Abstract
Background
Elevated levels of inflammatory biomarkers are associated with increased cardiovascular morbidity and mortality.
Objective
We sought to determine whether elevated concentrations of high-sensitivity troponin T (hs-TnT) and high-sensitivity C-reactive protein (hs-CRP) predict progression of coronary artery disease (CAD) as determined by coronary CT angiography (coronary CTA).
Methods
Patients presenting to the emergency department with acute chest pain who initially showed no evidence of an acute coronary syndrome underwent baseline and follow-up coronary CTA (median follow-up, 23.9 months) using identical acquisition and reconstruction parameters. Coronary CTA data of each major coronary artery were co-registered. Cross-sections were assessed for the presence of calcified and noncalcified plaques. Progression of atherosclerotic plaque and change of plaque composition from noncalcified to calcified plaque was evaluated and correlated to levels of hs-TnT and hs-CRP at the time of the baseline CT.
Results
Fifty-four patients (mean age, 54.1 years; 59% male) were included, and 6775 cross-sections were compared. CAD was detected in 12.2 ± 21.2 cross-sections per patient at baseline. Prevalence of calcified plaque increased by 1.5 ± 2.4 slices per patient (P < .0001) over the follow-up period. On average, 1.6 ± 3.6 slices with new noncalcified plaque were found per patient (P < .0001) and 0.7 ± 1.7 slices with pre-existing noncalcified plaque had progressed to calcified plaque (P < .0001). After multivariate adjustment, change of overall CAD burden was predicted by baseline hs-TnT and hs-CRP (r = 0.29; P = .039 and r = 0.40; P = .004). Change of plaque composition was associated with baseline hs-TnT (r = 0.29; P = .03).
Conclusion
Concentrations of hs-TnT and hs-CRP are weakly associated with a significant increase in CAD burden and change in plaque composition over 24 months independent of baseline risk factors.
Keywords: Coronary artery disease, Coronary atherosclerotic plaque, Plaque progression, Cardiac biomarker, Coronary CT angiography
1. Introduction
Major adverse cardiovascular events are often the initial manifestation of coronary artery disease (CAD).1 In recent years, several studies have demonstrated a link between circulating biomarkers of myocardial injury and inflammation and major adverse cardiovascular events.2,3 With the advent of coronary CT angiography (CCTA), it has been established that circulating biomarkers such as high-sensitivity troponin T (hs-TnT) and high-sensitivity C-reactive protein (hs-CRP) are associated with both the extent and composition of coronary atherosclerotic plaques as determined by CT.4 Moreover, it has been suggested that the release of such biomarkers is associated with a clinically silent rupture of atherosclerotic plaques.4,5 On the other hand, plaque rupture is believed to be the one factor determining the progression of coronary atherosclerosis.6 Thus, the purpose of this study was to assess whether circulating biomarkers, hs-TnT and hs-CRP, predict the progression of CAD as determined by CCTA in patients presenting with acute chest pain and whether this association is independent of baseline CAD and traditional risk factors.
2. Material and methods
The patient population of this study is a subset of patients prospectively enrolled into the Rule Out Myocardial Infarction using Computer Assisted Tomography (ROMICAT) I trial. The ROMICAT I trial is a prospective observational cohort study of patients presenting to the emergency department with chest pain but with normal conventional troponin and without ischemic changes on the initial electrocardiography who underwent CCTA before hospital admission. Detailed design and inclusion and exclusion criteria have been published elsewhere.7 The present study included consecutive patients who agreed to participate in a 2-year follow-up CCTA study during their index visit. Patients were contacted by phone, and the return visit included a clinical interview. Subjects with a creatinine clearance <50 mL/min or atrial fibrillation were excluded. Additionally, we excluded patients who had undergone bypass surgery from follow-up imaging. The study complied with local and federal regulations and was approved by the local institutional review board. All subjects provided written informed consent. All patients were examined using 64-slice scanner technology. Details of the CCTA acquisition and image analysis have been published elsewhere.8
Coronary CT data sets were anonymized. Curved multi-planar reformations of the left main coronary artery and the proximal 40 mm of the 3 major coronary arteries were generated as 1-mm thick cross-sections in 1-mm increments without gaps or overlaps. For the left circumflex artery, the larger of the actual circumflex and the obtuse marginal artery were used.
The data sets were co-registered using the first cross-section distal to the origin of the vessel as reference and advancing in 1-mm steps along the centerline of the vessel. Only cross-sections deemed evaluable at both baseline and follow-up were included in the analysis.
Atherosclerotic plaque burden and composition were assessed in identical sections and with identical window settings at baseline and follow-up (Fig. 1) using a semi-quantitative cross-section–based score. Calcified plaque was defined as any structure within the coronary vessel wall demonstrating a CT attenuation of >130 Hounsfield units (HU) that could be visually distinguished from the vessel lumen. The presence of any calcification within the corresponding cross-section rendered the entire cross-section calcified. Noncalcified plaque was defined as a structure in continuity with the coronary artery wall and a CT density greater than that of the surrounding tissue but lesser than that of the contrast-enhanced lumen and without any detectable calcification.11 This semiquantitative score has been shown to correlate well with the absolute plaque volumes determined on CCTA.8
Fig. 1.

Assessment of plaque burden and plaque composition. Multiplanar reformations (MPRs) derived from the baseline scan (A1 and B1) and the follow-up scan (A2 and B2) were co-registered and the presence of plaque and plaque morphology was evaluated. Slice A shows the development of a new noncalcified plaque in a slice without plaque at baseline. Slice B shows the change of plaque morphology from noncalcified to calcified plaque.
Based on the semiquantitative score, we assessed the change of plaque burden and plaque morphology in the co-registered data sets and defined 4 categories of plaque progression. Change of CAD burden was defined as any plaque (noncalcified or calcified) observed in slices without plaque at baseline. Incident noncalcified plaque was defined as new noncalcified plaque in slices without plaque at baseline, and incident calcified plaque was defined as new calcified plaque in slices without calcifications at baseline. Additionally, we assessed the change of plaque morphology from noncalcified plaque to calcified plaque.
Blood samples were collected during the index visit within 1 hour before the initial CT examination at a mean of 4.2 hours after the initial presentation. The samples were stored at −70°C until analysis. We decided to measure hs-TnT and hs-CRP as these have been shown to be involved in the pathophysiology and/or outcome of coronary atherosclerosis.3,5,12,13 All measurements were performed in an independent laboratory (Department of Cardiology, University of Ulm, Germany). The laboratory did not have any information on clinical or CT findings. The concentration of hs-CRP was determined nephelometrically on a BN II analyzer (Dade-Behring, Marburg, Germany) by latex-enhanced immunonephelometry (detection limit 0.16 mg/L). hs-TnT was assayed using a precommercial method (Roche Diagnostics, Penzberg, Germany) on an Elecsys 2010 platform (detection limit 3 ng/L). Interassay coefficients of variation were <5% for hs-CRP and < 6% at 24 ng/L for hs-TnT.
At baseline, information on the cardiovascular risk profile was collected for all patients during the index visit via interview or actual measurements. Measurements of blood pressure, serum lipids, and fasting blood glucose were obtained before the CT examination. History of CAD was defined as previous symptomatic CAD treated medically or with coronary revascularization (stent graft or bypass surgery). Family history of CAD was defined as the occurrence of myocardial infarction in a first-degree relative aged >55 years if a man or >65 years if a woman. Smoking was defined by current or previous daily cigarette use. The Framingham Risk Score (FRS) was calculated for each patient using an established regression model.14 Hypertension was defined by a systolic blood pressure >140 mm Hg or diastolic pressure >90 mm Hg or current antihypertensive medication. Hyperlipidemia was defined by a total cholesterol level >200 mg/dL or treatment with lipid-lowering medication and diabetes by fasting blood glucose >126 mg/dL or treatment with hypoglycemic medication. Left ventricular (LV) mass was calculated from the baseline CCTA scan.
Continuous variables were summarized as mean ± standard deviation and categorical variables by percentage unless otherwise specified. To determine differences between the entire cohort and the patients included in this subanalysis, we performed a 2-sided t test for continuous and the Fisher exact test for categorical variables. Interobserver and intraobserver variability for plaque detection and differentiation was assessed using the Kappa statistic. Change of plaque burden was assessed using the Wilcoxon matched pairs test. Correlations between blood biomarkers and change of plaque burden were determined using Spearman correlation coefficient. In a baseline model, correlations were adjusted for differences in follow-up time, age, and gender. Additional adjustment for the extent of baseline plaque, LV myocardial mass as determined by CT, FRS (plus body mass index [BMI]) at baseline as well as for acute coronary syndrome (ACS) during index hospitalization was performed using separated models. All statistical analyses were performed with SAS software (version 9.2; SAS Institute Inc, Cary, NC). A probability level < .05 was considered as statistically significant.
3. Results
Of 368 patients in the ROMICAT cohort, 69 patients agreed to take part in the follow-up study and underwent a follow-up CCTA. Of these, 15 patients had to be excluded because of missing biomarker data. Thus, we analyzed 54 patients (mean age, 54 ± 12 years; 59% male) who underwent follow-up CCTA after 2 years (23.9 months [interquartile range, 20.5–24.9 months]). Generally, baseline demographics, risk factors, and burden of CAD were similar between patients in the follow-up and the entire cohort. However, both baseline levels of hs-TnT and hs-CRP were different between the groups (Table 1).
Table 1.
Baseline demographics, status of coronary artery disease determined by coronary CT angiography and levels of biomarkers in the 54 patients undergoing repeat coronary CT angiography and a complete set of biomarkers vs. the remaining patients from the ROMICAT trial.
| Variables | Follow-up cohort (n=54) | Cohort without follow-up CCTA or incomplete biomarkers (n=314) | p-value |
|---|---|---|---|
| Median follow-up time (months) | 23.9 (20.5–24.9) | N/A | |
| Age (years) | 54.0 ± 12.1 | 53.6 ± 11.7 | .42 |
| men | 59% (32) | 62% (194) | .72 |
| Framingham Risk Score | 8.0 ± 7.8 | 7.0 ± 6.9 | .35 |
| Diabetes mellitus | 3 (6%) | 37 (12%) | .17 |
| Hypertension | 22 (41%) | 123 (39%) | .83 |
| Hyperlipidemia | 28 (52%) | 107 (34%) | .01 |
| Smoker | 10 (19%) | 83 (26%) | .22 |
| Fam. HxCAD | 15 (28%) | 74 (24%) | .50 |
| BMI (kg/m2) | 30.5 ± 6.8 | 28.7 ± 5.8 | .45 |
| ACS at index visit | 15% (8) | 7% (23) | .07 |
| ACS during follow-up | 0 | N/A | |
| CAD: any plaque | 61% (33) | 48% (152) | .11 |
| CAD: any stenosis | 17% (9) | 8% (25) | .07 |
| CAD: calcified plaque | 50% (27) | 45% (141) | .55 |
| hs-TnT (ng/L) | 3.46 (1.17–7.32) | 5.58 (2.82–8.69) | .03 |
| hs-CRP (mg/L) | 2.10 (0.95–4.20) | 1.29 (0.56–2.67) | .01 |
BMI, body mass index; CAD, coronary artery disease; CCTA, coronary CT angiography; hs-CRP, high-sensitivity C-reactive protein; hs-TnT, high-sensitivity troponin T; ROMICAT, Rule Out Myocardial Infarction using Computer Assisted Tomography; SD, standard deviation.
Values are presented as absolute numbers with percentages or mean SD. The Framingham Risk Score and the levels of bio markers are presented as median (interquartile range).
In the 54 patients, a total of 7177 cross-sections were co-registered between the baseline and the follow-up CCTAs. Of these, 402 were excluded because of insufficient image quality in either the baseline or the follow-up CCTA. Thus, a total of 6775 cross-sections (125.5 ± 18.8 slices per patient) were available for evaluation.
At baseline, an average of 12.2 ± 21.2 sections with CAD were detected per patient. Of these, 9.4 ± 18.0 contained calcified and 2.7 ± 5.3 noncalcified plaques.
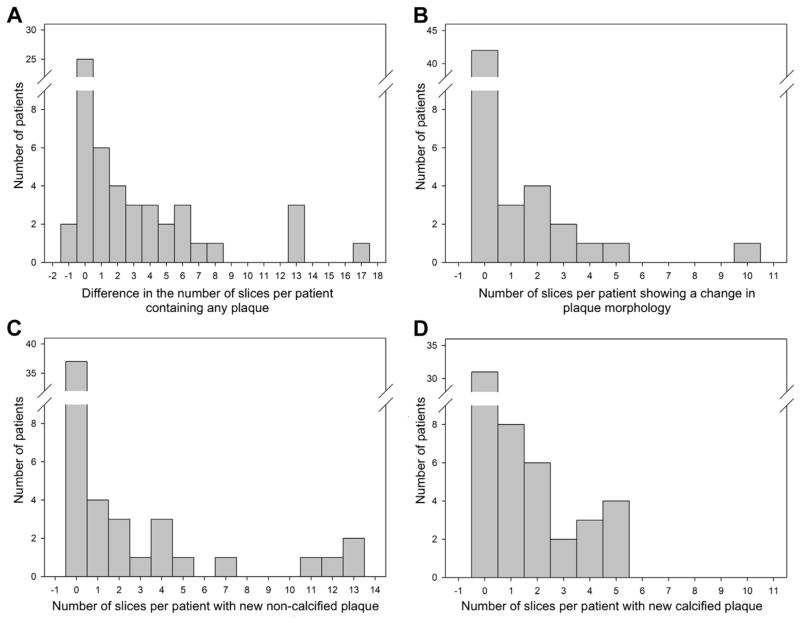
Follow-up CCTA demonstrated a significant increase in CAD burden (cross-sections with plaque per patient: 14.6 ± 22.6; P < .0001) corresponding to an increase of 2.4 ± 4.0 cross-sections per patient (P < .0001). The number of cross-sections with calcified plaque increased to 11.0 ± 19.3; P < .0001 corresponding to an increase of 1.5 ± 2.4 slices per patient (P < .001). On average, 1.6 ± 3.6 slices with new noncalcified plaque were detected per patient in the follow-up (P < .0001), and 0.7 ± 1.7 slices with noncalcified plaque per patient had progressed to calcified plaque (P < .0001). The net increase in slices containing noncalcified plaque was not significant (0.9 ± 3.8; P = .11; Fig. 2).
Fig. 2.
Histogram analysis showing the change in the number of slices per patient from the baseline to the follow-up scan exhibiting specific plaque qualities. (A) Any detectable plaque, (B) change of plaque morphology, (C) new noncalcified plaque, and (D) new calcified plaque. Note that in most patients no change in any of the 4 categories was found.
As previously reported, interobserver and intraobserver variability for the detection of calcified and noncalcified plaque was very low (κ = 0.97; 95% confidence interval, 0.96–0.98 for calcified plaque and κ = 0.73; 95% confidence interval, 0.65–0.81 for noncalcified plaque).8
We found a significant correlation between overall change of CAD burden and both hs-TnT and hs-CRP (r = 0.29; P = .039 and r = 0.40; P = .004; Table 2; Fig. 3). Adjustment for LV mass and FRS did not alter the association between hs-TnT or hs-CRP and overall change of CAD burden (Table 3). Similarly, the association of hs-CRP and overall change of CAD burden did not change significantly after additional adjustment for BMI (r = 0.41; P = .004), although hs-CRP and BMI were strongly correlated (r = 0.37; P = .007). Although the association between overall plaque progression and hs-CRP remained unchanged after adjustment for baseline CAD (r = 0.38; P = .007), the association with hs-TnT was slightly attenuated (r = 0.27; P = .06).
Table 2.
Correlation of biomarkers in the peripheral blood at baseline with change of plaque burden or morphology in the follow-up scan.
| Biomarker | Overall plaque change (n = 29/54 [54%])
|
New noncalcified plaque (n = 17/54 [31%])
|
New calcified plaque (n = 24/54 [44%])
|
Change of plaque morphology (n = 12/54 [22%])
|
||||
|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | |
| hs-TnT | 0.29 | .04 | 0.07 | .60 | 0.30 | .03 | 0.30 | .03 |
| hs-CRP | 0.40 | .004 | 0.23 | .10 | 0.14 | .32 | −0.23 | .10 |
hs-CRP, high-sensitivity C-reactive protein; hs-TnT, high-sensitivity troponin T; n, number of patients. All correlations are adjusted for age, gender and follow-up time.
Fig. 3.
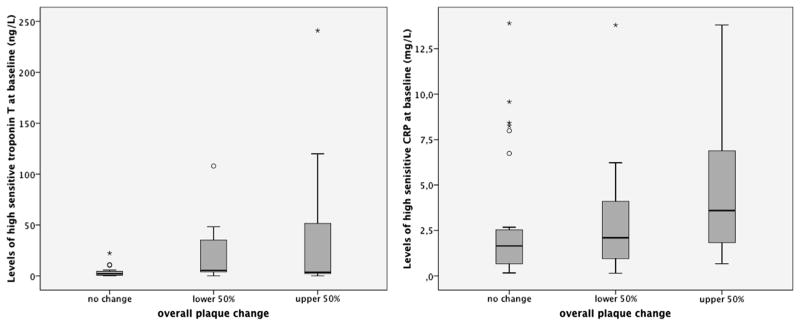
Relationship between the overall change of plaque burden from baseline to follow-up and baseline levels of high-sensitivity troponin T (left) and high-sensitivity C-reactive protein (CRP; right). The box plots refer to the patients with no change of plaque burden and the 50% of patients with the least and the greatest change of overall plaque burden.
Table 3.
Factors influencing the correlation between baseline levels of hs-TnT and hs-CRP at baseline and measures of plaque progression.
| Adjustment for | Correlation of hs-CRP with
|
Correlation of hs-TnT with
|
||||||
|---|---|---|---|---|---|---|---|---|
| Overall CAD change
|
Overall CAD change
|
New calcified plaque
|
Change of plaque morphology
|
|||||
| r | p | r | p | r | p | r | p | |
| Baseline plaque | 0.38 | .007 | 0.27 | .06 | 0.27 | .06 | 0.27 | .06 |
| LV mass | 0.37 | .008 | 0.29 | .04 | 0.31 | .04 | 0.29 | .04 |
| Baseline FRS | 0.41 | .003 | 0.30 | .04 | 0.31 | .03 | 0.34 | .02 |
| ACS at index hospitalization | 0.42 | .002 | 0.23 | .10 | 0.26 | .07 | 0.24 | .08 |
CAD, coronary artery disease; FRS, Framingham Risk Score; hs-CRP, high-sensitivity C-reactive protein; hs-TnT, high-sensitivity; LV, left ventricle.
Note that all correlations are additionally adjusted for age, gender and follow-up time.
We found a significant association between the overall incidence of calcified plaque and hs-TnT (r = 0.29; P = .034), which was maintained after adjusting for LV mass (r = 0.31; P = .042) or FRS (r = 0.31; P = .029) but was attenuated after adjusting for baseline CAD and ACS (r = 0.27; P = .055 and r = 0.26; P = .07). No association between circulating biomarkers and incident noncalcified plaque was found (Table 2). Further adjustment for baseline CAD burden, ACS, FRS, and LV mass did not alter these observations.
The progression from noncalcified plaque to calcified plaque was associated with hs-TnT (r = 0.30; P = .033), which was maintained after adjustment for LV mass and FRS (Table 3). This association, however, was attenuated after adjustment for baseline CAD (r = 0.27; P = .055) and ACS (r = 0.24; P = .08).
4. Discussion
Our study suggests that circulating levels of hs-TnT and hs-CRP are both associated with progression of CAD over time independent of other patient characteristics and follow-up time; hs-TnT was also associated with the transition from noncalcified to calcified plaque.
Cardiac troponins have been identified as markers of myocardial injury over 2 decades ago and are now routinely used in the diagnosis of myocardial infarction.15 Although standard assays allow the detection of larger quantities of troponin released from the contractile filaments of the myofibrils in gross myocardial infarction, newer more sensitive assays can detect much smaller quantities of troponin released in the early stages of myocardial infarction as well as in patients without myocardial infarction.12,16 Elevated levels of hs-TnT have been shown to have a good sensitivity and specificity for the diagnosis of ACS among low-to-intermediate risk patients presenting with chest pain.17 A recent study has also shown a positive association between the extent of CAD and levels of high-sensitivity troponin independent of traditional risk factors.5 Our study demonstrates hs-TnT and hs-CRP are not only associated with clinical outcomes but also associated with change of CAD burden over time independent of FRS and LV mass. Additional adjustment for BMI did not attenuate the correlation between hs-CRP and the change of CAD burden. Our results also confirm other studies reporting that plaque burden correlates with levels of hs-TnT.5,18 Furthermore, the concentration of hs-TnT in the peripheral blood are also raised in patients with very limited clinically silent myocardial injury and in patents with complex coronary atherosclerotic lesions and superficial thrombus formation, suggestive of plaque rupture.19,20
In CCTA, advanced atherosclerotic plaques usually present as large predominantly noncalcified lesions.21,22 A recent study by Korosoglou et al4 found an association between the volume of noncalcified plaque detected in CCTA and hs-TnT. In autopsy studies, small microscopically detectable calcifications are present in most advanced coronary atherosclerotic plaques; however, larger calcifications are almost always associated with signs of healed plaque ruptures in histopathology.23 Our results demonstrate a correlation between the development of calcified plaques in slices with only non-calcified plaque at baseline and levels of hs-TnT in the peripheral blood, suggesting that this change in plaque morphology is possibly related to plaque ruptures or ongoing remodeling of plaque, resulting in microvascular injury and elevation of troponin. As expected, this correlation attenuated after adjustment for ACS. However, ACS is usually triggered by plaque rupture with subsequent thrombus formation, and thus, this fact does not contradict our results.24 It has been noted that LV mass predicted levels of hs-TnT in patients without ACS; however, in the present study, the tie between the levels of troponin and change of plaque morphology only attenuated slightly after adjustment for LV mass.
Another biomarker that has been related to coronary atherosclerosis is CRP. This acute phase protein is mainly synthesized in the liver in response to trauma or infection, but it can also be detected in the coronary artery wall.25 Concentrations of CRP in the coronary artery wall correlate with the inflammatory activity in advanced atherosclerotic lesions and drop significantly in calcified plaques.26 Experimental data suggest that the expression of CRP accelerates atherogenesis.27 Although this might suggest that levels of CRP correlate with the incidence of noncalcified lesions, no such association was found in our study. In fact, in a study relating plaque characteristics to serum biomarkers, Korosoglou et al4 found increased hs-CRP values only in patients with advanced positively remodeled plaques. However, it has been shown that serum levels of hs-CRP correlate with the progression of coronary atherosclerotic plaques both in patients with ACS and unstable angina.28,29 This is in line with our observation that levels of hs-CRP correlate with overall plaque change.
The main limitation of the present study lies in a potential selection bias of patients presenting for a follow-up CT scan. Although no major differences were found between those undergoing a second CT scan and the entire ROMICAT cohort, our results must be interpreted considering the highly selective nature of this cohort. Furthermore, the relatively small number of patients may limit the generalizability of our findings. Also, we limited the assessment of coronary plaque burden to the proximal 40 mm of the coronary artery tree. The proximal 40 mm of the coronary arteries were chosen as in most patients presenting with ACS, culprit lesions are located in these sections of the coronary artery tree and the capability of CT to differentiate between different plaque types is better in the larger proximal segments of the vessel.9,10 However, we have to assume that more distal plaques also cause changes in blood biomarkers and thus possibly influence the results of this study. A further limitation is the fact that we simplified our scoring scheme to only 3 plaque categories. It is well known that spotty calcifications can be commonly found in plaques classified as culprit lesion in patients presenting with ACS.22,30 However, as we used a semiquantitative score on the level of slices reconstructed by the use of a multiplanar reformation, we could not account for the total size of calcifications.
5. Conclusions
In conclusion, elevated concentrations of hs-TnT and hs-CRP in the peripheral blood are associated with a significant increase in CAD burden and change in plaque composition over 24 months independent of LV mass and baseline risk factors.
Acknowledgments
This work was supported by the National Institutes of Health (R01 HL080053; Udo Hoffmann [principal investigator]) and in part by Siemens Medical Solutions and GE Healthcare. Dr Harald Seifarth was supported by Deutsche Forschungsgemeinschaft (grant SE 2029/1-1). Dr Christopher L. Schlett is supported in part by grants from the German Federal Ministry of Education and Research, as well as the Foundation of German Business. Dr James L. Januzzi reports having received research grant support from Roche Diagnostics, Siemens, and Critical Diagnostics and assay/reagent support from Siemens. He was also supported in part by the Balson Scholar Fund. Dr Quynh A. Truong is supported by National Institutes of Health (grants T32HL076136 and L30HL093896). Dr Udo Hoffmann has received grant support for the ROMICAT 1 and 2 trials from the National Institutes of Health and research grant support from GE Healthcare and Siemens Medical Systems.
References
- 1.Hackett D, Davies G, Maseri A. Pre-existing coronary stenoses in patients with first myocardial infarction are not necessarily severe. Eur Heart J. 1988;9:1317–1323. doi: 10.1093/oxfordjournals.eurheartj.a062449. [DOI] [PubMed] [Google Scholar]
- 2.Gerszten RE, Wang TJ. The search for new cardiovascular biomarkers. Nature. 2008;451:949–952. doi: 10.1038/nature06802. [DOI] [PubMed] [Google Scholar]
- 3.Koenig W, Breitling LP, Hahmann H, Wüsten B, Brenner H, Rothenbacher D. Cardiac troponin T measured by a high-sensitivity assay predicts recurrent cardiovascular events in stable coronary heart disease patients with 8-year follow-up. Clin Chem. 2012;58:1215–1224. doi: 10.1373/clinchem.2012.183319. [DOI] [PubMed] [Google Scholar]
- 4.Korosoglou G, Lehrke S, Mueller D, et al. Determinants of troponin release in patients with stable coronary artery disease: insights from CT angiography characteristics of atherosclerotic plaque. Heart. 2011;97:823–831. doi: 10.1136/hrt.2010.193201. [DOI] [PubMed] [Google Scholar]
- 5.Laufer EM, Mingels AMA, Winkens MHM, et al. The extent of coronary atherosclerosis is associated with increasing circulating levels of high sensitive cardiac troponin T. Arterioscler Thromb Vasc Biol. 2010;30:1269–1275. doi: 10.1161/ATVBAHA.109.200394. [DOI] [PubMed] [Google Scholar]
- 6.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262–1275. doi: 10.1161/01.atv.20.5.1262. [DOI] [PubMed] [Google Scholar]
- 7.Hoffmann U, Bamberg F, Chae CU, et al. Coronary computed tomography angiography for early triage of patients with acute chest pain: the Rule Out Myocardial Infarction using Computer Assisted Tomography (ROMICAT) trial. J Am Coll Cardiol. 2009;53:1642–1650. doi: 10.1016/j.jacc.2009.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lehman SJ, Schlett CL, Bamberg F, et al. Assessment of coronary plaque progression in coronary computed tomography angiography using a semiquantitative score. JACC Cardiovasc Imaging. 2009;2:1262–1270. doi: 10.1016/j.jcmg.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang JC. Coronary artery spatial distribution of acute myocardial infarction occlusions. Circulation. 2004;110:278–284. doi: 10.1161/01.CIR.0000135468.67850.F4. [DOI] [PubMed] [Google Scholar]
- 10.van der Giessen AG, Toepker MH, Donelly PM, et al. Reproducibility, accuracy, and predictors of accuracy for the detection of coronary atherosclerotic plaque composition by computed tomography: an ex vivo comparison to intravascular ultrasound. Invest Radiol. 2010;45:693–701. doi: 10.1097/RLI.0b013e3181e0a541. [DOI] [PubMed] [Google Scholar]
- 11.Achenbach S. Detection of calcified and noncalcified coronary atherosclerotic plaque by contrast-enhanced, submillimeter multidetector spiral computed tomography: a segment-based comparison with intravascular ultrasound. Circulation. 2004;109:14–17. doi: 10.1161/01.CIR.0000111517.69230.0F. [DOI] [PubMed] [Google Scholar]
- 12.Reichlin T, Hochholzer W, Bassetti S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361:858–867. doi: 10.1056/NEJMoa0900428. [DOI] [PubMed] [Google Scholar]
- 13.Melander O, Newton-Cheh C, Almgren P, et al. Novel and conventional biomarkers for prediction of incident cardiovascular events in the community. JAMA. 2009;302:49–57. doi: 10.1001/jama.2009.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 15.Alpert J, Thygesen K. Myocardial infarction redefined—a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. Eur Heart J. 2000;21:1502–1513. doi: 10.1053/euhj.2000.2305. [DOI] [PubMed] [Google Scholar]
- 16.Katus HA, Remppis A, Looser S, Hallermeier K, Scheffold T, Kübler W. Enzyme linked immuno assay of cardiac troponin T for the detection of acute myocardial infarction in patients. J Mol Cell Cardiol. 1989;21:1349–1353. doi: 10.1016/0022-2828(89)90680-9. [DOI] [PubMed] [Google Scholar]
- 17.Januzzi JL, Bamberg F, Lee H, et al. High-sensitivity troponin T concentrations in acute chest pain patients evaluated with cardiac computed tomography. Circulation. 2010;121:1227–1234. doi: 10.1161/CIRCULATIONAHA.109.893826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ndrepepa G, Braun S, Schulz S, Mehilli J, Schomig A, Kastrati A. High-sensitivity troponin T level and angiographic severity of coronary artery disease. AJC. 2011;108:639–643. doi: 10.1016/j.amjcard.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 19.Okamatsu K, Takano M, Sakai S, et al. Elevated troponin T levels and lesion characteristics in non-ST-elevation acute coronary syndromes. Circulation. 2004;109:465–470. doi: 10.1161/01.CIR.0000109696.92474.92. [DOI] [PubMed] [Google Scholar]
- 20.Omland T, de Lemos JA, Sabatine MS, et al. Prevention of Events with Angiotensin Converting Enzyme Inhibition (PEACE) Trial Investigators. A sensitive cardiac troponin T assay in stable coronary artery disease. N Engl J Med. 2009;361:2538–2547. doi: 10.1056/NEJMoa0805299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoffmann U, Moselewski F, Nieman K, et al. Noninvasive assessment of plaque morphology and composition in culprit and stable lesions in acute coronary syndrome and stable lesions in stable angina by multidetector computed tomography. J Am Coll Cardiol. 2006;47:1655–1662. doi: 10.1016/j.jacc.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 22.Pflederer T, Marwan M, Schepis T, et al. Characterization of culprit lesions in acute coronary syndromes using coronary dual-source CT angiography. Atherosclerosis. 2010;211:437–444. doi: 10.1016/j.atherosclerosis.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 23.Burke AP, Weber DK, Kolodgie FD, Farb A, Taylor AJ, Virmani R. Pathophysiology of calcium deposition in coronary arteries. Herz. 2001;26:239–244. doi: 10.1007/pl00002026. [DOI] [PubMed] [Google Scholar]
- 24.Davies MJ. The pathophysiology of acute coronary syndromes. Heart. 2000;83:361–366. doi: 10.1136/heart.83.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burke AP, Tracy RP, Kolodgie F, et al. Elevated C-reactive protein values and atherosclerosis in sudden coronary death: association with different pathologies. Circulation. 2002;105:2019–2023. doi: 10.1161/01.cir.0000015507.29953.38. [DOI] [PubMed] [Google Scholar]
- 26.Norja S, Nuutila L, Karhunen PJ, Goebeler S. C-reactive protein in vulnerable coronary plaques. J Clin Pathol. 2007;60:545–548. doi: 10.1136/jcp.2006.038729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paul A, Ko KWS, Li L, et al. C-reactive protein accelerates the progression of atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2004;109:647–655. doi: 10.1161/01.CIR.0000114526.50618.24. [DOI] [PubMed] [Google Scholar]
- 28.Takano M, Inami S, Ishibashi F, et al. Angioscopic follow-up study of coronary ruptured plaques in nonculprit lesions. J Am Coll Cardiol. 2005;45:652–658. doi: 10.1016/j.jacc.2004.09.077. [DOI] [PubMed] [Google Scholar]
- 29.Nakachi T, Kosuge M, Hibi K, et al. C-reactive protein elevation and rapid angiographic progression of nonculprit lesion in patients with non-ST-segment elevation acute coronary syndrome. Circ J. 2008;72:1953–1959. doi: 10.1253/circj.cj-08-0185. [DOI] [PubMed] [Google Scholar]
- 30.Motoyama S, Sarai M, Harigaya H, et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol. 2009;54:49–57. doi: 10.1016/j.jacc.2009.02.068. [DOI] [PubMed] [Google Scholar]