Abstract
Amblyomma maculatum (the Gulf Coast tick), an aggressive, human-biting, Nearctic and Neotropical tick, is the principal vector of Rickettsia parkeri in the United States. This pathogenic spotted fever group Rickettsia species has been identified in 8–52% of questing adult Gulf Coast ticks in the southeastern United States. To our knowledge, R. parkeri has not been reported previously from adult specimens of A. maculatum collected in Kansas or Oklahoma. A total of 216 adult A. maculatum ticks were collected from 18 counties in Kansas and Oklahoma during 2011–2014 and evaluated by molecular methods for evidence of infection with R. parkeri. No infections with this agent were identified; however, 47% of 94 ticks collected from Kansas and 73% of 122 ticks from Oklahoma were infected with “Candidatus Rickettsia andeanae” a spotted fever group Rickettsia species of undetermined pathogenicity. These preliminary data suggest that “Ca. R. andeanae” is well-adapted to survival in populations of A. maculatum in Kansas and Oklahoma, and that its ubiquity in Gulf Coast ticks in these states may effectively exclude R. parkeri from their shared arthropod host, which could diminish markedly or preclude entirely the occurrence of R. parkeri rickettsiosis in this region of the United States.
INTRODUCTION
Amblyomma maculatum (the Gulf Coast tick) is an aggressive, human-biting, Nearctic and Neotropical tick that is distributed widely across many countries in the Western Hemisphere. In the United States, A. maculatum is the principal vector of Rickettsia parkeri, a bacterial pathogen that causes a febrile, eschar- and rash-associated illness that clinically resembles Rocky Mountain spotted fever (RMSF) (Paddock and Goddard, 2015). Populations of A. maculatum occur throughout the southeastern and south-central states and along much of the eastern seaboard. Molecular surveys of Gulf Coast ticks collected from several locations in multiple states within its southern and eastern range reveal estimated rates of infection with R. parkeri in 8–52% of questing adult ticks (Sumner et al., 2007, Paddock et al., 2010, Fornadel et al., 2011, Wright et al., 2011, Varela-Stokes et al., 2011, Jiang et al., 2012, Ferrari et al., 2012, Nadolny et al., 2014, Florin et al., 2013, Pagac et al., 2014 and Florin et al., 2014). More than 35 cases of R. parkeri rickettsiosis have been identified in patients from 9 states (Paddock and Goddard, 2015).
“Candidatus Rickettsiae andeanae” was first described from specimens of A. maculatum and Ixodes boliviensis collected in Peru (Blair et al., 2004), and subsequently from Gulf Coast ticks in the United States (Paddock et al., 2010) and other tick species in Argentina, Brazil, and Chile (Pacheco et al., 2007, Abaraca et al., 2012 and Nieri-Bastos et al., 2014). “Ca R. andeanae” has been isolated recently in culture, although some difficulties remain in establishing continuously infected cell lines (Luce-Fedrow et al., 2012 and Ferrari et al., 2013). To our knowledge, no cases of R. parkeri rickettsiosis have been described from Kansas or Oklahoma, despite well-established populations of A. maculatum in those states which have existed for more than 40 years. In this study we used molecular techniques to evaluate adult Gulf Coast ticks collected from multiple sites in Kansas and Oklahoma for infections with R. parkeri or “Ca. R. andeanae”.
METHODS
Tick collection and processing
During 2011–2014, questing adult A. maculatum ticks were collected from vegetation by using cloth drags or flags at multiple sites in 9 counties of Kansas (Anderson, Butler, Crawford, Geary, Morris, Neosho, Osage, Riley, and Shawnee) and 9 counties of Oklahoma (Cleveland, Cotton, Kiowa, Lincoln, Payne, Osage, Tillman, Tulsa, and Washington). Field-collected specimens were placed in 70% ethanol and transported to the laboratory where these were air-dried, identified using a standard taxonomic key (Keirans and Litwak, 1989), transferred to individual 1.5 ml microcentrifuge tubes, and stored at −80 °C prior to DNA extraction.
Molecular analyses
Genomic DNA was extracted from tick specimens by using a DNA Minikit (Qiagen, Valencia, CA) and eluted in a final volume of 100 μL. Extracted samples were evaluated for DNA of R. parkeri and “Ca. R. andeanae” using the QuantiTect Multiplex PCR Kit (Qiagen) and primers and probes targeting sequence of the ompB gene (Jiang et al., 2012). For each real-time PCR assay, 2.5 μL of tick extract was mixed with 0.4 μM of the forward and reverse primers (Rpa129F and Rpa224R for R. parkeri, or Rand957F and Rand1062R for “Ca. R. andeanae”) and 0.2 μM of the FAM-labeled probe (Rpa188probe for R. parkeri, or Rand1003probe for “Ca. R. andeane”), in a final reaction volume of 25 μL. Cycling was performed on an Mx3005P thermal cycler (Agilent, Santa Clara, CA) and conditions consisted of 15 min at 95 °C, 45 cycles of 1 min at 95 °C, and 1 min at 60 °C. Ct values <40 were considered positive for the respective agent. Both assays were validated by testing a panel of DNA extracts of A. maculatum ticks naturally infected with R. parkeri or “Ca. R. andeanae”, confirmed previously by using a conventional ompA PCR assay and sequence analysis (Sumner et al., 2007 and Paddock et al., 2010), or identified negative for DNA of R. parkeri and “Ca. R. andeanae” by using a broad-range, Rickettsia species real-time PCR assay targeting sequence of the gltA gene (Stenos et al., 2005 and Denison et al., 2014).
As additional confirmatory steps, a subset of 5 tick extracts from Kansas and 5 from Oklahoma that tested positive for “Ca. R andeanae” by the real-time assay were evaluated by using a hemi-nested PCR assay with primers RR190.70 and RR190.701 in the primary reaction and RR190.70 and RR190.602 in the secondary reaction (Sumner et al., 2007), followed by sequence analysis of the amplified segments of the rickettsial ompA gene. A subset of 10 A. maculatum extracts representing ticks collected from 5 counties in Kansas, and 10 extracts representing ticks collected from 5 counties in Oklahoma, were selected for further analysis to verify the morphological species identification by using a conventional PCR assay with primers T1B and T2A (Beati and Keirans, 2001) and sequencing of the amplified segments of the ixodid mitochondrial 12S ribosomal DNA gene.
RESULTS
A total of 216 adult Gulf Coast ticks were evaluated, comprising 53 male and 41 female specimens from Kansas collected during 2012–2013, and 52 male and 70 female specimens collected from Oklahoma during 2011–2014 (Table 1). Of the Kansas specimens, 51 (54%) were obtained from multiple sites in Geary County during May-July 2013, whereas 87 (71%) of the total Oklahoma specimens were collected from 3 sites in Payne county during 2011–2013 (Fig. 1). Of the 20 tick extracts assessed by PCR and sequencing of a 313-bp segment the ixodid mitochondrial 12S rDNA gene, 6 samples from Kansas and 8 from Oklahoma were identical with each other and revealed 100% identity with A. maculatum (GenBank accession number JX192922). An additional 4 tick extracts from Kansas and 2 from Oklahoma were identical with each other, but differed from the other 14 mitochondrial 12S rDNA sequences by 3 nucleotide substitutions and revealed 99% identity with the corresponding GenBank bank sequence for A. maculatum.
Table 1.
Molecular evaluation for infection with “Ca. Rickettsia andeanae “and Rickettsia parkeri in questing adult Gulf Coast ticks (Amblyomma maculatum), Kansas and Oklahoma, 2011–2014.
| State (No. of counties) | Year | Number of ticks evaluated | Number (%) positive for | |
|---|---|---|---|---|
| “Ca. Rickettsia andeanae” | Rickettsia parkeri | |||
| Kansas | ||||
| (1) | 2012 | 6 | 2 (33) | 0 |
| (8) | 2013 | 88 | 42 (48) | 0 |
| Oklahoma | ||||
| (1) | 2011 | 18 | 14 (78) | 0 |
| (1) | 2012 | 26 | 20 (77) | 0 |
| (2) | 2013 | 44 | 37 (84) | 0 |
| (7) | 2014 | 34 | 18 (53) | 0 |
| Total | 216 | 133 (62) | 0 | |
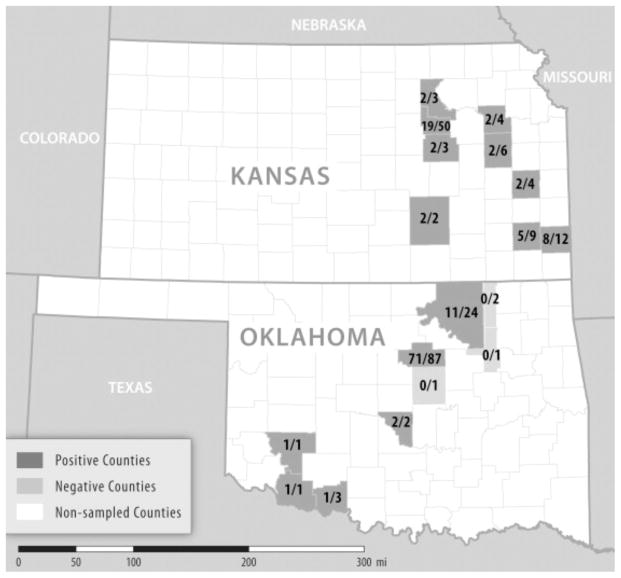
Fig. 1.
Frequency of infection with “Candidatus Rickettsia andeanae” among 216 questing adult Amblyomma maculatum ticks collected from Kansas (Anderson, Butler, Crawford, Geary, Morris, Neosho, Osage, Riley, and Shawnee counties) and Oklahoma (Cleveland, Cotton, Kiowa, Lincoln, Osage, Payne, Tillman, Tulsa, and Washington counties) during 2011–2014. Fractions represent the numbers of ticks infected with “Ca. R. andeanae” over the number of ticks that were evaluated by the real-time PCR assay. No molecular evidence of infection with Rickettsia parkeri was identified in any specimen of A. maculatum in either state.
Of the Kansas ticks, 44 (47%) were infected with “Ca. R andeanae”, including 29 (56%) of the male specimens and 15 (37%) of the female specimens. Of the ticks collected in Oklahoma, 89 (73%) were infected with “Ca. R. andeanae”, including 38 (73%) of male ticks and 51 (73%) of the female ticks. All 10 samples amplified by PCR for a segment the rickettsial ompA gene demonstrated 100% identity with the corresponding 597-bp segment of “Ca. R. andeanae” strain Agripino Enciso (GenBank accession number KF179352). No specimens from Kansas or Oklahoma demonstrated molecular evidence of infection withR. parkeri.
The performance of the real-time assays was assessed by evaluating 49 DNA extracts of A. maculatum determined by other molecular methods to be infected with one or neither of these Rickettsia species. Of 25 gltA-negative A. maculatum extracts, none were positive for R. parkeri or “Ca. R. andeanae” by the real-time assays. Of 14 ticks positive for “Ca. R. andeanae” by conventional ompA PCR and sequence analysis, all were positive for “Ca. R. andeanae”, and negative for R. parkeri using the real-time PCR assays for these rickettsiae. Of 10 Gulf Coast tick extracts from which R. parkeri had been confirmed previously byompA PCR and sequence analysis, all were negative for “Ca. R. andeanae” and positive for R. parkeri by the real-time assays.
DISCUSSION
In this investigation, we identified infections with “Ca. R. andeanae” in 62% of Gulf Coast ticks sampled from multiple locations throughout Kansas and Oklahoma during 2011–2014. Surprisingly, we found no evidence of infection with R. parkeri, despite evaluating more than 200 ticks collected from 18 counties in these states. These preliminary data suggest that “Ca. R. andeanae” is well-adapted to survival in populations of A. maculatum in Kansas and Oklahoma and that its ubiquity in Gulf Coast ticks in this region may effectively exclude R. parkeri from their shared arthropod host. From a public health perspective, this situation could diminish markedly or preclude entirely the occurrence of R. parkeri rickettsiosis in this region of the United States. It remains unknown whether “Ca. R. andeanae” elicits infection or disease in humans or animals. It has been suggested that other putative rickettsial symbionts, including Rickettsia amblyommii and Rickettsia montanensis cause abortive, transient, or subclinical infections in humans, dogs, and other animals (Apperson et al., 2008, Zanetti et al., 2008, McQuiston et al., 2012, Grasperge et al., 2014 and Barrett et al., 2014).
To our knowledge, R. parkeri has never been reported from adult A. maculatum specimens collected in Kansas or Oklahoma (Jiang et al., 2012 and Barrett et al., 2014; data herein). There is a single description of R. parkeri detected in an engorged nymph removed from a cotton rat (Sigmodon hispidus) in Pittsburgh County, Oklahoma (Sumner et al., 2007). Inasmuch as the sampling methods were designed only to survey for R. parkeri or “Ca. R. andeanae” at a point in time, the absence of R. parkeri among any adult Gulf Coast tick specimen from any location in these states is unexpected; in contrast, consistently high infection rates with R. parkeri have been documented in adult A. maculatum from at least 7 other U.S. states (Paddock and Goddard, 2015). For counties represented by larger sample sizes, such as Payne County in Oklahoma and Geary County in Kansas, specimens were collected from several widely separated locations over multiple points in time. Indeed, 77 tick specimens from Payne County were collected from multiple, widely separated sites over 3 years.
During the first half of the 20th century, the U.S. range of A. maculatum was defined as a relatively narrow band extending approximately 150 miles inland along the Gulf Coast and eastern seaboard, from Texas to South Carolina. Established populations of A. maculatum were subsequently identified in Oklahoma and Kansas during the 1970s (Semtner and Hair, 1973 and Goddard and Norment, 1983). It has been hypothesized that Gulf Coast ticks were introduced to these landscapes by tick-infested cattle transported by ranchers from the Gulf Coast for forage-rich pasturage of the upland prairies, or by migrating birds that follow the Central flyway, which extends through northeastern Kansas to the Gulf Coast of Texas (Ketchum et al., 2009). Although coastal and inland populations of A. maculatum are reproductively compatible (Ketchum et al., 2006), these populations differ in genetic haplotypes and seasonal phenology (Ketchum et al., 2009 and Teel et al., 2010) and, as suggested by this study, their predominant rickettsial associates. Our findings are also in agreement with a previous study which identified 2 distinct mitochondrial 12S rDNA haplotypes among Kansas and Oklahoma populations of A. maculatum (Ketchum et al., 2009).
Several species of birds that migrate from wintering areas in Central and South America to breeding areas in the United States using the Central and Mississippi flyways are infected with Rickettsia sp. Argentina (Mukherjee et al., 2013), which is now recognized as “Ca. R. andeanae” (Paddock et al., 2010). Importantly, many of these passerine species, including the indigo bunting (Passerina cyanea), the painted bunting (Passerina ciris), the common yellowthroat (Geothlypis trichas), the rose-breasted grosbeak (Pheucticus ludovicianus) and the wood thrush (Hylocichla mustelina) also serve as hosts for immature A. maculatum ticks (Teel et al., 2010 and Florin et al., 2014). In this context, it is possible that migratory birds deposit large numbers of “Ca. R. andeanae”-infected A. maculatum ticks across upland prairie habitats of Oklahoma and Kansas. It is also intriguing to speculate that specimens of A. maculatum from Kansas and Oklahoma represent ancestral remnants of larger Gulf Coast tick populations that existed throughout the expansive grasslands of the Great Plains prior to European settlement of the United States (Teel et al., 2010). In this context, the remarkably high prevalence of “Ca. R. andeanae” observed among these inland populations could reflect an ancient association, rather than a recent introduction of ticks infected predominantly or exclusively with “Ca. R. andeanae”.
The high rate of infection of A. maculatum with “Ca. R. andeanae” suggests its close association with Gulf Coast ticks as a facultative or secondary symbiont (Perlman et al., 2006). Similarly high rates of infection with “Ca. R. andeanae” (from 64–69%) have been reported in specimens of Amblyomma parvum collected in Argentina and Brazil (Pacheco et al., 2007 and Nieri-Bastos et al., 2014). These percentages are also consistent with the frequency of R. amblyommii, a vertically-transmitted rickettsial symbiont (Ponnusamy et al., 2014) detected in 37–57% of Amblyomma americanum ticks collected in Florida (Mixson et al., 2006 and Sayler et al., 2014), 45–60% in Georgia (Mixson et al., 2006 and Clay et al., 2008), 60–65% in Kentucky (Clay et al., 2008 and Jiang et al., 2010), 66–71% in Maryland (Jiang et al., 2010 and Zhang et al., 2012), 55–60% in North Carolina (Mixson et al., 2006, Clay et al., 2008 and Smith et al., 2010) and 70–82% in Virginia (Jiang et al., 2010 and Nadolny et al., 2014).
The importance of “Ca. R. andeanae” to the epidemiology of tick-borne rickettsioses in the Americas remains to be determined. Cultivation of “Ca. R. andeanae” from naturally infected embryonic cells of A. maculatum implies that “Ca. R. andeanae” is vertically transmitted in Gulf Coast ticks (Ferrari et al., 2013). Multiple studies suggest that hard ticks cannot maintain simultaneously separate Rickettsia species by vertical transmission, as demonstrated by the exclusion of transovarial transmission of Rickettsia rickettsiiby Rickettsia peacockii in Dermacentor andersoni (Burgdorfer et al., 1981), Rickettsia rhipicephali by R. montanensis in Dermacentor variabilis (Macaluso et al., 2002), and R. rickettsii by Rickettsia bellii inAmblyomma dubitatum (Sakai et al., 2014). The process whereby primary infection by one Rickettsia species excludes ovarian infection by another species has been termed rickettsial interference and this microbiological interaction can profoundly affect the distribution of tick-borne diseases. In 1981, Burgdorfer and colleagues identified a nonvirulent Rickettsia species in as many as 80% of D. andersoni ticks collected from the slopes of the Sapphire Mountains on the eastern side of the Bitterroot Valley (Burgdorfer et al., 1981). This Rickettsia species, initially designated as the East Side agent and later named R. peacockii, was detected at considerably lower frequencies (8–16%) on the western side of the valley. Importantly, historical data revealed that nearly all of the tick-derived isolates of R. rickettsii, and the great majority of human cases of RMSF, originated from the western slopes of the Bitterroot Valley (Niebylski et al., 1997). On a larger geographical scale, rickettsial interference between “Ca. R. andeanae” and R. parkeri is suggested by the observations in our study, and by surveys from other areas of the United States that demonstrate infrequency of “Ca. R. andeanae” among populations of A. maculatum where R. parkeri infections are common (Table 2). The relative distributions of these Rickettsia species among populations of A. maculatum from the Great Plains versus A. maculatum from southern coastal states may explain the disparate distribution of R. parkeri rickettsiosis between these two regions.
Table 2.
Molecular evaluation of adult Gulf Coast ticks (Amblyomma maculatum) in the United States for infection with “Candidatus Rickettsia andeanae” and Rickettsia parkeri, 2000–2014.
| State(s) | Year(s) | Number of ticks evaluated | Number (%) positive for | Reference | ||
|---|---|---|---|---|---|---|
| “Ca. R. andeanae” | R. parkeri | both Rickettsia spp. | ||||
| Florida | 2005–2007 | 128 | 2 (2) | 28 (22) | 0 | Paddock et al. 2010 |
| Mississippi | 2007 | 70 | 3 (4) | 27 (39) | 0 | Paddock et al. 2010 |
| 2008–2010 | 698 | 22 (3) | 118 (17) | 12 (2) | Ferrari et al. 2012 | |
| North Carolina | 2009–2010 | 234 | 9 (4) | 68 (29) | 1 (0.4) | Varela-Stokes et al. 2011 |
| Virginia | 2010–2012 | 293 | 1 (0.3) | 154 (52) | 0 | Naldony et al. 2013 |
| Kentucky | 2000–2009 | 10 | 0 | 3 (30) | 0 | Jiang et al. 2012 |
| Kentucky and Tennessee | 2012 | 105 | 0 | 15 (14) | 0 | Pagac et al. 2014 |
| Delaware | 2012–2013 | 26 | 0 | 2 (8) | 0 | Florin et al. 2013, Florin et al. 2014 |
| Kansas | 2000–2012 | 5 | 4 (80) | 0 | 0 |
Jiang et al. 2012 E.Y. Stromdahl, pers. comm |
| 2012–2013 | 94 | 44 (47) | 0 | 0 | Data herein | |
| Oklahoma | 2000–2009 | 1 | 1 (100) | 0 | 0 | Jiang et al. 2012 |
| 2011–2014 | 122 | 89 (73) | 0 | 0 | Data herein | |
Virulent spotted fever group Rickettsia species may adversely affect the survival and fecundity of the tick host (Burgdorfer and Brinton, 1975, Niebylski et al., 1999, Labruna et al., 2011 and Soares et al., 2012). Rickettsia parkeri negatively impacts the survival of infected Amblyomma triste nymphs (Nieri-Bastros et al., 2013). At present there are no data regarding the impact of R. parkeri or “Ca. R. andeanae” on the fitness of A. maculatum; however, it is recognized that vertical transmission places selective pressure on pathogens for low virulence (Fine, 1975 and Yamamura, 1993). Models that incorporate the epidemiology of vertical and horizontal transmission and host demography demonstrate that increasing levels of vertical transmission favor the evolution of lower virulence in pathogens, i.e., when strains of high vertical transmission and low virulence organisms exist in a host, these strains generally out-compete other strains (Lipsitch et al., 1996). Further study of the microbiological interactions between R. parkeri and “Ca. R. andeanae” that occur within A. maculatum could provide valuable insights into the natural history of spotted fever group rickettsioses in the United States.
Our study was limited by small sample sizes from several of the collection sites, with <5 specimens from 5 (56%) of the surveyed counties in Kansas, and 7 (78%) of the locations in Oklahoma (Fig. 1). This investigation focused on adult specimens and it is possible that directed sampling of immature Gulf Coast ticks in Kansas and Oklahoma could produce evidence of R. parkeri infecting A. maculatum in these states. It is also possible that sampling of A. maculatum ticks from counties not represented in our survey might reveal infections with R. parkeri, particularly in eastern Oklahoma, as R. parkeri-infected Gulf Coast ticks have been described recently from the adjacent state of Arkansas (Trout et al., 2010). Finally, it is possible that a genetic variant of R. parkeri exists among Kansas and Oklahoma tick populations that is not detected by the primers or probe used in the real-time assay; nonetheless, this assay has correctly identified strains of R. parkeri in tick and human hosts from widely separated locations in the United States, including Arizona, Florida, Mississippi, and North Carolina, and in multiple provinces of Argentina (Romer et al., 2014), suggesting strong conservation of the molecular target. Despite these limitations, these results demonstrate a need for future investigations that explore the dynamics among various spotted fever group Rickettsia species within their tick hosts, and how these interactions affect the epidemiology of rickettsioses in human and animal hosts.
Acknowledgments
We thank Anne Barrett, Jeff Gruntmeir, Eileen Johnson, Jaclyn Martin, Anna Noden, Ethan Noden, and Donald Mock for assistance with the collection of ticks evaluated in this study. Financial support for BHN came from the Oklahoma Agricultural Experiment Station (OKL-02909). The findings and conclusions in this article are those of the authors and do not necessarily reflect the views of the U.S. Department of Health and Human Services.
Footnotes
The findings and conclusions in this article are those of the authors and do not necessarily reflect the views of the U.S. Department of Health and Human Services.
References
- Abaraca K, Lopez J, Acosta-Jamett G, Lepe P, Soares JF, Labruna MB. A third Amblyomma species and the first tick-borne rickettsiosis in Chile. J Med Entomol. 2012;49:219–222. doi: 10.1603/me11147. [DOI] [PubMed] [Google Scholar]
- Apperson CS, Engber B, Nicholson WL, Mead DG, Engel J, Yabsley MJ, Dail K, Johnson J, Watson DW. Tick-borne diseases in North Carolina: is “Rickettsia amblyommii” a possible cause of rickettsiosis reported as Rocky Mountain spotted fever? Vector Borne Zoonotic Dis. 2008;8:597–606. doi: 10.1089/vbz.2007.0271. [DOI] [PubMed] [Google Scholar]
- Barrett A, Little SE, Shaw E. “Rickettsia amblyommii” and R. montanensis infection in dogs following natural exposure to ticks. Vector Borne Zoonotic Dis. 2014;14:20–25. doi: 10.1089/vbz.2013.1325. [DOI] [PubMed] [Google Scholar]
- Blair PJ, Jiang J, Schoeler GB, Moron C, Anaya E, Cespedes M, Cruz C, Felices V, Guevara C, Mendoza L, Villaseca P, Sumner JW, Richards AL, Olson JG. Characterization of spotted fever group rickettsiae in flea and tick specimens from northern Peru. J Clin Microbiol. 2004;42:4961–4967. doi: 10.1128/JCM.42.11.4961-4967.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanton LS, Mendell NL, Walker DH, Bouyer DH. “Rickettsia amblyommii” induces cross protection against lethal Rocky Mountain spotted fever in a guinea pig model. Vector Borne Zoonotic Dis. 2014;14:557–562. doi: 10.1089/vbz.2014.1575. [DOI] [PubMed] [Google Scholar]
- Burgdorfer W, Brinton LP. Mechanisms of transovarial infection of spotted fever rickettsiae in ticks. Ann N Y Acad Sci. 1975;266:61–72. doi: 10.1111/j.1749-6632.1975.tb35088.x. [DOI] [PubMed] [Google Scholar]
- Burgdorfer W, Hayes SF, Mavros AJ. Nonpathogenic rickettsiae in Dermacentor andersoni: a limiting factor for the distribution of Rickettsia rickettsii. In: Burgdorfer W, Anacker RL, editors. Rickettsiae and rickettsial diseases. Academic Press; New York: 1981. pp. 585–594. [Google Scholar]
- Clay K, Klyachko M, Grindle N, Civeitello D, Oleske D, Fuqua C. Microbial communities and interaticons in the lone star tick, Amblyomma americanum. Molec Ecol. 2008;17:4371–4381. doi: 10.1111/j.1365-294x.2008.03914.x. [DOI] [PubMed] [Google Scholar]
- Ferrari FA, Goddard J, Paddock CD, Varela-Stokes AS. Rickettsia parkeri and Candidatus Rickettsia andeanae in Gulf Coast ticks, Mississippi, USA. Emerg Infect Dis. 2012;18:1705–7. doi: 10.3201/eid1810.120250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari FA, Goddard J, Moraru GM, Smith WE, Varela-Stokes A. Isolation of “Candidatus Rickettsia andeanae” in embryonic cells of naturally infected Amblyomma maculatum. J Med Entomol. 2013;50:1118–1125. doi: 10.1603/me13010. [DOI] [PubMed] [Google Scholar]
- Ferrari FAG, Goddard J, Paddock CD, Varela-Stokes A. Ultrastructure of presumed “Candidatus Rickettsia andeanae” in the Gulf Coast tick, Amblyomma maculatum (Acari: Ixodidae) J Med Entomol. 2014 doi: 10.1603/ME14132. in press. [DOI] [PubMed] [Google Scholar]
- Fine PEM. Vectors and vertical transmission: an epidemiologic perspective. Ann N Y Acad Sci. 1975;266:173–194. doi: 10.1111/j.1749-6632.1975.tb35099.x. [DOI] [PubMed] [Google Scholar]
- Fitak RR, Kelly DJ, Daniels MK, Jiang J, Richards AL, Fuerst PA. The prevalence of rickettsial and ehrlichial organisms in Amblyomma americanum ticks collected from Ohio and surrounding areas between 2000 and 2010. Ticks Tick-Borne Dis. 2014;5:797–800. doi: 10.1016/j.ttbdis.2014.06.005. [DOI] [PubMed] [Google Scholar]
- Florin D, Jiang J, Robbins RG, Richards AL. Infection of the Gulf Coast tick, Amblyomma maculatum with Rickettsia parkeri: first report from the state of Delaware. Sys Appl Acarol. 2013;18:27–29. [Google Scholar]
- Florin DA, Brinkerhoff RJ, Gaff H, Jiang J, Robbins RG, Eickmeyer W, Butler J, Nielsen D, Wright C, White E, Gimpel ME, Richards AL. Additional collections of the Gulf Coast tick, Amblyomma maculatum (Acari: Ixodidae) from the State of Delaware, the first reported field collections of adult specimens from the State of Maryland, and data regarding the tick species from surveillance of migratory song birds in Maryland. Syst Appl Acarol. 2014;19:257–262. [Google Scholar]
- Fornadel CM, Zhang X, Smith JD, Paddock CD, Arias JR, Norris DE. High rates of Rickettsia parkeri infection in Gulf Coast ticks and identification of Candidatus Rickettsia andeanae from Fairfax County, Virginia. Vector Borne Zoonotic Dis. 2011;12:1535–1538. doi: 10.1089/vbz.2011.0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasperge BJ, Morgan TW, Paddock CD, Peterson KE, Macaluso KR. Feeding by Amblyomma maculatum (Acari: Ixodidae) enhances Rickettsia parkeri (Rickettsiales: Rickettsiaceae) infection in the skin. J Med Entomol. 2014;51:855–863. doi: 10.1603/me13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Yarina T, Miller MK, Stromdahl EY, Richards AL. Molecular detection of Rickettsia amblyommii in Amblyomma americanum parasitizing humans. Vector Borne Zoonotic Dis. 2010;10:329–340. doi: 10.1089/vbz.2009.0061. [DOI] [PubMed] [Google Scholar]
- Jiang J, Stromdahl EY, Richards AL. Detection of Rickettsia parkeri and Candidatus Rickettsia andeanae in Amblyomma maculatum Gulf Coast ticks collected from humans in the United States. Vector Borne Zoonotic Dis. 2012;12:175–182. doi: 10.1089/vbz.2011.0614. [DOI] [PubMed] [Google Scholar]
- Ketchum HR, Teel PD, Strey OF, Longnecker MT. Mating success of two geographically distinct populations of Gulf Coast ticks, Amblyomma maculatum Koch. Vet Parasitol. 2006;140:143–147. doi: 10.1016/j.vetpar.2006.03.020. [DOI] [PubMed] [Google Scholar]
- Ketchum HR, Teel PD, Coates CJ, Strey OF, Longnecker MT. Genetic variation in 12S and 16S mitochondrial rDNA genes our four geographically isolated populations of Gulf Coast ticks (Acari: Ixodidae) J Med Entomol. 2009;46:482–489. doi: 10.1603/033.046.0311. [DOI] [PubMed] [Google Scholar]
- Labruna MB, Ogrzewalska M, Soares JF, Martins TF, Soares Jmorares-Filho HS, Nieri-Bastos FA, Palmeida A, Pinter A. Experimental infection of Amblyomma aureolatum ticks with Rickettsia rickettsii. Emerg Infect Dis. 2011;17:829–834. doi: 10.3201/eid1705.101524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsitch M, Siller S, Nowak MA. The evolution of virulence in pathogens with vertical and horizontal transmission. Evolution. 1996;50:1729–1741. doi: 10.1111/j.1558-5646.1996.tb03560.x. [DOI] [PubMed] [Google Scholar]
- Luce-Fedrow A, Wright CL, Gaff HD, Sonenshine DE, Hynes WL, Richards AL. In vitro propagation of Candidatus Rickettsia andeanae isolated from Amblyomma maculatum. FEMS Immunol Med Microbiol. 2012;64:74–81. doi: 10.1111/j.1574-695X.2011.00905.x. [DOI] [PubMed] [Google Scholar]
- Macaluso KR, Sonenshine DE, Ceraul SM, Azad AF. Rickettsial infection in Dermacentor variabilis (Acari: Ixodidae) inhibits transovarial transmission of a second Rickettsia. J Med Entomol. 2002;39:809–813. doi: 10.1603/0022-2585-39.6.809. [DOI] [PubMed] [Google Scholar]
- McQuiston JH, Zemtsova G, Perniciaro J, Hutson M, Singleton J, Nicholson WL, Levin ML. Afebrile spotted fever group Rickettsia infection after a bite from a Dermacentor variabilis tick infected with Rickettsia montanensis. Vector Borne Zoonotic Dis. 2012;12:1059–1061. doi: 10.1089/vbz.2012.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mixson TR, Campbell SR, Gill JS, Ginsberg HS, Reichard MV, Schultze TL, Dasch GA. Prevalence of Ehrlichia, Borrelia, and rickettsial agents in Amblyomma americanum (Acari: Ixodidae) collected from nine states. J Med Entomol. 2006;43:1261–1268. doi: 10.1603/0022-2585(2006)43[1261:poebar]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Moncayo AC, Cohen SB, Fritzen CM, Huang E, Yabsley MJ, Freye JD, Dunlap BG, Huang J, Jones DGTF, Dunn JR. Absence of Rickettsia rickettsii and occurrence of other spotted fever group rickettsiae in ticks from Tennessee. Am J Trop Med Hyg. 2010;83:653–657. doi: 10.4269/ajtmh.2010.09-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee N, Beati L, Sellers M, Burton L, Adamson SW, Robbins RG, Moore F, Karim S. Importation of exotic ticks and tick-borne spotted fever group rickettsiae into the United States by migrating songbirds. Ticks Tick-Borne Dis. 2013;5:127–134. doi: 10.1016/j.ttbdis.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadolny RM, Wright CL, Sonenshine DE, Hynes WL, Gaff HD. Ticks and spotted fever group rickettsiae of southeastern Virginia. Ticks Tick-Borne Dis. 2014;5:53–57. doi: 10.1016/j.ttbdis.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niebylski ML, Peacock MG, Schwan TG. Lethal effect of Rickettsia rickettsii on its tick vector (Dermacentor andersoni) Appl Environ Microbiol. 1999;65:773–778. doi: 10.1128/aem.65.2.773-778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieri-Bastros FA, Szabó MPJ, Pacheco RC, Soares JF, Soares HS, Moraes-Filho J, Dias RA, Labruna MB. Comparative evaluation of infected and noninfected Amblyomma triste ticks with Rickettsia parkeri, the agent of an emerging rickettsiosis in the New World. Biomed Res Internat. 2013:402737. doi: 10.1155/2013/402737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieri-Bastos FA, Lopes MG, Duarte Cançado PH, Razera Rossa GA, Horácio Faccini JL, Gennari SM, Labruna MB. Candidatus Rickettsiae andeanae, a spotted fever group agent infecting Amblyomma parvum ticks in two Brazilian biomes. Mem Inst Oswaldo Cruz, Rio de Janeiro. 2014;10:1–3. doi: 10.1590/0074-0276140283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco RC, Moraes-Filho J, Nava S, Brandão PE, Richtzenhain LJ, Labruna MB. Detection of a novel spotted fever group rickettsia in Amblyomma parvum ticks from Argentina. Exp Appl Acarol. 2007;43:63–71. doi: 10.1007/s10493-007-9099-5. [DOI] [PubMed] [Google Scholar]
- Paddock CD, Fournier PE, Sumner JW, Goddard J, Elshenawy Y, Metcalfe MG, Loftis AD, Varela-Stokes A. Isolation of Rickettsia parkeri and identification of a novel spotted fever group Rickettsia sp. from Gulf Coast ticks (Amblyomma maculatum) in the United States. Appl Environ Microbiol. 2010;76:2689–2696. doi: 10.1128/AEM.02737-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddock CD, Goddard J. The evolving medical and veterinary importance of the Gulf Coast tick, Amblyomma maculatum Koch (Acari: Ixodidae) J Med Entomol. 2015 doi: 10.1093/jme/tju022. in press. [DOI] [PubMed] [Google Scholar]
- Ponnusamy L, Gonzalez A, Van Treuren W, Weiss S, Parobek CM, Juliano JJ, Knight R, Roe RM, Apperson CS, Meshnick SR. Diversity of Rickettsiales in the microbiome of the lone star tick, Amblyomma americanum. Appl Environ Microbiol. 2014;80:354–359. doi: 10.1128/AEM.02987-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai RK, Costa FB, Ueno TEH, Ramirez DG, Soares JF, Fonseca AH, Labruna MB, Barros-Battesti DM. Experimental infection with Rickettsia rickettsii in an Amblyomma dubitatum tick colony, naturally infected by Rickettsia bellii. Ticks Tick-Borne Dis. 2014;5:917–923. doi: 10.1016/j.ttbdis.2014.07.003. [DOI] [PubMed] [Google Scholar]
- Sayler KA, Wamsley HL, Pate M, Barbet AF, Alleman AR. Cultivation of Rickettsia amblyommii in tick cells, prevalence in Florida lone star ticks (Amblyomma americanum) Parasites Vectors. 2014;7:270. doi: 10.1186/1756-3305-7-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semtner PJ, Hair JA. Distribution, seasonal abundance, and hosts of the Gulf Coast tick in Oklahoma. Ann Entomol Soc Am. 1973;66:1264–1268. [Google Scholar]
- Smith MP, Ponnusamy L, Jiang J, Ayyash LA, Richards AL, Apperson CS. Bacterial pathogens in ixodid ticks in a Piedmont county in North Carolina: prevalence of rickettsial organisms. Vector Borne Zoonotic Dis. 2010;10:939–952. doi: 10.1089/vbz.2009.0178. [DOI] [PubMed] [Google Scholar]
- Soares JF, Soares HS, Barbieri AM, Labruna MB. Experimental infection of the tick Amblyomma cajennense, Cayenne tick, with Rickettsia rickettsii, the agent of Rocky Mountain spotted fever. Med Vet Entomol. 2012;26:139–151. doi: 10.1111/j.1365-2915.2011.00982.x. [DOI] [PubMed] [Google Scholar]
- Stenos J, Graves SR, Unsworth NB. A highly sensitive and specific real-time PCR assay for the detection of spotted fever and typhus group Rickettsiae. Am J Trop Med Hyg. 2005;73:1083–1085. [PubMed] [Google Scholar]
- Sumner JW, Durden LA, Goddard J, Stromdahl EY, Clark KL, Reeves WK, Paddock CD. Gulf Coast ticks (Amblyomma maculatum) and Rickettsia parkeri, United States. Emerg Infect Dis. 2007;13:751–753. doi: 10.3201/eid1305.061468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teel PD, Ketchum HR, Mock DE, Wright RE, Strey OF. The Gulf Coast tick: a review of the life history, ecology, distribution, and emergence as an arthropod of medical and veterinary importance. J Med Entomol. 2010;47:707–722. doi: 10.1603/me10029. [DOI] [PubMed] [Google Scholar]
- Varela-Stokes AS, Paddock CD, Engber B, Toliver M. Rickettsia parkeri in Amblyomma maculatum ticks, North Carolina, USA, 2009–2010. Emerg Infect Dis. 2011;17:2350–2353. doi: 10.3201/eid1712.110789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamura N. Vertical transmission and the evolution of mutualism from parasitism. Theor Popul Biol. 1993;44:95–109. [Google Scholar]
- Zanetti AS, Pornwiroon W, Kearny MT, Macaluso KR. Characterization of rickettsial infection in Amblyomma americanum (Acari: Ixodidae) by quantitative real-time polymerase chain reaction. J Med Entomol. 2008;45:267–275. doi: 10.1603/0022-2585(2008)45[267:coriia]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Zhang X, Ren X, Norris DE, Rasgon JL. Distribution and infection frequency of “Candidatus Rickettsia amblyommii” in Maryland populations of the lone star tick (Amblyomma americanum) and culture in an Anopheles gambiae mosquito cell line. Ticks Tick-Borne Dis. 2012;3:38–42. doi: 10.1016/j.ttbdis.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]