Abstract
The current application for many potential cell-based treatments for liver failure is limited by the low availability of mature functional hepatocytes. Although adult hepatocytes have a remarkable ability to proliferate in vivo, attempts to proliferate adult hepatocytes in vitro have been less successful. In this study, we investigated the effect of coculture cell type on the proliferative response and the functional activities of hepatocytes. We show, for the first time, a robust proliferative response of primary adult rat hepatocytes when cocultured with mouse 3T3-J2 fibroblasts. Hepatocytes cultured at low density on growth-arrested 3T3-J2 fibroblast feeder layers underwent significantly higher proliferation rates than when cultured on feeder layers made of four other cell types. Increasing colony size correlated with an increase in hepatocellular functions. The proliferating hepatocytes retained their morphologic, phenotypic, and functional characteristics. Using a cell patterning technique, we found that 3T3-J2 fibroblasts stimulate DNA synthesis in hepatocytes by short-range heterotypic cell–cell interactions. When hepatocytes that proliferated in cocultures were harvested and further subcultured either on 3T3-J2 fibroblast feeders or in the collagen sandwich configuration, their behavior was similar to that of freshly isolated hepatocytes. We conclude that adult rat hepatocytes can proliferate in vitro in a coculture cell type-dependent manner, and can be serially propagated by coculturing with 3T3-J2 fibroblasts while maintaining their differentiated characteristics. Our results also suggest that one of the major reasons for the functional differences in hepatocyte cocultures may be due to the different proliferative responses of hepatocytes as a function of coculture cell type. This study provides new insights in the roles of coculture cell types and cell–cell interactions in the modulation of hepatic proliferation and function.
Keywords: hepatocyte proliferation, coculture, DNA synthesis, cell patterning, subculture
Introduction
Orthotopic liver transplantation is a therapeutic modality for end-stage liver disease. Due to the severe shortage of donor organs, its potential complications, and high cost, many alternate approaches have been attempted to treat acute liver failure, including hepatocyte transplantation (Fox et al., 1998; Muraca et al., 2002; Strom et al., 1997), and bioartificial liver support systems (Chan et al., 2004; Demetriou et al., 2004). However, the current application of these potential therapies for liver failure is limited by the low availability of mature functional hepatocytes. Although for certain applications, hepatoma cells, which can be proliferated at will, can be used, they do not express the full range of liver-specific functions that is observed in adult primary hepatocytes.
Adult hepatocytes, the parenchymal cells of the liver, are normally quiescent and do not undergo cell division. However, after two-thirds partial hepatectomy, they rapidly undergo DNA synthesis and start to proliferate, followed by non-parenchymal cells, both of which participate in liver growth to its original size (Taub, 2004). This full regeneration process has been shown to occur even after 12 sequential hepatectomies (Stocker et al. 1973). Previous studies have also demonstrated that serially transplanted adult hepatocytes in a mouse model repopulated the liver (Overturf et al., 1997), indicating their high proliferative capacity in vivo.
Attempts to proliferate adult hepatocytes in vitro have not been nearly as successful. All published work using adult hepatocytes, whether for in vitro investigations or pre-clinical studies, has used primary cells isolated from an animal source. Although several previous studies have reported that DNA synthesis in cultured hepatocytes was enhanced by growth factors or coculturing with other cell types (Block et al., 1996; Kang et al., 2004; Michalopoulos et al., 1982; Mizuguchi et al., 2001; Richman et al., 1976; Shimaoka et al., 1987; Uyama et al., 2002), only in a few cases did these reports demonstrate actual cell division, and the proliferation was not precisely controlled or characterized.
Adult hepatocytes cocultured with other cell types have been shown to retain viability and function (Fraslin et al., 1985; Guguen-Guillouzo et al., 1983) through mechanisms dependent on heterotypic cell–cell contacts (Bhatia et al., 1998; Mesnil et al., 1987). Hepatocyte morphology and function varies somewhat depending on coculture cell type (Goulet et al., 1988; Khetani et al., 2004). In particular, murine 3T3-J2 fibroblasts appear to induce higher liver-specific functions in rat hepatocytes than any other coculture cell type (Bhatia et al., 1999). In addition, compared to the collagen gel sandwich configuration (Dunn et al., 1989), the hepatocyte/3T3-J2 fibroblast coculture has been shown to produce approximately 5–10 times higher liver-specific functions on a per cell basis, depending on hepatocyte to fibroblast seeding ratios. However, the reason for the seemingly very high expression of liver-specific function by hepatocytes cocultured with 3T3-J2 fibroblasts was not known.
Recently, we reported that hepatocytes increase in number in these cocultures, especially when the hepatocyte to fibroblast seeding ratio is low (Cho et al., 2007). Herein we characterized the proliferative response of adult rat hepatocytes cocultured with 3T3-J2 fibroblasts. We also investigated the impact of coculture cell type, and used a cell patterning technique to better understand the role of heterotypic interactions in the proliferative response. We found that adult rat hepatocytes proliferate on 3T3-J2 fibroblast feeder layers and that the proliferative responses of hepatocytes varied as a function of coculture cell type. Remarkably, hepatocytes undergoing proliferation maintained albumin expression so that total secretion in the culture increased continuously over time. Hepatocytes isolated from cocultures behaved similarly to freshly isolated hepatocytes from rat livers: when seeded onto new 3T3-J2 feeder layers, they continued to proliferate, and when seeded in a collagen sandwich, they stopped proliferating and maintained long-term stable function. These results demonstrate that 3T3-J2 fibroblasts not only maintain adult rat hepatocytes in a differentiated stage in culture, but also stimulate the proliferation of these cells in vitro.
Materials and Methods
Hepatocyte Isolation and Culture
Murine 3T3-J2 fibroblasts (purchased from Howard Green, Harvard Medical School, Boston, MA), were maintained in T175 tissue culture flasks in DMEM (Gibco, Gaithersburgh, MD) plus 10% FBS and 2% penicillin and streptomycin. After reaching confluence, 3T3-J2 fibroblasts were growth-arrested by treatment with 12 μg/mL of mitomycin-C (Sigma Chemical Co., St. Louis, MO) for 2.5 h. After incubation, the cells were washed with PBS (Sigma Chemical Co.), trypsinized, and plated into 35-mm tissue culture dishes at confluency (0.8 × 106 cells/35-mm dish) in 2 mL of fibroblast medium prior to seeding hepatocytes.
Hepatocytes were isolated from adult female Lewis rats (Charles River Laboratories, Wilmington, MA) weighing 150–200 g, using a two-step collagenase perfusion procedure as described previously (Dunn et al., 1989). Hepatocyte viability after isolation (approximately 200–300 million hepatocytes) was greater than 90% as determined by trypan blue exclusion. Hepatocytes were routinely seeded at a low density of 1.25 × 103 cells/cm2 on all feeder layers in 35-mm tissue culture dishes containing 1 mL of hepatocyte culture medium. In some cases (hepatocyte/3T3-J2 fibro-blast cocultures) a higher hepatocyte seeding density (15.63 × 103 cells/cm2) was used to study the effect of initial cell density on hepatocyte proliferation and functions. Culture medium was changed daily and medium samples were collected for functional analysis. Hepatocyte culture medium consisted of DMEM supplemented with 10% fetal bovine serum (Gibco), 7 ng/mL glucagon (Bedford Laboratories, Bedford, OH), 7.5 μg/mL hydrocortisone (Pharmacia Corporation, Kalamazoo, MI), 0.5 U/mL insulin (Eli Lilly, Indianapolis, IN), 20 ng/mL epidermal growth factor (Sigma Chemical Co.), 200 U/mL penicillin, and 200 μg/mL streptomycin (Gibco).
To assess the effect of coculture cell type, murine 3T3-J2 fibroblasts were substituted by murine NIH-3T3 fibroblasts (purchased from ATCC, Manassas, VA), murine embryonic fibroblasts (MEF; purchased from ATCC), rat heart micro-vascular endothelial cells (MVEC; purchased from VEC Technologies, Rensselaer, NY), or rat liver sinusoidal endothelial cells (LSEC) at the same seeding density (0.8 × 106 cells/35-mm dish). LSEC were isolated from the non-parenchymal fraction of the rat liver using a two-step Percoll gradient separation, following the procedure of Zhang et al. (1997). All these cells were maintained in fibroblast medium and were growth-arrested by mitomycin-C treatment prior to seeding hepatocytes, except for MVEC and LSEC, which were maintained in hepatocyte culture medium containing 10 ng/mL vascular endothelial growth factor (VEGF, R&D Systems, Minneapolis, MN) since they die if they are treated by mitomycin-C. As a control, hepatocytes were cultured alone at a low density of 1.25 × 103 cells/cm2 on collagen coated surfaces without cocultures. Type I collagen stock solution (1.1 μg/mL) was prepared from rat tail tendon as described previously (Dunn et al. 1989). To prepare collagen coated surfaces, tissue culture dishes (35-mm) were coated with 1 mL of type I rat tail collagen solution diluted 1:10 (0.11 mg/mL) in distilled water for 30 min at 378C. After rinsing the dishes twice with PBS, hepatocytes were seeded at the low density in 1 mL hepatocyte culture medium.
Cell Patterning With a Microfabricated PDMS Stencil
To better understand the role of heterotypic cell–cell interactions on hepatocyte proliferation in cocultures, a cell patterning technique was used. Polydimethylsiloxane (PDMS) stencils with different circular hole sizes (300 or 1,000 μm in diameter; ~300 μm thick) were made by the procedure modified from Ostuni et al. (2000). The PDMS prepolymer was made from a mixture of Sylgard 184 Silicone Elastomer Base and Curing Agent at 10:1 ratio (Sylgard 184 kit, Dow Corning, Midland, MI). The distance between the circular center holes was approximately 600 μm for 300 μm island patterns and 1,500 μm for 1,000 μm island patterns. Briefly, tissue culture dishes (35-mm) were coated with a solution of collagen type I for 30 min at 37°C and dried for 1hr within a laminar flow hood after washing with PBS. Stencils were applied to the dishes, covered with DMEM, and the air bubbles removed by gentle pipetting. Prior to cell seeding, DMEM was replaced with hepatocyte culture medium. Cell suspension (1 × 105 hepatocytes/mL) was added into the stencil, and incubated for 24 h at 37°C. After peeling off the stencil, cultures were washed with DMEM twice to remove unattached cells. The second cell suspension (growth-arrested 3T3-J2 fibroblasts) was seeded into the dishes at a density of 1 × 105 cells/cm2, and culture medium was changed daily.
Subculture of Proliferated Hepatocytes
Long-term cultures of hepatocytes (35 days after seeding) were treated with collagenase (1 mg/mL; Sigma Chemical Co.) and dispase (1 mg/mL; Gibco). The cell suspension (~10 mL) was placed in a 15 mL tube held vertically and the cells allowed to settle down at the bottom of the tube (about 2 min). The supernatant, which contained most of the nonparenchymal cells, was discarded. The cells at the bottom, mostly hepatocytes, were collected and treated with trypsin for 5 min with gentle pipetting to achieve a single-cell suspension, the cells were counted in a hemacytometer and replated either on another growth-arrested fibroblast feeder layer, or in a collagen-sandwich configuration (Dunn et al., 1989, 1991). For the feeder layer culture, the cells were handled similarly to freshly isolated hepatocytes.
To prepare collagen gel sandwich culture, 12-well plates were coated with 0.25 mL of a pre-mixed solution made of nine parts of type I rat tail collagen (1.1 mg/mL in 1 mM HCl) and 1 part 10× DMEM, and incubated for 1 h at 37°C to form a collagen gel. After gelation, approximately 0.5 × 106 hepatocytes/well in 0.5 mL hepatocyte culture medium were seeded, and incubated in 90% air/10% CO2 at 37°C. To achieve uniform cell attachment, the substrates were shaken every 15 min for the first hour after seeding. The following day, the culture medium was aspirated, and a second similar collagen gel was overlaid onto the hepatocytes and incubated for 1 h at 37°C. After gelation, 0.5 mL of hepatocyte culture medium was applied.
Immunofluorescence and Immunohistochemical Staining
For immunofluorescence staining, cultures in 35-mm dishes were washed twice with phosphate-buffered saline (PBS), fixed in 2% paraformaldehyde in PBS at room temperature for 20 min, washed twice in PBS, followed by addition of 0.2% Triton X-100 in PBS to permeabilize cells for intracellular staining. After 5 min of incubation at room temperature, the cells were washed twice in PBS and incubated in blocking buffer (PBS/20% FBS) for 60 min at room temperature to block non-specific antibody binding. After incubation, the cells were stained for 1 h at room temperature with rabbit anti-rat albumin (ICN Pharmaceuticals, Aurora, OH). After washing twice in blocking solution, the cells were incubated with FITC conjugated anti-rabbit IgG (ICN Pharmaceuticals) for 60 min at room temperature, and washed twice at room temperature. For the distribution of actin microfilaments, cells were stained by incubating fixed and permeabilized cultures with 0.1 μg/mL rhodamine phalloidin (Sigma Chemical Co.) for 30 min. In some cases, cells were counterstained with 4, 6-diamidino-2-phenylindole (DAPI; Invitrogen, Carlsbad, CA) for nuclear staining to count number of hepatocyte nuclei. Cells were visualized by fluorescence microscopy on a Zeiss 200 Axiovert microscope (Zeiss, Thornwood, NY) using an AxioCAM MRm digital camera.
For immunohistochemical staining, the fixed and permeabilized cultures were incubated with 0.3% hydrogen peroxide in methanol for 30 min followed by incubation in blocking buffer for 30 min. The primary antibody (rabbit anti-rat albumin) was applied for 30 min. For diaminobenzidine (DAB) staining, a biotinylated anti-rabbit secondary antibody (Vector Laboratories, Burlingame, CA) was used followed by a streptavidin conjugated horseradish peroxidase (Vector laboratories). Reaction was stopped with distilled water. All incubations were performed at room temperature. The glycogen storage was detected by periodic acid-Schiff (PAS) reaction (Sigma Chemical Co.).
BrdU Incorporation
The cells were incubated with 10 μM bromodeoxyuridine (BrdU; Sigma Chemical Co.) in the culture medium for 24 h at 37°C, fixed in 70% ethanol for 45 min at room temperature, then treated with 4N HCl for 20 min at room temperature to denature DNA. After incubation in blocking buffer for 30 min, the cells were stained for 60 min at 37°C with anti-BrdU-Alex594 (Invitrogen). The preparations were analyzed as described above.
Functional Assay
The culture medium samples were collected and stored at −20°C for analysis of albumin and urea content. The albumin content was determined by enzyme-linked immunosorbent assay (ELISA) using purified rat albumin and a peroxidase-conjugated antibody (MP Biomedicals, Aurora, OH). Urea content was determined with a commercially available kit (StanBio Laboratory, Boerne, TX). Standard curves were generated using purified rat albumin or urea dissolved in culture medium. Absorbances were measured with a Thermomax microplate reader (Molecular Devices, Sunnyvale, CA).
Quantification of Hepatocyte Proliferation
To distinguish between hepatocytes and fibroblasts in cocultures, hepatocytes were labeled with fluorescein isothiocyanate (FITC) for cytoplasmic albumin staining and with DAPI for nuclear staining. Individual hepatocytes could be easily distinguished as membrane borders were clearly visible. Cell proliferation was quantified by counting the number of hepatocyte nuclei in each hepatocyte colony on microscopic images captured during culture periods. Five different fields per dish were captured and analyzed, and the results averaged. Two to three independent experiments were performed in duplicate. A multinucleated hepatocyte was counted as a single cell. Counted cell numbers were normalized to average number of initial isolated cells seeded on feeder layer at day 1.
Statistical Analysis
Data are expressed as the mean ± standard deviation (SD). Statistical significance was determined by a two-tailed Student's t-test (*P < 0.05; **P < 0.005).
Results
To investigate the proliferative response and function of hepatocytes in coculture, isolated primary adult hepatocytes were seeded at low density on growth-arrested 3T3-J2 fibroblast feeder layers. Hepatocytes cultured alone on collagen coated dishes were included for comparison. Cultures were stained for cytoplasmic albumin and nuclear BrdU to identify differentiated and proliferating hepatocytes. Furthermore, cocultures were stained for filamentous actin with rhodamine phalloidin to assess the distribution of actin microfilaments, a rough indicator of cellular polarity and organization.
3T3-J2 Fibroblasts Stimulate the Proliferation of Primary Adult Hepatocytes
As shown in Figure 1A, hepatocytes cultured alone without 3T3-J2 cells on collagen coated surfaces tended to spread, and exhibited a dispersed distribution of stress fibers following which they deteriorate and die. Immunohisto-chemical analysis for albumin synthesis showed a very low level of albumin expression in the cytoplasm, indicating the loss of their hepatic characteristics. In contrast, hepatocytes cocultured with 3T3-J2 fibroblasts exhibited polygonal morphology with distinct nuclei and well-demarcated cell-cell borders. Notably, a highly proliferative response by hepatocytes was observed on 3T3-J2 fibroblast feeder layers (Fig. 1B). Hepatocyte colonies increased in size during culture periods and the number of hepatocytes per colony appeared to increase as well. To assess the hepatocellular characteristics of the proliferating hepatocytes, we performed immunofluorescence and immunohistochemical analysis for albumin synthesis and glycogen storage, as well as for actin filament distribution. The proliferating hepatocytes exhibited morphologic, phenotypic, and functional characteristics of mature hepatocytes. Expression of albumin (mature hepatocyte marker) was observed throughout the colonies and BrdU (DNA synthesis marker) incorporation was observed in many hepatocytes (Fig. 1C). As shown in Figure 1D, actin microfilaments within the hepatocytes were highly localized to the cell periphery, as it is the case in intact liver and other long-term stable hepatocyte culture systems. Immunohistochemical analysis after 27 days in culture showed strong and uniform albumin expression and glycogen storage in the cytoplasm, indicating the maintenance of their characteristic morphology and function. In contrast, conditioned media obtained from 3T3-J2 fibroblasts failed to maintain hepatic characteristics (data not shown), consistent with previous reports (Kuri-Harcuch and Mendoza-Figueroa, 1989; Shimaoka et al., 1987).
Figure 1.
Morphologic and phenotypic characteristics of hepatocytes cultured alone (A) and cocultured on growth arrested 3T3-J2 fibroblast feeder layers (B–D). Hepatocytes were sparsely seeded (1.25 × 103 cells/cm2) to better visualize individual hepatocyte colonies. A: Morphology, F-actin distribution, and albumin staining in hepatocytes cultured alone after 6 days of culture. B: Phase contrast images of the same single hepatocyte colony observed after 1, 6, and 14 days of culture in hepatocyte/3T3-J2 fibroblast coculture. Arrow points to hepatocyte colony 1 day after seeding. The dashed circles indicate the morphology of proliferating hepatocytes captured at the same location during culture periods. Scale bar, 100 μm. C: Albumin (mature hepatocyte marker) staining and BrdU (DNA synthesis marker) uptake after 8, 14, and 25 days of culture. Scale bar, 100 μm. D: Immunofluorescence and immunohistochemical analysis for actin filament distribution, albumin synthesis, and glycogen storage on day 27.
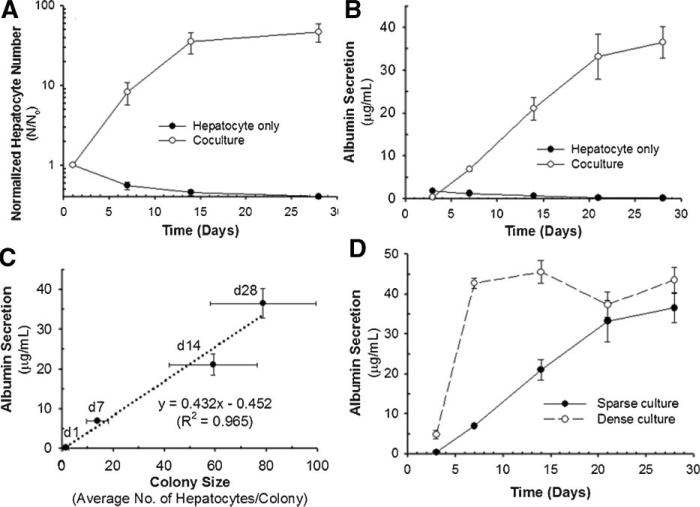
To quantify the proliferation of hepatocytes cocultured with 3T3-J2 fibroblasts, the average number of hepatocytes per colony was counted and normalized to initial cell number. Over the 28 days of culture, the highest growth rate was observed over the first 7 days of culture, with an 8.2-fold increase in hepatocyte number (Fig. 2A). This corresponds to about three population doublings, and 2.3 days per population doubling. In the first 14 days of culture, the proliferation rate observed was a 35-fold increase in hepatocyte number. This corresponds to about five population doublings, and 2.5 days per population doubling. The growth rate decreased after day 14 with the increase of colony size and cell density. In contrast, when the hepatocytes were cultured alone, there was a decrease in the number of hepatocytes throughout the culture period. The albumin secretion of hepatocytes when cocultured with 3T3-J2 fibroblasts increased continuously for up to 28 days of culture, which correlated with the increasing number of hepatocytes seen in these cultures (Fig. 2B). The hepatocytes cultured alone lost their functional characteristics rapidly, consistent with previous reports (Khetani et al., 2004). Figure 2C shows the relationship between hepatocyte colony size and albumin secretion by hepatocytes in coculture throughout the culture periods. There was a linear relationship between the colony size and function of hepatocytes in coculture. As the hepatocyte colony size increased, the albumin secretion increased. To examine how hepatocyte seeding density affects function and proliferation, hepatocyte cultures seeded in sparse culture were compared to those in dense culture. In dense culture, albumin secretion increased for only 7–10 days of culture and then reached a plateau (Fig. 2D). We observed high proliferation of hepatocytes in sparse culture, whereas dense culture presented low proliferative activity (data not shown), consistent with functional activities. These results in dense culture are consistent with our previous report (Cho et al., 2007), which showed that hepatocytes increase in number in these cocultures when the hepatocyte to fibroblast seeding ratio is low. Taken together, these results suggest that hepatocyte number and total albumin secretion increase over time and ultimately tend toward a maximum per culture. As a result, more proliferation is seen in sparsely seeded cocultures.
Figure 2.
The proliferation rate and function of hepatocytes cultured on growth arrested 3T3-J2 fibroblast feeder layers. Hepatocytes cultured alone on collagen coated dishes served as controls. A: The normalized hepatocyte number (N/No) over time in sparse culture (1.25 × 103 cells/cm2). The hepatocyte numbers (N) were normalized to the initial number of hepatocytes (No). B: Albumin secretion over time in sparse culture. C: Correlation between hepatocyte colony sizes and albumin secretion. D: Albumin secretion as a function of time for sparse (1.25 × 103 cells/cm2) and dense (15.63 × 103 cells/cm2) culture. Data shown are means ± SD of three independent experiments in duplicate.
Heterotypic Cell–Cell Contact Induces Hepatocellular DNA Synthesis
To investigate whether heterotypic cell–cell contact induces DNA synthesis in hepatocytes, hepatocytes were patterned as 1,000 μm islands using a PDMS stencil. After peeling off the stencil, growth-arrested 3T3-J2 fibroblasts were seeded in the dishes. The hepatocytes were double-stained for albumin and BrdU. A higher concentration of BrdU positive hepatocytes was observed at the boundaries of the hepatocyte islands, where hepatocytes directly contact the 3T3-J2 fibroblasts (Fig. 3B,C and E,F). Note that there was minimal BrdU staining outside of the hepatocyte areas (as delimited by albumin staining), which were covered with growth-arrested 3T3-J2 cells. Similar results were observed in a doughnut-shape hepatocyte island (Fig. 3G–L). These results suggest that hepatocyte DNA synthesis is likely induced by heterotypic cell–cell interactions between hepatocytes and 3T3-J2 fibroblasts.
Figure 3.
Role of heterotypic interface on hepatocyte DNA synthesis and albumin expression. Distribution of DNA synthetic activity, as reported by BrdU uptake, and albumin staining in a 1,000 μm diameter hepatocyte island (A–F) and in a doughnut-shape hepatocyte island (G–L). Scale bar, 500 μm (B,C and H,I) and 100 μm (D–F and J–L).
Among Five Coculture Cell Types, 3T3-J2 Fibroblasts Lead to the Greatest Hepatocyte Proliferative Response
To assess the effect of coculture cell type on the proliferative response and function of hepatocytes, five different cell types (3T3-J2, NIH-3T3, MEF, LSEC, and MVEC) were compared as feeder layers. Figure 4A shows the typical hepatocyte colony size stained by albumin on each feeder layer after culturing hepatocytes at low density for 14 days. The average number of hepatocytes per colony was significantly greater in 3T3-J2 cocultures compared to cocultures with four other cell types (Fig. 4B). Hepatocyte proliferation was unnoticeable in MVEC cocultures. The number of hepatocytes on the various feeder layer cell types (3T3-J2, NIH-3T3, MEF, LSEC, and MVEC) increased approximately 35-, 14-, 7-, 6-, and 1.5-fold, respectively, after 14 days (Fig. 4C). These correspond to about 5, 3.8, 2.8, 2.6, and 0.7 population doublings, respectively over the 14 days of culture. Total albumin secretion was also significantly higher in 3T3-J2 cocultures compared to the four other cocultures (Fig. 4D). Similar trends were observed for urea synthesis (Fig. 4E). Figure 4F and G shows the relationship between the hepatocyte colony sizes and the liver specific functions. There was a linear relationship between hepatocyte growth and albumin secretion or urea synthesis. These results indicate that increasing colony size correlates directly with an increase in hepatocellular functions.
Figure 4.
Effect of feeder layer cell types on hepatocyte proliferation and function. 3T3-J2, NIH-3T3, MEF, LSEC, and MVEC were used as feeder layers. A: Typical hepatocyte colony size and albumin staining in cocultures with the various feeder cell types on day 14. B: Average number of hepatocytes per colony in cocultures on day 14. C: Normalized hepatocyte number (N/No) in cocultures on day 14. D,E: Albumin secretion (D) and urea synthesis (E) by the hepatocytes cocultured with the various cell types on day 14. F,G: Correlation between hepatocyte colony sizes and liver-specific functions (albumin secretion and urea synthesis) in cocultures with the various feeder cell types on day 14. Data shown are means ± SD of two independent experiments in duplicate. *P < 0.05; **P < 0.005. Hepatocytes were seeded in sparse culture (1.25 × 103 cells/cm2) on all feeder layers. [Color figure can be seen in the online version of this article, available at www.interscience.wiley.com.]
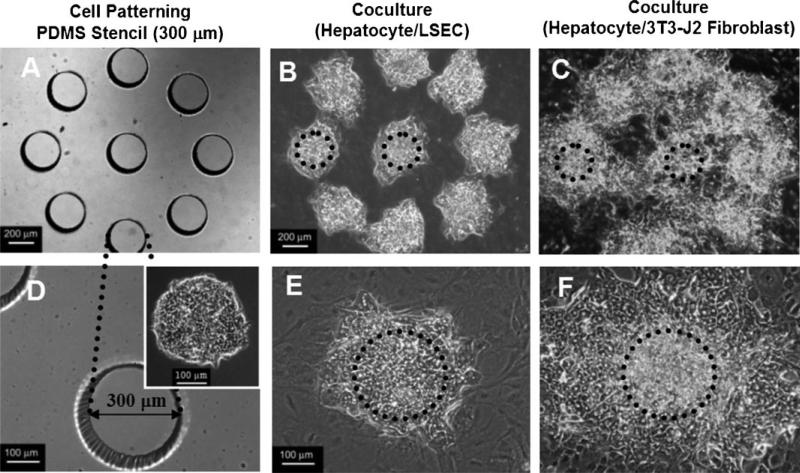
To precisely monitor the proliferative response of hepatocytes in these cocultures, hepatocytes were patterned as 300 μm islands using a PDMS stencil, and cocultured with 3T3-J2 fibroblasts or LSEC. After 13 days of culture, hepatocyte island sizes in LSEC cocultures were much smaller than in 3T3-J2 fibroblast cocultures (Fig. 5). Compared to the initial island size of 300 μm, the hepatocyte island size increased ~2.5-fold in LSEC cocultures and ~6.6-fold in 3T3-J2 cocultures. For a given cell type, similar hepatocyte proliferation was seen in all islands within a pattern (Fig. 5B and C).
Figure 5.
Comparison of hepatocyte proliferation between micropatterned cocultures on 3T3-J2 fibroblasts versus LSEC feeder layers. Hepatocytes were seeded as 300 μm diameter islands using PDMS stencils, and observed after 13 days of culture. A,D: Microfabricated PDMS stencil with 300 μm diameter holes. Inset in panel D shows the micropatterned hepatocytes on day 1. B,E: Hepatocyte and LSEC coculture on day 13. C,F: Hepatocyte and 3T3-J2 fibroblast coculture on day 13. Dashed circle lines indicate the original island size. Scale bar, 200 μm (upper panels) and 100 μm (lower panels).
Subcultured Hepatocytes Maintain Proliferative Capacity and Hepatocyte-Specific Characteristics
To investigate whether proliferated hepatocytes retain proliferative capacity and hepatocyte-specific characteristics, hepatocytes cocultured with 3T3-J2 cells for 35 days were isolated from the culture dishes by enzymatic treatment and replated either at low density on 3T3-J2 fibroblast feeder layers to assess proliferative capacity or at high density in the collagen sandwich configuration to assess albumin secretion. Hepatocytes replated in collagen gel sandwiches secreted albumin at a constant rate after 10 days in culture (Fig. 6A), and showed morphological characteristics similar to those seen in freshly isolated rat primary hepatocytes cultured in the same configuration (Fig. 6B). Hepatocytes replated on 3T3-J2 feeder layers started to proliferate by forming colonies that increased in size over time (Fig. 6C). Albumin secretion increased continuously for 30 days after subculture. These cultures also exhibited distinct nuclei, well-demarcated cell–cell borders, uniform albumin staining, and some cells were BrdU-labeled (Fig. 6D). Overall these subcultures were similar to those obtained by seeding freshly isolated hepatocytes onto 3T3-J2 feeder layers.
Figure 6.
Morphology and function of hepatocytes in subcultures. Hepatocytes were cocultured with 3T3-J2 fibroblasts for 35 days, detached from the substrate by dispase/collagenase treatment, dispersed in a single cell suspension by trypsin digestion, replated, and subcultured for another 30 days. Replating was either at high density (~125 × 103 cells/cm2) in a collagen sandwich configuration, or at low density (~1.25 × 103 cells/cm2) on growth arrested 3T3-J2 fibroblast feeder layers. A: Albumin secretion in the subcultures. B,C: Morphology of hepatocytes in the subcultures. Scale bar, 100 μm. D: Morphology of hepatocytes cocultured on 3T3-J2 fibroblast feeder layer in the subculture on day 39 (4 days after replating), day 47 (12 days after replating), and day 63 (28 days after replating), and double staining for albumin expression and BrdU incorporation on day 63 (28 days after replating). Scale bar, 100 μm.
Discussion
Results of this study indicate that 3T3-J2 fibroblasts stimulate a high level of proliferation of adult rat hepatocytes in vitro. The proliferating hepatocytes retained their morphologic, phenotypic, and functional characteristics. The proliferative responses of hepatocytes varied depending on coculture cell type. Using a cell patterning technique, we found that BrdU labeling of hepatocytes was primarily concentrated at the boundaries of hepatocyte islands, in the vicinity of the 3T3-J2 fibroblasts, suggesting a role for heterotypic cell–cell interactions in the hepatocyte proliferative response. When the hepatocytes that proliferated in cocultures were harvested and further subcultured either on 3T3-J2 fibroblast feeder layers or in the collagen sandwich configuration, their behavior was similar to that of freshly isolated hepatocytes. To our knowledge, this is the first report showing that adult rat hepatocytes can be serially propagated by coculture with 3T3-J2 fibroblasts while maintaining their differentiated characteristics in vitro.
It is known that hepatocyte morphology and function varies depending on coculture cell type (Bhatia et al., 1999; Goulet et al., 1988; Khetani et al., 2004). However, the reason for the differences in their morphologic and functional characteristics in cocultures is not known. Our results strongly suggest that one of the major reasons for the differences may be due to the different proliferative responses of hepatocytes as a function of coculture cell type. 3T3-J2 feeder layers induced significantly higher hepatocyte proliferation than feeder layers made of four other cell types. Increasing colony size correlated with an increase in hepatocellular functions. Other possible explanations for this phenomenon include variations in cell signaling, growth factor release, ECM deposition, and protein production. Further studies are necessary to address the underlying mechanisms that control hepatocyte proliferation in cocultures.
The normally quiescent liver has a remarkable ability to regulate its total mass. After two-thirds partial hepatectomy in the rat, the liver regenerates its original volume within 7–10 days, which involves 2–3 hepatocyte doublings (Fausto, 2001; Michalopoulos and DeFrances, 1997). Although liver regeneration is a well documented phenomenon, questions regarding the proliferative capacity of adult hepatocytes remain. Interestingly, hepatocyte transplantation experiments in mouse models suggest an enormous proliferative potential of adult hepatocytes (Overturf et al., 1997; Rhim et al., 1994). Serially transplanted hepatocytes in fumarylacetoacetate hydrolase (FAH)-deficient mice could repopulate more than 70 times, showing stem cell-like regenerative capacity. These findings raised the question whether hepatic stem cells with high regenerative capacity may be responsible for the serial repopulation. However, there was no evidence for a role of hepatic stem cells (Overturf et al., 1999). Furthermore, the results showed that small, mononucleated hepatocytes (~16 μm) repopulated significantly less well than larger and probably older hepatocytes (~27 μm). These findings suggested that adult hepatocytes have a remarkable ability to proliferate in vivo and that it may be possible to propagate these cells in vitro as well. In contrast with the in vivo data, prior in vitro studies have shown that small hepatocytes have a higher proliferative ability either in an enriched culture medium supplemented with nicotinamide and epidermal growth factor or when cocultured with nonparenchymal cells (Mitaka et al., 1995, 1999; Tateno et al., 2000). Block et al. (1996) reported that mature adult hepatocytes can undergo DNA synthesis in chemically defined medium while they lose hepatocyte-specific characteristics. However, these studies utilized different culture systems which involved either monolayer cultures or cocultures with different types of cells, and different culture media, and thus are difficult to compare with our own results.
Although adult hepatocytes have a remarkable ability to proliferate in vivo, attempts to proliferate adult hepatocytes in vitro have been less successful. Primary adult hepatocytes have been known to have low mitotic activity in vitro. The low availability of mature, functional hepatocytes is a major drawback for potential therapies using this cell type. Several culture systems have been utilized for the maintenance of hepatocyte-specific functions, including the collagen sandwich configuration (Dunn et al., 1989), spheroid culture (Landry et al., 1985), matrigel culture (Bissell et al., 1987), or dimethylsulfoxide (DMSO)-supplemented culture medium (Isom et al., 1985; Kost and Michalopoulos, 1991). However, these systems did not exhibit any significant rate of replication, and it appeared that hepatocytes were unable to fully respond to growth factor signals. Many studies have been focused on alternative sources of cells, such as hepatic progenitors (Wang et al., 2003), hematopoietic stem cells (Jiang et al., 2002; Schwartz et al., 2002), embryonic stem cells (Asahina et al., 2004; Chinzei et al., 2002; Cho et al., 2008; Hamazaki et al., 2001), immortalized hepatocytes (Kobayashi et al., 2000), or liver-tumor-derived cell lines (Ellis et al., 1996), but have had limited success, primarily due to insufficient functionality.
In our study, we found that adult hepatocytes cocultured with fibroblasts (3T3-J2) exhibited high proliferative activity. Based on Figure 2A, the highest proliferation rate was observed over the first 7 days of culture, with an 8.2-fold increase in cell number. This corresponds to about three population doublings, and 2.3 days per population doubling. Over the 14 days of culture, the proliferation rate was about five population doublings with a 35-fold increase in cell number, which corresponds to 2.5 days per population doubling. This proliferation rate is similar to estimated in vivo rates. For example, the rat liver takes 7–10 days for regeneration after two-thirds partial hepatectomy, yielding 2.5 days per population doubling. The proliferation rate decreased dramatically after day 14, which may reflect an inhibitory effect of increasing cell density, since brisk proliferation resumed upon subculture (Fig. 6). We envision that this culture system could have clinical application. For example, in designing a clinically scaled bioartificial liver device, approximately 1010 hepatocytes are necessary to support a patient with acute liver failure, which is a critical limitation in the application for many cell-based therapies for liver failure. Using this culture system, in vitro hepatocyte proliferation could potentially reduce the initial number of required hepatocytes.
In this study we showed that the proliferative activities of hepatocytes were high in sparse culture, but low in dense culture. It has been reported that the cell cycle of hepatocytes is regulated by cell density and cell–cell interactions (Cho et al., 2007; Corlu et al., 1997; Michalopoulos et al., 1982; Nakamura et al., 1983a; Sand et al., 1977). There was an inverse relationship between DNA synthesis and cell density. Nakamura et al. (1983b) suggested that the reciprocal regulations of cell growth and hepatic characteristics are mediated by a cell surface component of the plasma membrane via direct cell–cell contact. The mechanism of density-dependent regulation on hepatocyte growth might also consist of soluble inhibitors secreted by the hepatocytes into the culture medium. The molecular mechanisms whereby cell-cell contacts regulate cell growth are still unknown.
Micropatterning of cells and proteins has been widely used for studying and controlling cell behavior (Bhatia et al., 1999; Chen et al., 1997; Folch and Toner, 2000; Park et al., 2007; Singhvi et al., 1994). In this study, we employed cell patterning to better understand the role of heterotypic cell– cell interactions on hepatocyte proliferation in the coculture system. This patterning technique was extremely useful to demonstrate the role of heterotypic cell–cell interactions in DNA synthesis of hepatocytes. Interestingly, DNA synthesis of hepatocytes was mostly concentrated where hepatocytes are in close proximity to the 3T3-J2 fibroblasts, suggesting that 3T3-J2 stimulate DNA synthesis by heterotypic cell–cell interactions. This patterning technique can be a useful experimental tool for applications in basic studies, drug screening, and tissue engineering.
To apply this coculture system for clinical use, further development would be needed for expanding human hepatocytes while avoiding exposure to potential animal pathogens. For example, FACS sorting techniques after expanding hepatocytes in coculture systems could be used to isolate the hepatocytes and eliminate cells from other species. Preferably, the use of human fibroblast feeder layers as an alternative to mouse fibroblast feeder layers would be the next step to yield a methodology for expanding human hepatocytes in an animal-free culture system.
In summary, our results clearly show that it is possible to expand hepatocytes in vitro several fold without losing their liver-specific characteristics. Thus, our results suggest that hepatocyte culture on 3T3-J2 fibroblast feeder layers is a promising system for in vitro proliferation and the maintenance of differentiated functions of hepatocytes. The mature, functional hepatocytes obtained from such cocultures may become a reliable source of cells for hepatocyte transplantation therapies, the development of bioartificial liver support systems, drug screening, and gene therapy.
Acknowledgments
We thank Dr. Jaesung Park for providing PDMS stencils, and Dr. Yaakov Nahmias and Dr. Laurent Barbe for their assistance with LSEC isolation. This study was partially supported by the National Institutes of Health (grant numbers R01 DK43371, K08 DK066040, and K18 DK076819) and the Shriners Hospitals for Children.
References
- Asahina K, Fujimori H, Shimizu-Saito K, Kumashiro Y, Okamura K, Tanaka Y, Teramoto K, Arii S, Teraoka H. Expression of the liver-specific gene Cyp7a1 reveals hepatic differentiation in embryoid bodies derived from mouse embryonic stem cells. Genes Cells. 2004;9(12):1297–1308. doi: 10.1111/j.1365-2443.2004.00809.x. [DOI] [PubMed] [Google Scholar]
- Bhatia SN, Balis UJ, Yarmush ML, Toner M. Probing heterotypic cell interactions: Hepatocyte function in microfabricated co-cultures. J Biomater Sci Polym Ed. 1998;9(11):1137–1160. doi: 10.1163/156856298x00695. [DOI] [PubMed] [Google Scholar]
- Bhatia SN, Balis UJ, Yarmush ML, Toner M. Effect of cell-cell interactions in preservation of cellular phenotype: Cocultivation of hepatocytes and nonparenchymal cells. FASEB J. 1999;13(14):1883–1900. doi: 10.1096/fasebj.13.14.1883. [DOI] [PubMed] [Google Scholar]
- Bissell DM, Arenson DM, Maher JJ, Roll FJ. Support of cultured hepatocytes by a laminin-rich gel. Evidence for a functionally significant subendothelial matrix in normal rat liver. J Clin Invest. 1987;79(3):801–812. doi: 10.1172/JCI112887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block GD, Locker J, Bowen WC, Petersen BE, Katyal S, Strom SC, Riley T, Howard TA, Michalopoulos GK. Population expansion, clonal growth, and specific differentiation patterns in primary cultures of hepatocytes induced by HGF/SF, EGF and TGF alpha in a chemically defined (HGM) medium. J Cell Biol. 1996;132(6):1133–1149. doi: 10.1083/jcb.132.6.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C, Berthiaume F, Nath BD, Tilles AW, Toner M, Yarmush ML. Hepatic tissue engineering for adjunct and temporary liver support: Critical technologies. Liver Transpl. 2004;10(11):1331–1342. doi: 10.1002/lt.20229. [DOI] [PubMed] [Google Scholar]
- Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276(5317):1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- Chinzei R, Tanaka Y, Shimizu-Saito K, Hara Y, Kakinuma S, Watanabe M, Teramoto K, Arii S, Takase K, Sato C, et al. Embryoid-body cells derived from a mouse embryonic stem cell line show differentiation into functional hepatocytes. Hepatology. 2002;36(1):22–29. doi: 10.1053/jhep.2002.34136. [DOI] [PubMed] [Google Scholar]
- Cho CH, Park J, Nagrath D, Tilles AW, Berthiaume F, Toner M, Yarmush ML. Oxygen uptake rates and liver-specific functions of hepatocyte and 3T3 fibroblast co-cultures. Biotechnol Bioeng. 2007;97(1):188–199. doi: 10.1002/bit.21225. [DOI] [PubMed] [Google Scholar]
- Cho CH, Parashurama N, Park EY, Suganuma K, Nahmias Y, Park J, Tilles AW, Berthiaume F, Yarmush ML. Homogeneous differentiation of hepatocyte-like cells from embryonic stem cells: Applications for the treatment of liver failure. FASEB J. 2008;22(3):898–909. doi: 10.1096/fj.06-7764com. [DOI] [PubMed] [Google Scholar]
- Corlu A, Ilyin G, Cariou S, Lamy I, Loyer P, Guguen-Guillouzo C. The coculture: A system for studying the regulation of liver differentiation/proliferation activity and its control. Cell Biol Toxicol. 1997;13(4–5):235–242. doi: 10.1023/a:1007475122321. [DOI] [PubMed] [Google Scholar]
- Demetriou AA, Brown RS, Jr, Busuttil RW, Fair J, McGuire BM, Rosenthal P, Am Esch JS II, Lerut J, Nyberg SL, Salizzoni M, et al. Prospective, randomized, multicenter, controlled trial of a bioartificial liver in treating acute liver failure. Ann Surg. 2004;239(5):660–667. doi: 10.1097/01.sla.0000124298.74199.e5. discussion 667–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn JC, Yarmush ML, Koebe HG, Tompkins RG. Hepatocyte function and extracellular matrix geometry: Long-term culture in a sandwich configuration. FASEB J. 1989;3(2):174–177. doi: 10.1096/fasebj.3.2.2914628. [DOI] [PubMed] [Google Scholar]
- Dunn JC, Tompkins RG, Yarmush ML. Long-term in vitro function of adult hepatocytes in a collagen sandwich configuration. Biotechnol Prog. 1991;7(3):237–245. doi: 10.1021/bp00009a007. [DOI] [PubMed] [Google Scholar]
- Ellis AJ, Hughes RD, Wendon JA, Dunne J, Langley PG, Kelly JH, Gislason GT, Sussman NL, Williams R. Pilot-controlled trial of the extracorporeal liver assist device in acute liver failure. Hepatology. 1996;24(6):1446–1451. doi: 10.1002/hep.510240625. [DOI] [PubMed] [Google Scholar]
- Fausto N. Liver regeneration: From laboratory to clinic. Liver Transpl. 2001;7(10):835–844. doi: 10.1053/jlts.2001.27865. [DOI] [PubMed] [Google Scholar]
- Folch A, Toner M. Microengineering of cellular interactions. Annu Rev Biomed Eng. 2000;2:227–256. doi: 10.1146/annurev.bioeng.2.1.227. [DOI] [PubMed] [Google Scholar]
- Fox IJ, Chowdhury JR, Kaufman SS, Goertzen TC, Chowdhury NR, Warkentin PI, Dorko K, Sauter BV, Strom SC. Treatment of the Crigler-Najjar syndrome type I with hepatocyte transplantation. N Engl J Med. 1998;338(20):1422–1426. doi: 10.1056/NEJM199805143382004. [DOI] [PubMed] [Google Scholar]
- Fraslin JM, Kneip B, Vaulont S, Glaise D, Munnich A, Guguen-Guillouzo C. Dependence of hepatocyte-specific gene expression on cell-cell interactions in primary culture. EMBO J. 1985;4(10):2487–2491. doi: 10.1002/j.1460-2075.1985.tb03960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulet F, Normand C, Morin O. Cellular interactions promote tissue-specific function, biomatrix deposition and junctional communication of primary cultured hepatocytes. Hepatology. 1988;8(5):1010–1018. doi: 10.1002/hep.1840080506. [DOI] [PubMed] [Google Scholar]
- Guguen-Guillouzo C, Clement B, Baffet G, Beaumont C, Morel-Chany E, Glaise D, Guillouzo A. Maintenance and reversibility of active albumin secretion by adult rat hepatocytes co-cultured with another liver epithelial cell type. Exp Cell Res. 1983;143(1):47–54. doi: 10.1016/0014-4827(83)90107-6. [DOI] [PubMed] [Google Scholar]
- Hamazaki T, Iiboshi Y, Oka M, Papst PJ, Meacham AM, Zon LI, Terada N. Hepatic maturation in differentiating embryonic stem cells in vitro. FEBS Lett. 2001;497(1):15–19. doi: 10.1016/s0014-5793(01)02423-1. [DOI] [PubMed] [Google Scholar]
- Isom HC, Secott T, Georgoff I, Woodworth C, Mummaw J. Maintenance of differentiated rat hepatocytes in primary culture. Proc Natl Acad Sci USA. 1985;82(10):3252–3256. doi: 10.1073/pnas.82.10.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418(6893):41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- Kang YH, Berthiaume F, Nath BD, Yarmush ML. Growth factors and nonparenchymal cell conditioned media induce mitogenic responses in stable long-term adult rat hepatocyte cultures. Exp Cell Res. 2004;293(2):239–247. doi: 10.1016/j.yexcr.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Khetani SR, Szulgit G, Del Rio JA, Barlow C, Bhatia SN. Exploring interactions between rat hepatocytes and nonparenchymal cells using gene expression profiling. Hepatology. 2004;40(3):545–554. doi: 10.1002/hep.20351. [DOI] [PubMed] [Google Scholar]
- Kobayashi N, Fujiwara T, Westerman KA, Inoue Y, Sakaguchi M, Noguchi H, Miyazaki M, Cai J, Tanaka N, Fox IJ, et al. Prevention of acute liver failure in rats with reversibly immortalized human hepatocytes. Science. 2000;287(5456):1258–1262. doi: 10.1126/science.287.5456.1258. [DOI] [PubMed] [Google Scholar]
- Kost DP, Michalopoulos GK. Effect of 2% dimethyl sulfoxide on the mitogenic properties of epidermal growth factor and hepatocyte growth factor in primary hepatocyte culture. J Cell Physiol. 1991;147(2):274–280. doi: 10.1002/jcp.1041470212. [DOI] [PubMed] [Google Scholar]
- Kuri-Harcuch W, Mendoza-Figueroa T. Cultivation of adult rat hepatocytes on 3T3 cells: Expression of various liver differentiated functions. Differentiation. 1989;41(2):148–157. doi: 10.1111/j.1432-0436.1989.tb00742.x. [DOI] [PubMed] [Google Scholar]
- Landry J, Bernier D, Ouellet C, Goyette R, Marceau N. Spheroidal aggregate culture of rat liver cells: Histotypic reorganization, biomatrix deposition, and maintenance of functional activities. J Cell Biol. 1985;101(3):914–923. doi: 10.1083/jcb.101.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesnil M, Fraslin JM, Piccoli C, Yamasaki H, Guguen-Guillouzo C. Cell contact but not junctional communication (dye coupling) with biliary epithelial cells is required for hepatocytes to maintain differentiated functions. Exp Cell Res. 1987;173(2):524–533. doi: 10.1016/0014-4827(87)90292-8. [DOI] [PubMed] [Google Scholar]
- Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276(5309):60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- Michalopoulos G, Cianciulli HD, Novotny AR, Kligerman AD, Strom SC, Jirtle RL. Liver regeneration studies with rat hepatocytes in primary culture. Cancer Res. 1982;42(11):4673–4682. [PubMed] [Google Scholar]
- Mitaka T, Kojima T, Mizuguchi T, Mochizuki Y. Growth and maturation of small hepatocytes isolated from adult rat liver. Biochem Biophys Res Commun. 1995;214(2):310–317. doi: 10.1006/bbrc.1995.2289. [DOI] [PubMed] [Google Scholar]
- Mitaka T, Sato F, Mizuguchi T, Yokono T, Mochizuki Y. Reconstruction of hepatic organoid by rat small hepatocytes and hepatic nonparenchymal cells. Hepatology. 1999;29(1):111–125. doi: 10.1002/hep.510290103. [DOI] [PubMed] [Google Scholar]
- Mizuguchi T, Hui T, Palm K, Sugiyama N, Mitaka T, Demetriou AA, Rozga J. Enhanced proliferation and differentiation of rat hepatocytes cultured with bone marrow stromal cells. J Cell Physiol. 2001;189(1):106–119. doi: 10.1002/jcp.1136. [DOI] [PubMed] [Google Scholar]
- Muraca M, Gerunda G, Neri D, Vilei MT, Granato A, Feltracco P, Meroni M, Giron G, Burlina AB. Hepatocyte transplantation as a treatment for glycogen storage disease type 1a. Lancet. 2002;359(9303):317–318. doi: 10.1016/S0140-6736(02)07529-3. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Tomita Y, Ichihara A. Density-dependent growth control of adult rat hepatocytes in primary culture. J Biochem (Tokyo) 1983a;94(4):1029–1035. doi: 10.1093/oxfordjournals.jbchem.a134444. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Yoshimoto K, Nakayama Y, Tomita Y, Ichihara A. Reciprocal modulation of growth and differentiated functions of mature rat hepatocytes in primary culture by cell–cell contact and cell membranes. Proc Natl Acad Sci USA. 1983b;80(23):7229–7233. doi: 10.1073/pnas.80.23.7229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostuni E, Kane R, Chen C, Ingber D, Whitesides G. Patterning mammalian cells using elastomeric membranes. Langmuir. 2000;16:7811–7819. [Google Scholar]
- Overturf K, al-Dhalimy M, Ou CN, Finegold M, Grompe M. Serial transplantation reveals the stem-cell-like regenerative potential of adult mouse hepatocytes. Am J Pathol. 1997;151(5):1273–1280. [PMC free article] [PubMed] [Google Scholar]
- Overturf K, Al-Dhalimy M, Finegold M, Grompe M. The repopulation potential of hepatocyte populations differing in size and prior mitotic expansion. Am J Pathol. 1999;155(6):2135–2143. doi: 10.1016/S0002-9440(10)65531-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Cho CH, Parashurama N, Li Y, Berthiaume F, Toner M, Tilles AW, Yarmush ML. Microfabrication-based modulation of embryonic stem cell differentiation. Lab Chip. 2007;7(8):1018–1028. doi: 10.1039/b704739h. [DOI] [PubMed] [Google Scholar]
- Rhim JA, Sandgren EP, Degen JL, Palmiter RD, Brinster RL. Replacement of diseased mouse liver by hepatic cell transplantation. Science. 1994;263(5150):1149–1152. doi: 10.1126/science.8108734. [DOI] [PubMed] [Google Scholar]
- Richman RA, Claus TH, Pilkis SJ, Friedman DL. Hormonal stimulation of DNA synthesis in primary cultures of adult rat hepatocytes. Proc Natl Acad Sci USA. 1976;73(10):3589–3593. doi: 10.1073/pnas.73.10.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sand T, Condie R, Rosenberg A. Metabolic crowding effect in suspension of cultured lymphocytes. Blood. 1977;50(2):337–346. [PubMed] [Google Scholar]
- Schwartz RE, Reyes M, Koodie L, Jiang Y, Blackstad M, Lund T, Lenvik T, Johnson S, Hu WS, Verfaillie CM. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Invest. 2002;109(10):1291–1302. doi: 10.1172/JCI15182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimaoka S, Nakamura T, Ichihara A. Stimulation of growth of primary cultured adult rat hepatocytes without growth factors by coculture with nonparenchymal liver cells. Exp Cell Res. 1987;172(1):228–242. doi: 10.1016/0014-4827(87)90109-1. [DOI] [PubMed] [Google Scholar]
- Singhvi R, Kumar A, Lopez GP, Stephanopoulos GN, Wang DI, Whitesides GM, Ingber DE. Engineering cell shape and function. Science. 1994;264(5159):696–698. doi: 10.1126/science.8171320. [DOI] [PubMed] [Google Scholar]
- Stocker E, Wullstein HK, Brau G. Capacity of regeneration in liver epithelia of juvenile, repeated partially hepatectomized rats. Autoradiographic studies after continous infusion of 3H-thymi-dine (author's transl). Virchows Arch B Cell Pathol. 1973;14(2):93–103. [PubMed] [Google Scholar]
- Strom SC, Fisher RA, Thompson MT, Sanyal AJ, Cole PE, Ham JM, Posner MP. Hepatocyte transplantation as a bridge to orthotopic liver transplantation in terminal liver failure. Transplantation. 1997;63(4):559–569. doi: 10.1097/00007890-199702270-00014. [DOI] [PubMed] [Google Scholar]
- Tateno C, Takai-Kajihara K, Yamasaki C, Sato H, Yoshizato K. Heterogeneity of growth potential of adult rat hepatocytes in vitro. Hepatology. 2000;31(1):65–74. doi: 10.1002/hep.510310113. [DOI] [PubMed] [Google Scholar]
- Taub R. Liver regeneration: From myth to mechanism. Nat Rev Mol Cell Biol. 2004;5(10):836–847. doi: 10.1038/nrm1489. [DOI] [PubMed] [Google Scholar]
- Uyama N, Shimahara Y, Kawada N, Seki S, Okuyama H, Iimuro Y, Yamaoka Y. Regulation of cultured rat hepatocyte proliferation by stellate cells. J Hepatol. 2002;36(5):590–599. doi: 10.1016/s0168-8278(02)00023-5. [DOI] [PubMed] [Google Scholar]
- Wang X, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Grompe M. The origin and liver repopulating capacity of murine oval cells. Proc Natl Acad Sci USA. 2003;100(Suppl 1):11881–11888. doi: 10.1073/pnas.1734199100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Borderie D, Sogni P, Soubrane O, Houssin D, Calmus Y. NO-mediated vasodilation in the rat liver. Role of hepatocytes and liver endothelial cells. J Hepatol. 1997;26(6):1348–1355. doi: 10.1016/s0168-8278(97)80471-0. [DOI] [PubMed] [Google Scholar]