Abstract
Several characteristics of Streptococcus pneumoniae (pneumococcus) combine to make it a particularly problematic pathogen. Firstly, the pneumococcus has the capacity to cause disease through the expression of virulence factors such as its polysaccharide capsule and pore-forming toxin. In addition, the pneumococcus is highly adaptable as demonstrated by its ability to acquire and disseminate resistance to multiple antibiotics. Although the pneumococcus is a major cause of disease, the organism is most commonly an “asymptomatic” colonizer of its human host (the carrier state), with transmission occurring exclusively from this reservoir of commensal organisms. Thus, it is unclear how the organism’s virulence and adaptability promote its persistence or host to host spread during its carrier state. This review summarizes current understanding of how these characteristics may contribute to the commensal lifestyle of the pneumococcus.
Keywords: Colonization, Virulence factor, Neutrophil, Pneumococcus
Introduction
Streptococcus pneumoniae (pneumococcus) remains a major pathogen responsible for over a million deaths each year. In addition, treatment of pneumococcal infection has been complicated by the acquisition and widespread dissemination of resistance to β-lactams and other common antibiotics [1]. The pneumococcus is a leading cause of both common mucosal infections, including otitis media and pneumonia, as well as invasive diseases, including sepsis and meningitis. None of these disease states, however, promote the dissemination of the organism to new hosts [2]. In fact, pneumococcal infection that disables or kills a proportion of its hosts will have a biological cost to the organism by reducing its opportunities for transmission. So, why has the pneumococcus evolved or maintained its capacity to cause disease?
Although interest in the pneumococcus is primarily due to its importance as a pathogen, the main biological niche for this organism is the upper respiratory tract of humans where it colonizes the mucosal surfaces lining the nasopharynx [3]. Disease occurs when resident organisms from the upper respiratory tract gain access to normally sterile spaces in the middle ear, lung, or bloodstream, and therefore, colonization is thought to be the initial step in the pathogenesis of all pneumococcal disease [4]. There is serial and simultaneous colonization by multiple strains with an individual isolate generally being carried transiently for weeks to months before it is cleared. Pneumococcal carriage rates are highest in the first 2 years of life and may exceed 50%, but decline thereafter. Person to person spread occurs through direct contact with secretions of carriers [2].
Since the vast reservoir of pneumococci in the population is found in the commensal state in the upper airway, which is the site where the transmission occurs exclusively, the selective pressures driving its behavior must reflect the requirements of colonization. Thus, the attributes that allow for its virulence and pathogenicity must somehow promote its commensal lifestyle. The focus of this review is to discuss how pneumococcal characteristics that make it a threat to public health also contribute to the biology of colonization. Factors promoting the acquisition of virulence traits and the adaptability of the pneumococcus are summarized in the Table 1.
Table 1.
Environmental pressures selecting for pneumococcal virulence and adaptations during carriage
| Factors promoting pathogenicity | Pneumococcal virulence determinant |
|---|---|
| Evasion of mucosal inflammatory response | Capsule blocking opsonophagocytosis |
| Pneumolysin interfering with leukocytes | |
| Exoglycosidases acting on opsonins | |
| Competition between species | Capsule inhibiting clearance triggered by other members of the micoflora |
| Generation of hydrogen peroxide to eliminate other microbes | |
| Evasion of mucosal immunity | IgA1 protease |
| Mechanical clearance | Pilus and other adhesins |
| Factors promoting adaptability | Pneumococcal adaptation |
| Oxidative stress | Genetic exchange to maintain fitness |
| Intraspecies competition | Acquisition of pneumocins/immunity factors |
| Antibiotics | Acquisition of resistance traits |
| Humoral immunity | Antigenic variation |
| Immunity to capsular polysaccharide vaccines | Serotype replacement |
Pneumococcal virulence determinants
The combined efforts of many investigators over many decades have identified a number of pneumococcal virulence factors important in facilitating the disease process. Recent genomic analysis has revealed that many of these individual factors are also found among closely related oral streptococcal species [5]. The maintenance of these virulence factors among streptococci with a similar commensal lifestyle but much less potential to cause disease suggests a primary function related to colonization. Chief among these virulence factors is the capsule. With rare exception, disease isolates obtained from normally sterile sites are encapsulated, and unencapsulated mutants are avirulent in animal models of infection [6]. Capsular polysaccharide is the major antigen on the bacterial surface, and different isolates are able to express at least 91 structurally and immunologically unique capsules or “types.” Clearance of pneumococci is generally thought to require opsonophagocytosis, a process made more efficient after the development of type-specific antibody to the capsular polysaccharide. Prior to the generation of type-specific antibody, the coating of the organism by a thick layer of polysaccharide limits recognition of underlying surface features by complement and antibody opsonins that would otherwise promote phagocytosis and killing by professional phagocytes.
The pneumococcus produces a single toxin, pneumolysin, which is a member of the large family of cholesterol binding cytolysins expressed by gram-positive pathogens [7]. Administration of the pneumolysin alone may be sufficient to recapitulate many of the key pathologic features of pneumococcal pneumonia [8]. Oligomerization of the protein toxin leads to pore-formation. In epithelial cells studied in culture, the osmotic stress of toxin insertion into the membrane triggers proinflammatory signaling events [9]. In addition, the toxin impairs both the function and viability of host cells including cells needed for clearance and also activates the classical pathway of complement.
The pneumococcus expresses three surface attached exoglycosidases: neuraminidase (NanA), β-galactosidase (BgaA), and N-acetylglucosaminidase (StrH) [10]. These enzymes sequentially remove terminal sugars common to many human glycoconjugates. Deglycosylation of host molecules may expose cell-surface receptors, inhibit mechanisms of clearance that require these glycoproteins, or provide nutrition for the organism.
Like many other mucosal pathogens that reside in the upper airway, the pneumococcus secretes a protease that cleaves the hinge region of human IgA1, the most abundant immunoglobulin expressed at this host site [11]. As a result, the organism is left coated with fragments lacking Fc domains and thus, evades recognition by Fc receptors or complement.
Inflammatory events during pneumococcal colonization
Pneumococcal colonization has been studied in its natural host following intranasal inoculation of healthy adult volunteers [12]. These experimental studies provided an understanding of the inoculum dose, duration of carriage, and immune response during an individual colonization event. These studies also provided the basis for the adaptation of a murine model of carriage in which the inoculum dose, duration of carriage, and immune response during colonization are similar in mice and man for the same strain tested in humans [13, 14]. This murine model has facilitated the analysis of host-mediated events during colonization.
Within hours of inoculation in the murine model, bacteria transit to the glycocalyx overlying the respiratory and olfactory epithelium along the ventral (anterior) nasal spaces [15]. This results in the activation of the epithelial surface with toll-like receptor 2 (TLR2)-dependent signaling that involves both p38 MAP kinase and TGFβ pathways [16]. These signaling events initiate inflammatory responses and promote the opening of the epithelial barrier at cellular junctions allowing for the egress of inflammatory mediators and cells from the tissues to the lumen [17]. Opening of the epithelial barrier may also be permissive for bacterial invasion if these host responses do not control the infection at the mucosal surface. Direct seeding of the bloodstream from the nasopharynx, a process referred to as occult bacteremia, is a well-recognized complication of pneumococcal carriage particularly in early childhood.
By 24 h postinoculation, there is a mild suppurative rhinitis characterized by an influx of neutrophils into the nasal spaces (Fig. 1). Dense collections of pneumococci are seen associated with these luminal neutrophils during days 1 to 3 after acquisition of the organism. This histopathologic process in the upper airway resembles that which is seen in the lungs during pneumococcal pneumonia. Interestingly, if a pneumolysin-deficient mutant or point mutant deficient in pore-formation is tested, this inflammatory response is greatly diminished [18, 19]. In the absence of this robust inflammatory response, subsequent development of adaptive immunity is delayed and carriage persists for a longer duration. This observation suggests that the pneumolysin-dependent inflammatory response provides an advantage for the pneumococcus that must outweigh the negative consequences associated with more rapid clearance. Perhaps, secretions associated with the acute inflammatory response during early carriage are important as a vehicle for host to host spread. In this regard, during experimental pneumococcal colonization of humans, many of the participants developed a mild rhinorrhea without other symptoms. Together, these observations provide evidence that initiation of pneumococcal colonization is an inflammatory rather than a quiescent process.
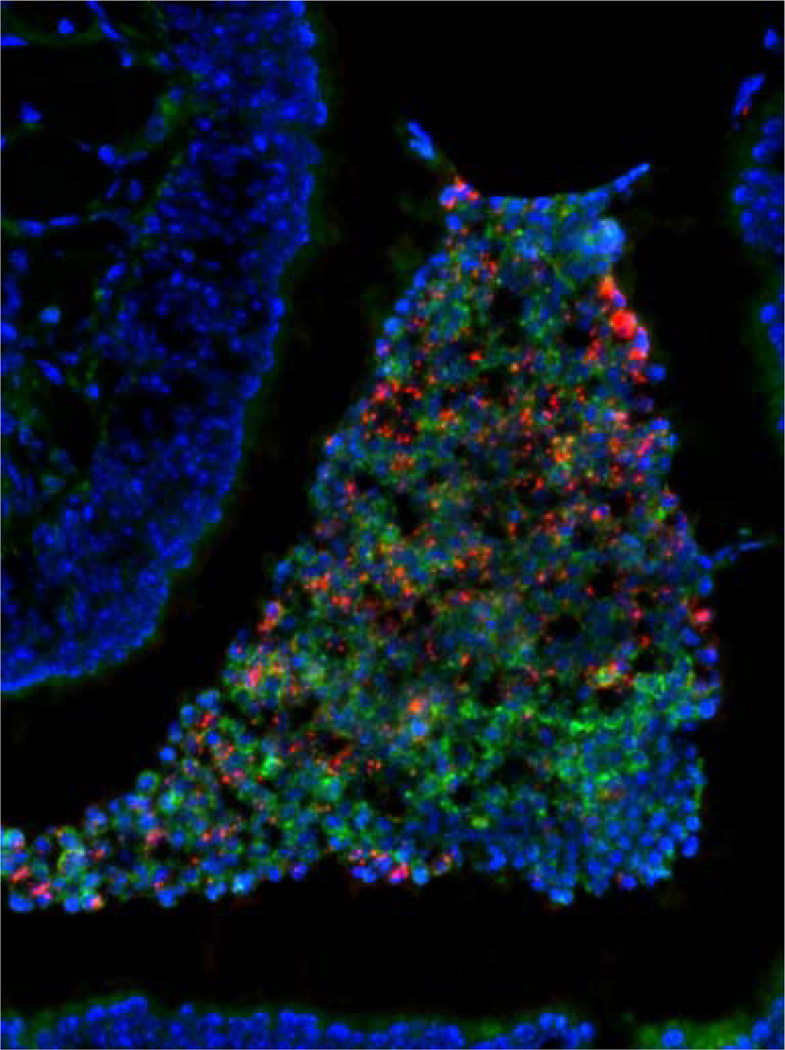
Fig. 1.
Nasal colonization of C57Bl/6 mice with a serotype 4 pneumococcal isolate. Forty-eight hours following intranasal inoculation bacteria are seen in association with neutrophils that have migrated into the nasal spaces. Bacteria (red) were detected using type-specific antisera. Mouse tissue was stained using DAPI (4′,6-diamidino-2-phenylindole; blue). Neutrophils are stained (green) with a mAb to mouse Ly6G. Magnification 200×. Images courtesy of A. Roche, University of Pennsylvania. (figure published in Infection and Immunity, American Society for Microbiology Publications, January 2007, volume 75. The copyright remains with ASM. ASM grants a nonexclusive license number 2252091449539 to use this material.) 149 × 200 mm (300 × 300 DPI)
Clearance of pneumococcal colonization
The brisk influx of neutrophils during primary infection does not clear the organism; pneumococci persist along the epithelial surfaces long after these inflammatory cells are no longer detected. Although neutrophil-depletion predisposes mice to develop bloodstream infection, it has minimal impact on the density of colonizing pneumococci [18]. Colonization is eventually resolved over a period of several weeks. This requires a more gradual recruitment of monocytes/macrophages into the nasal spaces [20]. The recruitment of macrophages is dependent on TLR2 signaling but also requires cellular immune responses involving CD4+ T cells and IL-17. During secondary pneumococcal infection, when these cellular immune responses are primed, the recruitment of neutrophils is more robust and effective at reducing the burden of colonizing organisms. This may be because specific antibody is available to enhance neutrophil-mediated opsonophagocytic killing, whereas, during primary infection the neutrophilic infiltrate has resolved by the time opsonic antibody is generated.
Evasion of clearance during acute inflammation
The events previously described demonstrate that the ability of the pneumococcus to survive and persist during the acute inflammatory response it induces is critical to its success as a commensal. This ability may also underlie its success as a pathogen, since there is also a marked inflammatory response during infections such as acute pneumococcal pneumonia. The critical attribute that allows the pneumococcus to resist killing by neutrophils is its antiphagocytic capsular polysaccharide. In the animal model, unencapsulated mutants colonize poorly [15]. In ex vivo assays, once taken up by neutrophils, the organism is rapidly killed by serine proteases released into the phagolysosome [21]. The relative effectiveness of structurally different capsular polysaccharides in inhibiting phagocytic uptake varies. These differences may affect the fitness of pneumococcal types during colonization and explain, at least in part, why a limited number of types are consistently shown to be more prevalent in carriage surveys [22]. Differences in resistance to phagocytosis may also be a factor in the attack rates for invasive infection, which varies by type in as much as 60-fold [23, 24]. In this regard, a more complete understanding of population biology of the organism may allow greater insight into why some strains may be associated with disease more than others. For example, genomic analysis and whole genome sequencing of multiple isolates has identified virulence determinants, such as pneumococcal pili adhesin, associated with a clone of greater pathogenicity [25].
Additionally, the pneumococcus is preprogrammed to meet the differing demands its commensal lifestyle and events during an inflammatory response. Most pneumococcal isolates display phase variation between two forms as distinguished by their opaque or transparent colony morphology [26]. During initial colonization, transparent variants with a thinner capsule layer and other characteristics that promote binding to host tissues prevail [15, 27]. In both mice models and in humans, opaque variants that express increased amounts of capsular polysaccharide per cell and are more resistant to opsonophagocytic killing are selected for in the transition from the mucosal surface to bloodstream [28, 29].
It appears that there may be additional mechanism employed by the pneumococcus to evade clearance by neutrophils beyond the expression of capsule. Ongoing studies conducted in this laboratory have taken a whole genomic approach to identify pneumococcal genes that affect killing by human neutrophils, complement and natural antibody. These studies demonstrate that exoglycosidases act on human complement components to diminish their opsonic activity. Deglycosylation of human secretory IgA and C-reactive protein, which may also act as opsonins, has previously been described [10]. It is likely that other pneumococcal products also act to inhibit neutrophil-mediated clearance. Thus, the pneumococcus has the ability to trigger inflammatory events and then resist clearance by the responses it induces.
Competitive interactions during colonization
Recent experience with the pneumococcal conjugate vaccine has revealed the importance of intraspecies competition among pneumococci to become established in the population. The vaccine reduces carriage of targeted types and has allowed formerly uncommon types not included in the vaccine formulation to become more common in a process referred to as serotype replacement [30]. There is now some understanding of the biological basis of this intraspecies competition. Pneumococci express bacteriocins (pneumocins) that target other members of the same species not expressing a cognate immunity factor [31]. Both pneumocins and immunity factors are encoded by the blp locus and are regulated in response to a quorum-sensing pheromone also generated by this locus [32]. The blp locus is among the most heterogeneous regions on the genome suggesting that it is a focus of intense selective pressure. The requirements of intraspecies competition and the need to acquire pneumocins and/or immunity factors may be a driving force in the marked ability of this species to adapt by homologous gene transfer.
In addition to competition among cocolonizing pneumococci, the organism must cope with other species competing for the same niche on the mucosal surface. This laboratory has modeled these interactions during cocolonization with Haemophilus influenzae [33]. Although both S. pneumoniae and H. influenzae colonize mice when given individually, coinoculation results in increased inflammation and subsequent clearance of the pneumococcus. H. influenzae stimulated clearance requires both neutrophils and complement and is dependent on recognition of peptidoglycan fragments of H. influenzae through the Nod1 signaling pathway [34]. These observations demonstrate that one species can benefit from the host inflammatory response to eliminate a competitor. A further implication is that the resistance of pneumococci to neutrophils recruited to the nasal spaces can be overcome by appropriate signals. Moreover, these results predict that pneumococcal types, which are more resistant to neutrophil-mediated killing, may be selected during interspecies competition mediated by the host inflammatory response. Thus, survival of competitive interactions among the flora during colonization may be another driving force in the evolution of “virulence determinants” that enhance resistance to opsonophagocytic clearance.
Implications of pneumococcal adaptation
Pneumococci are adept at taking up DNA from their environment. Because incorporation of sequences requires homologous recombination, genetic exchange occurs primarily between pneumococci or closely related oral streptococci. Natural competence involves a large proportion of the pneumococcal genome (>100 genes) and is controlled by a quorum-sensing pheromone in a manner similar to pneumocins [35]. Why does the pneumococcus seem to devote so much of its compact genome to genetic exchange? One reason may be that the adaptation to life in the oxygen-rich environment of the airway surface results in a high rate of spontaneous mutation caused by oxidative damage and that natural competence allows for the correction of deleterious mutations. Pneumococci use oxygen in very limited ways. One is the aerobic metabolism of pyruvate, which when oxidized to generate acetate, produces additional ATP [36]. The byproduct of pyruvate oxidation by SpxB is hydrogen peroxidase, which pneumococci can generate at concentrations equivalent to those produced by neutrophils. Enough hydrogen peroxide is generated as a consequence of its normal metabolic activity to damage host tissues or kill other organisms that compete for the same niche [37–41]. High concentrations of endogenously produced hydrogen peroxide leads to oxidative damage to DNA through the generation of 8-oxoguanine and its mismatch repair [42]. There is evidence that oxidative stress contributes to spontaneous mutation in vivo. Resistance to fluoroquinolone antibiotics occurs by the accumulation of point mutations in DNA gyrase and topoisomerase. For patients developing resistance while on therapy, most of these mutations involve mismatch repair of guanine residues [43]. Therefore, the pneumococcus is uniquely positioned to maintain beneficial mutations in the presence of the selective pressure of antibiotics and to gain function by horizontal gene transfer. For example, the natural competence of the species has contributed to the acquisition of sequences conferring reduced affinity for β-lactam drugs [44].
Conclusion
The ability of the pneumococcus to colonize its host, adapt through genetic exchange, and express virulence factors are inherent properties that contribute to its success as a commensal. The acquisition of these characteristics is largely driven by its metabolism, the need to evade the local mucosal immune response, and competitive interactions among the local flora. Many of these same selective pressures have led to the acquisition of traits that simultaneously allow for efficient colonization as well as invasive disease. These attributes combine to make the pneumococcus a particularly problematic pathogen.
Acknowledgments
The author is indebted to the current and former members of his laboratory contributing to these studies. This work was supported by grants from the U.S. Public Health Service (AI44231, AI38446, and AI78538).
Footnotes
Conflict of interest statement The author declares no conflict of interest.
References
- 1.Tomasz Pneumococcus at the gates. New Eng J Med. 1995;333:514–515. doi: 10.1056/NEJM199508243330810. [DOI] [PubMed] [Google Scholar]
- 2.Musher D. How contagious are common respiratory tract infections? N Engl J Med. 2003;348:1256–1266. doi: 10.1056/NEJMra021771. [DOI] [PubMed] [Google Scholar]
- 3.Austrian R. Some aspects of the pneumococcal carrier state. J Antimicrob Chemother. 1986;18:35–45. doi: 10.1093/jac/18.supplement_a.35. [DOI] [PubMed] [Google Scholar]
- 4.Bogaert D, de Groot R, Hermans P. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis. 2004;4:144–154. doi: 10.1016/S1473-3099(04)00938-7. [DOI] [PubMed] [Google Scholar]
- 5.Kilian M, Poulsen K, Blomqvist T, Håvarstein L, Bek-Thomsen M, Tettelin H, Sørensen U. Evolution of Streptococcus pneumoniae and its close commensal relatives. PLoS One. 2008;3:e2683. doi: 10.1371/journal.pone.0002683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watson DA, Musher DM. Interuption of capsule production in Streptococcus pneumoniae serotype 3 by insertion of transposon Tn916. Infect Immun. 1990;58:3135–3138. doi: 10.1128/iai.58.9.3135-3138.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marriott H, Mitchell T, Dockrell D. Pneumolysin: a double-edged sword during the host-pathogen interaction. Curr Mol Med. 2008;8:497–550. doi: 10.2174/156652408785747924. [DOI] [PubMed] [Google Scholar]
- 8.Maus U, Srivastava M, Paton J, Mack M, Everhart M, Blackwell T, Christman J, Schlondorff D, Seeger W, Lohmeyer J. Pneumolysin-induced lung injury is independent of leukocyte trafficking into the alveolar space. J Immunol. 2004;173:1307–1312. doi: 10.4049/jimmunol.173.2.1307. [DOI] [PubMed] [Google Scholar]
- 9.Ratner A, Hippe K, Aguilar J, Bender M, Nelson A, Weiser J. Epithelial cells are sensitive detectors of bacterial pore-forming toxins. J Biol Chem. 2006;281:12994–12998. doi: 10.1074/jbc.M511431200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.King S, Hippe K, Weiser J. Deglycosylation of human glycoconjugates by the sequential activities of exoglycosidases expressed by Streptococcus pneumoniae. Mol Microbiol. 2006;59:961–974. doi: 10.1111/j.1365-2958.2005.04984.x. [DOI] [PubMed] [Google Scholar]
- 11.Bender MH, Weiser JN. The atypical amino-terminal LPNTG-containing domain of the pneumococcal human IgA1-specific protease is required for proper enzyme localization and function. Mol Microbiol. 2006;61:526–543. doi: 10.1111/j.1365-2958.2006.05256.x. [DOI] [PubMed] [Google Scholar]
- 12.McCool T, Cate T, Moy G, Weiser J. The immune response to pneumococcal proteins during experimental human carriage. J Exp Med. 2002;195:359–365. doi: 10.1084/jem.20011576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCool T, Weiser J. Limited role of antibody in clearance of Streptococcus pneumoniae in a murine model of colonization. Infect Immun. 2004;72:5807–5813. doi: 10.1128/IAI.72.10.5807-5813.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu H, Virolainen A, Mathews B, King J, Russell M, Briles D. Establishment of a Streptococcus pneumoniae nasopharyngeal colonization model in adult mice. Microb Pathog. 1997;23:127–137. doi: 10.1006/mpat.1997.0142. [DOI] [PubMed] [Google Scholar]
- 15.Nelson A, Roche A, Gould J, Chim K, Ratner A, Weiser J. Capsule enhances pneumococcal colonization by limiting mucus-mediated clearance. Infect Immun. 2007;75:83–90. doi: 10.1128/IAI.01475-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beisswenger C, Lysenko E, Weiser J. Early bacterial colonization induces toll-like receptor-dependent transforming growth factor beta signaling in the epithelium. Infect Immun. 2009;77:2212–2220. doi: 10.1128/IAI.01224-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beisswenger C, Coyne C, Shchepetov M, Weiser J. Role of p38 MAP kinase and transforming growth factor-beta signaling in transepithelial migration of invasive bacterial pathogens. J Biol Chem. 2007;282:28700–28708. doi: 10.1074/jbc.M703576200. [DOI] [PubMed] [Google Scholar]
- 18.Matthias K, Roche A, Standish A, Shchepetov M, Weiser J. Neutrophil-toxin interactions promote antigen delivery and mucosal clearance of Streptococcus pneumoniae. J Immunol. 2008;180:6246–6254. doi: 10.4049/jimmunol.180.9.6246. [DOI] [PubMed] [Google Scholar]
- 19.van Rossum A, Lysenko E, Weiser J. Host and bacterial factors contributing to the clearance of colonization by Streptococcus pneumoniae in a murine model. Infect Immun. 2005;73:7718–7726. doi: 10.1128/IAI.73.11.7718-7726.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Z, Clarke T, Weiser J. Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. J Clin Invest. 2009;119:1899–1909. doi: 10.1172/JCI36731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Standish A, Weiser JN. Human neutrophils kill Streptococcus pneumoniae via serine proteases. J Immunol. 2009;183:2602–2609. doi: 10.4049/jimmunol.0900688. [DOI] [PubMed] [Google Scholar]
- 22.Weinberger D, Trzciński K, Lu Y, Bogaert D, Brandes A, Galagan J, Anderson P, Malley R, Lipsitch M. Pneumococcal capsular polysaccharide structure predicts serotype prevalence. PLoS Pathog. 2009;5:e1000476. doi: 10.1371/journal.ppat.1000476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sleeman K, Griffiths D, Shackley F, Diggle L, Gupta S, Maiden M, Moxon E, Crook D, Peto T. Capsular serotype-specific attack rates and duration of carriage of Streptococcus pneumoniae in a population of children. J Infect Dis. 2006;194:682–688. doi: 10.1086/505710. [DOI] [PubMed] [Google Scholar]
- 24.Brueggemann AB, Griffiths DT, Meats E, Peto T, Crook DW, Spratt BG. Clonal relationships between invasive and carriage Streptococcus pneumoniae and serotype- and clone-specific differences in invasive disease potential. J Infect Dis. 2003;187:1424–1432. doi: 10.1086/374624. [DOI] [PubMed] [Google Scholar]
- 25.Sjöström K, Blomberg C, Fernebro J, Dagerhamn J, Morfeldt E, Barocchi M, Browall S, Moschioni M, Andersson M, Henriques F, et al. Clonal success of piliated penicillin nonsusceptible pneumococci. Proc Natl Acad Sci U S A. 2007;104:12907–12912. doi: 10.1073/pnas.0705589104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weiser J, Austrian R, Sreenivasan P, Masure H. Phase variation in pneumococcal opacity: relationship between colonial morphology and nasopharyngeal colonization. Infect Immun. 1994;62:2582–2589. doi: 10.1128/iai.62.6.2582-2589.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cundell DR, Weiser JN, Shen J, Young A, Tuomanen EI. Relationship between colonial morphology and adherence of Streptococcus pneumoniae. Infect Immun. 1995;63:757–761. doi: 10.1128/iai.63.3.757-761.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim JO, Romero-Steiner S, Sørensen U, Blom J, Carvalho M, Barnardi S, Carlone G, Weiser JN. Relationship between cell-surface carbohydrates and intrastrain variation on opsonophagocytosis of Streptococcus pneumoniae. Infec Immun. 1999;67:2327–2333. doi: 10.1128/iai.67.5.2327-2333.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weiser J, Bae D, Epino H, Gordon S, Kapoor M, Zenewicz L, Shchepetov M. Changes in availability of oxygen accentuate differences in capsular polysaccharide expression by phenotypic variants and clinical isolates of Streptococcus pneumoniae. Infect Immun. 2001;69:5430–5439. doi: 10.1128/IAI.69.9.5430-5439.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klugman K. The significance of serotype replacement for pneumococcal disease and antibiotic resistance. Adv Exp Med Biol. 2009;634:121–128. doi: 10.1007/978-0-387-79838-7_11. [DOI] [PubMed] [Google Scholar]
- 31.Dawid S, Roche A, Weiser J. The blp bacteriocins of Streptococcus pneumoniae mediate intraspecies competition both in vitro and in vivo. Infect Immun. 2007;75:443–451. doi: 10.1128/IAI.01775-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dawid S, Sebert M, Weiser J. Bacteriocin activity of Streptococcus pneumoniae is controlled by the serine protease HtrA via posttranscriptional regulation. J Bacteriol. 2009;191:1509–1518. doi: 10.1128/JB.01213-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lysenko E, Ratner A, Nelson A, Weiser J. The role of innate immune responses in the outcome of interspecies competition for colonization of mucosal surfaces. PLoS Pathogens. 2005;1:1–9. doi: 10.1371/journal.ppat.0010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lysenko E, Clarke T, Shchepetov M, Ratner A, Roper D, Dowson C, Weiser J. Nod1-signaling overcomes resistance of Streptococcus pneumoniae to opsonophagocytic killing. PLoS Pathog. 2007;3:1073–1081. doi: 10.1371/journal.ppat.0030118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peterson SN, Sung CK, Cline R, Desai BV, Snesrud EC, Luo P, Walling J, Li H, Mintz M, Tsegaye G, et al. Identification of competence pheromone responsive genes in Streptococcus pneumoniae by use of DNA microarrays. Mol Microbiol. 2004;51:1051–1070. doi: 10.1046/j.1365-2958.2003.03907.x. [DOI] [PubMed] [Google Scholar]
- 36.Spellerberg B, Cundell DR, Sandros J, Pearce BJ, Idanpaan-Heikkila I, Rosenow C, Masure HR. Pyruvate oxidase, as a determinant of virulence in Streptococcus pneumoniae. Mol Microbiol. 1996;19:803–813. doi: 10.1046/j.1365-2958.1996.425954.x. [DOI] [PubMed] [Google Scholar]
- 37.Pericone C, Overweg K, PW H, Weiser J. Inhibitory and bactericidal effects of hydrogen peroxide production by Streptococcus pneumoniae on other inhabitants of the upper respiratory tract. Infect Immun. 2000;68:3990–3997. doi: 10.1128/iai.68.7.3990-3997.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Regev-Yochay G, Trzcinsk IK, Thompson C, Malley R, Lipsitch M. Interference between Streptococcus pneumoniae and Staphylococcus aureus: in vitro hydrogen peroxide-mediated killing by Streptococcus pneumoniae. J Bacteriol. 2006;188:4996–5001. doi: 10.1128/JB.00317-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park B, Nizet V, Liu G. Role of Staphylococcus aureus catalase in niche competition against Streptococcus pneumoniae. J Bacteriol. 2008;190:2275–2278. doi: 10.1128/JB.00006-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoffmann O, Zweigner J, Smith S, Freyer D, Mahrhofer C, Dagand E, Tuomanen E, Weber J. Interplay of pneumococcal hydrogen peroxide and host-derived nitric oxide. Infect Immun. 2006;74:5058–5066. doi: 10.1128/IAI.01932-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feldman C, Anderson R, Cockeran R, Mitchell T, Cole P, Wilson R. The effects of pneumolysin and hydrogen peroxide, alone and in combination, on human ciliated epithelium in vitro. Respir Med. 2002;96:580–585. doi: 10.1053/rmed.2002.1316. [DOI] [PubMed] [Google Scholar]
- 42.Pericone C, Bae D, Shchepetov M, McCool T, Weiser J. Short-sequence tandem and nontandem DNA repeats and endogenous hydrogen peroxide production contribute to genetic instability of Streptococcus pneumoniae. J Bacteriol. 2002;184:4392–4399. doi: 10.1128/JB.184.16.4392-4399.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davidson R, Cavalcanti R, Brunton J, Bast D, De Azavedo J, Kibsey P, Fleming C, Low D. Resistance to levofloxacin and failure of treatment of pneumococcal pneumonia. N Engl J Med. 2002;346:747–750. doi: 10.1056/NEJMoa012122. [DOI] [PubMed] [Google Scholar]
- 44.Dowson C, Coffey T, Spratt B. Origin and molecular epidemiology of penicillin-binding-protein-mediated resistance to beta-lactam antibiotics. Trends Microbiol. 1994;2:361–366. doi: 10.1016/0966-842x(94)90612-2. [DOI] [PubMed] [Google Scholar]