigation.
MATERIALS AND METHODS
Mice
Animals used in this study have been previously described (8). Briefly, female mice, approximately 8 weeks old, weighing 15–25 g each, were purchased from the Jackson Laboratory (Bar Harbor, ME). Berkeley sickle cell transgenic mice (Tg(Hu-miniLCRα1GγAγδβS) Hba−/− Hbb−/−) expressing human HBA and HBBS and no longer expressing mouse Hba and Hbb were used as a murine model of SCD. The stock background of this strain is a mixture of FVB/N, 129, DBA/2, Black Swiss and >50% C57Bl/6 genomes. It was backcrossed to C57Bl/6 one generation after importation to The Jackson Laboratory. Hemizygous controls of the Berkeley transgenic SCD mouse (generated on the same mixed background of strains) that express no mouse Hba, but do express one copy of mouse Hbb, human HBA and HBBS giving rise to a hemizygous genotype were used as a second control arm. All mice were conventionally housed in plastic cages with corncob bedding. The housing facility was maintained at 22–24°C with a 12 hr light/dark cycle. Chow and water were given ad libitum. The Animal Care Committee at the University of Connecticut Health Center approved all mouse experiments.
Vaccinations
Mice were vaccinated by either the intraperitoneal (IP) or intramuscular (IM) route once per week for three weeks. A suspension of 25 μg of OVA (grade V, Sigma Chemical, St. Louis, MO) with 2 mg of aluminum hydroxide (alum) in 500 μl of saline was used for IP inoculations, while a final volume of 50 μl was used for IM inoculations (one ½ dose injection into each hindlimb).
Collection of Serum and BAL fluid
One week after the third injection, mice were humanely euthanized with an overdose of ketamine/xylazine and whole blood was immediately collected via cardiac puncture and placed into non-heparinized tubes for serum purification. Blood was allowed to clot at room temperature for approximately 30–60 min and then spun for 10 min at 4000 RPM in an Eppendorf 5415 C centrifuge. Serum was then aliquoted and stored at −80°C until used. After sacrifice, a bronchoalveolar lavage (BAL) was performed on mouse lungs with five 1-ml aliquots of physiologic saline. The BAL was then centrifuged at 1700 × g using a Thermo Scientific Sorvall ST 40R centrifuge with a swinging bucket rotor and the supernatant (BAL fluid) was removed and concentrated 10x using Amicon Centriplus YM-10 filtration devices (Millipore, Bedford, MA), per the manufacturer’s instructions. BAL fluid samples were stored at −80°C until used.
Analysis of Cytokines and Antibodies using Luminex Assays
Analysis of serum chemokines/cytokines and antibody class/sub-class were conducted using Milliplex kits (Millipore), as previously described (8). Cytokines were analyzed using a 1:2 dilution while immunoglobulins were analyzed at a 1:25,000 dilution (concentrations were adjusted to account for dilution). Serum IgA fell below the detection limit at this dilution for all animals and was not considered for analysis. BAL fluid cytokines were processed and analyzed using the same technique as serum, but sterile PBS was used as a diluent for the standard curve. For samples in which an analyte could not be detected above the minimum detection limit (MDL) surrogate values of 0.1 or 1 less than the MDL (depending on the number of significant figures used) were used. For BAL fluid samples, post-assay sample concentration and limits of detection were reported taking into consideration the 10X sample concentration prior to analysis. Fold-change values were calculated using data from naïve animals published in a previous report (8). Calculations are based on groups of 11 C57Bl/6 mice, 11 hemizygous mice, and 9 SCD mice.
OVA-specific Serum IgE Levels
An OVA-specific sandwich ELISA was used to measure serum levels of IgE, as has been previously described (29). Results were interpolated from a standard curve. Calculations are based on groups of 8 C57Bl/6 mice, 9 hemizygous mice, and 8 SCD mice.
Statistics
Statistical comparisons between groups were made with one-way analysis of variance and pairwise comparisons were conducted utilizing the Dunnett’s post-hoc test with SCD serving as the control group. Analyses were conducted using GraphPad Prism version 5 software (GraphPad software, San Diego, CA) unless no variance was recorded within a group, then JMP statistical software version 5.1 (SAS Institute, Cary, NC) was used. All data were expressed as means +/− standard error of the mean. Differences were considered significant if p ≤ 0.05.
Acknowledgments
STATEMENT OF FINANCIAL SUPPORT: Connecticut Institute for Clinical and Translational Science (CICATS) Clinical and Translational Scholars K12 Award; Lea’s Foundation for Leukemia Research, Inc.; and National Institiute of Allergy and Infectious Disease grant RO1 AI04357-11.
References
- 1.Overturf GD. Infections and immunizations of children with sickle cell disease. Adv Pediatr Infect Dis. 1999;14:191–218. [PubMed] [Google Scholar]
- 2.Adamkiewicz TV, Sarnaik S, Buchanan GR, et al. Invasive pneumococcal infections in children with sickle cell disease in the era of penicillin prophylaxis, antibiotic resistance, and 23-valent pneumococcal polysaccharide vaccination. J Pediatr. 2003;143:438–44. doi: 10.1067/S0022-3476(03)00331-7. [DOI] [PubMed] [Google Scholar]
- 3.Halasa NB, Shankar SM, Talbot TR, Arbogast PG, Mitchel EF, Wang WC, et al. Incidence of invasive pneumococcal disease among individuals with sickle cell disease before and after the introduction of the pneumococcal conjugate vaccine. Clin Infect Dis. 2007;44(11):1428–33. doi: 10.1086/516781. [DOI] [PubMed] [Google Scholar]
- 4.McCavit TL, Quinn CT, Techasaensiri C, Rogers ZR. Increase in invasive Streptococcus pneumoniae infections in children with sickle cell disease since pneumococcal conjugate vaccine licensure. J Pediatr. 2011;158(3):505–7. doi: 10.1016/j.jpeds.2010.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bjornson AB, Lobel JS. Direct evidence that decreased serum opsonization of Streptococcus pneumoniae via the alternative complement pathway in sickle cell disease is related to antibody deficiency. J Clin Invest. 1987;79(2):388–98. doi: 10.1172/JCI112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ballester OF, Abdallah JM, Prasad AS. Impaired IgM antibody responses to an influenza virus vaccine in adults with sickle cell anemia. Am J Hematol. 1985;20(4):409–12. doi: 10.1002/ajh.2830200413. [DOI] [PubMed] [Google Scholar]
- 7.Rautonen N, Martin NL, Rautonen J, Rooks Y, Mentzer WC, Wara DW. Low number of antibody producing cells in patients with sickle cell anemia. Immunol Lett. 1992;34(3):207–11. doi: 10.1016/0165-2478(92)90215-a. [DOI] [PubMed] [Google Scholar]
- 8.Szczepanek SM, McNamara JT, Secor ER, et al. Splenic morphologic changes are accompanied by altered baseline immunity in a mouse model of sickle cell disease. Am J Pathol. 2012;181(5):1725–34. doi: 10.1016/j.ajpath.2012.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Purohit S, Alvarez O, O’Brien R, Andreansky S. Durable immune response to inactivated H1N1 vaccine is less likely in children with sickle cell anemia receiving chronic transfusions. Pediatr Blood Cancer. 2012;59:1280–3. doi: 10.1002/pbc.24206. [DOI] [PubMed] [Google Scholar]
- 10.Hambidge SJ, Ross C, McClure D, Glanz J VSD team. Trivalent inactivated influenza vaccine is not associated with sickle cell hospitalizations in adults from a large cohort. Vaccine. 2011;29(46):8179–81. doi: 10.1016/j.vaccine.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Hambidge SJ, Ross C, Glanz J, et al. Trivalent inactivated influenza vaccine is not associated with sickle cell crises in children. Pediatrics. 2012;129(1):e54–9. doi: 10.1542/peds.2011-1294. [DOI] [PubMed] [Google Scholar]
- 12.Hambidge SJ, Glanz JM, France EK, et al. Safety of trivalent inactivated influenza vaccine in children 6 to 23 months old. JAMA. 2006;296(16):1990–7. doi: 10.1001/jama.296.16.1990. [DOI] [PubMed] [Google Scholar]
- 13.Wallace KL, Marshall MA, Ramos SI, et al. NKT cells mediate pulmonary inflammation and dysfunction in murine sickle cell disease through production of IFN-gamma and CXCR3 chemokines. Blood. 2009;114(3):667–76. doi: 10.1182/blood-2009-02-205492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holtzclaw JD, Jack D, Aguayo SM, Eckman JR, Roman J, Hsu LL. Enhanced pulmonary and systemic response to endotoxin in transgenic sickle mice. Am J Respir Crit Care Med. 2004;169(6):687–95. doi: 10.1164/rccm.200302-224OC. [DOI] [PubMed] [Google Scholar]
- 15.Nandedkar SD, Feroah TR, Hutchins W, et al. Histopathology of experimentally induced asthma in a murine model of sickle cell disease. Blood. 2008;112(6):2529–38. doi: 10.1182/blood-2008-01-132506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pritchard KA, Jr, Feroah TR, Nandedkar SD, et al. Effects of experimental asthma on inflammation and lung mechanics in sickle cell mice. Am J Respir Cell Mol Biol. 2012;46(3):389–96. doi: 10.1165/rcmb.2011-0097OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Secor ER, Carson WF, Singh A, et al. Oral bromelain attenuates inflammation in an ovalbumin-induced murine model of asthma. Evid Based Complement Alternat Med. 2008;5(1):61–9. doi: 10.1093/ecam/nel110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Unkeless JC, Scigliano E, Freedman VH. Structure and function of human and murine receptors for IgG. Annu Rev Immunol. 1988;6:251–81. doi: 10.1146/annurev.iy.06.040188.001343. [DOI] [PubMed] [Google Scholar]
- 19.Hord J, Windsor B, Koehler M, Blatt J, Janosky J, Mirro J. Diminished antibody response to hepatitis B immunization in children with sickle cell disease. J Pediatr Hematol Oncol. 2002;24(7):548–9. doi: 10.1097/00043426-200210000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Narni-Mancinelli E, Campisi L, Bassand D, et al. Memory CD8+ T cells mediate antibacterial immunity via CCL3 activation of TNF/ROI+ phagocytes. J Exp Med. 2007;204(9):2075–87. doi: 10.1084/jem.20070204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wanjalla CN, Goldstein EF, Wirblich C, Schnell MJ. A role for granulocyte-macrophage colony-stimulating factor in the regulation of CD8(+) T cell responses to rabies virus. Virology. 2012;426(2):120–33. doi: 10.1016/j.virol.2012.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He S, Cao Q, Yoneyama H, Ge H, Zhang Y. MIP-3alpha and MIP-1alpha rapidly mobilize dendritic cell precursors into the peripheral blood. J Leukoc Biol. 2008;84(6):1549–56. doi: 10.1189/jlb.0708420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dawson TC, Beck MA, Kuziel WA, Henderson F, Maeda N. Contrasting effects of CCR5 and CCR2 deficiency in the pulmonary inflammatory response to influenza A virus. Am J Pathol. 2000;156(6):1951–9. doi: 10.1016/S0002-9440(10)65068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chies JA, Hutz MH. High frequency of the CCR5delta32 variant among individuals from an admixed Brazilian population with sickle cell anemia. Braz J Med Biol Res. 2003;36(1):71–5. doi: 10.1590/s0100-879x2003000100010. [DOI] [PubMed] [Google Scholar]
- 25.Moffitt KL, Gierahn TM, Lu YJ, et al. T(H)17-based vaccine design for prevention of Streptococcus pneumoniae colonization. Cell Host Microbe. 2011;9(2):158–65. doi: 10.1016/j.chom.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller ML, Gao G, Pestina T, Persons D, Tuomanen E. Hypersusceptibility to invasive pneumococcal infection in experimental sickle cell disease involves platelet-activating factor receptor. J Infect Dis. 2007;195(4):581–4. doi: 10.1086/510626. [DOI] [PubMed] [Google Scholar]
- 27.Jonker MA, Hermsen JL, Gomez FE, Sano Y, Kudsk KA. Injury induces localized airway increases in pro-inflammatory cytokines in humans and mice. Surg Infect (Larchmt) 2011;12(1):49–56. doi: 10.1089/sur.2010.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wanderer AA. Rationale for IL-1beta targeted therapy for ischemia-reperfusion induced pulmonary and other complications in sickle cell disease. J Pediatr Hematol Oncol. 2009;31(8):537–8. doi: 10.1097/MPH.0b013e3181acd89d. [DOI] [PubMed] [Google Scholar]
- 29.Kabbur PM, Carson WFt, Guernsey L, Secor ER, Jr, Thrall RS, Schramm CM. Interleukin-10 does not mediate inhalational tolerance in a chronic model of ovalbumin-induced allergic airway disease. Cell Immunol. 2006;239(1):67–74. doi: 10.1016/j.cellimm.2006.04.004. [DOI] [PubMed] [Google Scholar]