Abstract
Spices are rich in natural antioxidants and have been shown to be potent inhibitors of lipid peroxidation during cooking of meat. Turmeric contains unique conjugated curcuminoids with strong antioxidant activity. Piperine, one of the main constituents of black pepper, is known to increase the bioavailability of curcuminoids in mouse and human studies when consumed with turmeric. We investigated whether adding black pepper to turmeric powder may further inhibit lipid peroxidation when added to meat patties prior to cooking. The addition of black pepper to turmeric significantly decreased the lipid peroxidation in hamburger meat. When investigating the antioxidant activity of the main chemical markers, we determined that piperine did not exhibit any antioxidant activity. Therefore, we conclude that other black pepper ingredients are responsible for the increased antioxidant activity of combining black pepper with turmeric powder.
Keywords: Black pepper, hamburger, lipid peroxidation, malondialdehyde (MDA), turmeric
Introduction
Depending on the fat content, hamburger meat patties are susceptible to lipid oxidation during cooking (Esterbauer, 1993). In addition, digestion in the stomach, which can act as a bioreactor, can further enhance lipid oxidation and the production of secondary products including aldehydes, acids, alcohols and ketones (Halliwell et al., 2000; Kanner et al., 2012). Furthermore, the aldehydes such as hexanal, pentanal and 2-octenal can produce toxic compounds including free radicals, hydroperoxides and malonaldehyde by oxidation of unsaturated fatty acids (Eckl et al., 1993).
We previously demonstrated that a mixture of spices added to hamburger meat during cooking not only decreased malondialdehyde (MDA) in the meat, but healthy volunteers consuming hamburgers cooked with spices excreted significantly less urinary malondialdehyde, a marker of oxidative damage, compared to volunteers ingesting hamburgers cooked without added spices (Li et al. 2010, 2013). The spice mix contained ground cloves (4.3%), cinnamon (4.3%), Mediterranean oregano (26.2%), rosemary (4.3%), ginger (10.9%), black pepper (6.5%), paprika (30.4%) and garlic powder (13.0%) (Li et al., 2010).
The spice mixture used in our previous experiment contained a wide variety of chemical compounds with strong antioxidant activity (Li et al., 2010). In a preliminary experiment we determined that among the eight spices in the mixture, on an equal weight basis, turmeric exhibited the strongest inhibition of lipid peroxidation. Turmeric is the dried rhizome powder of Curcuma longa, a perennial herb of the Zingiberaceae family. Curcumin is the main yellow pigment of turmeric, a popular spice, which is widely used as a food colorant (Govindarajan, 1980). The main components of turmeric powder are curcuminoids with strong antioxidant activity (Ruby et al., 1995) and have received attention as promising components of designer foods for their health-promoting benefits (Kelloff et al., 1996). Curcuminoids have a unique conjugated structure including methoxylated phenols and an enol form of β-diketone (Figure 1). The structure of curcuminoids confers oxygen radical-trapping capacity as a chain-breaking antioxidant. Limited bioavailability decreases the potential of curcumin in the prevention of chronic disease (Metzler et al., 2013). It has been demonstrated that combining curcumin with piperine from black pepper increased the bioavailability of curcuminoids in animal and human studies (Pawar et al., 2012; Sehgal et al., 2011, 2012). This effect in vivo was mainly explained through the ability of piperine to inhibit hepatic and intestinal glucuronidation of curcuminoids and inhibition of release of curcuminoid into the intestine leading to an increase in bioavailability (Berginc et al., 2012). However, we were interested to determine whether other chemical interactions between turmeric and black pepper may increase the availability and antioxidant activity in our in vitro hamburger model system.
Figure 1.
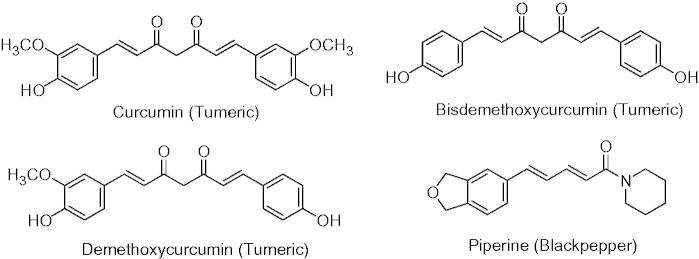
Structures of curcuminoids and piperine.
Black pepper is one of the most commonly used spices in the preparation of hamburger to enhance flavor and aroma. Black pepper (Piper nigrum), known as the king of spices, is one of the oldest and the most important spices in the world (Srinivasan, 2007). The characteristic pungent aroma of black pepper is due to a mixture of several compounds. A methanol extraction of black pepper powder mainly contains piperine (2–4% by weight), an alkaloid contributing to the pungency of black pepper (Figure 1) (Krchnak et al., 2011; Musenga et al., 2007). Recently, piperine has been shown to have anti-inflammatory in several in vitro and animal models (Rinwa et al., 2013; Umar et al., 2013; Ying et al., 2013). Distillation of pepper in a current of steam yields an essential oil (0.06%) containing a number of aromatic and terpenic constituents such as β-caryophyllene, limonene, β-pinene, aphellandrene and α-humulene, as well as minor constituents such as 3-carene, sabinene, β-bisabolene and caryophyllene oxide (Kapoor et al., 2009). Essential oil and ethanol-extracted oleoresin fractions from black pepper have been reported to have some antioxidant activity and may enhance the antioxidant activity of curcuminoids in vitro (Kapoor et al., 2009). Since turmeric and black pepper are commonly used together in flavoring meat, we determine the effect of combining curcuminoids and piperine on the antioxidant activity and the effect of combining turmeric and black pepper on lipid peroxidation during the preparation of hamburger meat.
Materials and methods
Reagents and spice samples
All solvents were of high-performance liquid chromatography (HPLC) grade and purchased from Fisher Scientific Co. (Tustin, CA). The standard chemicals were purchased from ChromaDex (Curcumin, Lot#00003926-1103; Demethoxycurcumin, Lot#00004230-112; Bisdemethoxycurcumin, Lot#04231-531; Piperine, Lot#16870-W03). All the chemical reagents were purchased from Sigma-Aldrich (St. Louis, MO) including perchloric acid, orthophosphoric acid, 2-thiobarbituric acid (TBA), 1,1,3,3-tetramethoxypropane (TMP). Pure water was prepared using a Millipore water system in our laboratory (Millipore, Billerica, MA). Turmeric (Curcuma longa) powder and black pepper (Piper nigrum) powder were purchased from McCormick Spices, Inc. (Hunt Valley, MD).
Free radical scavenging capacity
The free radical scavenging capacity was analyzed by the 1,1-dipenyl-2-picryl-hydrazyl (DPPH) assay (Kedare & Singh, 2011). DPPH is a purple stable organic free radical with an unpaired valence electron. Upon reduction by an antioxidant, the solution color fades and the reaction can be monitored by spectrophotometer at 517 nm. The decrease in absorbance is proportional to the antioxidant capacity and can be measured in comparison to Trolox as standard. All samples and standard were diluted in methanol. Aliquots (20 µL) from the test compounds were mixed with 200 µL of DPPH (610 µmol/L) in methanol and the change in optical density (517 nm) was monitored after 30 min using a VersaMax plate reader (Molecular Devices, Sunnyvale, CA).
Quantification of the curcuminoids in the turmeric powder and piperine in black pepper powder and the cooked hamburgers by HPLC-UV
Sample preparation for HPLC analysis
Turmeric powder (150 mg) was shaken with 30 mL of MeOH in a 50 mL centrifuge tube for 30 min on an orbital shaker at 250 rpm. The mixture of the sample and solvent was centrifuged at 13 900 rpm for 10 min. The supernatant was diluted 20 times by MeOH–H2O (1:1, v:v) and subjected to HPLC analysis. Black pepper powder (200 mg) was shaken with 30 mL of MeOH in a 50 mL centrifuge tube for 30 min on an orbital shaker at 250 rpm. The mixture of the sample and solvent was centrifuged at 13 900 rpm for 10 min. The supernatant was diluted 20 times by MeOH–H2O (1:1, v:v) and subjected to HPLC analysis.
Sample preparation for HPLC analysis of curcuminoids and piperine in cooked hamburgers
Hamburger patties were cut into four sections and three sections were further cut into smaller chunks and homogenized in a blender until meat was ground uniformly. One gram of ground meat was shaken with 40 mL of MeOH–H2O (80:20, v:v) in a 50 mL centrifuge tube on an orbital shaker for 30 min, and centrifuged at 13 900 rpm for 10 min. The supernatant was subjected to HPLC analysis.
HPLC conditions for analysis of curcuminoids and piperine
The HPLC system consisted of a Waters Alliance 2695 Module with a 996 photodiode array detector, controlled by Waters Empower 2 Software (Waters, Milford, MA). The mobile phase, solvent A (acetonitrile) and solvent B (0.4% aqueous phosphoric acid), was used under linear gradient condition as follows: 0–60 min, 15–60% solvent A in solvent B; with a flow rate of 1.0 mL/min. All samples were filtered (0.22 µm) and loaded (25 µL injection volume) and analyzed on a Phenomenex Gemini-NX C18, 110A 4.6 × 250 mm, 5 µm column with a guard column (C18 5 µm, 3.9 × 20 mm). The monitored wavelength was 420 nm for detection and quantification of curcumin, demethoxycurcumin and bisdemethoxycurcumin (Figure 2), and 330 nm for piperine. The retention time for curcumin, demethoxycurcumin, bisdemethoxycurcumin and piperine at above HPLC condition are 7.04, 10.97, 12.65 and 23.58 min, respectively.
Figure 2.
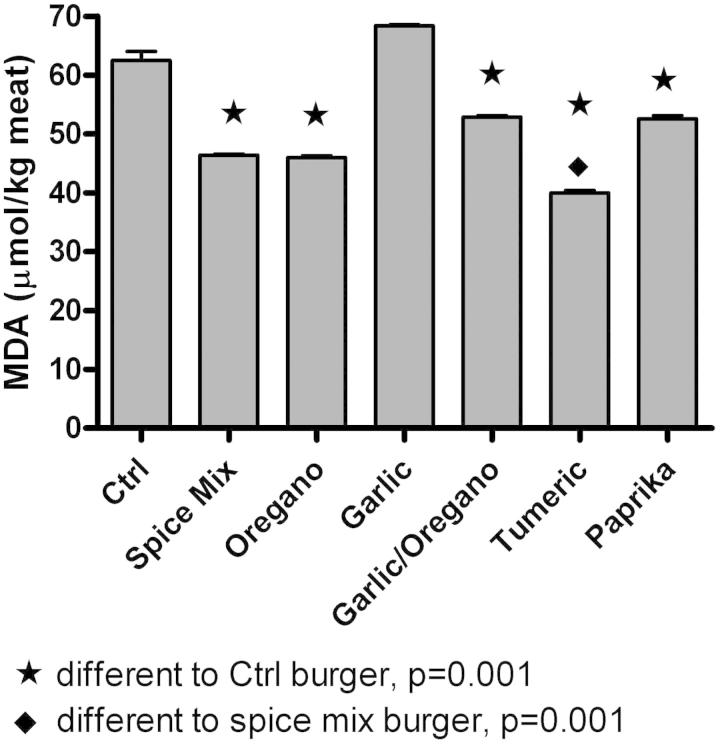
Effect of individual spices and salt on the malondialdehyde content of cooked hamburgers compared to hamburgers prepared with salt or spice mix and salt (data: mean ± std, n = 3).
Hamburger preparation
Hamburger patties were prepared in the research kitchen of the UCLA Clinical and Translational Science Institute. The beef (10% of fat) was weighed, minced in a commercial blender (Kitchen-Aid) for 2 min on the lowest setting and mixed with ingredients in Table 1. All ingredients were weighed and mixed at the Center for Human Nutrition kitchen. After blending with a paddle attachment for 1 min, a 5.75-in ring mold was used to divide the meat into flattened 250 g patties. Three burger patties were mixed with each combination of ingredients as listed in Table 1. The spice mix was mixed into each of the burger patties. As there were 16 possible combinations of spices studied in triplicate, a total of 48 patties were made. The burger patties were labeled and cooked to an internal temperature of 77 °C, frozen and packaged in the kitchen of the UCLA Clinical and Translational Science Institute. The burgers were then delivered frozen to the UCLA Center for Human Nutrition and kept at −20 °C until tested.
Table 1. Hamburger preparation and remaining weight after cooking.
| # | Salt added to the hamburger (g) | Turmeric added to the hamburger (g) | Black pepper added to the hamburger (g) | Hamburger weight after cooking (g) |
|---|---|---|---|---|
| 1 | 2.5 | 1.5 | 0 | 176 ± 7 |
| 2 | 2.5 | 3.0 | 0 | 172 ± 7 |
| 3 | 2.5 | 6.0 | 0 | 182 ± 3 |
| 4 | 2.5 | 0 | 0.1 | 168 ± 8 |
| 5 | 2.5 | 0 | 0.4 | 170 ± 7 |
| 6 | 2.5 | 0 | 1.1 | 176 ± 7 |
| 7 | 2.5 | 1.5 | 0.1 | 174 ± 3 |
| 8 | 2.5 | 3.0 | 0.1 | 178 ± 7 |
| 9 | 2.5 | 6.0 | 0.1 | 178 ± 6 |
| 10 | 2.5 | 1.5 | 0.4 | 176 ± 10 |
| 11 | 2.5 | 3.0 | 0.4 | 179 ± 8 |
| 12 | 2.5 | 6.0 | 0.4 | 180 ± 10 |
| 13 | 2.5 | 1.5 | 1.1 | 177 ± 8 |
| 14 | 2.5 | 3.0 | 1.1 | 179 ± 10 |
| 15 | 2.5 | 6.0 | 1.1 | 184 ± 9 |
| 16 | 2.5 | 0 | 0 | 173 ± 6 |
The indicated amount of turmeric, black pepper and salt was used in 250 g of hamburger meat for the preparation of the meat patties. Data: mean ± std, n = 3.
Hamburger patty preparation for initial selection of spices
A spice comparison was performed to investigate different spices' individual potential to prevent the formation of MDA. Hamburger patties were prepared in the kitchen at the UCLA Center for Human Nutrition. All ingredients were used in the same proportions, and the same meat and salt were used as in the intervention patties. The 99 g of meat was mixed for 2 min on the lowest setting with 1 g salt alone (control) or 94.5 g of meat was mixed with 1 g of salt and 4.5 g spice. The spices used in comparison were: oregano, garlic, oregano/garlic, turmeric, paprika and spice mixture. The meat was formed into 100 g patties and patties were fried in a non-stick pan on medium heat for 7 min on each side, then frozen and analyzed the following day.
Malondialdehyde solution preparation
1,1,3,3-Tetramethoxypropane (TMP) was used to prepare a malondialdehyde stock solution. A volume of 17 µL of TMP was diluted in 0.1 N HCl (10 mL) and incubated at 40 °C for 60 min to convert TMP into malondialdehyde (MDA). The concentration of MDA was determined by measuring its absorbance at 245 nm (ɛ = 13 700). This stock solution was stored at 4 °C and freshly prepared on a weekly basis.
2-Thiobarbituric acid solution preparation
2-Thiobarbituric acid (TBA) (0.6%, w/v) was prepared by dissolving 0.6 g of TBA in approximately 80 mL of water, with stirring on a hot plate (50–55 °C). After cooling down to room temperature the volume was adjusted to 100 mL with pure water.
HPLC conditions for the analysis of MDA in hamburger meat
The HPLC system consisted of a Waters Alliance 2695 Module with a Waters 474 Scanning Fluorescence Detector, controlled by Waters Empower 2 Software (Waters). The mobile phase, solvent A (acetonitrile) and solvent B, was used under linear gradient condition as follows: 0–20 min, 5–35% solvent A in solvent B; with a flow rate of 1.0 mL/min. All samples were filtered (0.22 µm) and loaded (25 µL injection volume) and analyzed on a Waters Symmetry C18, 4.6 × 100 mm, 3.5 µm column with a guard column (C18 5 µm, 3.9 × 20 mm). The excitation and emission wavelength were 515 and 550 nm for detection and quantification of MDA (Figure 2). The retention time for MDA at above HPLC condition is 11.3 min.
MDA calibration curve
Orthophosphoric acid solution (3 mL), 0.4 mL water and 0.6 mL of MDA standard (dilute 1000, 2000, 4000, 8000 and 16 000 times from stock solution) were vortex mixed after which 1 mL of TBA solution, and 0.6 mL of 3.86% perchloric acid solution were added. The solutions were heated in an oven at 100 °C for 1 h. At the end of the heating, samples were cooled on ice and centrifuged at 14 000 g. The supernatant was transferred to glass HPLC vials and 25 µL were injected into the column for analysis.
Statistical analysis
Each determination was performed in triplicate. Data were expressed as mean ± std. Statistics were performed using the StatView 5.0 software (SAS Institute Inc., Cary, NC). Differences were evaluated by ANOVA followed by the Fisher post-hoc analysis. Levels of statistical significance were p < 0.05.
Results and discussion
The physiological effects of spices are of great interest when studied in amounts used in culinary practice due to their presumed safety and the potential antioxidant, nutritional and therapeutic effects. An area that has received less attention is the interaction of different spices to affect bioavailability, metabolism or physiological effects. The first step in such studies where there is a known interaction in vivo as with turmeric and black pepper is to account for any interactions occurring during cooking.
Preliminary antioxidant study
Our preliminary studies demonstrated that turmeric exhibited the strongest antioxidant activity (Figure 2) among eight different spices commonly used in the flavoring of hamburger patties. It has been demonstrated in human and animal studies that the addition of black pepper to curcumin was associated with enhanced curcumin bioavailability (Anand et al., 2007). However this in vivo effect is mainly based on the inhibition of the glucuronidase enzyme activity and inhibition of transport proteins such as MRP1 by piperine from black pepper (Berginc et al., 2012). During cooking, however, this effect will not contribute to any interaction between turmeric and black pepper. We therefore determined whether other interactions between black pepper and turmeric will increase the recovery and antioxidant activity of the marker chemicals of black pepper (piperine) and turmeric (curcumioids) after cooking. The turmeric powder used in the present studies contained 42.3 ± 0.1 mg of curcuminoids (curcumin, demethoxycurcumin and bisdemethoxycurcumin) per gram of turmeric powder and black pepper contained 40.1 ± 0.2 mg of piperine per gram of black pepper powder.
Marker chemical recovery
Table 2 shows the content and recovery of curcuminoids from burger patties prepared with increasing amounts of black pepper powder combined with increasing amounts of turmeric powder. The recovery of curcuminoids from hamburger meat after cooking remained about 60% independent of the amount of turmeric added. The addition of increasing amounts of black pepper did not affect the recovery of curcuminoids from the burgers after cooking. The recovery of piperine, however, increased as more black pepper was added, regardless of the amount of curcuminoids present, with 96–98% recovery at the highest amounts of added black pepper (1.08 g added) (Table 3).
Table 2. Curcuminoid recovery (in %) from cooked hamburgers prepared with different combinations of turmeric and black pepper.
| Piperine added (mg/kg) | Curcuminoids added (mg/kg) | Curcuminoids measured (mg/kg) | Recovered (%) |
|---|---|---|---|
| 0 | 251.6 | 153.2 ± 1.6 | 60.9 |
| 0 | 505.6 | 304.4 ± 1.2 | 60.2 |
| 0 | 1013.6 | 666.0 ± 11.2 | 65.7 |
| 19.2 | 251.6 | 141.6 ± 4.0 | 56.3 |
| 19.2 | 505.6 | 272.4 ± 6.0 | 53.9 |
| 19.2 | 1013.6 | 638.4 ± 2.4 | 63.0 |
| 57.2 | 251.6 | 121.2 ± 1.6 | 48.2 |
| 57.2 | 505.6 | 274.4 ± 3.6 | 54.3 |
| 57.2 | 1013.6 | 571.2 ± 6.0 | 56.4 |
| 172.4 | 251.6 | 142 ± 2.0 | 56.4 |
| 172.4 | 505.6 | 288 ± 1.2 | 57.0 |
| 172.4 | 1013.6 | 616 ± 8.4 | 60.8 |
The indicated amount of black pepper and curcuminoids was used in combination with 2.5 g of salt in 250 g of hamburger meat for the preparation of the meat patties. Data: mean ± std, n = 3.
Table 3. Piperine recovery from cooked hamburgers prepared with different combinations of turmeric and black pepper.
| Curcumoids added (mg/kg) | Piperine added (mg/kg) | Piperine measure in cooked hamburger (mg/kg) | Piperine recovered (%) |
|---|---|---|---|
| 0 | 19.2 | 11.5 ± 0.0 | 60 |
| 0 | 57.2 | 31.2 ± 0.4 | 65 |
| 0 | 172.4 | 134.5 ± 1.2 | 78 |
| 251.6 | 19.2 | 15.4 ± 1.2 | 80 |
| 505.6 | 19.2 | 14.6 ± 0.4 | 76 |
| 1013.6 | 19.2 | 13.4 ± 0.0 | 70 |
| 251.6 | 57.2 | 47.5 ± 0.8 | 83 |
| 505.6 | 57.2 | 42.9 ± 0.9 | 75 |
| 1013.6 | 57.2 | 46.9 ± 0.8 | 82 |
| 251.6 | 172.4 | 168.9 ± 0.4 | 98 |
| 505.6 | 172.4 | 168.9 ± 0.8 | 98 |
| 1013.6 | 172.4 | 165.5 ± 0.0 | 96 |
The indicated amount of black pepper and curcuminoids was used in combination with 2.5 g of salt in 250 g of hamburger meat for the preparation of the meat patties. Data: mean ± std, n = 3.
Antioxidant activity in hamburger meat
We further investigated the antioxidant effect of increasing amounts of turmeric and black pepper or whether the various combinations of both influenced the formation of lipid peroxides during the cooking process of hamburger meat. While increasing amounts of curcumin as shown in Table 4 led to reduced amounts of lipid peroxidation during cooking, this was not true for black pepper. However, when black pepper (0.12, 0.36 and 1.08 g per burger) was added to turmeric root powder at 3.0 and 6.0 g per patty, it resulted in a synergistic inhibition of MDA formation compared to the same amount of curcumin used without black pepper when adjusted for differences in patty weight after cooking (Table 4).
Table 4. Dose response of combinations of three different concentrations of turmeric and pepper on the malondialdehyde (MDA) content in cooked hamburgers adjusted for meat patty weight.
| Sample # | MDA (µg/Kg) | Patty weight (g) | Adjusted MDA/patty (µg/meat patty) | |
|---|---|---|---|---|
| 1 | T1 | 222.2 ± 6.5a | 176 | 39.1 ± 1.1 |
| 2 | T2 | 204.8 ± 3.1a | 172 | 35.2 ± 0.5 |
| 3 | T3 | 221.7 ± 1.4a | 182 | 40.2 ± 0.1 |
| 4 | BP1 | 315.2 ± 4.4 | 168 | 53.6 ± 0.4 |
| 5 | BP2 | 280.3 ± 2.1 | 170 | 47.5 ± 0.5 |
| 6 | BP3 | 271.9 ± 6.3 | 176 | 48.7 ± 0.4 |
| 7 | T1 + BP1 | 231.2 ± 2.2 | 174 | 40.3 ± 0.02 |
| 8 | T2 + BP1 | 228.7 ± 5.0 | 178 | 41.3 ± 0.7 |
| 9 | T3 + BP1 | 210.5 ± 4.1 | 178 | 36.9 ± 0.1 |
| 10 | T1 + BP2 | 151.1 ± 3.0b | 176 | 26.6 ± 0.6 |
| 11 | T2 + BP2 | 174.0 ± 1.5b | 179 | 31.3 ± 0.2 |
| 12 | T3 + BP2 | 150.2 ± 3.2b | 180 | 27.3 ± 0.5 |
| 13 | T1 + BP3 | 168.1 ± 2.7b | 177 | 29.5 ± 0.6 |
| 14 | T2 + BP3 | 153.4 ± 6.5b | 179 | 26.5 ± 0.5 |
| 15 | T3 + BP3 | 162.0 ± 4.5b | 184 | 29.3 ± 0.7 |
| 16 (Control) | Salt | 289.6 ± 6.5b | 173 | 50.4 ± 0.2 |
Turmeric (T): T1 = 1.5, T2 = 3, T3 = 6 g added and black pepper (BP): BP1 = 0.12, BP2 = 0.36 and BP3 = 1.08 g of black pepper powder added. Salt control had no other spices. Data: mean ± std, n = 3.
aDifferent from control.
bDifferent from corresponding meat patty with turmeric alone.
Antioxidant activity of chemical markers
To investigate the potential mechanism of this increase in antioxidant activity by combining black pepper and turmeric, we determined the capacity of scavenging free radicals by the marker chemical compounds of turmeric (curcuminoids) and black pepper (piperine). Using the DPPH scavenging assay, we demonstrated that the antioxidant activity of a methanol extract of black pepper (648 ± 13 µmol TE/100 g) was very small (20-fold lower) compared to a methanol extract of turmeric (12 745 ± 548 µmol TE/100 g). In addition, piperine, the marker compound in black pepper did not exhibit any DPPH scavenging activity, while curcumin (2 50 160 ± 11 216 µmol TE/100 g) clearly is the main antioxidant contributor in turmeric powder (Figure 3A). The same combination of curcumin and piperine as added to the hamburger meat in form of turmeric and black pepper did not increase the radical scavenging activity (Figure 3B).
Figure 3.
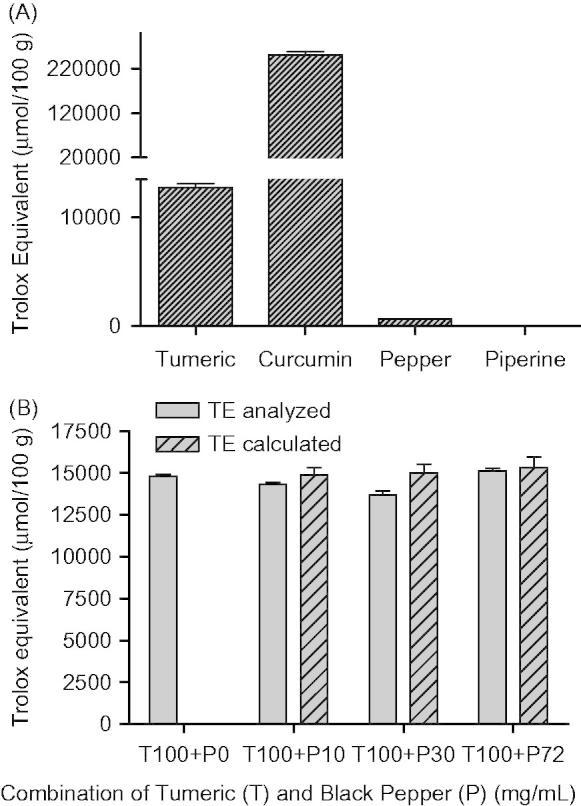
Antioxidant activity of turmeric, curcumin, black pepper and piperine and their combinations using the DPPH assay (data: mean ± std, n = 3).
Until now most of the investigations of black pepper are focused on the contribution of piperine. Since our findings showed that piperine is not contributing antioxidant activity, most likely other chemical components (aromatic and terpenic constituents such as β-caryophyllene, limonene, β-pinene, aphellandrene and α-humulene) of black pepper may contribute to enhance the antioxidant activity of turmeric.
Postprandial changes on oxidative stress, inflammation and endothelial function have been observed after the consumption of high-fat and carbohydrate meals (Phillips et al., 2010; Sies et al., 2005). Mixing turmeric and black pepper together significantly increased the recovery of piperine that may then increase the bioavailability of curcuminoids.
Conclusion
The present study provides evidence that mixtures of spices will lead to an increase in antioxidant activity, leading to a decrease in lipid peroxidation during the preparation of high-fat foods. As demonstrated by our laboratory and other groups, the reduction of lipid peroxidation in foods by the addition of spices or other sources of polyphenols (red wine powder or roasted coffee) lead to the reduction of postprandial changes such as oxidative damage, inflammatory processes and vasoconstriction (Gorelik et al., 2008; Sirota et al., 2013) and may be beneficial in reducing the development of atherosclerosis, coronary heart disease and other age-related chronic diseases. Future studies are warranted to determine whether the combination of black pepper and turmeric powder will enhance the postprandial health benefits of turmeric in human consumption of high-fat and carbohydrate meals.
Declaration of interest
The chemical analyses were supported by Division Funds of the Department of Medicine of the David Geffen School of Medicine at UCLA. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the manuscript.
References
- Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- Berginc K, Trontelj J, Basnet NS, Kristl A. Physiological barriers to the oral delivery of curcumin. Pharmazie. 2012;67:518–524. [PubMed] [Google Scholar]
- Eckl PM, Ortner A, Esterbauer H. Genotoxic properties of 4-hydroxyalkenals and analogous aldehydes. Mutat Res. 1993;290:183–192. doi: 10.1016/0027-5107(93)90158-c. [DOI] [PubMed] [Google Scholar]
- Esterbauer H. Cytotoxicity and genotoxicity of lipid-oxidation products. Am J Clin Nutr. 1993;57:779S–785S. doi: 10.1093/ajcn/57.5.779S. [DOI] [PubMed] [Google Scholar]
- Gorelik S, Ligumsky M, Kohen R, Kanner J. A novel function of red wine polyphenols in humans: prevention of absorption of cytotoxic lipid peroxidation products. FASEB J. 2008;22:41–46. doi: 10.1096/fj.07-9041com. [DOI] [PubMed] [Google Scholar]
- Govindarajan VS. Turmeric – chemistry, technology, and quality. Crit Rev Food Sci Nutr. 1980;12:199–301. doi: 10.1080/10408398009527278. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Zhao K, Whiteman M. The gastrointestinal tract: a major site of antioxidant action? Free Radic Res. 2000;33:819–830. doi: 10.1080/10715760000301341. [DOI] [PubMed] [Google Scholar]
- Kanner J, Gorelik S, Roman S, Kohen R. Protection by polyphenols of postprandial human plasma and low-density lipoprotein modification: the stomach as a bioreactor. J Agric Food Chem. 2012;60:8790–8796. doi: 10.1021/jf300193g. [DOI] [PubMed] [Google Scholar]
- Kapoor IP, Singh B, Singh G, De Heluani CS, De Lampasona MP, Catalan CA. Chemistry and in vitro antioxidant activity of volatile oil and oleoresins of black pepper (Piper nigrum) J Agric Food Chem. 2009;57:5358–5364. doi: 10.1021/jf900642x. [DOI] [PubMed] [Google Scholar]
- Kedare SB, Singh RP. Genesis and development of DPPH method of antioxidant assay. J Food Sci Technol. 2011;48:412–422. doi: 10.1007/s13197-011-0251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelloff GJ, Crowell JA, Hawk ET, Steele VE, Lubet RA, Boone CW, Covey JM, et al. Strategy and planning for chemopreventive drug development: clinical development plans II. J Cell Biochem Suppl. 1996;26:54–71. doi: 10.1002/jcb.240630705. [DOI] [PubMed] [Google Scholar]
- Krchnak V, Zajicek J, Miller PA, Miller MJ. Selective molecular sequestration with concurrent natural product functionalization and derivatization: from crude natural product extracts to a single natural product derivative in one step. J Org Chem. 2011;76:10249–10253. doi: 10.1021/jo201361s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Henning SM, Zhang Y, Rahnama N, Zerlin A, Thames G, Tseng CH, Heber D. Decrease of postprandial endothelial dysfunction by spice mix added to high-fat hamburger meat in men with Type 2 diabetes mellitus. Diabet Med. 2013;30:590–595. doi: 10.1111/dme.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Henning SM, Zhang Y, Zerlin A, Li L, Gao K, Lee RP, et al. Antioxidant-rich spice added to hamburger meat during cooking results in reduced meat, plasma, and urine malondialdehyde concentrations. Am J Clin Nutr. 2010;91:1180–1184. doi: 10.3945/ajcn.2009.28526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler M, Pfeiffer E, Schulz SI, Dempe JS. Curcumin uptake and metabolism. Biofactors. 2013;39:14–20. doi: 10.1002/biof.1042. [DOI] [PubMed] [Google Scholar]
- Musenga A, Mandrioli R, Ferranti A, D'Orazio G, Fanali S, Raggi MA. Analysis of aromatic and terpenic constituents of pepper extracts by capillary electrochromatography. J Sep Sci. 2007;30:612–619. doi: 10.1002/jssc.200600456. [DOI] [PubMed] [Google Scholar]
- Pawar YB, Purohit H, Valicherla GR, Munjal B, Lale SV, Patel SB, Bansal AK. Novel lipid based oral formulation of curcumin: development and optimization by design of experiments approach. Int J Pharm. 2012;436:617–623. doi: 10.1016/j.ijpharm.2012.07.031. [DOI] [PubMed] [Google Scholar]
- Phillips LK, Peake JM, Zhang X, Hickman IJ, Kolade O, Sacre JW, Huang BE, et al. The effect of a high-fat meal on postprandial arterial stiffness in men with obesity and type 2 diabetes. J Clin Endocrinol Metab. 2010;95:4455–4459. doi: 10.1210/jc.2010-0413. [DOI] [PubMed] [Google Scholar]
- Rinwa P, Kumar A, Garg S. Suppression of neuroinflammatory and apoptotic signaling cascade by curcumin alone and in combination with piperine in rat model of olfactory bulbectomy induced depression. PLoS One. 2013;8:e61052. doi: 10.1371/journal.pone.0061052. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ruby AJ, Kuttan G, Babu KD, Rajasekharan KN, Kuttan R. Anti-tumour and antioxidant activity of natural curcuminoids. Cancer Lett. 1995;94:79–83. doi: 10.1016/0304-3835(95)03827-j. [DOI] [PubMed] [Google Scholar]
- Sehgal A, Kumar M, Jain M, Dhawan DK. Combined effects of curcumin and piperine in ameliorating benzo(a)pyrene induced DNA damage. Food Chem Toxicol. 2011;49:3002–3006. doi: 10.1016/j.fct.2011.07.058. [DOI] [PubMed] [Google Scholar]
- Sehgal A, Kumar M, Jain M, Dhawan DK. Synergistic effects of piperine and curcumin in modulating benzo(a)pyrene induced redox imbalance in mice lungs. Toxicol Mech Methods. 2012;22:74–80. doi: 10.3109/15376516.2011.603392. [DOI] [PubMed] [Google Scholar]
- Sies H, Stahl W, Sevanian A. Nutritional, dietary and postprandial oxidative stress. J Nutr. 2005;135:969–972. doi: 10.1093/jn/135.5.969. [DOI] [PubMed] [Google Scholar]
- Sirota R, Gorelik S, Harris R, Kohen R, Kanner J. Coffee polyphenols protect human plasma from postprandial carbonyl modifications. Mol Nutr Food Res. 2013;57:916–919. doi: 10.1002/mnfr.201200557. [DOI] [PubMed] [Google Scholar]
- Srinivasan K. Black pepper and its pungent principle-piperine: a review of diverse physiological effects. Crit Rev Food Sci Nutr. 2007;47:735–748. doi: 10.1080/10408390601062054. [DOI] [PubMed] [Google Scholar]
- Umar S, Golam Sarwar AH, Umar K, Ahmad N, Sajad M, Ahmad S, Katiyar CK, Khan HA. Piperine ameliorates oxidative stress, inflammation and histological outcome in collagen induced arthritis. Cell Immunol. 2013;284:51–59. doi: 10.1016/j.cellimm.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Ying X, Yu K, Chen X, Chen H, Hong J, Cheng S, Peng L. Piperine inhibits LPS induced expression of inflammatory mediators in RAW 264.7 cells. Cell Immunol. 2013;285:49–54. doi: 10.1016/j.cellimm.2013.09.001. [DOI] [PubMed] [Google Scholar]