Abstract
Background
The measurement of brain perfusion may provide valuable information for assessment and treatment of newborns with hypoxic-ischemic encephalopathy (HIE). While arterial spin labeled perfusion (ASL) magnetic resonance imaging (MRI) provides noninvasive and direct measurements of regional cerebral blood flow (CBF) values, it is logistically challenging to obtain. Near-infrared spectroscopy (NIRS) might be an alternative, as it permits noninvasive and continuous monitoring of cerebral hemodynamics and oxygenation at the bedside.
Objective
The purpose of this study is to determine the correlation between measurements of brain perfusion by NIRS and by MRI in term newborns with HIE treated with hypothermia.
Design/Methods
In this prospective cohort study, ASL-MRI and NIRS performed during hypothermia were used to assess brain perfusion in these newborns. Regional cerebral blood flow values (CBF), measured from 1–2 MRI scans for each patient, were compared to mixed venous saturation values (SctO2) recorded by NIRS just before and after each MRI. Analysis included groupings into moderate versus severe HIE based on their initial background pattern of amplitude-integrated electroencephalogram.
Results
Twelve concomitant recordings were obtained of seven neonates. Strong correlation was found between SctO2 and CBF in asphyxiated newborns with severe HIE (r = 0.88; p value = 0.0085). Moreover, newborns with severe HIE had lower CBF (likely lower oxygen supply) and extracted less oxygen (likely lower oxygen demand or utilization) when comparing SctO2 and CBF to those with moderate HIE.
Conclusions
NIRS is an effective bedside tool to monitor and understand brain perfusion changes in term asphyxiated newborns, which in conjunction with precise measurements of CBF obtained by MRI at particular times, may help tailor neuroprotective strategies in term newborns with HIE.
Keywords: brain, hypoxic-ischemic encephalopathy, magnetic resonance imaging, newborn, near-infrared spectroscopy, perfusion
INTRODUCTION
Hypoxic-ischemic encephalopathy (HIE) remains the most common cause of brain injury in term newborns. Induced hypothermia is currently the only existing treatment to minimize brain injury in these newborns, with decreased death and disability rates at 12–18 months and beyond (Azzopardi et al., 2009; Eicher et al., 2005; Gluckman et al., 2005; Jacobs et al., 2007; Shankaran et al., 2005, 2012). However, some newborns still develop brain injury despite this treatment (Barks, 2008; Higgins et al., 2006; Higgins and Shankaran, 2009). It has been demonstrated that hyperperfusion may be present early on in these newborns, despite treatment with induced hypothermia (Wintermark et al., 2011), and was probably a manifestation of reperfusion injury. The measurement of brain perfusion is thus valuable for the early assessment and potentially the management of newborns with HIE, even if treated with hypothermia.
Many approaches have been applied to measure brain perfusion in newborns, and especially newborn with HIE. Most techniques provide only discrete, episodic data and are limited in assessing dynamic changes in cerebral circulation (Levene et al., 1989; Minhas et al., 2003). Arterial spin labeled perfusion magnetic resonance imaging (ASL-MRI) is currently the only method that provides noninvasive and direct measurements of regional cerebral blood flow (CBF) values in multiple brain regions (Biagi et al., 2007; Huisman and Sorensen, 2004; Miranda et al., 2006; Wang et al., 2006a, 2006b; Wintermark P, AJNR 2011). However, obtaining MRI scans in critically ill newborns, is logistically challenging, expensive and not widely available. Near-infrared spectroscopy (NIRS) might prove to be an attractive alternative (Ancora et al., 2009, 2011; Grant et al., 2009; Gucuyener et al., 2012; Toet et al., 2006; Toet and Lemmers, 2009; Wolfberg and du Plessis, 2006), as it permits continuous bedside monitoring of cerebral hemodynamics and oxygenation, by measuring changes in the concentration of oxygenated and deoxygenated hemoglobin (Marin and Moore, 2011; Meek et al., 1999; Pellicer and del Carmen Bravo, 2011; Soul and du Plessis, 1999; Wyatt, 1993). It thus does not provide direct measurement of CBF in different brain regions, but records regional mixed venous saturation (SctO2), which are representative of oxygen supply/demand ratio. NIRS monitoring is also much less expensive, and easily allows serial measures to be taken over time, which may be highly valuable in disorders such as HIE where brain perfusion and oxygen metabolism change over the course of the illness. NIRS monitoring is now becoming widely used in premature newborns (Greisen et al., 2011), as it provides valuable insights on the impact of intensive care on early brain development (Greisen et al., 2011; Roche-Labarbe et al., 2012). For example, SctO2 has been demonstrated to correlate with chronological age (Roche-Labarbe et al., 2012). NIRS has permitted to identify premature newborns with impaired cerebrovascular autoregulation, and thus with a high likelihood of severe brain injury (Tsuji et al., 2000). NIRS measurements were also showed to be highly influenced by routine caregiving procedures (Limperopoulos et al., 2008). Such extended use needs probably to be extended to the asphyxiated term newborns.
This study aimed to compare measurements of brain perfusion by NIRS and by MRI at specific time-points in term newborns with HIE treated with therapeutic hypothermia to determine whether NIRS provides a clinically useful measure of brain perfusion and oxygenation, as validated by ASL-MRI measures.
MATERIALS AND METHODS
Patients
We conducted a prospective cohort study of term newborns with HIE admitted to the neonatal intensive care unit (NICU) within six hours of life, who met criteria for therapeutic hypothermia: (1) gestational age ≥ 36 weeks and birth weight ≥ 2000 g; (2) evidence of fetal distress, e.g., history of acute perinatal event or cord pH ≤ 7.0; (3) evidence of neonatal distress, such as Apgar score ≤ 5 at 10 minutes, postnatal blood gas pH obtained within the first hour of life ≤ 7.0, or need for ventilation initiated at birth and continued for at least 10 minutes; (4) evidence of neonatal encephalopathy by physical examination and by abnormal initial amplitude-integrated electroencephalogram (aEEG) background pattern. Newborns who met the above criteria received whole-body cooling to an esophageal temperature of 33.5°C, initiated by 6 h of life, continued for 72 h (unless contraindications developed), and were then slowly rewarmed over 12 hours per standard protocol. This study was approved by the local Institutional Review Board, and parental consent was obtained.
The aEEG recording was started as soon as the patient was admitted to the neonatal intensive care unit. Asphyxiated newborns treated with induced hypothermia were categorized as moderately or severely abnormal according to their initial background pattern of aEEG (al Naqueeb et al., 1999; Gluckman et al., 2005). Initial background pattern was considered as moderately abnormal if lower margin less than 5 mcV, upper margin more than 10 mcV, and absence of sleep-wake cycling (al Naqueeb et al., 1999). Initial background pattern was considered as severely abnormal if lower margin less than 5 mcV, upper margin less than 10 mcV, and presence of periodic bursts of higher voltage electrical activity (spikes) (al Naqueeb et al., 1999).
Other variables influencing brain perfusion (temperature, partial pressure of carbon dioxide (pCO2), fraction of inspired oxygen (FiO2), hematocrit levels, mechanical ventilation, pressor support, sedation, antiepileptic treatment) were also recorded on the day of the MR imaging.
Near-infrared spectroscopy (NIRS)
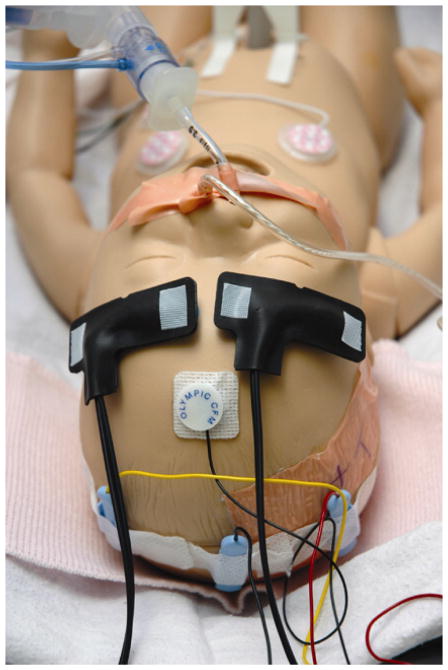
NIRS monitor (FORE-SIGHT Cerebral Oximeter; CAS Medical Systems, Branford, CT) was used for non-invasive continuous monitoring at the bedside during the 72-hour of hypothermia treatment and the rewarming period. Two neonatal sensors were placed respectively on each side of the newborn’s forehead, over the area of frontal lobes (FIGURE 1). Regional cerebral oxygen saturation (SctO2) was recorded continuously.
FIGURE 1. Positions of the near-infrared spectroscopy (NIRS) sensors.
For the purpose of monitoring continuously mixed venous saturation (SctO2), two neonatal NIRS sensors were placed respectively on each side of the newborn’s forehead, over the area of frontal lobes.
Brain magnetic resonance imaging (MRI)
Asphyxiated newborns underwent MRI once or twice within the first 72 hours after birth, i.e. during the hypothermia treatment, on day 1 (i.e. within 24 hours of birth) and/or on day 2 (24–48 hours after birth). Patients continued to receive hypothermia treatment during the MR imaging without any adverse events (Wintermark et al., 2010). Any ventilatory support, pressor support requirement, or sedation used for treatment in the NICU was also maintained during the MRI acquisition, but additional sedation was avoided. Only asphyxiated newborns, who were hemodynamically stable, underwent the MRI scans during hypothermia treatment. MR imaging was performed with a 3T Magnetom Trio scanner (Siemens, Erlangen, Germany), using a 32-channel head coil in most cases (Siemens) (Wiggins et al., 2006) or a standard 12-channel head coil. A pulsed arterial spin labeling (PASL) sequence (Luh et al., 1999) (with TR/TE, 2400/13 ms; matrix size, 64 × 64; FOV, 192 mm; 15 axial sections with a section thickness of 4.9 mm) was acquired with high-spatial-resolution anatomic T1- and T2-weighted images and diffusion-weighted imaging (DWI). Regional cerebral blood flow (CBF) maps were obtained from the PASL Postprocessing Functor (Siemens). Quantitative estimates of regional CBF were made by using the formula described by Wang et al. (Wang et al., 2003):
M0 was estimated by measuring the fully relaxed signal intensity and ΔM by the average difference in signal intensity between control and tag acquisitions. The conversion efficiency α was assumed to be 95% and the blood/tissue water partition coefficient λ, to be 1.2 mL/g, with TI1/TI2 = 700/1400 ms and the longitudinal relaxation time of blood T1a = 1500 ms (Cavusoglu et al., 2009). The imaging sections were positioned axially covering the brain, and the acquisition order was ascending (inferior to superior) to reduce the required TI2. The labeling slab had a thickness of 50 mm and was positioned with a gap of 10 mm below the most proximal section. The labeling slab thus covered the lower part of the head and the neck, but not the upper part of the chest so as to exclude the heart. Quantification of regional CBF was performed by using the CBF maps (FIGURE 2).
FIGURE 2. Example of axial cerebral blood flow (CBF) (mL/100g/min) maps obtained at different brain levels with the described pulsed arterial spin labeling (PASL) MRI sequence in a normal term newborn.

Perfusion was higher in the gray matter and in the basal ganglia compared to the white matter.
For the purpose of this study, manually drawn regions of interest were placed in the gray matter of frontal lobes bilaterally, by looking at the same time at the ASL data and the corresponding T2-weighted imaging; coregistration was not used. Measurements were obtained in the right and left side of the cerebrum in these tissue regions; sizes of the regions of interest were always similar. Motion during MRI may cause severe artifacts in the acquired images, especially for PASL. These were minimized by wrapping the neonates in an MRI-compatible vacuum cushion. In addition, a 3D prospective acquisition-correction technique was used with the PASL sequence to reduce motion-induced effects on magnetization history.
Conventional sequences obtained during the same or later MRI studies were used to define the presence and extent of brain injury in these patients. Neuroradiologists interpreted the brain conventional MR images (T1- and T2-weighted images and diffusion-weighted images).
Estimation of relative cerebral metabolic rate of oxygen (rCMRO2)
To estimate rCMRO2 (mLO2/100g/min) from our NIRS measurements of SctO2 and MRI measurements of CBF, we used the formula described by Roche-Labarbe et al. (Roche-Labarbe et al., 2012):
Hb (g/L) is the hemoglobin concentration in the blood. CBF (mL/100g/min) is the cerebral blood flow, measured by MRI in this study. SaO2 (%) is the arterial hemoglobin oxygenation, estimated with the pulse oximeter. SctO2 (%) is the cerebral oxygen saturation measured by NIRS in this study. MWHb (64500 g/Mol) is the molecular weight of hemoglobin.
Statistical analysis
SctO2 measurements for each hemisphere were performed 10 minutes before and 10 minutes after each MRI and were averaged between the left and right sides and correlated to the CBF values averaged from both frontal lobes obtained by PASL. After this first comparison, we explored the relationship between SctO2 and CBF in the subgroups based on initial aEEG severity, i.e., moderate vs. severe encephalopathy. The association between SctO2 values and CBF values between each group was explored using spearman correlations, as the data were not normally distributed. Analysis was performed using SAS 9.1 (Carey, NC).
RESULTS
Twelve concomitant recordings were obtained in seven asphyxiated newborns treated with hypothermia. Five of these seven patients had brain MRIs on day 1 and 2; the remaining two had only one MRI during hypothermia on day 2. Mean CBF value averaged from both frontal lobes measured on each MRI was correlated with averaged SctO2 measured just before and just after the MRI (TABLE 1). For all patients for whom measurements were performed on both days 1 and 2, SctO2 and CBF values increased from day 1 to day 2 (FIGURE 3).
Table 1.
Regional cerebral oxygen saturation (SctO2) and cerebral blood flow (CBF) of the study patients.
| Left SctO2 % | Right SctO2 % | Average SctO2 % | Left frontal CBF mL/100g/min | Right frontal CBF mL/100g/min | Average frontal CBF mL/100g/min | Hb g/L | rCMRO2 mLO2/100g/min | |
|---|---|---|---|---|---|---|---|---|
| Patient 1 | ||||||||
| Day 1 | 78.7 ± 1.0 | 81.9 ± 1.0 | 80.3 ± 0.7 | 24.2 ± 2.1 | 23.7 ± 2.1 | 23.9 ± 1.9 | 133.0 | 0.72 |
| Day 2 | 89.0 ± 2.9 | 86.7 ± 0.6 | 87.8 ± 1.2 | 27.5 ± 1.4 | 28.4 ± 0.7 | 28.0 ± 1.1 | 129.5 | 0.40 |
| Patient 2 | ||||||||
| Day 1 | 72.8 ± 5.5 | 67.2 ± 5.6 | 70.0 ± 5.5 | 22.5 ± 4.1 | 17.6 ± 3.3 | 20.3 ± 5.9 | 178.5 | 1.40 |
| Day 2 | 76.1 ± 3.2 | 76.8 ± 3.5 | 76.5 ± 3.4 | 26.0 ± 2.5 | 24.9 ± 4.3 | 25.5 ± 3.2 | 175.0 | 1.28 |
| Patient 3 | ||||||||
| Day 1 | 75.9 ± 2.6 | 71.2 ± 0.3 | 73.5 ± 1.4 | 22.9 ± 5.4 | 22.5 ± 4.3 | 22.7 ± 4.3 | 168.0 | 1.27 |
| Day 2 | 85.5 ± 5.8 | 81.3 ± 1.2 | 83.5 ± 3.5 | 24.3 ± 5.0 | 26.5 ± 4.4 | 25.4 ± 4.4 | 140.0 | 0.63 |
| Patient 4 | ||||||||
| Day 1 | 70.7 ± 2.7 | 72.4 ± 2.2 | 71.5 ± 2.6 | 21.3 ± 1.1 | 24.0 ± 2.8 | 22.7 ± 2.4 | 133.0 | 1.10 |
| Patient 5 | ||||||||
| Day 1 | 73.8 ± 4.5 | 74.7 ± 3.6 | 74.2 ± 4.5 | 34.9 ± 2.9 | 37.3 ± 2.4 | 36.1 ± 2.8 | 122.5 | 1.43 |
| Day 2 | 78.8 ± 2.3 | 79.8 ± 3.0 | 79.3 ± 2.7 | 34.8 ± 3.9 | 39.9 ± 4.7 | 37.3 ± 4.3 | 129.5 | 1.18 |
| Patient 6 | ||||||||
| Day 1 | 74.8 ± 2.7 | 72.2 ± 0.7 | 73.5 ± 1.7 | 37.2 ± 2.1 | 38.0 ± 2.1 | 37.7 ± 1.9 | 133.0 | 1.67 |
| Day 2 | 75.2 ± 2.5 | 74.5 ± 1.9 | 74.9 ± 2.2 | 39.9 ± 1.0 | 38.3 ± 4.6 | 39.1 ± 3.1 | 126.0 | 1.54 |
| Patient 7 | ||||||||
| Day 2 | 82.9 ± 2.6 | 86.3 ± 4.2 | 84.6 ± 3.9 | 37.5 ± 1.5 | 36.2 ± 0.9 | 36.9 ± 1.3 | 147.0 | 0.87 |
SctO2 = regional cerebral oxygen saturation; CBF = cerebral blood flow; Hb = hemoglobin concentration in the blood; rCMRO2 = relative cerebral metabolic rate of oxygen Values presented as mean ± standard deviation
FIGURE 3. Comparison between cerebral blood flow (CBF) (mL/100g/min) measured by ASL-MRI and mixed venous saturation (SctO2) (%) measured by NIRS in asphyxiated newborns treated with hypothermia during the first days of life.
Both SctO2 and CBF increased from day 1 to 2 in all term newborns with HIE, despite hypothermia treatment. Newborns with severe encephalopathy had lower CBF than newborns with moderate encephalopathy. A strong correlation (r = 0.88, p < 0.01) between SctO2 and CBF was found in asphyxiated newborns with initially severe encephalopathy. Newborns who developed HI brain injury had higher SctO2 than newborns who did not develop brain injury.
Three of the asphyxiated newborns treated with hypothermia enrolled in the study had moderate encephalopathy on the initial aEEG, while the other four had severe encephalopathy. Differences were found between newborns with moderate versus severe HIE. Newborns with severe encephalopathy had lower CBF compared to those with moderate encephalopathy, despite similar SctO2 values, as demonstrated in FIGURE 3. Mean CBF was 24.07 ± 2.49 mL/100g/min in newborns with severe encephalopathy versus 37.42 ± 1.11 mL/100g/min in newborns with moderate encephalopathy (p <0.001). Mean SctO2 was 77.59 ± 6.57 % in newborns with severe encephalopathy versus 77.30 ± 4.67 % in newborns with moderate encephalopathy (p = 0.93). Also asphyxiated newborns with an initially severe encephalopathy showed a greater increase in SctO2 and more increased CBF from day 1 to day 2 than those presenting with an initially moderate HIE. Mean difference in CBF from day 1 to day 2 was 8.00 ± 1.80 mL/100g/min in newborns with severe encephalopathy versus 3.25 ± 2.62 mL/100g/min in newborns with moderate encephalopathy. Mean difference in SctO2 from day 1 to day 2 was 4.43 ± 1.92 % in newborns with severe encephalopathy versus 1.30 ± 0.14 % in newborns with moderate encephalopathy.
Mean rCMRO2 was lower in newborns with severe encephalopathy compared to newborns with moderate encephalopathy: respectively 0.97 ± 0.38 mLO2/100g/min versus 1.34 ± 0.32 mLO2/100g/min. Estimated rCMRO2 decreased from day 1 to day 2 in both groups of newborns: from 1.13 ± 0.29 mLO2/100g/min to 0.77 ± 0.45 mLO2/100g/min in newborns with severe encephalopathy, and from 1.55 ± 0.17 mLO2/100g/min to 1.20 ± 0.33 mLO2/100g/min in newborns with severe encephalopathy. Mean difference in rCMRO2 from day 1 to day 2 was lower in newborns with severe encephalopathy compared to newborns with moderate encephalopathy: respectively 0.36 ± 0.26 mLO2/100g/min versus 0.19 ± 0.08 mLO2/100g/min.
Statistical analysis using Spearman correlations showed no significant correlation (r = 0.34, p = 0.27, 12 measurements) between SctO2 measured by NIRS and CBF measured by MRI when all data for both moderate and severe encephalopathy groups were analyzed together. However, when data were separated according to severity of encephalopathy, a strong correlation (r = 0.88, p < 0.01, 7 measurements) between SctO2 and CBF was found in asphyxiated newborns with initially severe HIE (FIGURE 3). No such significance was found in the ones with an initially moderate HIE (r = −0.20, p = 0.74, 5 measurements), but only 5 different measurements were available and SctO2 and CBF measurements were around the same ranges in the same patients (i.e. not enough variation to find correlation).
Among these seven patients, five developed hypoxic-ischemic (HI) brain injury (Patients # 1, 2 and 3 with initial severe encephalopathy, as well as Patients # 5 and 7 with initial moderate encephalopathy) and two did not (Patient # 4 with initial severe encephalopathy and Patient # 6 with initial moderate encephalopathy). HI brain injury was located mainly in bilateral basal ganglia in three patients (Patients # 1, 2 and 3 with initial severe encephalopathy) and in bilateral anterior and posterior white matter in the other two patients (Patients # 5 and 7 with initial moderate encephalopathy). Mean CBF was 28.66 ± 6.90 mL/100g/min in newborns who developed HI brain injury versus 33.17 ± 9.09 mL/100g/min in newborns who did not develop brain injury (p = 0.49). Mean SctO2 was 78.86 ± 5.82 % in newborns who developed HI brain injury versus 73.30 ± 1.71 % in newborns who did not develop brain injury (p = 0.03).
DISCUSSION
Lowering body temperature is thought to decrease brain perfusion uniformly (Laptook and Corbett, 2002; Polderman, 2008). In our five cases with measurements on both day 1 and day 2, SctO2 and CBF values increased over time during hypothermia treatment, even though body temperature remained the same, consistent with a previous study (Wintermark et al., 2011). These findings suggest that CBF (and likely oxygen supply) increases over time and extraction of oxygen (and likely oxygen demand or utilization) decreases over time in asphyxiated newborns during hypothermia treatment. Estimated rCMRO2 also showed a decreased from day 1 to day 2 in all our asphyxiated newborns treated with hypothermia. It is unclear whether this increase in CBF represents the normal evolution of brain perfusion in encephalopathic patients during hypothermia or a manifestation of luxury perfusion described in asphyxia (Perlman, 2006). The observation that the two patients who did not develop any radiologically detectable brain injury showed this evolution suggests that it may not represent luxury perfusion, but this study is too small to be definitive about this interpretation.
Differences were found between newborns with moderate versus severe encephalopathy. Newborns with severe encephalopathy had lower CBF (likely lower oxygen supply) and seemed to extract less oxygen (lower oxygen demand or utilization). This may reflect intrinsic mechanisms to minimize further injury by lowering oxygen supply and oxygen demand in the most asphyxiated newborns. Estimated rCMRO2 was also lower in newborns with severe encephalopathy compared to newborns with moderate encephalopathy. This could be either the manifestation or the cause of the underlying severity of HIE. Previous studies in term neonates with severe HIE (not treated with hypothermia) using NIRS demonstrated changes in CBF and cerebral blood volume predicting an adverse outcome (Ancora et al., 2011; Archer et al., 1986; Toet et al., 2006; Van Bel et al., 1987; Wyatt, 1993). Moreover, the increase in CBF and SctO2 (and the decrease in estimated rCMRO2) from day 1 to day 2 was more pronounced in the severely asphyxiated newborns. This suggests that CBF (and likely oxygen supply) increases more over time and extraction of oxygen (and likely oxygen demand or utilization) decreases more over time in severely asphyxiated newborns during hypothermia treatment compared to moderately asphyxiated newborns. This may be because they started at lower oxygen supply and demand on the first day because of a more severe asphyxial insult compared to the moderately asphyxiated newborns. More data are needed to confirm these results in newborns treated with hypothermia to determine if the changes in oxygen supply and demand could serve as a predictor of long-term outcome. Detailed analysis of the 72-hour recording SctO2 would also be necessary to understand better how SctO2 changes during hypothermia treatment, how these changes correlate with the progress of injury, and how they are influenced by the degree of encephalopathy.
Previous studies have shown that asphyxiated newborns who developed HI brain injury despite hypothermia treatment displayed hypoperfusion followed by hyperperfusion during hypothermia in the brain regions later confirmed to be injured (Wintermark et al., 2011). A strong correlation between SctO2 and CBF was found in severely asphyxiated newborns treated with hypothermia. For lower CBF values (likely lower oxygen supply), there was more extraction of oxygen (likely higher oxygen demand) in these patients. Conversely, for higher CBF values (likely higher oxygen supply), there was less extraction of oxygen (likely lower oxygen demand or utilization). This correlation supports NIRS as a bedside measure proxy for brain perfusion in these newborns, as suggested also by other studies (Ancora et al., 2011; Toet et al., 2006). These patients derive less benefit from hypothermia treatment (Azzopardi et al., 2009; Gluckman et al., 2005), and have the worse neurologic outcomes. Therefore they are a high priority group to better understand the underlying pathophysiological mechanisms, in hopes of adjusting their treatment to improve their outcome. Such conclusion cannot be drawn in the moderate encephalopathy group in this study, as only 5 different measurements were available and SctO2 and CBF measurements were all in a similar range with inadequate variation to establish a correlation. It may be that these patients have an adequate oxygen supply to meet oxygen demand or utilization, but more data are needed to better understand these patients.
Among these seven patients, five developed hypoxic-ischemic brain injury and two did not. Patients developing brain HI injury tended to have higher SctO2 (and lower estimated rCMRO2), suggesting that they extracted less oxygen (likely lower oxygen demand or utilization), but the sample size was too small to draw any definitive conclusions.
As previously noted, perfusion MR imaging is the only existing method that provides direct and noninvasive measurements of brain perfusion in different brain regions (Wintermark et al., 2011). However, it cannot provide continuous measurements because MRI only provides measurements at specific time-points. NIRS is a bedside tool that can continuously monitor changes in brain perfusion. If correlated with exact measurements of CBF obtained by MRI at specific time-points like in our study, NIRS might be used in term asphyxiated newborns to tailor neuroprotective or neurorestorative strategies (Liem and Greisen, 2010) and potentially improve prediction of outcome at the bedside.
NIRS-measured SctO2 reflects the average of arterial, capillary and venous blood hemoglobin oxygen saturation (Toet and Lemmers, 2009; Van Bel et al., 2008). However, in cerebral circulation, arteries are estimated to represent 10–20% of the total vessel volume, capillaries 5% and veins the remaining 75–85% (Van Bel et al., 2008; Marin and Moore, 2011), thus SctO2 predominantly represents the venous saturation in the brain (Benni et al., 2005; Tachtsidis et al., 2008). SctO2 reflects the balance between the oxygen that is delivered to the brain tissue (i.e. cerebral blood flow or oxygen supply) and the oxygen that is extracted at the brain tissue level (i.e. oxygen demand or utilization) (Van Bel et al., 2008; Marin and Moore, 2011). SctO2 is thus a complex variable that is affected by the arterial venous blood volume ratio, the cerebral metabolic rate of oxygen (CMRO2) and inversely proportional to the cerebral blood flow and the blood hemoglobin concentration (Banaji et al., 2008; Roche-Labarbe et al., 2010; Tisdall et al, 2009). It is influenced by the arterial oxyhemoglobin saturation and the end-tidal carbon dioxide tension, and, to a lesser extent, by the mean arterial blood pressure and the cerebral blood volume (Banaji et al., 2008; Roche-Labarbe et al., 2010; Tisdall et al, 2009). Clinicians taking care of newborns need to be aware of these parameters that can affect SctO2, in order to interpret correctly changes in this variable during clinical monitoring (Banaji et al., 2008; Roche-Labarbe et al., 2010; Tisdall et al, 2009). In this study, we combine our NIRS and MRI measurements to try to estimate CMRO2, in order to get more information about metabolism and oxygen consumption.
The main limitation of our study is our small number of concomitant recordings (n=12) with both NIRS and MRI. However, to our knowledge, this is the only study correlating bedside NIRS measurements of oxygenation and MRI measurements of brain perfusion, especially in asphyxiated newborns treated with hypothermia. Also, we did not adjust our results for other variables influencing brain perfusion (such as temperature, pCO2, FiO2, hematocrit levels, mechanical ventilation, pressor support, sedation or antiepileptic treatment), as they were similar in all the newborns at the different time-points. However, our data suggest an expanded role of NIRS in the evaluation of brain perfusion and oxygenation for newborns with HIE (Greisen, 2006; Liem and Greisen, 2010; Toet and Lemmmers, 2009; Wolfberg and du Plessis, 2006). Further data are needed to determine whether the observations in this study hold true in a larger sample and whether early measurements of brain perfusion and oxygenation by NIRS can predict long-term outcome or even be used to tailor neuroprotective therapies.
Correlation between NIRS measurements of oxygenation and MRI measurements of brain perfusion were performed only over the area of frontal lobes, as NIRS was placed respectively on each side of the newborn’s forehead during the duration of hypothermia treatment. Several studies have shown hemispheric and regional variations of SctO2 and CBF values in newborns (Lin et al., 2012; Wintermark et al., 2008a, 2008b). It would thus be interesting in a future study to study the correlation between NIRS measurements of oxygenation and MRI measurements of brain perfusion in different regions of the brain.
CONCLUSION
In conclusion, both SctO2 and CBF increase from day 1 to 2 in all term newborns with HIE, despite treatment with hypothermia. SctO2 and CBF are highly correlated in newborns with severe encephalopathy. Newborns with severe encephalopathy have lower CBF than newborns with moderate encephalopathy. Newborns developing brain HI injury have higher SctO2 than newborns not developing brain injury. Correlating bedside monitoring measurements of SctO2 using NIRS and ASL-MRI measurements of CBF may thus improve our understanding of brain perfusion and oxygenation changes in asphyxiated newborns treated with hypothermia and help us understand better how NIRS could be more widely used in these newborns to tailor therapeutic strategies.
Highlights.
We study term asphyxiated newborns treated with hypothermia.
We show correlation between measurements of brain perfusion by NIRS and by MRI.
NIRS is an effective bedside tool to monitor brain perfusion in these newborns.
NIRS and MRI may help tailor neuroprotective strategies in these newborns.
Acknowledgments
The authors thank the families and their newborns for participating in this study. Special thanks are also due to the NICU nurses and MRI technicians who have made this study possible.
FUNDING.
Pia Wintermark receives research grant funding from the Thrasher Research Fund Early Career Award Program and the William Randolph Hearst Fund Award. The work of Simon K. Warfield is supported by NIH grants R01 RR021885, R01 EB008015, and R01 LM010033. The Fore-sight™ cerebral oximeters (NIRS) were available for the study through in-kind support from Casmed.
Abbreviations
- aEEG
amplitude-integrated electroencephalogram
- CBF
Cerebral Blood Flow
- HIE
Hypoxic-Ischemic Encephalopathy
- MRI
Magnetic Resonance Imaging
- NICU
Neonatal Intensive Care Unit
- NIRS
Near-Infrared Spectroscopy
- PASL
Pulsed Arterial Spin Labeling
- SctO2
mixed venous saturation
Footnotes
Conflict of interest: This manuscript has been contributed to, seen and approved by all the authors. There is no conflict of interest. All the authors fulfill the authorship credit requirements
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al Naqeeb N, Edwards AD, Cowan FM, Azzopardi D. Assessment of neonatal encephalopathy by amplitude-integrated electroencephalography. Pediatrics. 1999;103:1263–1271. doi: 10.1542/peds.103.6.1263. [DOI] [PubMed] [Google Scholar]
- Ancora G, Maranella E, Locatelli C, Pierantoni L, Faldella G. Changes in cerebral hemodynamics and amplitude integrated EEG in an asphyxiated newborn during and after cool cap treatment. Brain Dev. 2009;31:442–444. doi: 10.1016/j.braindev.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Ancora G, Maranella E, Grandi S, Sbravati F, Coccolini E, Savini S, Faldella G. Early predictors of short-term neurodevelopmental outcome in asphyxiated cooled infants. A combined brain amplitude-integrated electroencephalography and near infrared spectroscopy study. Brain Dev. 2011 doi: 10.1016/j.braindev.2011.09.008. [DOI] [PubMed] [Google Scholar]
- Archer LN, Levene MI, Evans DH. Cerebral artery doppler ultrasonography for prediction of outcome after perinatal asphyxia. Lancet. 1986;2:1116–1118. doi: 10.1016/s0140-6736(86)90528-3. [DOI] [PubMed] [Google Scholar]
- Azzopardi DV, Strohm B, Edwards AD, Dyet L, Halliday HL, Juszczak E, Kapellou O, Levene M, Marlow N, Porter E, Thoresen M, Whitelaw A, Brocklehurst P TOBY Study Group . Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361:1349–1358. doi: 10.1056/NEJMoa0900854. [DOI] [PubMed] [Google Scholar]
- Banaji M, Mallet A, Elwell CE, Nicholls P, Cooper CE. A model of brain circulation and metabolism: NIRS signal changes during physiological challenges. PLoS Comput Biol. 2008;4:e1000212. doi: 10.1371/journal.pcbi.1000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barks JD. Current controversies in hypothermic neuroprotection. Semin Fetal Neonatal Med. 2008;13:30–34. doi: 10.1016/j.siny.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Benni PB, Chen B, Dykes FD, Wagoner SF, Heard M, Tanner AJ, Young TL, Rais-Bahrami K, Rivera O, Short BL. Validation of the CAS neonatal NIRS system by monitoring vv-ECMO patients: preliminary results. Adv Exp Med Biol. 2005;566:195–201. doi: 10.1007/0-387-26206-7_27. [DOI] [PubMed] [Google Scholar]
- Biagi L, Abbruzzese A, Bianchi MC, Alsop DC, Del Guerra A, Tosetti M. Age dependence of cerebral perfusion assessed by magnetic resonance continuous arterial spin labeling. J Magn Reson Imaging. 2007;25:696–702. doi: 10.1002/jmri.20839. [DOI] [PubMed] [Google Scholar]
- Cavusoglu M, Pfeuffer J, Ugurbil K, Uludağ K. Comparison of pulsed arterial spin labeling encoding schemes and absolute perfusion quantification. Magn Reson Imaging. 2009;27:1039–1045. doi: 10.1016/j.mri.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Eicher DJ, Wagner CL, Katikaneni LP, Hulsey TC, Bass WT, Kaufman DA, Horgan MJ, Languani S, Bhatia JJ, Givelichian LM, Sankaran K, Yager JY. Moderate hypothermia in neonatal encephalopathy: efficacy outcomes. Pediatr Neurol. 2005;32:11–17. doi: 10.1016/j.pediatrneurol.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, Polin RA, Robertson CM, Thoresen M, Whitelaw A, Gunn AJ. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365:663–670. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- Grant PE, Roche-Labarbe N, Surova A, Themelis G, Selb J, Warren EK, Krishnamoorthy KS, Boas DA, Franceschini MA. Increased cerebral blood volume and oxygen consumption in neonatal brain injury. J Cereb Blood Flow Metab. 2009;29:1704–1713. doi: 10.1038/jcbfm.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greisen G. Is near-infrared spectroscopy living up to its promises? Semin. Fetal Neonatal Med. 2006;11:498–502. doi: 10.1016/j.siny.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Greisen G, Leung T, Wolf M. Has the time come to use near-infrared spectroscopy as a routine clinical tool in preterm infants undergoing intensive care? Philos Transact A Math Phys Eng Sci. 2011;369:4440–4451. doi: 10.1098/rsta.2011.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gucuyener K, Beken S, Ergenekon E, Soysal S, Hirfanoglu I, Turan O, Unal S, Altuntas N, Kazanci E, Kulali F, Koc E, Turkyilmaz C, Onal E, Atalay Y. Use of amplitude-integrated electroencephalography (aEEG) and near infrared spectroscopy findings in neonates with asphyxia during selective head cooling. Brain Dev. 2012;34:280–286. doi: 10.1016/j.braindev.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Higgins RD, Raju TN, Perlman J, Azzopardi DV, Blackmon LR, Clark RH, Edwards AD, Ferriero DM, Gluckman PD, Gunn AJ, Jacobs SE, Eicher DJ, Jobe AH, Laptook AR, LeBlanc MH, Palmer C, Shankaran S, Soll RF, Stark AR, Thoresen M, Wyatt J. Hypothermia and perinatal asphyxia: executive summary of the National Institute of Child Health and Human Development workshop. J Pediatr. 2006;148:170–175. doi: 10.1016/j.jpeds.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Higgins RD, Shankaran S. Hypothermia for hypoxic-ischemic encephalopathy in infants > or = 36 weeks. Early Hum Dev. 2009;85:S49–S52. doi: 10.1016/j.earlhumdev.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman TA, Sorensen AG. Perfusion-weighted magnetic resonance imaging of the brain: techniques and application in children. Eur Radiol. 2004;14:59–72. doi: 10.1007/s00330-003-1972-y. [DOI] [PubMed] [Google Scholar]
- Jacobs S, Hunt R, Tarnow-Mordi W, Inder T, Davis P. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 2007;4:CD003311. doi: 10.1002/14651858.CD003311.pub2. [DOI] [PubMed] [Google Scholar]
- Laptook AR, Corbett RJ. The effects of temperature on hypoxic-ischemic brain injury. Clin Perinatol. 2002;29:623–649. doi: 10.1016/s0095-5108(02)00057-x. [DOI] [PubMed] [Google Scholar]
- Levene MI, Fenton AC, Evans DH, Archer LN, Shortland DB, Gibson NA. Severe birth asphyxia and abnormal cerebral blood-flow velocity. Dev Med Child Neurol. 1989;31:427–434. doi: 10.1111/j.1469-8749.1989.tb04020.x. [DOI] [PubMed] [Google Scholar]
- Liem KD, Greisen G. Monitoring of cerebral haemodynamics in newborn infants. Early Human Dev. 2010;86:155–158. doi: 10.1016/j.earlhumdev.2010.01.029. [DOI] [PubMed] [Google Scholar]
- Lin PY, Roche-Labarbe N, Dehaes M, Fenoglio A, Grant PE, Franceschini MA. Regional and hemispheric asymmetries of cerebral hemodynamic and oxygen metabolism in newborns. Cereb Cortex. 2013;23:339–348. doi: 10.1093/cercor/bhs023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limperopoulos C, Gauvreau KK, O’Leary H, Moore M, Bassan H, Eichenwald EC, Soul JS, Ringer SA, Di Salvo DN, du Plessis AJ. Cerebral hemodynamic changes during intensive care of preterm infants. Pediatrics. 2008;122:e1006–1013. doi: 10.1542/peds.2008-0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luh WM, Wong EC, Bandettini PA, Hyde JS. QUIPSS II with thin-slice TI1 periodic saturation: a method for improving accuracy of quantitative perfusion imaging using pulsed arterial spin labeling. Magn Reson Med. 1999;41:1246–1254. doi: 10.1002/(sici)1522-2594(199906)41:6<1246::aid-mrm22>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Marin T, Moore J. Understanding Near-Infrared Spectroscopy. Adv Neonatal Care. 2011;11:382–388. doi: 10.1097/ANC.0b013e3182337ebb. [DOI] [PubMed] [Google Scholar]
- Meek JH, Elwell CE, McCormick DC, Edwards AD, Townsend JP, Stewart AL, Wyatt JS. Abnormal cerebral haemodynamics in perinatally asphyxiated neonates related to outcome. Arch Dis Child Fetal Neonatal Ed. 1999;81:F110–F115. doi: 10.1136/fn.81.2.f110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minhas PS, Menon DK, Smielewski P, Czosnyka M, Kirkpatrick PJ, Clark JC, Pickard JD. Positron emission tomographic cerebral perfusion disturbances and transcranial Doppler findings among patients with neurological deterioration after subarachnoid hemorrhage. Neurosurgery. 2003;52:1017–1022. [PubMed] [Google Scholar]
- Miranda MJ, Olofsson K, Sidaros K. Noninvasive measurements of regional cerebral perfusion in preterm and term neonates by magnetic resonance arterial spin labeling. Pediatr Res. 2006;60:359–363. doi: 10.1203/01.pdr.0000232785.00965.b3. [DOI] [PubMed] [Google Scholar]
- Pellicer A, del Carmen Bravo M. Near-infrared spectroscopy: A methodology-focused review. Semin Fetal Neonatal Med. 2011;16:42–49. doi: 10.1016/j.siny.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Perlman JM. Summary proceedings from the neurology group on hypoxic- ischemic encephalopathy. Pediatrics. 2006;117:S28–S33. doi: 10.1542/peds.2005-0620E. [DOI] [PubMed] [Google Scholar]
- Polderman KH. Induced hypothermia and fever control for prevention and treatment of neurological injuries. Lancet. 2008;371:1955–1969. doi: 10.1016/S0140-6736(08)60837-5. [DOI] [PubMed] [Google Scholar]
- Roche-Labarbe N, Carp SA, Surova A, Patel M, Boas DA, Grant PE, Franceschini MA. Noninvasive optical measures of CBV, StO(2), CBF index, and rCMRO(2) in human premature neonates’ brains in the first six weeks of life. Hum Brain Mapp. 2010;31:341–352. doi: 10.1002/hbm.20868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche-Labarbe N, Fenoglio A, Aggarwal A, Dehaes M, Carp SA, Franceschini MA, Grant PE. Near-infrared spectroscopy assessment of cerebral oxygen metabolism in the developing premature brain. J Cereb Blood Flow Metab. 2012;32:481–488. doi: 10.1038/jcbfm.2011.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, Fanaroff AA, Poole WK, Wright LL, Higgins RD, Finer NN, Carlo WA, Duara S, Oh W, Cotton CM, Stevenson DK, Stoll BJ, Lemons JA, Guillet R, Jobe AH National Institute of Child Health, Human Development Neonatal Research Network . Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- Shankaran S, Pappas A, McDonald SA, Vohr BR, Hintz SR, Yolton K, Gustafson KE, Leach TM, Green C, Bara R, Petrie Huitema CM, Ehrenkranz RA, Tyson JE, Das A, Hammond J, Peralta-Carcelen M, Evans PW, Heyne RJ, Wilson-Costello DE, Vaucher YE, Bauer CR, Dusick AM, Adams-Chapman I, Goldstein RF, Guillet R, Papile LA, Higgins RD Eunice Kennedy Shriver NICHD Neonatal Research Network . Childhood outcomes after hypothermia for neonatal encephalopathy. N Engl J Med. 2012;366:2085–2092. doi: 10.1056/NEJMoa1112066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soul JS, du Plessis AJ. New technologies in pediatric neurology. Near-infrared spectroscopy. Semin Pediatr Neurol. 1999;6:101–110. doi: 10.1016/s1071-9091(99)80036-9. [DOI] [PubMed] [Google Scholar]
- Tachtsidis I, Tisdall M, Delpy DT, Smith M, Elwell CE. Measurement of cerebral tissue oxygenation in young healthy volunteers during acetazolamide provocation: a transcranial Doppler and near-infrared spectroscopy investigation. Adv Exp Med Biol. 2008;614:389–396. doi: 10.1007/978-0-387-74911-2_43. [DOI] [PubMed] [Google Scholar]
- Tisdall MM, Taylor C, Tachtsidis I, Leung TS, Elwell CE, Smith M. The effect on cerebral tissue oxygenation index of changes in the concentrations of inspired oxygen and end-tidal carbon dioxide in healthy adult volunteers. Anesth Analg. 2009;109:906–913. doi: 10.1213/ane.0b013e3181aedcdc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toet MC, Lemmers PMA, van Shelven LJ, van Bel F. Cerebral Oxygenation and Electrical Activity After Birth Asphyxia: Their Relation to Outcome. Pediatrics. 2006;117:333–339. doi: 10.1542/peds.2005-0987. [DOI] [PubMed] [Google Scholar]
- Toet MC, Lemmers PMA. Brain monitoring in neonates. Early Hum Dev. 2009;85:77–84. doi: 10.1016/j.earlhumdev.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Tsuji M, Saul JP, du Plessis A, Eichenwald E, Sobh J, Crocker R, Volpe JJ. Cerebral intravascular oxygenation correlates with mean arterial pressure in critically ill premature infants. Pediatrics. 2000 Oct;106(4):625–32. doi: 10.1542/peds.106.4.625. [DOI] [PubMed] [Google Scholar]
- van Bel F, van de Bor M, Stijnen T, Baan J, Ruys JH. Cerebral blood flow velocity pattern in healthy and aspxhyaited newborns: a controlled study. Eur J Pediatr. 1987;146:461–467. doi: 10.1007/BF00441595. [DOI] [PubMed] [Google Scholar]
- van Bel F, Lemmers P, Naulaers G. Monitoring Neonatal Regional Cerebral Oxygen Saturation in Clinical Practice: Value and Pitfalls. Neonatology. 2008;94:237–244. doi: 10.1159/000151642. [DOI] [PubMed] [Google Scholar]
- Wang J, Licht DJ, Jahng GH, Liu CS, Rubin JT, Haselgrove J, Zimmerman RA, Detre JA. Pediatric perfusion imaging using pulsed arterial spin labeling. J Magn Reson Imaging. 2003;18:404–413. doi: 10.1002/jmri.10372. [DOI] [PubMed] [Google Scholar]
- Wang J, Licht DJ, Silvestre DW, Detre JA. Why perfusion in neonates with congenital heart defects is negative - technical issues related to pulsed arterial spin labeling. Magn Reson Imaging. 2006;24:249–254. doi: 10.1016/j.mri.2005.10.031. [DOI] [PubMed] [Google Scholar]
- Wang J, Licht DJ. Pediatric perfusion MR imaging using arterial spin labeling. Neuroimaging Clin N Am. 2006;16:149–167. doi: 10.1016/j.nic.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Wiggins GC, Triantafyllou C, Potthast A, Reykowski A, Nittka M, Wald LL. 32-channel 3 Tesla receive-only phased-array head coil with soccer-ball element geometry. Magn Reson Med. 2006;56:216–223. doi: 10.1002/mrm.20925. [DOI] [PubMed] [Google Scholar]
- Wintermark P, Moessinger AC, Gudinchet F, Meuli R. Perfusion-weighted magnetic resonance imaging patterns of hypoxic-ischemic encephalopathy in term neonates. J Magn Reson Imaging. 2008;28:1019–1025. doi: 10.1002/jmri.21525. [DOI] [PubMed] [Google Scholar]
- Wintermark P, Moessinger AC, Gudinchet F, Meuli R. Temporal evolution of MR perfusion in neonatal hypoxic-ischemic encephalopathy. J Magn Reson Imaging. 2008;27:1229–1234. doi: 10.1002/jmri.21379. [DOI] [PubMed] [Google Scholar]
- Wintermark P, Labrecque M, Warfield SK, DeHart S, Hansen A. Can induced hypothermia be assured during brain MRI in neonates with hypoxic-ischemic encephalopathy? Pediatr. Radiol. 2010;40:19550–19554. doi: 10.1007/s00247-010-1816-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintermark P, Hansen A, Gregas MC, Soul J, Labrecque M, Robertson RL, Warfield SK. Brain Perfusion in Asphyxiated Newborns Treated with Therapeutic Hypothermia. AJNR Am J Neuroradiol. 2011;32:2023–2029. doi: 10.3174/ajnr.A2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfberg AJ, du Plessis AJ. Near-Infrared Spectroscopy in the Fetus and Neonate. Clin Perinatol. 2006;33:707–728. doi: 10.1016/j.clp.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Wyatt JS. Near-infrared spectroscopy in asphyxial brain injury. Clin Perinatol. 1993;20:369–378. [PubMed] [Google Scholar]