Abstract
Activation of JAK2, frequently as a result of the JAK2V617F mutation, is a characteristic feature of the classical myeloproliferative neoplasms (MPN) polycythemia vera, essential thrombocythemia and myelofibrosis and is thought to be responsible for the constitutional symptoms associated with these diseases. BMS-911543 is a JAK2 selective inhibitor that induces apoptosis in JAK2-dependent cell lines and inhibits the growth of CD34+ progenitor cells from patients with JAK2V617F - positive MPN. To explore the clinical potential of this inhibitor, we tested BMS-911543 in a murine retroviral transduction – transplantation model of JAK2V617F MPN. Treatment was initiated at two dose levels (3 mg/kg and 10 mg/kg) when the hematocrit exceeded 70%. Following the first week, white blood cell counts were reduced to normal in the high dose group and were maintained well below the vehicle-treated mice throughout the study. However, BMS-911543 had no effect on red blood cell parameters. After 42 days of treatment, the proportion of JAK2V617F - positive cells in hematopoietic tissues was identical or slightly increased compared to controls. Plasma concentrations of IL-6, IL-15, and TNFα were elevated in MPN mice and reduced in the high dose treatment group, while other cytokines were unchanged. Inhibitor activity after dosing was confirmed in a cell culture assay using the plasma of dosed mice and pSTAT5 flow cytometry. Collectively, these results show that BMS-911543 has limited activity in this murine model of JAK2V617F – driven MPN and suggest that targeting JAK2 alone may be insufficient to achieve effective disease control.
Keywords: Polycythemia vera, Myelofibrosis, Janus kinase, pSTAT5
Introduction
An activating mutation in the JAK2 gene (JAK2V617F) is common in patients with myeloproliferative neoplasms (MPN), including over 90% of patients with polycythemia vera (PV) and 30–50% of patients with primary myelofibrosis (PMF) or essential thrombocythemia (ET) (1–6). Ruxolitinib, the only JAK inhibitor thus far approved for clinical use in PMF, is equipotent against JAK1 and JAK2 (Table 1), but is less active against the remaining JAK family members TYK2 and JAK3 (7–12). While ruxolitinib effectively controls MF symptoms, it has no significant impact on disease burden, raising the question whether more potent and specific inhibitors of JAK2 may target the MPN clone more effectively. BMS-911543 is a highly selective inhibitor of JAK2. In kinase assays, BMS-911543 is 356-fold more potent against JAK2 compared to JAK1, 73-fold more potent against JAK3 and 66-fold more potent against TYK2 (12). BMS-911543 was shown to selectively inhibit the proliferation of JAK2-dependent cell lines and to reduce colony growth by V617F+ patient samples at submicromolar concentrations (12). BMS-911543 is bioavailable in mice and has been shown to inhibit pSTAT5 activity in xenografts using the JAK2V617F heterozygous SET2 cell line or BaF3 cells co-expressing JAK2V617F and EpoR, with IC50 values achieved at ~2 mg/kg and up to 90% suppression of pSTAT5 at 7 hours post dosing (12). As effects on tumor growth were not reported and xenografts are imperfect models of human MPN, we decided to test BMS-911543 in a retroviral transduction – transplantation model of MPN. This model closely resembles human PV and progresses to secondary MF (13).
Table 1.
Summary of JAK family inhibitors.
| Inhibitor name | Company | JAK1 | JAK2 | JAK3 | TYK2 | Citation |
|---|---|---|---|---|---|---|
| BMS-911543 | Bristol-Myers Squibb | 356 | 1 | 73 | 66 | Purandare et al., 2012 |
| ruxolitinib (INCB018424) | Novartis | 3.3 | 2.8 | 428 | 19 | Quintas-Cardama et al., 2010 |
| TG101209 | Sanofi-Aventis | ND | 6 | 169 | ND | Pardanani et al., 2007 |
| tofacitinib (CP-960,550) | Pfizer | 112 | 20 | 1 | ND | Changelian et al., 2003 |
| pacritinib (SB1518) | S*BIO | 1280 | 23 | 520 | 50 | William et al., 2011 |
| momelotinib (CYT387) | Gilead | 11 | 18 | 155 | 17 | Tyner et al., 2010 |
Methods
Induction of MPN, study design and drug administration
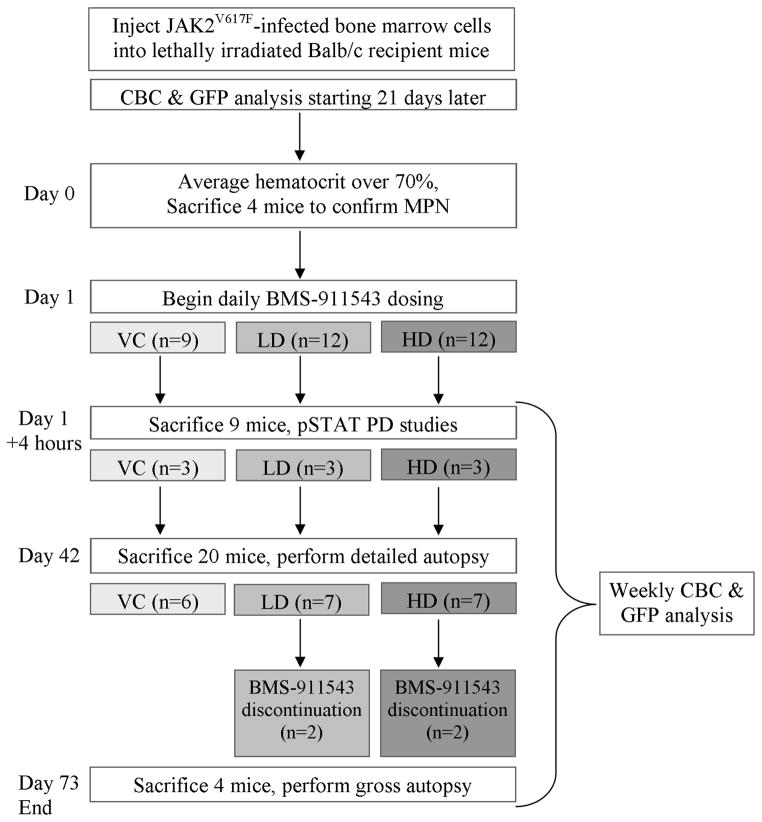
A PV-like myeloproliferative neoplasm (MPN) was induced in female Balb/c mice as previously described (11, 13). Once the average hematocrit of the cohort exceeded 70%, four mice were euthanized and examined to confirm MPN through the presence of splenomegaly and GFP+ cells in the spleen and bone marrow. Mice were randomly assigned to three treatment groups; vehicle control, 3 mg/kg (low dose, LD), and 10 mg/kg (high dose, HD)). The 3 and 10 mg/kg doses were chosen based on the near complete suppression of STAT5 phosphorylation observed following 2, 5, and 10 mg/kg dosing. (12). BMS-911543{(N,N-dicyclopropyl-4-((1,5-dimethyl-1H-pyrazol-3-yl)amino)-6-ethyl-1-methyl-1,6-dihydroimidazo[4,5-d]pyrrolo[2,3b]pyridine-7-carboxamide)} was prepared in a solution of 20% citrate/80% PEG400 with brief sonication and aliquots were stored at −20°C. Mouse weight was recorded weekly and drug dilutions were made according to the average weight of each group. Details of the compound, including structure and IC50 values, are provided elsewhere (12). Pharmacodynamic studies were performed on three mice per group (nine total) four hours after administration of the first dose of BMS-911543. Mice were dosed by oral gavage (100 μl/dose) once daily for 42 days, at which point one healthy control, six vehicle-treated mice and seven mice per treatment group were harvested for examination. Two mice from each drug treatment group were observed for an additional 32 days after discontinuation of drug. Complete blood counts were performed once or twice weekly on a Heska HemaTrue (Loveland, CO) and GFP percentage (as a proxy for disease burden) was determined weekly on a Guava 6HT flow cytometer (Millipore, Billerica, MA). A schematic of the study design is presented in Figure 1. Mouse procedures were carried out according to guidelines approved by the Institutional Animal Care and Use Committee (IACUC) at The University of Utah.
Figure 1. A schematic of the research plan.
Donor mice received 5FU and bone marrow cells were harvested five days later. These cells were infected with MIG-JAK2V617F and injected into lethally irradiated recipient mice. Dosing with BMS-911543 began when the average hematocrit exceeded 70% and continued for 42 days. VC - vehicle control, LD - low dose (3 mg/kg), HD - high dose (10 mg/kg), PD - pharmacodynamics, CBC - complete blood count, GFP - green fluorescent protein
Histology and reticulocyte counts
Six vehicle-treated, seven low dose, and seven high dose mice were evaluated. Livers, lungs, and spleens were dissected and placed in freshly prepared 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) in phosphate buffered saline for 24 hours then moved to 70% ethanol. Bones were placed in 4% paraformaldehyde in phosphate buffered saline overnight, moved to Formical-2000 (Decal Chemical Corp., Tallman, NY) for 7–9 days, then placed in 70% ethanol. Tissues were embedded in paraffin, sectioned at 5 micrometers, and stained with H&E. Separate bone marrow sections were also stained with Chandler’s Reticulin Stain Kit (American MasterTech, Lodi, CA). For reticulin scoring, three images per mouse were captured at 20x and blindly scored according to the 0–3 semi-quantitative method described by Thiele et al. (14). Reticulocyte counts were performed on 10 μl whole blood after 10 minutes incubation with 8 μl 0.5% new methylene blue solution (Ricca Chemical Company, Arlington, TX). Two hundred red blood cells were scored.
Flow cytometry and antibodies
For pSTAT analysis, blood, spleen, and bone marrow cells were harvested 4 hours after treatment with vehicle, 3 mg/kg or 10 mg/kg BMS-911543 (N=3 for each group), fixed in 2% paraformaldehyde at 37°C for 10 minutes, stored on ice for one minute, permeabilized with 90% cold methanol for 30 minutes on ice and stored at −20°C until analyzed. Cells were washed twice in PBS supplemented with 0.5% bovine serum albumin (PBS/BSA) and then resuspended in 100 μl PBS/BSA at room temperature. Following incubation for 10 minutes, antibody was added at 1:50 for one hour. After two washes in PBS/BSA, the cells were analyzed on a BD FACS Canto. Alexa Fluor 647 conjugated antibodies directed against pSTAT1Y701, pSTAT3Y705 and pSTAT5Y694 were purchased from Cell Signaling Technology (Danvers, MA; catalog numbers 8009S, 4324S, 9365S, respectively). Data was analyzed for each pSTAT as well as separately for GFP+ and GFP− cells. Ba/F3 cells were cytokine starved overnight then simultaneously stimulated with IL-3 (50 ng/ml) and IL-6 (100 ng/ml) for 5 minutes to serve as species-specific controls. HEL cells served as an additional control. Lineage antibodies were purchased from BD Biosciences (San Jose, CA; Ter119-V450, CD19-PE-Cy7, cKit-PerCP-Cy5.5, CD34-Alexa Fluor 647, CD19-PE-Cy7, CD41-PE, CD3-V450, CD71-PE) and eBiosciences (San Diego, CA; Gr1-PerCP-Cy5.5, B220-APC, Fc block). Aqua Live/Dead (Life Technologies, Carlsbad, CA) was included at 1:100 per sample and only live (negative) cells were analyzed. For detection of lineage markers, freshly isolated cells were incubated in a 96 well plate in PBS/BSA and 0.5 μg Fc block for 10 minutes, antibody for 30 minutes, washed twice with PBS, fixed with 2% paraformaldehyde and analyzed on a BD FACS Canto the following day. Compensation was set with OneComp eBeads from eBiosciences.
Cytokine profiling and statistics
Cytokine profiling was performed with the Milliplex Mouse Cytokine/Chemokine kit by Millipore and analyzed with a MAGPIX instrument (Luminex Corporation, Austin, TX). Cytokine quantifications more than threefold above or below the data set median were not used for analysis. All data sets in this publication were compared in Microsoft Excel with a student’s t-test using two-tailed distribution and two-sample equal variance. Significance was assigned to p values ≤ 0.05.
Indirect plasma drug activity assay
Given that BMS-911543 was minimally effective at altering the natural course of murine MPN, we sought to determine whether the drug was bioavailable. We dosed four mice at 30 mg/kg and harvested plasma 90 minutes later. Plasma was centrifuged twice at 21,000 g for 10 minutes to remove all cellular debris, incubated with 1.8 mg/mL of Proteinase K (Qiagen, Netherlands) at 37°C for 1 hour to digest proteins (including cytokines), and centrifuged through an Amicon Ultra-0.5 centrifugal filter unit with Ultracel-3 membrane (3 kDa nominal molecular weight cut off, Millipore) to ensure removal all cytokines. Ba/F3 cells (1 × 105/well, 96 well plate, 100 μL) were IL-3 starved overnight, incubated for 30 minutes with 50 μL of either vehicle plasma (n=3) or with plasma from mice dosed with BMS-911543 at 30 mg/kg (n=4). This dose was chosen to accommodate the need to dilute the plasma 1:3 in cell culture medium (100 μL plasma plus 50 μL plasma) while maintaining a sufficiently high concentration to allow for a clear effect to be observed. Following the 30 minute incubation period, murine IL-3 (50 ng/mL, Peprotech, Rocky Hill, NJ) was added to each well, incubated for 5 minutes at 37°C, then cells were prepared for flow cytometry following standard fixation/permeabilization steps and anti-pSTAT5 (Y694)-Alexa Fluor 647 (Cell Signaling Technology). Data was collected on a BD FACSCanto and analyzed with FlowJo X (Treestar, Ashland, OR).
Results & Discussion
Induction of MPN
JAK2V617F-positive MPN was induced in lethally irradiated Balb/c mice by transplantation of bone marrow cells retrovirally transduced with MIG-JAK2V617F, as described (Figure 1) (13). An average hematocrit over 70% was predefined as the trigger for the initiation of treatment. Twenty-one days after transplant, the hematocrit was 74±6%, red blood cells 10.7±0.8×1012/L, hemoglobin 195±11.6 g/L and white blood cells 31.0±27.9×109/L, with 27.7±3.0% granulocytes and 68.4±5.8% lymphocytes. FACS revealed 34.2±12.2% GFP+ cells in the peripheral blood. The following day, four mice were sacrificed and subjected to autopsy and histopathology. Spleen weights were increased to 304.5±46.9 mg (healthy control=76.8 mg), bone marrow was hypercellular and splenic architecture was effaced. GFP+ cells in bone marrow and spleen were 26.1±7.6% and 16.7±2.9%, respectively. Together, these parameters established a diagnosis of MPN. On the following day, BMS-911543 treatment was initiated at two dose levels [low dose (LD) = 3 mg/kg and high dose (HD) = 10 mg/kg, once daily by oral gavage] based on the pharmacokinetic data and maintained for 42 days (except in mice harvested for pharmacodynamic studies). Vehicle control mice received carrier only.
BMS-911543 suppresses leukocytosis but not erythrocytosis in JAK2V617F-induced MPN
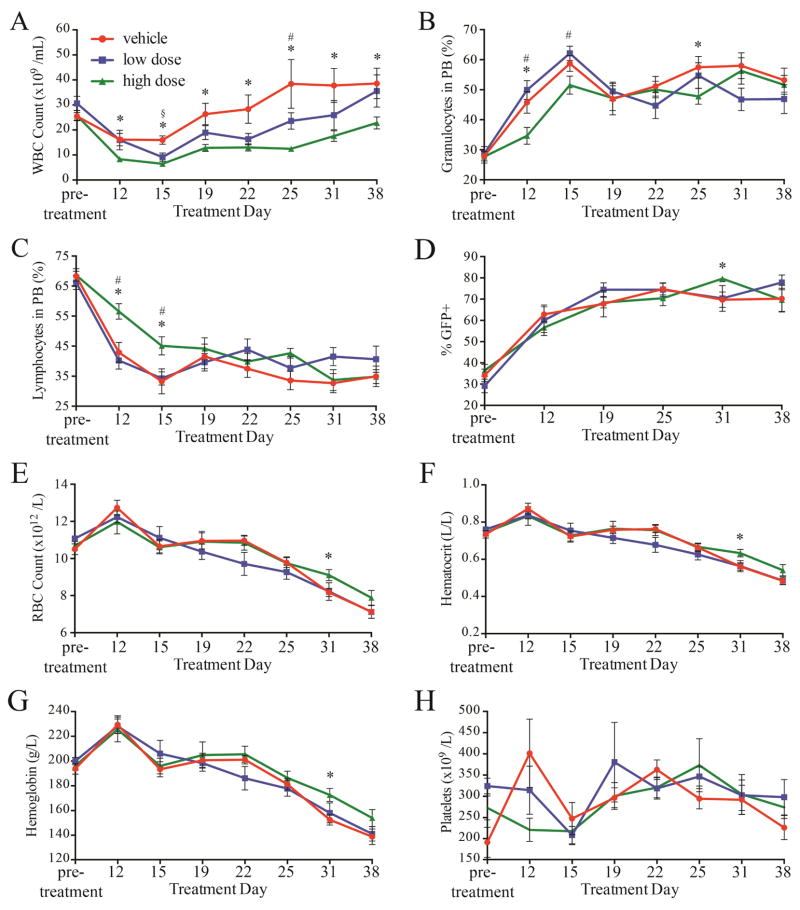
Four hours after the first treatment, three mice per group were euthanized for pSTAT analysis. Spleen, bone marrow, and blood were harvested and cells were analyzed by flow cytometry to evaluate pSTAT1Y701, pSTAT3Y705, and pSTAT5Y694. pSTAT5Y694 was reduced in the peripheral blood of treated mice compared to controls, both in GFP+ and GFP− cells, although the difference did not quite reach statistical significance (p<0.10 for each, Supplemental Figure 1). In contrast, pSTAT5Y694 was identical in the marrow and spleen. pSTAT1Y701 and pSTAT3Y705 in treated mice were generally comparable to controls (Supplemental Figures 1–3). The mice were followed by daily inspection and 1–2 weekly complete blood counts (CBC). After an initial decrease in all groups (Figure 2), the white blood cells (WBC) in the vehicle treated group steadily increased, reaching 40×109/L on day 25 of treatment. In contrast, the WBC in the LD and, more so in the HD treatment groups, were reduced, although differences became smaller toward the end of the study. Relative proportions of granulocytes decreased over the treatment period. Erythrocytosis, hemoglobin and hematocrit gradually decreased into the normal range, but were not different between treatment groups and controls (Figure 2). Reticulocyte counts were also identical between treated mice and controls (Supplemental Figure 4), and platelet counts remained in the normal range, as previously described with this model (13). Thus, BMS-911543 suppressed the leukocytosis that is associated with JAK2V617F MPN, without affecting red cell parameters. Following the 42-day treatment period, two randomly selected high dose and two low dose mice were observed for an additional 32 days after discontinuation of BMS-911543. All mice showed a further increase in WBC that was maintained for the 32-day observation period (Supplemental Figure 5). Red cell parameters remained stable, probably reflecting the fact that the disease had entered the ‘spent phase’ and transformed to myelofibrosis. At autopsy, spleens were enlarged but their weight was reduced (~184 mg; data not shown) compared to the spleens harvested at day 1 (~300 mg) or day 42 (~230 mg) (Supplemental Figure 4). Five healthy mice were dosed with 10 mg/kg BMS-911543 and five with vehicle in a similar, separate experiment and no changes in spleen mass, spleen or bone marrow histology (Supplemental Figure 6), or blood parameters (Supplemental Figure 7) were observed. Gr1+ cells were reduced from 11.6 to 8.6% (p=0.026) in the BMS-911543 group while B and T cell populations were not altered (data not shown, compare to Figure 4), suggestive of mild splenic and/or myeloid toxicity.
Figure 2. BMS-911543 lowered and maintained white blood cell counts in murine MPN.
(A) The high dose group maintained white cell counts that were nearly normal and well below the vehicle-treated group for the treatment period. (B) Granulocytes, as a percentage of white blood cells, were reduced and (C) relative lymphocyte counts were elevated by high dose BMS-911543 early in the treatment period, but all groups became indistinguishable after 15 days of treatment. (D) The percentage of peripheral white cells positive for GFP increased steadily in all groups throughout the treatment period and were slightly higher in the HD group at day 31. (E, F, G) Red blood cells, hematocrit, and hemoglobin decreased steadily throughout the treatment period. (H) Platelet counts did not vary between groups. Data from ten vehicle, ten low dose, and nine high dose mice are shown. * p≤0.05 high dose vs. vehicle; § p≤0.05 low dose vs. vehicle; # p≤0.05 low dose vs. high dose. Error bars represent standard error of the mean.
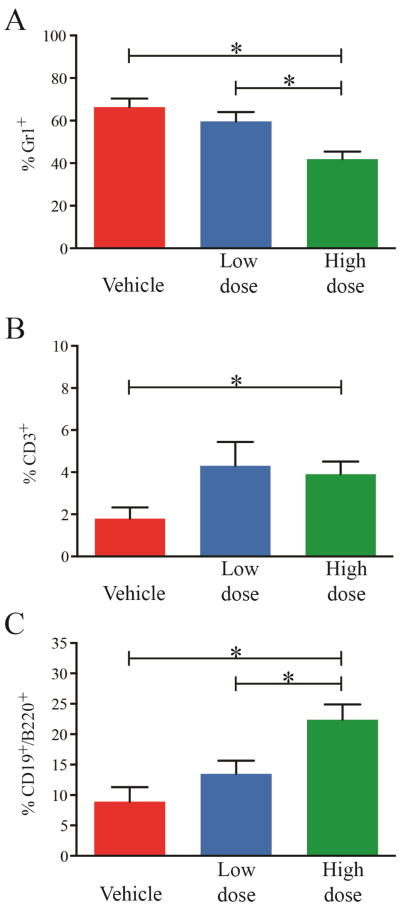
Figure 4. Effect of BMS-911543 on hematopoietic cell lineages in MPN.
Following 42 days of treatment, high dose BMS-911543 reduced the granulocytes (A, Gr1+) and increased the T (CD3+) and B (CD19+/B220+) cells (B and C, respectively) in the spleen, but not the blood or bone marrow (not shown). Flow cytometry data from six vehicle, seven low dose, and seven high dose mice are shown. Error bars represent standard error of the mean. *p≤0.05
BMS-911543 treatment does not significantly alter MPN histology
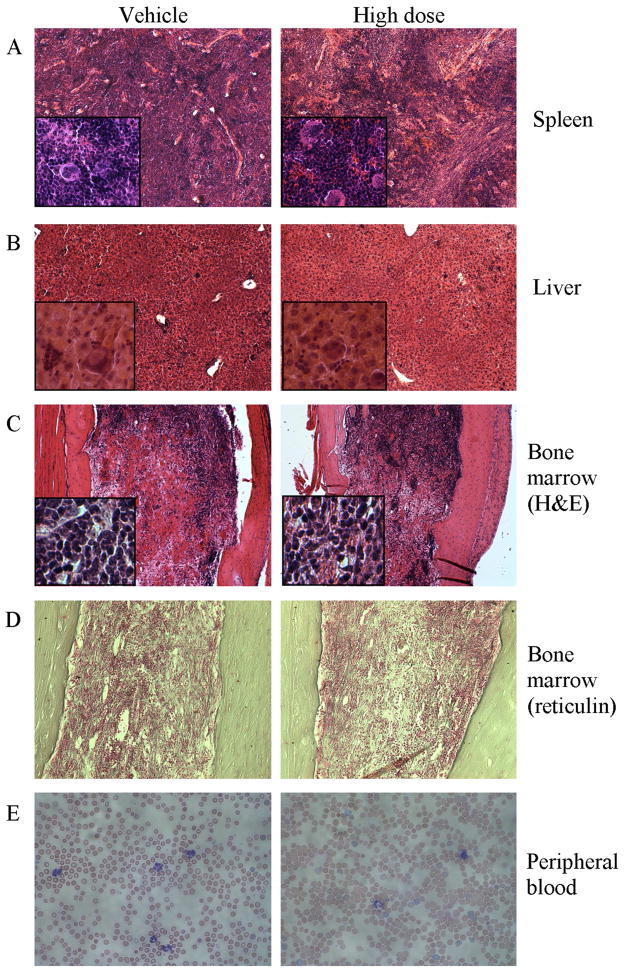
On treatment day 42, spleens, livers and femurs were harvested from seven mice in each of the two treatment groups and six vehicle controls. Spleen weight was similar in all groups (Supplemental Figure 4). Histology showed trilineage hematopoiesis with prominent megakaryocytes (Figure 3). Spleens from the vehicle and LD (not shown) groups exhibited complete effacement of splenic architecture, while the HD group showed some restoration of germinal centers (Figure 3). Liver histology revealed megakaryocytes and extramedullary hematopoiesis, especially near portal veins, without significant differences between groups. Bone marrows were uniformly hypocellular and reticulin staining showed severe fibrosis in each group, with average scores near 2.5 on the 0–3 scale (Supplemental Figure 4) (14). We observed a higher percentage of total GFP+ cells (JAK2V617F – positive) in the spleen (85.1±0.3% vs. 75.6±8.3%; p=0.014) and bone marrow (67.1±6.7% vs. 57.2±7.3%; p=0.026) of mice treated with HD BMS-911543 compared to the vehicle control group (Supplemental Figure 4).
Figure 3. Histopathology after 42 days of treatment with BMS-911543.
Representative histological sections are shown for (A) spleen, (B) liver, (C) bone marrow stained with H&E, (D) bone marrow stained with Chandler’s reticulin stain, each at Day 42, and (E) peripheral blood (Wright’s stain) at Day 35. A–C are 10x with 40x insets, D is 20x, and E is 40x.
BMS-911543 partially restores lineage composition of spleen but not marrow or blood
Spleen, marrow, and peripheral blood were harvested after 42 days of treatment and analyzed by flow cytometry for lineage markers. A dose dependent reduction of granulocytes (Gr1+) and partial restoration of B (B220+, CD19+) and T (CD3+) cell distributions was observed in the spleen (Figure 4), but not in the blood or bone marrow (Supplemental Figure 8). No significant differences were observed in other lineage markers, except a small reduction of peripheral blood B cells in the HD group (1.3±0.4 vs. 0.8±0.3%, p<0.05). To evaluate the impact of BMS-911543 treatment on MPN burden we measured GFP expression as well as immunophenotypically defined subsets of blood, bone marrow, and spleen cells. BMS-911543 had little effect on the percentage of GFP+ cells in each cellular subset or even led to a mild increase (e.g. GFP+ bone marrow granulocytes were increased to 20.3±4.8% in the HD group compared to 15.0±3.2% in the vehicle group, p=0.0377 (Supplemental Figure 9). These results suggest that BMS-911543 fails to provide JAK2 wild-type cells with a competitive advantage over MPN cells throughout all cell compartments analyzed.
BMS-911543 partially normalizes serum cytokine concentrations
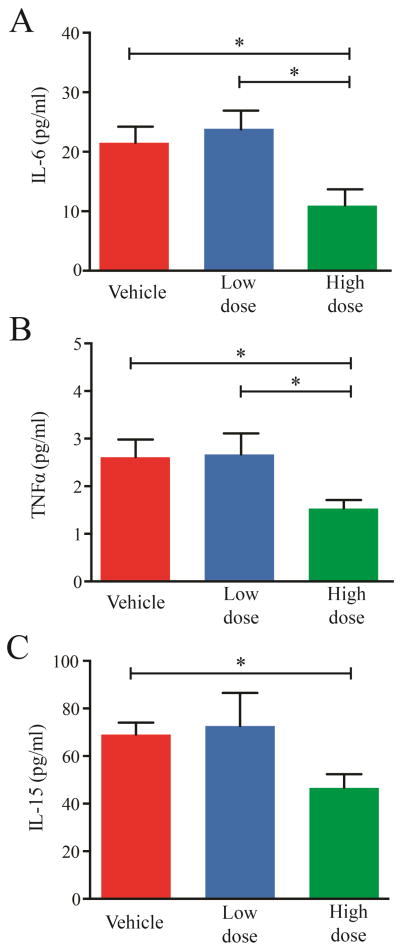
JAK family inhibitors like TG101209 or momelotinib (formerly Cytopia387) have been shown to partially normalize cytokine concentrations in mice with JAK2V617F-induced MPN (13, 15). We therefore measured serum cytokine profiles after 42 days of treatment with BMS-911543. TNFα, IL-6, and IL-15 concentrations were significantly reduced in the HD group compared to the vehicle control, with TNFα and IL-15 reaching the normal range (Figure 5). G-CSF and KC concentrations were reduced in MPN mice compared to normal mice, with further reduction upon treatment with BMS-911543 (Supplemental Figure 10). IL-10, IL-17, and VEGF concentrations were also elevated in MPN mice but not reduced by treatment (Supplemental Figure 10).
Figure 5. Effect of BMS-911543 on cytokine concentrations in MPN.
Serum was evaluated after the 42 day treatment period and IL-6 (A), TNFα (B), and IL-15 (C) were elevated compared to normal mice and reduced in the high dose group compared to the vehicle-treated group. Data from six vehicle, seven low dose, and seven high dose mice are shown. Error bars represent standard error of the mean. *p≤0.05
BMS-911543 is present and active in the plasma of treated mice
The limited efficacy of this inhibitor mandated verification of the presence of BMS-911543 in the plasma of mice following oral dosing. While this has been reported elsewhere (12), we sought to confirm bioavailability in our system. To this end we developed a functional, indirect plasma drug activity assay, using plasma from mice treated with BMS-911543 in a cell culture assay with phosphorylation of STAT5 (Y694) as the end point. At a dose of BMS-911543 equivalent to 10 mg/kg we observed a 48% reduction of pSTAT5 MFI compared to controls (p=0.002; Supplemental Figure 11), confirming the presence of active drug.
Our data show that BMS-911543 has limited efficacy in our murine model of JAK2V617F - induced MPN. Although we did not perform a direct comparison, the results are clearly inferior to those of momelotinib tested in the same disease model (11). As momelotinib is equipotent against JAK1 and JAK2, but BMS-911543 is highly selective for JAK2, it is conceivable that JAK1 inhibitory activity is required for more profound effects in this mouse model of MPN. Direct in vivo validation of this notion would require treating mice with drug concentrations that are equipotent toward JAK2, which would be challenging due to differences in their pharmacokinetic properties. Similar to CYT387 (11), treatment was initiated when the hematocrit of the cohort exceeded 70%. Given that this is a late model of MPN, more profound effects may have been observed with earlier treatment initiation. The limited activity of BMS-911543 against the MPN phenotype is reflected by PD studies. BMS-911543 reduced pSTAT5Y694 only in the peripheral white blood cells, but not the bone marrow or spleen cells, even at high doses (10 mg/kg). This is in contrast to its strong effects in cell line xenografts, where a 90% suppression of pSTAT5Y694 was reported after a single dose of 10 mg/kg (12), suggesting that pSTAT5Y694 in these key disease sites is not, or is not exclusively, under the control of JAK2, and that the physiological microenvironmental context is critical for PD assessment. Similarly, BMS-911543 did not reduce pSTAT3Y705, suggesting that JAK1 inhibition may be required to suppress this key regulator of inflammatory cytokine responses. A phase 1/2 study of BMS-911543 is ongoing and it will be interesting to see whether limited activity of the compound in murine JAK2V617F-induced MPN model is reflected clinically (16)
Supplementary Material
Highlights.
A highly selective JAK2 inhibitor was tested in a murine model of JAK2V617F+ MPN
Despite high preclinical potential, BMS-911543 is minimally effective in murine MPN
JAK2 inhibition is not sufficient to control the symptoms of murine MPN
Selective inhibition of JAK2 may be detrimental to healthy cells and/or advantageous to malignant cells
Acknowledgments
Funding for this work was provided to MWD by Bristol-Myers Squibb. His laboratory is also funded by Novartis, Celgene, Genzyme, and Gilead and ADP, AME, AVS, MSZ, and TO work in his laboratory. He is a consultant/advisory board member of Bristol-Myers Squibb, Novartis, Pfizer, ARIAD Pharmaceuticals, and Incyte.
Funding for this work was provided to MWD by Bristol-Myers Squibb. This work was supported by the University of Utah Flow Cytometry Facility in addition to the National Cancer Institute through Award Number 5P30CA042014-24. This work was supported The Leukemia & Lymphoma Society’s Translational Research Program Award (Grant 6086-12 to MWD) and The Leukemia & Lymphoma Society’s Specialized Center of Research Program and the Oregon Health Sciences University (Grant GCNCR0314A-UTAH to Brian J. Druker). Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number R01CA178397 (TO and MWD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. A.M.E. is a Fellow of the Leukemia and Lymphoma Society (grant 5090-12 to M.W.D.) and was supported by T32CA093247 (Donald E. Ayer) from the National Cancer Institute. The authors thank Dr. Clint C. Mason for assistance with statistics and Dr. Ashok V. Purandare of Bristol-Myers Squibb for pharmacokinetic data.
Footnotes
Authorship: ADP designed experiments and implemented the study, performed analyzes, and prepared the manuscript. AME assisted with experiments and provided manuscript critiques. AVS assisted with experiments. MSZ provided assistance with the manuscript. JEM designed and performed flow cytometry. JTP provided guidance regarding the model and manuscript critiques. TO provided project direction and manuscript critiques. MWD is the principal investigator, provided project direction, technical guidance, manuscript critiques, and takes primary responsibility for this manuscript.
No potential conflicts of interest were disclosed by the other authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005 Mar 19–25;365(9464):1054–61. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 2.James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005 Apr 28;434(7037):1144–8. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 3.Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005 Apr 28;352(17):1779–90. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 4.Levine RL, Loriaux M, Huntly BJ, Loh ML, Beran M, Stoffregen E, et al. The JAK2V617F activating mutation occurs in chronic myelomonocytic leukemia and acute myeloid leukemia, but not in acute lymphoblastic leukemia or chronic lymphocytic leukemia. Blood. 2005 Nov 15;106(10):3377–9. doi: 10.1182/blood-2005-05-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer cell. 2005 Apr;7(4):387–97. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 6.Jones AV, Kreil S, Zoi K, Waghorn K, Curtis C, Zhang L, et al. Widespread occurrence of the JAK2 V617F mutation in chronic myeloproliferative disorders. Blood. 2005 Sep 15;106(6):2162–8. doi: 10.1182/blood-2005-03-1320. [DOI] [PubMed] [Google Scholar]
- 7.Quintas-Cardama A, Vaddi K, Liu P, Manshouri T, Li J, Scherle PA, et al. Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: therapeutic implications for the treatment of myeloproliferative neoplasms. Blood. 2010 Apr 15;115(15):3109–17. doi: 10.1182/blood-2009-04-214957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Changelian PS, Flanagan ME, Ball DJ, Kent CR, Magnuson KS, Martin WH, et al. Prevention of organ allograft rejection by a specific Janus kinase 3 inhibitor. Science. 2003 Oct 31;302(5646):875–8. doi: 10.1126/science.1087061. [DOI] [PubMed] [Google Scholar]
- 9.William AD, Lee AC, Blanchard S, Poulsen A, Teo EL, Nagaraj H, et al. Discovery of the macrocycle 11-(2-pyrrolidin-1-yl-ethoxy)-14,19-dioxa-5,7,26-triaza-tetracyclo[19.3.1.1(2,6). 1(8,12)]heptacosa-1(25),2(26),3,5,8,10,12(27),16,21,23-decaene (SB1518), a potent Janus kinase 2/fms-like tyrosine kinase-3 (JAK2/FLT3) inhibitor for the treatment of myelofibrosis and lymphoma. Journal of medicinal chemistry. 2011 Jul 14;54(13):4638–58. doi: 10.1021/jm200326p. [DOI] [PubMed] [Google Scholar]
- 10.Pardanani A, Hood J, Lasho T, Levine RL, Martin MB, Noronha G, et al. TG101209, a small molecule JAK2-selective kinase inhibitor potently inhibits myeloproliferative disorder-associated JAK2V617F and MPLW515L/K mutations. Leukemia: official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2007 Aug;21(8):1658–68. doi: 10.1038/sj.leu.2404750. [DOI] [PubMed] [Google Scholar]
- 11.Tyner JW, Bumm TG, Deininger J, Wood L, Aichberger KJ, Loriaux MM, et al. CYT387, a novel JAK2 inhibitor, induces hematologic responses and normalizes inflammatory cytokines in murine myeloproliferative neoplasms. Blood. 2010 Jun 24;115(25):5232–40. doi: 10.1182/blood-2009-05-223727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Purandare AV, McDevitt TM, Wan H, You D, Penhallow B, Han X, et al. Characterization of BMS-911543, a functionally selective small-molecule inhibitor of JAK2. Leukemia: official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2012 Feb;26(2):280–8. doi: 10.1038/leu.2011.292. [DOI] [PubMed] [Google Scholar]
- 13.Bumm TG, Elsea C, Corbin AS, Loriaux M, Sherbenou D, Wood L, et al. Characterization of murine JAK2V617F-positive myeloproliferative disease. Cancer Res. 2006 Dec 1;66(23):11156–65. doi: 10.1158/0008-5472.CAN-06-2210. [DOI] [PubMed] [Google Scholar]
- 14.Thiele J, Kvasnicka HM, Facchetti F, Franco V, van der Walt J, Orazi A. European consensus on grading bone marrow fibrosis and assessment of cellularity. Haematologica. 2005 Aug;90(8):1128–32. [PubMed] [Google Scholar]
- 15.Geron I, Abrahamsson AE, Barroga CF, Kavalerchik E, Gotlib J, Hood JD, et al. Selective inhibition of JAK2-driven erythroid differentiation of polycythemia vera progenitors. Cancer cell. 2008 Apr;13(4):321–30. doi: 10.1016/j.ccr.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 16.Pardanani A, Roberts AW, Seymour JF, Burbury K, Verstovsek S, Kantarjian HM, Begna K, Yoshitsugu H, Gestone TA, Phillips P, Xing G, Peltz G, Lorenzi MV, Alland L, Woolfson A, Tefferi A. BMS-911543, A Selective JAK2 Inhibitor: A Multicenter Phase 1/2a Study In Myelofibrosis. American Society of Hematology. 2013 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.