Abstract
Aim and background. The Nordic Liver Transplant Registry (NLTR) accounts for all liver transplants performed in the Nordic countries since the start of the transplant program in 1982. Due to short waiting times, donor liver allocation has been made without considerations of the model of end-stage liver disease (MELD) score. We aimed to summarize key outcome measures and developments for the activity up to December 2013. Materials and methods. The registry is integrated with the operational waiting-list and liver allocation system of Scandiatransplant (www.scandiatransplant.org) and accounted at the end of 2013 for 6019 patients out of whom 5198 were transplanted. Data for recipient and donor characteristics and relevant end-points retransplantation and death are manually curated on an annual basis to allow for statistical analysis and the annual report. Results. Primary sclerosing cholangitis, acute hepatic failure, alcoholic liver disease, primary biliary cirrhosis and hepatocellular carcinoma are the five most frequent diagnoses (accounting for 15.3%, 10.8%, 10.6%, 9.3% and 9.0% of all transplants, respectively). Median waiting time for non-urgent liver transplantation during the last 10-year period was 39 days. Outcome has improved over time, and for patients transplanted during 2004–2013, overall one-, five- and 10-year survival rates were 91%, 80% and 71%, respectively. In an intention-to-treat analysis, corresponding numbers during the same time period were 87%, 75% and 66%, respectively. Conclusion. The liver transplant program in the Nordic countries provides comparable outcomes to programs with a MELD-based donor liver allocation system. Unique features comprise the diagnostic spectrum, waiting times and the availability of an integrated waiting list and transplant registry (NLTR).
Key Words: end-stage liver disease, indication, liver transplantation, organ allocation, outcome, registry
Introduction
In 1983, the National Institutes of Health declared liver transplantation (LTX) as an accepted therapy for end-stage liver disease, and it is still the only curative treatment for both acute and chronic terminal liver failure [1, 2]. Driven by an improvement in surgical techniques, critical care medicine and immunosuppression, the annual number of transplantations has increased steadily over the last three decades [3]. Recent data from the European Liver Transplant Registry (ELTR) indicate that almost 6000 LTX are currently performed in Europe each year [4]. There are substantial differences between countries regarding the prevalence of the underlying liver disease leading to terminal liver failure [5, 6, 7, 8, 9]. This is, at least to some extent, reflected in the observed differences in the number of LTX performed for the various indications in different countries and at different time periods [4, 10].
The outcome after LTX is dependent on a number of factors including the underlying liver disease [11], age of the recipient [12], age of donor [13], the preoperative condition of the recipient and surgical complications [14, 15]. All these factors are likely to vary between different transplant programs, between countries, and potentially even between different centres in the same country. However, publication of regional and national data on patient and graft survival is scarce. Such information is important for quality control of the activity in a program-restricted manner and to provide the patients with accurate information regarding risks and prognosis.
There are large intra-European variations in prevalence and incidence of both acute and chronic liver diseases [3]. The Nordic countries have an especially high prevalence of autoimmune liver diseases; primary sclerosing cholangitis (PSC), primary biliary cirrhosis (PBC) and autoimmune hepatitis [16, 17, 18]. The incidence of post hepatitis-C cirrhosis, on the other hand, is much lower than in most other European countries [19]. Since the beginning of 2000, the MELD scoring system [20] for prioritization of liver transplant recipients has been implemented in USA and most European countries [21]. The MELD score system was originally created, at the Mayo clinic, to predict survival following elective placement of transjugular intrahepatic portosystemic shunt [22]. It was subsequently modified and validated by the United Network for Organ Sharing and used as a predictor of survival in patients on the waiting list for LTX [23]. The Nordic countries have generally had a favorable organ donation rate with short waiting lists. For this reason, the MELD score is currently not used to prioritize patients for LTX in our region. In some instances, it is used locally at the individual centres to help match an available organ with the patient in greatest need.
In this paper, we present the total activity and the results following LTX in the five Nordic countries (Denmark, Finland, Iceland, Norway and Sweden), since the beginning of the program in 1982 to the end of 2013. All organ transplantations in these five countries are organized within the common organ exchange organization Scandiatransplant, which also provides a network for close collaboration between the centres. The Nordic Liver Transplant Registry (NLTR), which is an integrated part of the Scandiatransplant database [24], contains complete data from the LTX program at all transplantation centres in all the Nordic countries since the first LTX was performed in 1982 [18]. This publication aims to serve as an expanded version of the annual report from the NLTR, which has previously only been made available as a summary document at the Scandiatransplant webpages.
Materials and methods
Patients
All the Nordic transplant centres participate in Scandiatransplant organ exchange organization and registration of every patient listed for transplantation is obligatory. This implicates that all patients undergoing LTX in the five Nordic countries are included in the system, although singular cases of system entry errors cannot be formally excluded. The data collection is performed through a standardized questionnaire, including in the first part, information regarding date of acceptance, diagnosis, previous malignancy, events (encephalopathy, variceal bleeding, ascites, hospitalization, ventilator use), biochemistry and serology. The second part includes pre- and post-LTX histopathology results, donor characteristics and serology, type of operation (whole/partial/split), ischemia times and immunosuppression. Data for patients that underwent LTX before 1990 have been acquired retrospectively (constituting 3.4% of the total number of transplanted patients), while from 1st of January 1990, all patients listed for LTX in the Nordic countries have been recorded prospectively in the NLTR. In September 1994, the NLTR was integrated with the Scandiatransplant waiting list and organ allocation system, meaning complete waiting list data are available from all patients being listed for LTX from this point in time, regardless of whether they were transplanted or not, along with lifelong follow-up. The inclusion of all patients listed for LTX offers the unique possibility to also perform intention-to-treat (ITT) analysis. Entry of missing data and correction of errors were performed by transplant coordinators at all centres prior to the final data extraction. Data are stored centrally at the Scandiatransplant office in Aarhus, Denmark (www.scandiatransplant.org). In accordance with previous annual reports, the data was anonymized and analyzed in its entirety.
Liver transplant centers
The first Nordic LTX was performed in Helsinki, Finland in 1982, except for a few experimental procedures in Norway in the early 1970s [25, 26]. In Oslo, Norway, and Stockholm, Sweden, the first transplantation was carried out 2 years later, in 1984. This was followed by Gothenburg, Sweden, in 1985 and Copenhagen, Denmark, in 1990. LTX was also temporarily performed in Aarhus, Denmark, during 1993 and 1994 and in Uppsala, Sweden, between 1994 and 2010. Icelandic patients have previously undergone LTX in Sweden, Denmark and Norway, and are currently transplanted in Sweden.
Period of transplantation
In order to account for changes in the LTX programs in the Nordic countries over time, we initially considered all LTX since 1982 to the end of 2013. However, in the subsequent analyses, the patients were divided according to two 10-year time periods: (1) from January 1994 to the end of 2003 (2028 listed patients, 1735 transplanted), (2) from January 2004 to the end of 2013 (3214 listed patients, 2787 transplanted). The years prior to 1994 were characterized by greater variation in indications from year to year, low annual numbers and must also be considered a pioneering period with respect to surgical techniques and perioperative treatment. The most recent period was chosen to provide an evaluation of the current indications and results of LTX in the Nordic countries.
Organ allocation
The Scandiatransplant cooperation relies on center-based allocation of organs. In addition, there is extensive organ sharing between the centers. As early as 1985, four liver grafts were sent from Helsinki to Sweden. This was made possible due to the efforts of the recently formed Nordic Liver Transplant Group (NLTG), initiated in May 1985 by colleagues from the four Nordic countries. NLTG have two meetings annually to discuss and decide on the policy of common Nordic clinical issues on organ donation and transplantation. These decisions have usually been approved unchanged by the Scandiatransplant board, which represents the formal governance body of the Scandiatransplant system. There is no common waiting list in Scandinavia; however, an agreement embodied in Scandiatransplant ensures that high-urgency candidates are entitled to the first available liver graft from any member-country within 3 days. The highly urgent status is based on the diagnosis, clinical status and evaluation made by the hepatologist together with the transplant surgeon and anesthesiologist. The highly urgent-call status remains active for a maximum of 3 days. Urgent calls can also be used for patients in need of an acute retransplantation (due to primary nonfunction or vascular thrombosis) within 14 days of the primary transplantation. The MELD score has not been formally used for organ allocation, but has for several years been used, together with clinical judgment, to evaluate the prognosis of patients of patients on the list in some of the transplantation centres in the Nordic region. Still, we have included this in our analysis, and MELD score was calculated for patients undergoing LTX from the beginning of 2001 when the international normalized ratio (INR) was uniformly implemented in clinical assessments at the Nordic centres. In this study, only the laboratory MELD score was calculated, i.e. no alterations to the score was done for patients with hepatocellular carcinoma (HCC) or metabolic liver disorders such as familial amyloidotic polyneuropathy (FAP).
Intention to treat (ITT) analysis
In the ITT analysis, the first date of acceptance to the waiting list was considered as day 0 and survival was calculated from this time point. The patients were followed until death or 31 December 2013, regardless of transplantation status or re-listing for transplantation.
Statistical analysis
Anonymized data was extracted from the Scandiatransplant database. Statistical analyses were performed using SPSS 21.0 (Statistical Package for Social Sciences version, Chicago, Illinois, USA). Kaplan–Meier plots were used to visualize survival distributions and the Log-Rank test was used to compare groups. Life-tables were used to calculate actuarial survival. Predictors of patient and graft survival were evaluated using Cox-regressions. p-Values ≤ 0.05 were considered statistically significant.
Results
Transplantation activity
From 1982 to 31st of December 2013, data from a total of 6019 patients from the five Nordic countries (Sweden, Norway, Denmark, Iceland and Finland) had been entered into the NLTR. Of these, 5198 patients (86.4% of the total; 2267 females [44%] and 2931 males [56%]) had undergone a total of 5812 LTX. Five hundred and twenty three patients (10%) had been transplanted more than once, and 92 patients (2%) had been transplanted more than twice. The total number of patients that have undergone LTX per country was as follows; Sweden: 2489 (48%), Norway: 983 (19%), Finland: 916 (18%) and Denmark: 810 (16%).
For the entire period, there were 2391 (46%) recipients with blood group A, 1858 (36%) with blood group O, 638 (12%) with blood group B and 311 (6%) recipients with blood group AB. A total of 74 living donor LTX have been performed, of which the majority (86.5%) were performed in Sweden. Children (below 18 years of age) constituted 605 (11.6%) of the recipients in the registry.
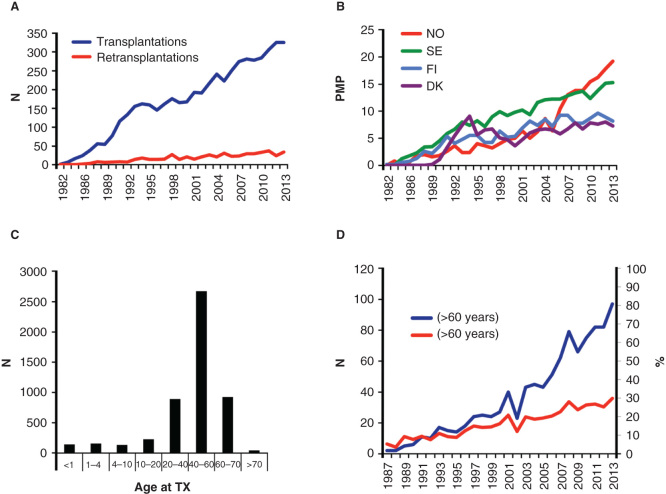
As shown in Figure 1A, the annual number of first-LTX has increased steadily throughout the entire period. The annual number of LTX per million of the population (pmp) in the four different countries demonstrates considerable variability (Figure 1B). In Norway, there has been a substantial increase in transplant numbers during the last decade, and now the liver transplant rate is close to 20 LTX pmp. The lowest level of LTX in Scandinavia is seen in Denmark (7.5 pmp).
Figure 1.
(A) The total number of first liver transplantations (LTX) performed in the Nordic countries (Denmark, Sweden, Finland and Norway) since 1982 along with the annual number of retransplantations in the same period. (B) The distribution of LTX per. 1 million of the country population (PMP) of the different Nordic countries from 1982 to 2013. (C) Age distribution of recipients at first LTX through the period from 1982 to 2013 for all the Nordic countries combined. (D) The total number of liver recipients older than 60 years (blue), and the same group as the fraction of the total LTX ctivity in the period from 1987 to 2013.
Indications for LTX in the Nordic countries
The major indications for LTX in the Nordic countries for the entire period between 1982 and 2013 are displayed in Table I. PSC, acute hepatic failure, alcoholic liver disease, PBC and HCC are the five most frequent diagnoses (representing 15.3%, 10.8%, 10.6%, 9.3% and 9.0%, respectively). However, there have been considerable changes in the relative rank of indications for LTX over time. Most clearly has been seen a significant increase in transplantations for HCC; from 4.8% of the total between 1994 and 2004 to 12.0% between 2004 and 2013 (Table II). In 2013, HCC accounted for as many as 19.1% of all indications. Of the 62 patients transplanted in 2013 with a primary diagnosis of HCC, slightly less than half of the patients (n = 27) were registered with a positive history of hepatitis C infection. Counting these, hepatitis-C-associated disease accounted for a total of 18.8% of all transplantations.
Table I.
Primary indications for LTX in the Nordic countries and corresponding graft and patient survival for the entire period between 1982 and 2013 (first transplantations).
| Survival rate year (%) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indication for LTX | No. of patients | (%) | 1 |
5 |
10 |
15 |
20 |
|||||
| Graft | Patient | Graft | Patient | Graft | Patient | Graft | Patient | Graft | Patient | |||
| PSC | 796 | 15.3% | 87 | 91 | 75 | 82 | 64 | 71 | 54 | 65 | 44 | 57 |
| PBC | 485 | 9.3% | 85 | 88 | 77 | 82 | 67 | 72 | 58 | 63 | 48 | 50 |
| Autoimmune cirrhosis | 214 | 4.1% | 82 | 85 | 75 | 80 | 66 | 69 | 51 | 58 | 32 | 38 |
| Alcoholic cirrhosis | 549 | 10.6% | 85 | 87 | 73 | 77 | 55 | 59 | 36 | 38 | 23 | 25 |
| Acute hepatic failure | 559 | 10.8% | 71 | 77 | 60 | 68 | 53 | 62 | 48 | 58 | 34 | 46 |
| Metabolic diseases | 378 | 7.3% | 87 | 88 | 76 | 79 | 67 | 69 | 60 | 62 | 50 | 56 |
| Posthepatitis B cirrhosis | 110 | 2.1% | 82 | 87 | 72 | 78 | 67 | 71 | 60 | 65 | 46 | 51 |
| Posthepatitis C cirrhosis | 433 | 8.3% | 81 | 86 | 62 | 69 | 47 | 53 | 34 | 38 | 25 | 29 |
| Cryptogenic cirrhosis | 152 | 2.9% | 79 | 81 | 71 | 74 | 53 | 58 | 40 | 41 | 24 | 29 |
| Cirrhosis other causes | 201 | 3.9% | 85 | 87 | 71 | 73 | 61 | 64 | 45 | 49 | 28 | 28 |
| Polycystic liver disease | 73 | 1.4% | 89 | 90 | 86 | 87 | 76 | 78 | 72 | 74 | 47 | 55 |
| Hepatocellular carcinoma | 468 | 9.0% | 79 | 82 | 54 | 57 | 38 | 40 | 27 | 30 | 20 | 24 |
| Cholangiocellular carcinoma | 42 | 0.8% | 70 | 77 | 34 | 38 | 22 | 25 | 13 | 25 | 0 | 0 |
| Secondary liver tumors | 60 | 1.1% | 84 | 90 | 53 | 55 | 39 | 41 | 23 | 24 | 0 | 0 |
| Other liver malignancies | 81 | 1.6% | 77 | 81 | 58 | 62 | 46 | 50 | 39 | 44 | 24 | 29 |
| Budd-Chiari | 75 | 1.4% | 71 | 80 | 64 | 72 | 62 | 68 | 66 | 66 | 38 | 48 |
| Biliary atresia | 201 | 3.9% | 74 | 77 | 66 | 71 | 63 | 71 | 60 | 67 | 52 | 59 |
| Cholestatic disease in children | 53 | 1.0% | 84 | 88 | 79 | 83 | 67 | 71 | 62 | 66 | 51 | 54 |
| Other liver diseases | 268 | 5.2% | 76 | 82 | 66 | 73 | 57 | 66 | 53 | 60 | 43 | 47 |
| Total number of first-LTX | 5198 | |||||||||||
Abbreviations: LTX = liver transplantation; PSC = primary sclerosing cholangitis; PBC = primary biliary cirrhosis; No = number.
Table II.
Primary indications for LTX in the Nordic countries and corresponding graft and patient survival for two consecutive 10-year periods (1994–2004 vs 2004–2013). The number of patients transplanted according to the various indications during 2013, is displayed in the far right column.
| From 1994 to 2004 |
From 2004 to 2013 |
2013 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-year survival (%) |
5-year survival (%) |
1-year survival |
5-year survival (%) |
|||||||||||
| Indication for LTX | No. of patients | (%) | Graft | Patient | Graft | Patient | No. of patients | (%) | Graft | Patient | Graft | Patient | No. of patients | (%) |
| PSC | 283 | 16.3% | 83 | 89 | 74 | 81 | 444 | 15.9% | 93 | 96 | 80 | 87 | 34 | 10.5% |
| PBC | 169 | 9.7% | 86 | 91 | 80 | 85 | 180 | 6.5% | 92 | 94 | 82 | 87 | 16 | 4.9% |
| Autoimmune cirrhosis | 66 | 3.8% | 76 | 80 | 71 | 77 | 127 | 4.6% | 87 | 90 | 80 | 85 | 20 | 6.2% |
| Alcoholic cirrhosis | 211 | 12.2% | 81 | 82 | 72 | 74 | 311 | 11.2% | 89 | 92 | 78 | 81 | 35 | 10.8% |
| Acute hepatic failure | 206 | 11.9% | 73 | 80 | 63 | 69 | 263 | 9.4% | 75 | 82 | 67 | 77 | 31 | 9.5% |
| Metabolic diseases | 110 | 6.3% | 80 | 82 | 73 | 75 | 199 | 7.1% | 93 | 94 | 84 | 86 | 32 | 9.8% |
| Post-hepatitis B cirrhosis | 53 | 3.1% | 85 | 92 | 72 | 74 | 44 | 1.6% | 88 | 90 | 79 | 85 | 5 | 1.5% |
| Post-hepatitis C cirrhosis | 129 | 7.4% | 70 | 81 | 53 | 81 | 290 | 10.4% | 87 | 89 | 69 | 73 | 34 | 10.5% |
| Cryptogenic cirrhosis | 70 | 4.0% | 77 | 80 | 71 | 74 | 50 | 1.8% | 88 | 90 | 78 | 80 | 0 | - |
| Cirrhosis other causes | 50 | 2.9% | 88 | 88 | 68 | 70 | 142 | 5.1% | 86 | 89 | 76 | 78 | 23 | 7.1% |
| Polycystic liver disease | 25 | 1.4% | 86 | 86 | 80 | 80 | 43 | 1.5% | 92 | 98 | 92 | 95 | 3 | 0.9% |
| Hepatocellular carcinoma | 83 | 4.8% | 75 | 78 | 47 | 49 | 335 | 12.0% | 86 | 88 | 63 | 66 | 62 | 19.1% |
| Cholangiocellular carcinoma | 11 | 0.6% | 73 | 73 | 55 | 55 | 25 | 0.9% | 72 | 84 | 27 | 42 | 0 | - |
| Secondary liver tumors | 9 | 0.5% | 78 | 78 | 56 | 56 | 46 | 1.7% | 89 | 94 | 58 | 60 | 5 | 1.5% |
| Other liver malignancies | 34 | 2.0% | 71 | 74 | 56 | 56 | 33 | 1.2% | 84 | 90 | 60 | 72 | 3 | 0.9% |
| Budd-Chiari | 34 | 2.0% | 74 | 79 | 74 | 76 | 30 | 1.1% | 75 | 89 | 60 | 73 | 3 | 0.9% |
| Biliary atresia | 83 | 4.8% | 75 | 77 | 67 | 72 | 86 | 3.1% | 80 | 83 | 72 | 79 | 7 | 2.2% |
| Cholestatic disease in children | 16 | 0.9% | 100 | 100 | 100 | 100 | 31 | 1.1% | 76 | 83 | 67 | 74 | 5 | 1.5% |
| Other liver diseases | 93 | 5.4% | 75 | 86 | 65 | 75 | 108 | 3.9% | 85 | 89 | 81 | 87 | 7 | 2.1% |
| Total number of LTX | 1735 | 2787 | 325 | |||||||||||
Abbreviations: LTX = liver transplantation; PSC = primary sclerosing cholangitis; PBC = primary biliary cirrhosis; No = number.
When comparing the two decades (1994–2004 vs. 2004–2013) LTX for cirrhosis due to HCV infection has increased from 7.4% in the first period to 10.4% in the latter. The percentage of PSC patients has remained high (15–16 %) through the entire period, while PBC has shown a decrease in terms of the fraction of the total number of first LTX performed, from 9.7% to 6.5%. However, the absolute number of LTX on the basis of PBC has remained stable (n = 169 vs. n = 180).
When investigating the primary indications among first liver allograft recipients during the last 10 years within the specific countries, some notable regional differences appear (Table III). LTX for alcoholic cirrhosis comprises 16.1% of the liver transplanted patients in Denmark and 15.7% in Finland, but only 8.6% in Sweden and 10.1% in Norway. There is also a distinct difference in the number of patients undergoing LTX for acute hepatic failure, being highest in Finland (15.7%) and Denmark (16.1%) and lowest in Sweden (6.3%). PSC is consistently one of the three main indications in all the Nordic countries, most frequent in Norway and Sweden (18.0% and 15.0%, respectively). Very few patients have been transplanted due to post-hepatitis C cirrhosis in Finland during the last decade (3%), while this has been one of the most frequent indications in Sweden during the same period (17.3%). The proportion of recipients with malignant liver disease also differs considerably between the four countries. HCC is one of the major indications in Sweden (14.3%) and Norway (13.7%), but represents only a small proportion of the Danish patients transplanted the last 10 years (4.9%) (Table III).
Table III.
Primary indications for LTX (first transplantations) during the last 10 years (2004–2013), subdivided according to the different Nordic countries.
| Indication | 2004–2013 n (% of total for each country) |
|||
|---|---|---|---|---|
| Sweden | Norway | Finland | Denmark | |
| PSC | 189 (15.0) | 121 (18.0) | 74 (16.1) | 60 (15.3) |
| PBC | 67 (5.3) | 45 (6.7) | 39 (8.5) | 29 (7.4) |
| Autoimmune cirrhosis | 51 (4.0) | 35 (5.2) | 23 (5.0) | 18 (4.6) |
| Alcoholic liver cirrhosis | 108 (8.6) | 68 (10.1) | 72 (15.7) | 63 (16.1) |
| Acute hepatic failure | 80 (6.3) | 56 (8.3) | 70 (15.2) | 57 (14.6) |
| Metabolic diseases | 114 (8.9) | 31 (4.6) | 22 (4.8) | 32 (8.2) |
| Post-hepatitis B cirrhosis | 28 (2.2) | 9 (1.3) | 2 (0.4) | 5 (1.3) |
| Post-hepatitis C cirrhosis | 218 (17.3) | 46 (6.8) | 14 (3.0) | 12 (3.1) |
| Cryptogenic cirrhosis | 24 (1.9) | 6 (0.9) | 12 (2.6) | 8 (2) |
| Cirrhosis other cause | 40 (3.2) | 44 (6.5) | 28 (6.1) | 30 (7.7) |
| Polycystic liver disease | 12 (1.0) | 11 (1.6) | 7 (1.5) | 13 (3.3) |
| Hepatocellular carcinoma | 181 (14.3) | 92 (13.7) | 43 (9.3) | 19 (4.9) |
| Cholangiocarcinoma | 12 (1) | 12 (1.8) | 0 (0) | 1 (0.3) |
| Secondary liver tumors | 8 (0.6) | 36 (5.3) | 1 (0.2) | 1 (0.3) |
| Other liver malignancies | 17 (1.3) | 7 (1.0) | 6 (1.3) | 3 (0.8) |
| Budd-Chiari | 11 (0.9) | 7 (1.0) | 8 (1.7) | 4 (1.0) |
| Biliary atresia | 30 (2.4) | 25 (3.7) | 17 (3.7) | 14 (3.6) |
| Cholestatic disease in children | 11 (0.9) | 5 (0.7) | 12 (2.6) | 3 (0.8) |
| Other liver diseases | 62 (4.9) | 17 (2.4) | 10 (2.2) | 19 (4.9) |
| Total number of LTX | 1263 | 673 | 460 | 391 |
Abbreviations: LTX = liver transplantation; PSC = primary sclerosing cholangitis; PBC = primary biliary cirrhosis.
Time on waiting list
The median waiting time for all patients listed for a first LTX (excluding urgent patients) during the last 10 years was 39 days. The waiting time has remained relatively stable during this period. The median waiting time for patients undergoing a non-urgent LTX in 2003–2004 was 31 days, and in 2012–2013 it was 34 days. The median waiting time for the entire period since 1982 was 35 days. The pooled median waiting time for all the Nordic countries according to the different blood groups for the last ten years is displayed in Table IV, and stratified by transplantation centre for the last two years in Table V. The number of deaths on the waiting list in 2013 was 20 (Denmark 1, Sweden 11, Finland 0, Norway 8), the corresponding numbers for 2012, 2011 and 2010 were 15, 21 and 19, respectively. There has been no consistent increase in waiting times for any blood group the last 10 years (Table V).
Table IV.
Median time on waiting list (days) in the Nordic centres combined, for patients receiving a first liver allograft since year 2004 (patients listed as highly urgent are excluded from the calculations).
| 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | |
|---|---|---|---|---|---|---|---|---|---|---|
| All blood types | 40 | 41 | 41 | 51 | 58 | 44 | 64 | 46 | 39 | 41 |
| Blood type A | 29 | 38 | 26 | 33 | 56 | 24 | 33 | 27 | 26 | 27 |
| Blood type 0 | 71 | 60 | 105 | 62 | 76 | 80 | 119 | 98 | 81 | 67 |
| Blood type AB | 10 | 23 | 42 | 52 | 44 | 24 | 36 | 14 | 29 | 45 |
| Blood type B | 44 | 44 | 28 | 63 | 84 | 83 | 90 | 67 | 38 | 61 |
Table V.
Median time on waiting list (days) for patients receiving first liver allograft in 2013, subdivided according to the different Nordic countries (patients listed as highly urgent are excluded from the calculations, numbers in brackets are 2012 numbers for comparison)
| Copenhagen | Gothenburg | Helsinki | Oslo | Stockholm | |
|---|---|---|---|---|---|
| Blood type A | 65 (65) | 19 (40) | 6 (21) | 24 (9) | 36 (30) |
| Blood type 0 | 102 (187) | 166 (257) | 13 (79) | 43 (26) | 171 (99) |
| Blood type AB | 108 (16) | 40 (NA) | 19 (29) | 24 (6) | 67 (18) |
| Blood type B | 155 (27) | 39 (69) | 16 (4) | 92 (33) | 189 (118) |
Recipient age at transplantation
The recipient age distribution throughout the period 1982–2013 is shown in Figure 1C. One hundred and forty-one children (2.7%) less than 1 year old have undergone a first LTX; 158 children (3.0%) were between 1 and 4 years when receiving their first liver graft; 925 patients (17.8%) were between 60 and 70 years and only 44 patients (0.8%) were more than 70 years at the time of LTX. The annual number of liver recipients older than 60 years has gradually increased over the last two decades (Figure 1D) and now accounts for approximately 30% of all the LTX.
Donor characteristics
The median donor age has increased throughout the period between 1982 and 2013 (30 years in 1993, 47 years in 2003 and 54 years in 2013, respectively). The donor male and female percentages during the total period were 56% and 44%, respectively. An increasing proportion of livers have been procured from donors older than 60 years (0% in 1993, 17% in 2003 and 40% in 2013). During the last 3 years, 13% of the donors were older than 70 years and the oldest donor was 86 years.
Patient survival after LTX
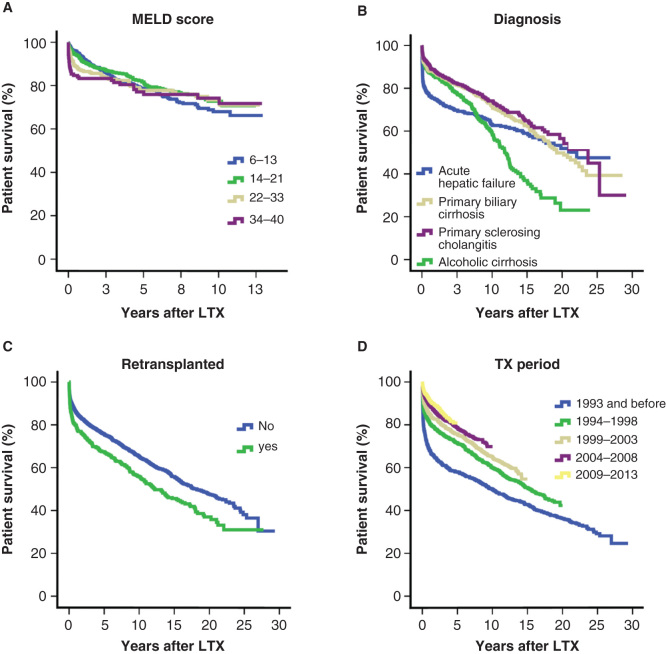
When all indications were included for the entire study period, the overall survival rates after first LTX in the Nordic countries were 85 % at 1 year, 75% at 5 years and 64% at 10 years. When analyzing patients transplanted within the 10 last years (2004–2013) the corresponding figures were 91%, 80% and 71%, respectively. Patient survival rates for the four major patient groups are shown in Figure 2B. The patient survival has improved significantly over the years as is displayed in Figure 2D, which shows the survival rates during five different time periods. Of note, survival curves for patients undergoing LTX during the two latest five-year time periods are practically identical. There are distinct differences in patient survival rates according to diagnosis (Table II). Inferior long time survival is notable for patients receiving a liver allograft on the basis of HCV with a 5-year survival of 73% and malignant disease; HCC and cholangiocarcinoma (CCA), with 5-year survival rates of 66% and 42%, respectively (Table II). Recipients above the age of 60 years operated within the last 10 years have on average 1-, 3- and 5-year survival probabilities of 88%, 78% and 73%, respectively. During this period, 32 patients above the age of 70 years have received a first liver graft with 1- and 3-year patient survival rates of 97% and 81%, respectively.
Figure 2.
(A) Patient Kaplan–Meier survival after first liver transplantation (LTX) between 2001 and 2013, subdivided according to model of end-stage liver disease (MELD) score. (B) Patient Kaplan–Meier survival curves per disease category for first LTX during the period of 1982 and 2013. (C) Patient Kaplan–Meier survival curves stratified by retransplantation status for LTX performed between 1982 and 2013. (D) Patient Kaplan–Meier survival curves according to different time periods between 1982 and 2013.
Laboratory MELD was possible to calculate for 67% of our patients (after 2001 when INR was uniformly implemented). The median laboratory MELD score at listing since 2001 was 15 (range 6–40) and 46% of the patients had a score below 14 (Table VI). Figure 2A shows survival curves according to different MELD scores. Cox regression analysis showed no significant impact from MELD on patient survival in our data. Recipients with MELD scores of 6–13, 14–21 and 22–33, respectively, had survival outcomes of 92.6%, 91.0% and 89.1% at 1 year; 78.7%, 81.7% and 82.0% at 5 years; and 68%, 72.8% and 82% at 10 years. Recipients with the highest MELD score, that is a MELD score of 34 or greater, showed survival estimates of 83.3%, 77.1% and 74.2% at 1, 5 and 10 years, respectively.
Table VI.
Median age and median MELD score (at listing) for patients transplanted during 2004–2013. Retransplantation rates for the same period and for the total period are given for each diagnosis.
| |
2004–2013 |
2004–20013 |
2004–2013 |
1982–2013 |
|---|---|---|---|---|
| Indication | Median age (range) | Median MELD (range) | Re-LTX(%) | Re-LTX(%) |
| Acute liver failure | 44.9 (0.1–74.1) | 36.6 (6–40) | 12.2 | 12.0 |
| Alcoholic liver cirrhosis | 57.4 (22.0–72.8) | 17.5 (7–40) | 4.2 | 4.5 |
| Autoimmune cirrhosis | 43.6 (10.6–70.0) | 16.2 (7–40) | 7.9 | 9.1 |
| Biliary atresia | 1.0 (0.4–40.1) | 17.9 (8–33) | 9.3 | 11.9 |
| Budd-Chiari | 34.5 (2.8–65.2) | 17.8 (11–31) | 20.0 | 15.4 |
| Cholangiocarcinoma | 53.8 (28.3–70.7) | 9.5 (6–24) | 15.4 | 6.8 |
| Hepatocellular carcinoma | 58.8 (3.9–74.1) | 10.6 (6–40) | 4.2 | 4.8 |
| Metabolic liver disease | 50.7 (0.7–71.8) | 10.3 (6–38) | 2.6 | 7.5 |
| PBC | 57.5 (24.3–73.2) | 15.1 (6–40) | 6.7 | 7.9 |
| Post-hepatitis B cirrhosis | 51.8 (8.3–67.9) | 14.7 (7–34) | 6.8 | 8.3 |
| Post-hepatitis C cirrhosis | 53.9 (17.0–73.0) | 14.7 (6–40) | 7.6 | 9.3 |
| PSC | 43.0 (14.3–73.4) | 12.5 (6–40) | 7.9 | 11.4 |
| SECA | 55.2 (31.6–71.2) | 7.5 (6–40) | 6.9 | 4.0 |
Abbreviations: LTX = liver transplantation; PSC = primary sclerosing cholangitis; PBC = primary biliary cirrhosis; MELD = model of end-stage liver disease; SECA = secondary liver cancer.
Retransplantations
In total, 698 (13.4%) patients were listed for retransplantation and the procedure was performed in 523 (10.1%) of these patients. Hence, 74.9% of all patients listed for a first retransplantation actually underwent retransplantation. The retransplantation rate, for all centres combined, has fluctuated between 8 and 15% during the last two decades (data not shown). A total of 77 patients were transplanted a third time, 14 patients a fourth time and 1 patient a fifth time during the entire period. As expected, patient survival in the subgroup of patients having undergone at least one retransplantation is worse than if no retransplantation is performed (Figure 2C).
Pediatric transplantation
The most frequent diagnosis for LTX in children (under 18 years of age) is biliary atresia (Table I), which accounts for 31.7% of all first LTX in this patient category. The median annual number of patients listed under this diagnosis the last 10 years is 9, and this has remained relatively stable over the last two decades, ranging from 5 to 12 patients per year.
Predictors of patient and graft survival
Potential predictors of patient and graft survival were evaluated using univariate cox-regressions. Sex, donor-age, recipient age, LTX period, waiting time and listing for retransplantation significantly affected both patient and graft survival (Table VII and VIII). Listing for LTX in the context of a highly urgent call was not associated with long-term overall patient and graft-survival. When the significant predictors were tested in a multivariate model, only waiting time did not remain an independent predictive factor (Tables VII and VIII).
Table VII.
Univariate and multivariate Cox regressions evaluating patient survival after first liver transplantation in the Nordic countries.
| Univariate |
Multivariate |
|||||
|---|---|---|---|---|---|---|
| p-Value | HR | 95% CI for HR | p-Value | HR | 95% CI for HR | |
| Female gender | <0.0001 | 0.84 | 0.77–0.92 | 0.006 | 0.93 | 0.88–0.98 |
| Tx period 2009–2013 (ref) | <0.0001 | <0.0001 | ||||
| Tx period 1982–1993 | <0.0001 | 2.63 | 2.2–3.15 | <0.0001 | 2.54 | 1.98–3.26 |
| Tx period 1994–1998 | <0.0001 | 2.01 | 1.68–2.4 | <0.0001 | 1.89 | 1.55–2.29 |
| Tx period 1999–2003 | <0.0001 | 1.66 | 1.38–1.99 | <0.0001 | 1.58 | 1.30–1.91 |
| Tx period 2004–2008 | 0.002 | 1.34 | 1.12–1.61 | 0.007 | 1.29 | 1.07–1.56 |
| Blood type 0 (ref) | 0.95 | |||||
| Blood type AB | 0.67 | 1.04 | 0.89–1.22 | |||
| Blood type A | 0.72 | 1.04 | 0.83–1.32 | |||
| Blood type B | 0.57 | 1.05 | 0.89–1.22 | |||
| Waiting time | 0.001 | 1.0001 | 1.00005–1.002 | 0.56 | 1.00 | 1.00–1.00 |
| MELD score 6–13 (ref) | 0.64 | |||||
| MELD score 14–21 | 0.63 | 0.91 | 0.64–1.32 | |||
| MELD score 22–33 | 0.28 | 0.82 | 0.56–1.18 | |||
| MELD score 34–40 | 0.70 | 0.92 | 0.61–1.39 | |||
| Donor age | <0.0001 | 1.01 | 1.01–1.01 | <0.0001 | 1.01 | 1.01–1.02 |
| Recipient age | <0.0001 | 1.01 | 1.01–1.02 | <0.0001 | 1.01 | 1.01–1.02 |
| Listed as highly urgent | 0.82 | 0.98 | 0.80–1.18 | |||
| Kidney dialysis at entry | 0.12 | 1.19 | 0.19–1.47 | |||
| Listed for retransplantation | <0.0001 | 1.71 | 1.53–1.92 | <0.0001 | 1.74 | 1.52–1.98 |
Abbreviations: HR = hazard ratio; Tx = transplantation; MELD = model for end stage liver; CI = confidence interval.
Table VIII.
Univariate and multivariate Cox regressions evaluating graft survival after first liver transplantation in the Nordic countries.
| Univariate |
Multivariate |
|||||
|---|---|---|---|---|---|---|
| p-Value | HR | 95% CI for HR | p-Value | HR | 95% CI for HR | |
| Female gender (ref) | 0.001 | 0.86 | 0.79–0.94 | 0.04 | 0.96 | 0.91–1.00 |
| Tx period 2009–2013 (ref) | <0.0001 | <0.0001 | ||||
| Tx period 1982–1993 | <0.0001 | 2.28 | 1.94–2.68 | <0.0001 | 1.53 | 1.22–1.93 |
| Tx period 1994–1998 | <0.0001 | 1.85 | 1.58–2.17 | <0.0001 | 1.40 | 1.77–2.03 |
| Tx period 1999–2003 | <0.0001 | 1.54 | 1.31–1.81 | 0.02 | 1.23 | 1.04–1.46 |
| Tx period 2004–2008 | 0.002 | 1.28 | 1.09–1.51 | 0.02 | 1.11 | 0.94–1.31 |
| Blood type 0 (ref) | 0.89 | |||||
| Blood type AB | 0.71 | 1.02 | 0.94–1.09 | |||
| Blood type A | 0.75 | 1.01 | 0.94–1.09 | |||
| Blood type B | 0.84 | 1.01 | 0.88–1.17 | |||
| Waiting time | 0.032 | 1.00008 | 1.000007–1.00001 | 0.43 | 1.00 | 1.00–1.00 |
| MELD score 6–13 (ref) | 0.71 | |||||
| MELD score 14–21 | 0.84 | 0.71 | 0.66–1.22 | |||
| MELD score 22–33 | 0.41 | 0.65 | 0.50–1.11 | |||
| MELD score 34–40 | 0.77 | 0.75 | 0.51–1.41 | |||
| Donor age | <0.0001 | 1.01 | 1.01–1.01 | <0.0001 | 1.01 | 1.00–1.02 |
| Recipient age | <0.0001 | 1.01 | 1.00–1.01 | <0.0001 | 1.01 | 1.01–1.02 |
| Listed as highly urgent | 0.72 | 0.94 | 0.70–1.16 | |||
| Kidney dialysis at entry | 0.15 | 1.19 | 0.17–1.49 | |||
| Listed for retransplantation | <0.0001 | 3.64 | 3.09–4.24 | <0.0001 | 3.14 | 3.01–4.54 |
Abbreviations: HR = Hazard ratio; Tx = Transplantation; MELD = Model for end stage liver; CI = Confidence interval.
Outcome of patients listed for LTX (intention to treat)
Data from the time of listing were available from 1994 to 2013, and we could perform an ITT analysis using 75.1% (n = 4522) of the dataset. During this period, 262 patients (5.8%) died while waiting for a liver graft. Four hundred and thirty-three patients were permanently withdrawn from the list after a median waiting time of 37 days (range 0 to 1352), of these patients 297 (68.6%) died within a median of 49 days (0 to 5295), and the rest were alive at the end of follow-up (n = 136). Nine patients were lost to follow-up after they were permanently withdrawn from the waiting list (mainly patients from outside of the Nordic countries). Patients listed for acute hepatic failure or alcoholic cirrhosis was most likely to die on the waiting list accounting for 21.4% and 16.8% of the deaths, respectively. From time of listing (between 1994 and 2013) the 1-, 3- and 5- year survival in the ITT analyses were 80%, 73% and 69%, respectively. During the same time period, the corresponding survival figures for patients undergoing LTX were 88%, 82% and 77%, respectively. Similar to what was seen for the post-LTX survival rates, the ITT analysis displayed considerable improvement in overall survival over the last time periods and 1- and 5 years survival increased from 82% and 72%, respectively, in the time period of 1994–1998 to 93% and 80%, respectively, for the period of 2009–2013. In the ITT analysis, the survival of patients with acute liver failure at 1-, 3- and 5 years was 69%, 65% and 62%, respectively, compared to the survival following LTX at 1-, 3- and 5 years of 78%, 74% and 70%, respectively.
Patients listed with HCC had shorter time on the waiting list. It is also small differences in survival rates in the ITT analysis (1, 3 and 5 year of 82%, 64% and 55%, respectively, compared with corresponding figures following LTX of 86%, 70% and 62%, respectively). For patients listed for CCA or secondary liver tumors (mainly colorectal liver metastases), the difference was more pronounced. In the ITT analysis, the survival of patients with CCA at 1-, 3- and 5 years was 68%, 47% and 36%, respectively compared to the survival following LTX at 1-, 3- and 5 years of 88%, 63% and 48%, respectively. While the corresponding figures for patients listed for secondary liver tumors was 82%, 57% and 48% in the ITT analysis and 92%, 67% and 60% following LTX.
Discussion
With data on more than 6000 patients listed for LTX, the NLTR provides a unique opportunity for evaluating the development and results of LTX in the Nordic countries. The mandatory participation in the registry ensures inclusion of data from all patients listed and transplanted within the Scandiatransplant network. The main aim of this report was to neutrally describe key features and outcomes of the Nordic LTX program. The results have been aligned with established factors that affect the outcome after LTX (e.g. MELD, age, donor characteristics etc.) without drawing conclusions as to the validity of the current practice.
In Denmark, Finland and Norway, all LTX are performed at a single centre, whereas Sweden has two liver transplant centres. There is a close and well-functioning collaboration between the centers, including an invaluable organ exchange program within Scandiatransplant. The close cooperation between the centres has also ensured common guidelines and a quite similar practice regarding operative technique, postoperative care, immunosuppressive protocols and long-term follow-up [18] and represents a major strength of the program and associated outcomes as herein presented. The social welfare system is well developed and robust in all the Nordic countries, ensuring all patients the benefit of follow-up at regular intervals as well as reliable and essentially free access to medications or hospitalization when needed.
The number of first LTX has increased annually; however, during the last 2 years, the number of first LTX have remained stable (n = 325 both years) and it will be interesting to observe if the Nordic program is about to reach a similar plateau as seen in the rest of Europe in recent years [4]. Like in the rest of the world, a plateau is related to limited availability of donors and not due to lack of liver recipients [27]. When analyzing the total patient population in 5-year intervals, there was a significant increase in LTX activity in all the Nordic countries. However, over the last few years the annual number of transplantations seems to have stabilized. This is most pronounced in Denmark and Finland while Sweden and Norway have continued to have a slight increase. The retransplantation rate has remained stable around 10% since the beginning of the 1990s and thus has had no major impact on the number of transplants performed.
None of the transplant programs within NLTR use the MELD-score [28] as a criterion for organ-allocation, but within each individual centre the MELD score are sometimes used to determine the recipients of greatest need. Still the data within the NLTR allowed us to calculate the laboratory MELD at time of listing for the majority of the patients. This analysis revealed that our population has a relatively low MELD, to which HCC patients, patients transplanted for FAP, neuroendocrine tumors and colorectal metastases without MELD score compensation contribute. Since MELD was designed as a score predicting risk of death within 3 months if not transplanted [22], the score may have limited validity in the Scandinavian setting, particularly in countries with very short waiting times (i.e. Finland and Norway).
The LTX survival rates found in the present analysis compare favorably with any centre in the rest of Europe and the United States [4, 29, 30]. There has been a dramatic improvement in survival since the start of our programs. This is mainly due to improved survival within the first year, which is difficult to significantly improve. To what extent the apparent lack of further improvement in overall results during the last 10 years is due to changes in age and in the spectrum of diagnoses, or a general lack of improvement in long-term management, warrants further investigation. In a recent publication by NLTR, the survival beyond 1 year was demonstrated in more details. In this analysis, it was suggested that in order to improve long-term survival, increased attention should be on cancer screening in high-risk patients, reducing the occurrence and impact of kidney disease, improving early detection and management of infections, depression and suicidal behavior, and supporting alcohol abstinence [31]. Further improvements in the immunosuppressive regimens will also be important.
The fact that the NLTR has follow-up data on all patients transplanted and close to complete data on all patients listed provided a unique opportunity to assess the survival after LTX in an ITT analysis. This analysis includes all patients in need for a transplant at a given time-point irrespective of whether they underwent LTX or not. As expected, the overall survival was only slightly decreased compared to the survival following LTX and probably reflects the short waiting time. It is worth noting that the ITT survival for HCC was in practice similar to the post-LTX survival indicating a low degree of withdrawals due to cancer progression and deaths on the waiting list.
Recipient age has continued to increase throughout the period 1982 to 2013. The proportion of patients over 60 years of age now accounts for nearly 30% of all first LTX. Our results show a highly satisfactory survival among these patients with a 1-, 3- and 5 year survival of 88%, 79% and 71%, respectively, during the last decade. The annual number of children between 1 and 4 years receiving a first liver graft increased considerably during the early nineties due to the introduction of partial LTX. During the last two decades, the number have remained relatively stable, fluctuating between 4 and 11 transplantations per year reflecting that there has not been an increase in the recipient pool for LTX in children of this age group. This corresponds well with data from the European Biliary Atresia Registries, with an estimated incidence of biliary atresia of 1:18,000 live births [32]. The annual number of new patients in the Nordic countries would thus be in the range of 10–20. The majority of these children will sooner or later need a liver graft [33]. The good outcomes in this group are encouraging, given the fact that LTX in children inevitably is a low volume activity. In the adult patient population, PSC has been the most frequent indication for LTX in the Nordic countries. The survival after transplantation in this group is excellent, despite the lack of predictability of the disease process, including the risk of developing cholangiocarcinoma [34]. The number of patients with alcoholic liver disease has remained stable throughout the entire period. When evaluating the listing diagnosis for the entire material alcoholic liver disease represents almost 11% of the indications. However, we must take into account that some patients listed with for instance HCC or hepatitis C infection could also have a significant consumption of alcohol contributing to the disease. A study conducted in Gothenburg showed that as many as 19% of first LTX performed between 1988 and 2003 were due to alcoholic liver disease [35]. Of these, 35% of the patients also had hepatitis C. It is therefore reasonable to assume that the number of patients where alcohol consumption is contributing to the disease is higher than what is given by the primary diagnoses of listing available from the NLTR. In Denmark, alcoholic cirrhosis has been the major indication (16%) in the last 10-year period, while in Finland the second largest indication (16%). Of note, the long-term (>10 years) survival for this group of patients is worse than for several other cirrhotic indications and in contrast to the beneficial 5-year survival. This finding warrants further detailed studies as well as confirmation in other series.
The prevalence of HCV and HBV has historically been low in the Nordic countries [36, 37] and cirrhosis due chronic viral infection (HCV and HBV) has consequently accounted for only a small proportion of LTX in the Nordic countries (in contrast to the situation in most Western countries) [38, 39, 40]. However, as shown by the present assessments there has been a steadily increasing number of patients referred for LTX due to HCV cirrhosis over the recent years. The Nordic countries jointly considered, HCV cirrhosis now accounts for more than 10% of all LTX. In Sweden, it has become the second most frequent indication for LTX, only surpassed by HCC. The recent developments might indicate that the HCV epidemic in Scandinavia has a time-shift as compared with the rest of the Western world.
Detailed data on tumor burden in transplanted HCC patients is not recorded in the NLTR and represent an important limitation when evaluating this group of patients. Nevertheless, due to the good access to organs, a considerable number of patients outside of the inclusion criteria like Milan [41] or UCSF [42] have been transplanted with acceptable results in the group as a whole [43]. In Norway, since 2005, the local criteria for acceptance of HCC patients on the waiting list has been 1) single tumor up to 10 cm, 2) five tumors up to 4 cm and 3) if more than five tumors all should be less than 2 cm (“Oslo Criteria”). This practice has been facilitated by unique access to donor organs and the short waiting times and may suggest that further stratification of LTX in HCC patients with tumors exceeding standard acceptance criteria warrants investigation [44, 45]. In contrast, patients with cholangiocarcinoma exhibit a 5-year survival rate of 39% during the last 10 year period. In selected patients (i.e. TNM<2 and CA 19–9 < 100), patients transplanted after 1995 had a 5-year survival of 58% in a previous study from the NLTR [46]. LTX for secondary liver tumors has been considerably higher in Norway (5.3%) than in the other Nordic countries during the last 10-year period, where LTX on this indication is well below 1%. The marked difference is explained by the Norwegian study on LTX for colorectal liver metastases (CLMs) which was initiated in 2006 [47]. In the rest of the world, CLMs are currently considered an absolute contraindication for LTX. The first results of the Norwegian study was published in 2013 and showed 1-, 3- and 5 years patient survival of 95%, 68% and 60%, respectively [47], further suggesting the utility of LTX in highly selected cases of different types of liver malignancies.
The integration of NLTR with the Scandiatransplant waiting-list system was completed in 1994. The registry would not have existed without prior pioneering efforts over the preceding 5 years period (1989–1994) in establishing the prospective registration (efforts driven mainly by Susanne Keiding, Krister Höckerstedt, Erik Schrumpf and Bo-Göran Ericzon, in Denmark, Finland, Norway and Sweden, respectively). The integration with the Scandiatransplant waiting list system is unique in providing 100% coverage of donors and recipients, as well as comprehensive waiting list data and donor information. The Nordic countries also provide a subset of data for processing in the ELTR; http://www.eltr.org/), but this input only accounts for transplanted patients. Importantly, the NLTR forms a hub for the NLTG collaboration. It provides an essential basis for volume and quality monitoring, has served dozens of collaborative scientific projects and warrants support and continuation.
In conclusion, the Nordic transplant program shows a distinct spectrum of key diagnoses of underlying liver diseases and delivers outcomes comparable to the rest of Europe and the United States. The access to organs has been very good with short waiting times, low waitlist mortality and the possibility to transplant patients with extended indications. The number of transplants may be about to reach a plateau, and work to maintain the unique donor access is warranted.
Acknowledgments
The maintenance of the Oracle system has been performed by Scandiatransplant. We are sincerely grateful for the help and support by Frank Pedersen, Christian Mondrup and Ilse Duus Weinreich and the Scandiatransplant team in Aarhus. A large number of physicians, nurses and transplant coordinators at all the Nordic transplant centers have provided invaluable support in the data collection and data curation for the Scandiatransplant system and the NLTR. The ongoing support by Stian Angelsen, Oslo, Norway, in the maintenance of the NLTR is appreciated.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- National Institutes of Health Consensus Development Conference: liver transplantation. R I Med J. 1984;67:73–6. [PubMed] [Google Scholar]
- Klein KB, Stafinski TD, Menon D. Predicting survival after liver transplantation based on pre-transplant MELD score: a systematic review of the literature. PLoS ONE. 2013;8:e80661. doi: 10.1371/journal.pone.0080661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blachier M, Leleu H, Peck-Radosavljevic M, Valla DC, Roudot-Thoraval F. The burden of liver disease in Europe: a review of available epidemiological data. J Hepatol. 2013;58:593–608. doi: 10.1016/j.jhep.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Adam R, Karam V, Delvart V, O’Grady J, Mirza D, Klempnauer J, et al. Evolution of indications and results of liver transplantation in Europe. A report from the European Liver Transplant Registry (ELTR) J Hepatol. 2012;57:675–88. doi: 10.1016/j.jhep.2012.04.015. [DOI] [PubMed] [Google Scholar]
- Boberg KM, Aadland E, Jahnsen J, Raknerud N, Stiris M, Bell H. Incidence and prevalence of primary biliary cirrhosis, primary sclerosing cholangitis, and autoimmune hepatitis in a Norwegian population. Scand J Gastroenterol. 1998;33:99–103. doi: 10.1080/00365529850166284. [DOI] [PubMed] [Google Scholar]
- Escorsell A, Pares A, Rodes J, Solis-Herruzo JA, Miras M, de la Morena E. Epidemiology of primary sclerosing cholangitis in Spain. Spanish Association for the Study of the Liver. J Hepatol. 1994;21:787–91. doi: 10.1016/s0168-8278(94)80240-8. [DOI] [PubMed] [Google Scholar]
- Bellentani S, Tiribelli C, Saccoccio G, Sodde M, Fratti N, De MC, et al. Prevalence of chronic liver disease in the general population of northern Italy: the Dionysos Study. Hepatology. 1994;20:1442–9. doi: 10.1002/hep.1840200611. [DOI] [PubMed] [Google Scholar]
- van der Poel CL, Ebeling F. Hepatitis C virus: epidemiology, transmission and prevention. Curr Stud Hematol Blood Transfus. 1998;62:208–36. doi: 10.1159/000060480. [DOI] [PubMed] [Google Scholar]
- Christenson B, Bottiger M, Grillner L. The prevalence of hepatitis B in Sweden; a statistical serosurvey of 3381 Swedish inhabitants. Epidemiol Infect. 1997;119:221–5. doi: 10.1017/s0950268897007838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bismuth H, Castaing D, Ericzon BG, Otte JB, Rolles K, Ringe B, et al. Hepatic transplantation in Europe. First Report of the European Liver Transplant Registry. Lancet. 1987;2:674–6. doi: 10.1016/s0140-6736(87)92453-6. [DOI] [PubMed] [Google Scholar]
- Roberts MS, Angus DC, Bryce CL, Valenta Z, Weissfeld L. Survival after liver transplantation in the United States: a disease-specific analysis of the UNOS database. Liver Transpl. 2004;10:886–97. doi: 10.1002/lt.20137. [DOI] [PubMed] [Google Scholar]
- Levy MF, Somasundar PS, Jennings LW, Jung GJ, Molmenti EP, Fasola CG, et al. The elderly liver transplant recipient: a call for caution. Ann Surg. 2001;233:107–13. doi: 10.1097/00000658-200101000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano D, Grosso G, Vizzini G, Spada M, Cintorino D, Malaguarnera M, et al. Recipient-donor age matching in liver transplantation: a single-center experience. Transplant Proc. 2013;45:2700–6. doi: 10.1016/j.transproceed.2013.07.039. [DOI] [PubMed] [Google Scholar]
- Cholongitas E, Marelli L, Shusang V, Senzolo M, Rolles K, Patch D, et al. A systematic review of the performance of the model for end-stage liver disease (MELD) in the setting of liver transplantation. Liver Transpl. 2006;12:1049–61. doi: 10.1002/lt.20824. [DOI] [PubMed] [Google Scholar]
- McElroy LM, Daud A, Davis AE, Lapin B, Baker T, Abecassis MM, et al. A meta-analysis of complications following deceased donor liver transplant. Am J Surg. 2014;208:605–18. doi: 10.1016/j.amjsurg.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boberg KM. Prevalence and epidemiology of autoimmune hepatitis. Clin Liver Dis. 2002;6:635–47. doi: 10.1016/s1089-3261(02)00021-1. [DOI] [PubMed] [Google Scholar]
- Bjoro K, Brandsaeter B, Foss A, Schrumpf E. Liver transplantation in primary sclerosing cholangitis. Semin Liver Dis. 2006;26:69–79. doi: 10.1055/s-2006-933565. [DOI] [PubMed] [Google Scholar]
- Bjoro K, Friman S, Hockerstedt K, Kirkegaard P, Keiding S, Schrumpf E, et al. Liver transplantation in the Nordic countries, 1982-1998: changes of indications and improving results. Scand J Gastroenterol. 1999;34:714–22. doi: 10.1080/003655299750025930. [DOI] [PubMed] [Google Scholar]
- Melum E, Schrumpf E, Bjoro K. Liver TX for hepatitis C cirrhosis in a low prevalence population: risk factors and status at evaluation. Scand J Gastroenterol. 2006;41:592–6. doi: 10.1080/00365520500347113. [DOI] [PubMed] [Google Scholar]
- Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91–6. doi: 10.1053/gast.2003.50016. [DOI] [PubMed] [Google Scholar]
- Cholongitas E, Burroughs AK. The evolution in the prioritization for liver transplantation. Ann Gastroenterol. 2012;25:6–13. [PMC free article] [PubMed] [Google Scholar]
- Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–70. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- Kamath PS, Kim WR. The model for end-stage liver disease (MELD) Hepatology. 2007;45:797–808. doi: 10.1002/hep.21563. [DOI] [PubMed] [Google Scholar]
- Grunnet N, Bodvarsson M, Jakobsen A, Kyllonen L, Olausson M, Pfeffer P, et al. Scandiatransplant report. Transplant Proc. 2009;2010;42:4429–31. doi: 10.1016/j.transproceed.2010.09.111. [DOI] [PubMed] [Google Scholar]
- Aune S, Schistad G, Skulberg A. Human liver transplantation without azathioprine. Surg Gynecol Obstet. 1972;135:727–8. [PubMed] [Google Scholar]
- Aune S. Orthotopic liver transplantation. Ann Chir Gynaecol Fenn. 1973;62:175–7. [PubMed] [Google Scholar]
- Abouna GM. Organ shortage crisis: problems and possible solutions. Transplant Proc. 2008;40:34–8. doi: 10.1016/j.transproceed.2007.11.067. [DOI] [PubMed] [Google Scholar]
- Martin AP, Bartels M, Hauss J, Fangmann J. Overview of the MELD score and the UNOS adult liver allocation system. Transplant Proc. 2007;39:3169–74. doi: 10.1016/j.transproceed.2007.04.025. [DOI] [PubMed] [Google Scholar]
- Stepanova M, Wai H, Saab S, Mishra A, Venkatesan C, Younossi Z. The outcomes of adult liver transplants in the united states from 1987 to 2013. Liver Int. 2015 doi: 10.1111/liv.12779. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Jain A, Reyes J, Kashyap R, Dodson SF, Demetris AJ, Ruppert K, et al. Long-term survival after liver transplantation in 4,000 consecutive patients at a single center. Ann Surg. 2000;232:490–500. doi: 10.1097/00000658-200010000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aberg F, Gissler M, Karlsen TH, Ericzon BG, Foss A, Rasmussen A, et al. Differences in long-term mortality among liver transplant recipients and the general population: A population-based Nordic study. Hepatology. 2015;61:668–77. doi: 10.1002/hep.27538. [DOI] [PubMed] [Google Scholar]
- Petersen C, Harder D, Abola Z, Alberti D, Becker T, Chardot C, et al. European biliary atresia registries: summary of a symposium. Eur J Pediatr Surg. 2008;18:111–16. doi: 10.1055/s-2008-1038479. [DOI] [PubMed] [Google Scholar]
- Kumagi T, Drenth JP, Guttman O, Ng V, Lilly L, Therapondos G, et al. Biliary atresia and survival into adulthood without transplantation: a collaborative multicentre clinic review. Liver Int. 2012;32:510–18. doi: 10.1111/j.1478-3231.2011.02668.x. [DOI] [PubMed] [Google Scholar]
- Boberg KM, Lind GE. Primary sclerosing cholangitis and malignancy. Best Pract Res Clin Gastroenterol. 2011;25:753–64. doi: 10.1016/j.bpg.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Bjornsson E, Olsson J, Rydell A, Fredriksson K, Eriksson C, Sjoberg C, et al. Long-term follow-up of patients with alcoholic liver disease after liver transplantation in Sweden: impact of structured management on recidivism. Scand J Gastroenterol. 2005;40:206–16. doi: 10.1080/00365520410009591. [DOI] [PubMed] [Google Scholar]
- Cornberg M, Razavi HA, Alberti A, Bernasconi E, Buti M, Cooper C, et al. A systematic review of hepatitis C virus epidemiology in Europe, Canada and Israel. Liver Int. 2011;31:30–60. doi: 10.1111/j.1478-3231.2011.02539.x. [DOI] [PubMed] [Google Scholar]
- Rimseliene G, Nilsen O, Klovstad H, Blystad H, Aavitsland P. Epidemiology of acute and chronic hepatitis B virus infection in Norway, 1992-2009. BMC Infect Dis. 2011;11:153. doi: 10.1186/1471-2334-11-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgard O, Jeansson S, Skaug K, Raknerud N, Bell H. Hepatitis C in the general adult population of Oslo: prevalence and clinical spectrum. Scand J Gastroenterol. 2003;38:864–70. doi: 10.1080/00365520310004542. [DOI] [PubMed] [Google Scholar]
- Hoffmann G, Berglund G, Elmstahl S, Eriksson S, Verbaan H, Widell A, et al. Prevalence and clinical spectrum of chronic viral hepatitis in a middle-aged Swedish general urban population. Scand J Gastroenterol. 2000;35:861–5. doi: 10.1080/003655200750023246. [DOI] [PubMed] [Google Scholar]
- Scaglione S, Kliethermes S, Cao G, Shoham D, Durazo R, Luke A, et al. The Epidemiology of Cirrhosis in the United States: A Population-based Study. J Clin Gastroenterol. 2014 doi: 10.1097/MCG.0000000000000208. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Mazzaferro V, Bhoori S, Sposito C, Bongini M, Langer M, Miceli R, et al. Milan criteria in liver transplantation for hepatocellular carcinoma: an evidence-based analysis of 15 years of experience. Liver Transpl. 2011;17:S44–57. doi: 10.1002/lt.22365. [DOI] [PubMed] [Google Scholar]
- Unek T, Karademir S, Arslan NC, Egeli T, Atasoy G, Sagol O, et al. Comparison of Milan and UCSF criteria for liver transplantation to treat hepatocellular carcinoma. World J Gastroenterol. 2011;17:4206–12. doi: 10.3748/wjg.v17.i37.4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Line PD, Karlsen TH, Boberg KM, Schrumpf E, Fosby B, Bentdal Ø, et al. Outcome after liver transplantation for hepatocellular carcinoma (HCC) on extended criteria. 2012 Abstract presented at The Scandinavian Transplantation Society XXVI Congress, Reykjavik, Iceland, May 9-12. [Google Scholar]
- Koschny R, Schmidt J, Ganten TM. Beyond Milan criteria--chances and risks of expanding transplantation criteria for HCC patients with liver cirrhosis. Clin Transplant. 2009;23:49–60. doi: 10.1111/j.1399-0012.2009.01110.x. [DOI] [PubMed] [Google Scholar]
- Piardi T, Audet M, Odeh M, Panaro F, Cag M, El-ahmar J, et al. Liver transplantation exceeding UCSF criteria: case report of a late recurrence treated by surgery and review of the literature. Eur Surg Res. 2010;44:52–5. doi: 10.1159/000264635. [DOI] [PubMed] [Google Scholar]
- Friman S, Foss A, Isoniemi H, Olausson M, Hockerstedt K, Yamamoto S, et al. Liver transplantation for cholangiocarcinoma: selection is essential for acceptable results. Scand J Gastroenterol. 2011;46:370–5. doi: 10.3109/00365521.2010.533384. [DOI] [PubMed] [Google Scholar]
- Hagness M, Foss A, Line PD, Scholz T, Jorgensen PF, Fosby B, et al. Liver transplantation for nonresectable liver metastases from colorectal cancer. Ann Surg. 2013;257:800–6. doi: 10.1097/SLA.0b013e3182823957. [DOI] [PubMed] [Google Scholar]