Abstract
Cervical cancer continues to cause significant morbidity and mortality worldwide, making prophylactic cervical cancer vaccines an important focus for cervical cancer prevention. The increasing accessibility of these vaccines worldwide has the potential to greatly decrease the incidence and burden of disease in the future. However, current prophylactic vaccines offer no therapeutic benefit for persons already infected with human papillomavirus types targeted by vaccines or persons with precancerous lesions or cervical cancer. The protection offered by current vaccines is primarily against human papillomavirus types used to derive the vaccine, although partial cross-protection for related virus types has been observed. Herein, we describe findings from preclinical and clinical studies that employ vaccine strategies that have the potential to shape the future of vaccines against cervical cancer. Modalities include prophylactic strategies to target more oncogenic virus types by using the minor capsid antigen L2 and/or by increasing the number of types used to derive virus-like particle vaccines. Therapeutic strategies include the development of vaccines against human papillomavirus early proteins (targets for cellular immunity) for the resolution of precancerous lesions and cervical cancer. Future applications of existing VLP-based vaccines are also discussed.
Introduction
As the etiologic agent of genital warts, as well as precancerous lesions and carcinomas of the cervix, vagina, and vulva, human papillomavirus (HPV) infections continue to cause great morbidity and mortality. In the United States (US), it is estimated that 24.9 million women between the ages of 14 and 59 years are currently infected with HPV.[1] It is also estimated that more than 80% of women will acquire a genital HPV infection by 50 years of age.[2] Of the 15 oncogenic HPV types, HPV 16 and 18 account for approximately 52% of cervical intraepithelial neoplasia (CIN) 2+ cases and 70% of cervical cancer cases worldwide, as well as 54% of CIN 2+ cases and 77% of all cervical cancer cases in the US.[3] In addition, an estimated 10% of men and women will develop anogenital warts during their lifetime, of which approximately 90% are caused by benign HPV types 6 and 11.[4]
HPVs contain eight open reading frames, including the early genes, E1 to E7 (except E3), and the late genes L1 and L2.[5] E5, E6, and E7 proteins regulate specific events involved in oncogenesis, but E6 and E7 are generally considered the major transforming proteins.[6] L1 and L2 are capsid proteins produced for assembly of complete virions.[5] L1 self-assembles to form virus-like particles (VLPs), which can also incorporate L2 if co-expressed. However, the L2 protein does not form a VLP when expressed on its own. Prophylactic vaccination against L1 of types 16 and 18 is currently available for the prevention of cervical cancer and precancerous lesions. To reduce the healthcare burden of cervical cancer, it is necessary to continue developing better vaccination strategies for the control and treatment of genital HPV.
Prophylactic Vaccines Available for Cervical Cancer
The two vaccines designed to prevent cervical cancer are composed of empty VLPs generated by expression systems for recombinant capsid antigen L1.[7, 8] VLPs do not contain viral DNA and are non-infectious. Recombinant L1 VLPs strongly resemble authentic papilloma virions in both structure and immunogenicity.[9] Gardasil® (Merck and Company; Whitehouse Station, NJ, USA), is quadrivalent and consists of L1 VLP derived from HPV 6, 11, 16, and 18. Cervarix® (GlaxoSmithKline Biologicals; Rixensart, Belgium), is bivalent and composed of L1 proteins for HPV 16 and 18. Gardasil® is produced by yeast expression of L1 and is formulated with Merck’s amorphous aluminum hydroxyphosphate sulfate adjuvant.[8] Cervarix® uses a Baculovirus-based L1 expression system in insect cells and the proprietary ASO4 adjuvant, a mixture of aluminum hydroxide and monophosphoryl lipid A.[10] ASO4 has been shown to induce higher antibody responses than aluminum hydroxide, although the clinical significance of this is unclear.[9]
Passive transfer studies in animal models suggest the importance of serum antibodies specific for L1 VLPs in mediating protection.[11, 12] Results for seropositivity and geometric mean titers (GMTs) of L1 VLP-specific antibodies have been obtained in patients for both vaccines; however, different assays were used, making it difficult to directly compare the studies. After three years, Gardasil® recipients exhibited 96%, 97%, 99%, and 68% seropositivity for HPV 6, 11, 16, and 18, respectively.[13] After a 15-month pre-specified planned analysis of an ongoing Phase 3 trial, Cervarix® recipients were 99.5% seropositive against both HPV 16 and 18.[14] After three years, Phase 2 studies for Gardasil® showed that GMTs induced against HPV 16 and 18 were 17 and 2 times greater, respectively, than levels induced after natural infection.[15] At five years, GMTs against HPV 16 declined by 22% and were at natural infection levels for HPV 18, although clinical efficacy is apparently maintained.[15–17] At seven months during a Phase 3 interim analysis, Cervarix® was shown to have GMTs that were 313 and 211 times higher than levels induced after natural infection with HPV 16 and 18, respectively.[14] Results from Phase 2 studies suggest that Cervarix®-induced GMT levels can be sustained for at least 5½ years.[18]
In a Phase 2 study, Gardasil® showed 96.6% (95% CI, 79.2% to 99.9%) and 90.6% (95% CI, 35.6% to 99.8%) efficacy against persistent infection caused by HPV 16 and 18, respectively, in the per-protocol group after three years.[17] In a Phase 3 study, Cervarix® showed 84.1% (97.9% CI, 73.5% to 91.1%) and 74.0% (97.9% CI, 49.1% to 87.8%) efficacy against six-month persistent infections with oncogenic types 16 and 18, respectively.[14] Gardasil® was 98% effective in preventing CIN 2+ lesions associated with HPV 16 and 18.[13] Results from the 15-month pre-specified planned analysis showed that Cervarix® was 90.4% effective in preventing CIN 2+ lesions associated with HPV 16 and 18.[14] The 15-month planned analysis also showed cross-protection, as it prevented 59.9% (97.9% CI, 2.6% to 85.2% [p = 0.0165]), 36.1% (97.9% CI, 0.5% to 59.5% [p = 0.0173]), and 31.6% (97.9% CI, 3.5% to 51.9% [p = 0.0093]) of six-month persistent infections with oncogenic types 45, 31, and 52, respectively.[14] Cross-protection data for Gardasil® have also been reported. Post-hoc analysis showed that Gardasil® had 38% (95% CI, 6% to 60%) efficacy against CIN 2+ or AIS associated with 10 oncogenic HPV types not included in the vaccine, and as high as 62% (95% CI, 10% to 85%) efficacy for types 45 and 31 together.[19] Neither study was powered to show cross-protective efficacy against CIN with individual non-vaccine types. Additional analysis of Gardasil® and Cervarix® in larger populations is needed to establish cross-protective efficacy against CIN for each non-vaccine type.[19]
Recent clinical studies with L1 VLP-based vaccines have primarily revolved around the prevention of cancer associated with the female genital tract, including cervical cancer. L1 VLP vaccines may also prevent additional HPV-associated cancers. HPV has been detected in 25.9% of head and neck squamous cell carcinomas, of which HPV 16 was responsible for 86.7% of oropharyngeal, 68.2% of oral, and 69.2% of laryngeal squamous cell carcinomas.[20] HPV-DNA has also been detected in 80.9% of anal squamous cell carcinomas (86.8% were HPV 16 positive).[21]
Considerations for the Continued Development of Cervical Cancer Vaccines
Although prophylactic vaccines against high-risk HPV types have been highly effective, unresolved issues may shape the future of cervical cancer vaccines. Current vaccines must contain intact L1 VLPs, which promote the generation of strong, type-restricted, neutralizing IgG antibody responses. The minimum antibody titer needed for protection in humans is unknown, though the importance of antibody responses to prophylactic VLP vaccination has been suggested by studies in preclinical models. A denatured L1 VLP does not induce protection in the cottontail rabbit papillomavirus or canine oral papillomavirus (COPV) challenge models, suggesting the importance of L1 conformation-dependent antibody responses.[11, 12] Suzich et al. also reported that protective L1 immune responses could be dose-dependent.[11] Increased COPV L1-specific antibody titers were associated with protection from papillomas, suggesting that there is a certain level of humoral immunity associated with protection against papilloma viruses.[11, 12] In other studies, passive transfer experiments with purified IgG, or sera, have further demonstrated the protective effects of neutralizing L1 VLP-specific antibodies.[11, 12] Although the mechanism is currently unclear, it is likely that high and sustained antibody levels are needed in the serum to ensure transudation of antibodies to the cervical epithelium (site of infection) or exudation at sites of microtrauma during intercourse.[10]
Both vaccines contain L1 VLPs from oncogenic types 16 and 18. Gardasil® also contains L1 VLPs from the benign types 6 and 11. Since the findings to date suggest that cross-protection against related HPV types is partial, it remains important to broaden protection to the remaining oncogenic HPV types. A potential strategy is to simply combine many types of L1 VLPs to prevent infection with multiple oncogenic types. Assuming type specificity and 100% efficacy, a polyvalent vaccine containing L1 VLP types 16, 18, 35, 33, 31, 45, 52, and 58 could potentially prevent 96% of cervical cancer cases in the US.[3] Merck has performed clinical trials with an octavalent HPV VLP vaccine, including benign L1 VLP types 6 and 11, and six oncogenic HPV types (16, 18, 31, 45, 52, and 58).[22] However, data have not yet been released and it is unclear how this program will advance. Increasing valency complicates vaccine development and cost. Antigenic competition may potentially complicate dose selection or limit the amount of protein that can be injected.
Conversely, preclinical studies have demonstrated that L2-based vaccines can be used to generate broad spectrum cross-neutralizing antibodies and could reduce the number of VLP types required for protection.[23, 24] An L2-based vaccine is in development for early phase clinical trials at the University of Alabama at Birmingham.
Immunization against HPV will potentially have its greatest impact in developing countries where 80% of the global cervical cancer burden occurs, which may be due to a lack of effective Pap screening programs. The current cost and cold chain storage strategy (at 4°C) required for VLP vaccines may challenge the ability to immunize women in developing countries. Alternate pricing structures for current vaccines and new vaccination technologies that allow for lower cost and easier delivery and storage are important for the global fight against cervical cancer.
Cervical Cancer Vaccination Strategies in Development
Prophylactic (VLP-based), antibody-generating, vaccines do not eliminate pre-existing persistent infections;[13, 25] however, therapeutic vaccination could have an immediate impact in reducing the incidence of cervical cancer.
L1 and L2 late capsid proteins are not expressed in the basal cells that harbor infection in precancerous tissues or cancerous tissues,[26] suggesting that they are not good targets for therapeutic vaccines.[25] Conversely, E6 and E7 proteins are very promising target proteins for therapeutic vaccines, as they are the only viral proteins constitutively expressed in cervical cancer cells and are required to maintain the disease phenotype.[27, 28] During natural infection, cell-mediated or T-cell responses are important for the recognition of specific HPV antigens present in the lesion. CIN 2/3 lesions contain increased numbers of activated T-cells in the stroma and dysplastic epithelium.[29] Recipients of an organ transplant or individuals co-infected with human immunodeficiency virus have been shown to have a significantly increased likelihood of developing cervical cancer.[30] E7-specific cell-mediated immune responses are significantly correlated with regression of CIN 2/3 disease and resolution of viral infection within 12 months.[31] T-cell responses against multiple early proteins and L1 are associated with the maintenance of normal cytology.[32] Topical treatment with A-007, an aryl hydrazone capable of directly upregulating T-cell activation and agglutination, was shown to increase T-cell infiltrate in precancerous lesions. A-007 is currently being evaluated in Phase 2 studies.[33] Such results stress the importance of cellular immune responses in controlling CIN and subsequent cervical cancer. Augmenting these responses with therapeutic vaccines may prevent the progression of invasive cervical cancer and potentially clear HPV infections and related intraepithelial neoplasia.
Peptide Immunization
Recombinant proteins, and smaller, chemically synthesized peptides are one option for therapeutic vaccination.[34] However, peptide-based vaccines designed to induce effective T-cell responses against established cancers have had limited clinical success to date, but continue to be improved.[34] In a nonrandomized, Phase 1 clinical trial, 12 women were vaccinated with HPV 16 E7 lipopeptide. No clinical responses or adverse events were observed; however, antigen-specific cell-mediated immune responses in patients with advanced cervical cancer were demonstrated.[35] In another study, HPV 16 positive women with CIN 2 were treated with a lipidated E7 peptide emulsified with an adjuvant. Limited clinical responses were observed in patients after vaccination, but notable increases in E7-specific T-cell responses were observed.[36] Recently, non-responder patients with HPV 16-induced cervical cancer or vulvar intraepithelial neoplasia immunized with long, overlapping peptides (30–35 amino acids) from E6 and E7 in an oil-in-water emulsion were converted into robust T-cell responders against E6 and E7. Completed Phase 2 study reports of clinical responses are forthcoming.[37] A complete clinical response was observed in 4 of 11 patients, and a complete virological response was observed in three of the four patients.
Recombinant Protein Vaccines
Many groups have focused on recombinant protein strategies for therapeutic immunization because peptides lack the entire protein sequence and therefore have the potential to limit the pool of relevant epitopes that may be necessary for widespread use. The fusion of recombinant proteins of interest with heat shock proteins (Hsp) has been shown to generate a stronger immune response than the protein expressed alone.[38] In the absence of an adjuvant, a recombinant E6 and E7 protein fused to the Hsp70 protein of M. tuberculosis was capable of protecting mice against challenge with HPV 16 E6/E7 transformed tumor cells.[38]
A therapeutic vaccine consisting of a Hsp65 and HPV 16 E7 fusion protein (HspE7) was evaluated in women with CIN 3.[39] While HspE7 vaccination appeared to provide clinical benefits in some women with CIN 3, it is unclear whether this response was due to natural regression or to the effect of treatment. A large randomized controlled trial is needed to better define efficacy.[39] To further evaluate recombinant protein therapeutic vaccines, a multicenter, nonrandomized, open-label, uncontrolled Phase 1 safety study of HspE7 administered concomitantly with an adjuvant in subjects with precancerous lesions is underway.[40]
An alternative immunization strategy involves the delivery of antigens directly to autologous antigen presenting cells, such as dendritic cells, in vitro and then re-administration to the patient to potentially illicit immune responses. Cervical cancer patients who reacted to immunization with dendritic cells pulsed with recombinant HPV 16 or 18 E7 proteins experienced a slower tumor progression (13-month survival) than unresponsive patients who died within five months of the beginning of therapy.[41] A Phase 1 study is in progress to evaluate the safety and efficacy of HPV 16 E7 peptide-pulsed autologous dendritic cells in cervical cancer patients.[42]
Recombinant protein vaccines have also been developed to include both prophylactic and therapeutic components. In preclinical studies, a chimeric HPV 16 L1 VLP combined with full-length HPV 16 L2, E7, and E2 tandem fusion protein (VLP-E7E2) has shown therapeutic potential, in mice, without diminishing the antibody response against L1 VLPs.[43] A similar construct, L1-E7 fusion VLP, was tested in a Phase 1 trial in 39 CIN 2/3 patients mono-infected with HPV 16. The vaccine induced high antibody titers against HPV 16 L1 and low titers against E7, as well as cellular immune responses against both proteins. Non-significant trends for histological improvement to CIN 1 or normal and HPV DNA status were observed.[44]
Clinical studies have also evaluated the use of a HPV 16 fusion protein consisting of L2, E7, and E6 (L2E7E6) without adjuvant. [45, 46] Vaccination of healthy volunteers induced L2-specific HPV 16 and 18 neutralizing antibodies. Immunogen-specific cellular responses were detected in healthy volunteers, specifically against E6 and E7.[45, 46] HPV L2E6E7 fusion protein (TA-CIN) was shown in a Phase 1 study to induce both antibody responses and oncoprotein-specific T-cell responses.[45] However this approach has produced little evidence of efficacy in patients with HPV 16 positive anogenital intraepithelial neoplasia.[47–49]
Plasmid DNA Vaccines
In comparison to conventional vaccines, DNA vaccines offer several potential advantages.[50] DNA encoding a viral antigen is delivered either naked, on gold beads, via electroporation, or encapsulated in a biodegradable polymer coat to antigen presenting cells that express and present the antigen of interest to lymphocytes. Although therapeutic targets, E6 and E7 are thought to be poor immunogens.[51] Multiple constructs evaluated in preclinical studies have been designed to increase the immunogenicity of E6 and E7 DNA vaccines by expressing or cross-priming the oncoproteins with various immunomodulating and cellular targeting proteins.[51–56] To improve the safety of genetic immunization with HPV oncoproteins, E6 and E7 vaccines have been designed to retain essential epitopes, while inactivating oncogenic potential.[50, 57] The use of DNA vaccination to obtain protective antibody responses against a mixture of different L1 VLPs has also been investigated and shown to induce neutralizing antibodies in mice and protection in rabbits.[58] A Phase 1 trial was recently conducted to evaluate the safety, efficacy, and optimal dose of a DNA vaccine, SigE7HSP70 (containing a secretion signal sequence), in treating HPV 16 positive CIN 2+ patients and the analysis is ongoing.[59]
Viral Vector Vaccines
Viral vector vaccines can be used to challenge the host and induce the endogenous synthesis of viral and recombinant proteins, promoting strong T-cell responses. Viral vectors can also be used to display surface antigens that may induce strong antibody responses. The vaccinia virus vector is frequently used in preclinical vaccine studies as genetic manipulation is straightforward and vaccination is immunogenic and relatively safe in immunocompetent individuals. A live recombinant vaccinia virus expressing modified forms of HPV 16 and 18 E6 and E7 was shown to be safe and immunogenic.[60] After a single immunization, cervical cancer patients developed both HPV-specific T-cell and serologic responses.[60] Additional studies in women with HPV 16 positive anogenital intraepithelial neoplasia with this vaccine combined with the HPV 16 L2E7E6 fusion protein showed hints of efficacy, but no clear evidence.[47] The development and commercialization of a modified vaccinia virus Ankara (MVA) expressing modified HPV 16 E6 and E7 and the autocrine T-cell cytokine, IL-2, has further demonstrated the potential of these therapeutic vaccines. Results of a Phase 2 study reported CIN 2/3 regression in 10 of 21 women vaccinated after six months. Six of the patients evaluated to date remained negative for CIN 2/3 after 12 months.[61]
Immune responses to E2 may also be important in controlling disease. A Phase 1/2 study comparing a recombinant vaccinia virus, expressing bovine papillomavirus E2 (MVA E2), injected into the uterus once weekly for six weeks, against cryosurgery for treatment of CIN 1 to 3, showed complete elimination of 94% of precancerous lesions. The vaccine was well tolerated, with no apparent side effects in any of the patients.[62] A similar Phase 2 clinical trial evaluated an E2 recombinant vaccinia virus for treatment of CIN 2/3. Patients were treated with either an E2 therapeutic vaccine (n = 34) injected directly into the uterus or with conization (n = 20).[63] All immunized patients developed antibody and T-cell responses, in addition to a significant reduction in viral DNA load.[63] By colposcopy, 56% of patients showed no lesions, and lesions were significantly reduced in 15 patients.[63] It should be noted that E2 is typically lost from the genome during progression to cancer, suggesting that targeting E2 is not appropriate in cancer patients.[5]
Although no clinical trials evaluating the use of adenovirus vectors have occurred to date, it is possible that this modality could be used in the near future for cervical cancer treatment, as gene delivery by recombinant adenovirus is safe and immunogenic.[64] Several preclinical studies have been conducted to increase the efficacy of immune responses against E7, including the combined administration in tumor sites of adenovirus expressing interleukin 12 (to enhance immunogenicity and anti-tumor responses), and adenovirus expressing E7.[65] Human adenovirus vectors encoding a fusion of E7 to the carboxyl terminus of hepatitis B virus surface antigen (HBsAg) or calreticulin have induced strong cellular immunity and resistance to tumors.[65–67] In addition to cellular immunity targets, adenovirus capable of expressing HPV 16 L1 VLP in culture have also been produced.[68] These results may eventually lead to a more cost-effective delivery system for prophylactic VLP vaccines.
The Future of Cervical Cancer Vaccines
As demonstrated in this review, there are several promising methodologies to generate prophylactic and therapeutic cervical cancer vaccines (Table 1). However, many studies are still only in preclinical development, and translation of results from small animals to humans is far from certain. This may be a result of a lack of understanding of the mechanisms of immune evasion or suppression employed by HPV and issues related to targeting the immune response at the tumor site. Understanding of tumor immunology is rapidly improving, and numerous approaches are being developed to overcome these phenomena. An effective cervical cancer vaccine should protect against many oncogenic HPV types, the necessary cause of cervical cancer and precancerous lesions. Additionally, an effective cervical cancer vaccine should provide a strong and sustained immune response to protect women throughout their lives.
Table 1.
Novel prophylactic and therapeutic vaccine strategies for cervical cancer.
| Strategy | Response | Advantage(s) | Disadvantage(s) |
|---|---|---|---|
| Peptide | Prophylactic (L2) and therapeutic | Safe; easily synthesized and purified | Only contains selected epitopes, therefore can be less effective for a diverse population; small size may reduce immunogenicity |
| Recombinant protein | Prophylactic and therapeutic | Can induce strong antibodies against L1 or L2 and weak cellular immune responses | Cost, storage, and purification |
| Dendritic cell delivery of novel vaccines | Therapeutic | Direct targeting of vaccine to professional antigen presenting cells | Each immunization is labor intensive and can only be performed in a limited number of facilities |
| DNA | Prophylactic and therapeutic | Inexpensive; easy storage | Has had limited immunogenicity in humans and has the potential to transform cells |
| Recombinant vaccinia virus | Therapeutic | Induces strong and protective cellular immune responses | Could cause disease in immunocompromised individuals |
| Recombinant adenovirus | Prophylactic and therapeutic | Induces strong and protective cellular immune responses | Previously existing natural immunity can limit efficacy |
One unanswered question is how long VLP-induced protection will last. Cervarix® immunogenicity and clinical efficacy has been measured up to five and a half years.[18] A conventional antibody decay model reported for women 16 to 23 years of age immunized with monovalent HPV 16 L1 VLP predicted antibody titers above those of natural infection for 12 years.[69] However, a model modified to better fit the experimental data estimated that levels above those associated with reduction in natural infection could be lifelong in as many as 76% of immunized subjects.[69] However, long-term clinical efficacy of antibody titers at or below the natural infection threshold is unknown, and antibodies generated during the course of infection have not been shown to significantly reduce the likelihood re-infection with the same HPV type.[70] Females immunized at 16 to 23 years of age could have antibody titers similar to natural infection by 28 to 35 years of age, which may leave them at risk for infection and potentially require booster immunization. It is also difficult to predict how this antibody decay model will apply to females immunized at nine years of age. In 2002–2005, Nordic HPV vaccine trials began for both Gardasil® and Cervarix®. Following participants through the Nordic healthcare registry system, will be able to show the impact of vaccination on CIN3+ lesions by 2015–2020.[71]
Current indications for females 9 to 26 years of age are based on cost effectiveness, clinical efficacy and safety, and maximum public health benefit. Further studies of vaccination of women currently outside of the indicated age are ongoing (Figure 1). Gardasil® immunogenicity and efficacy has been evaluated in women 24 to 45 years of age.[72] Women between the ages of 16 to 23 had a seroconversion rate of 100% for types 16 and 18. Subjects between 24 to 34 years of age had seroconversion rates of 100% and 98%, for types 16 and 18, respectively, and women between the ages of 35 to 45 had seroconversion rates of 98% and 96% for types 16 and 18, respectively.[72] Combined reduction of disease incidence in these subjects caused by persistent infection, CIN, and external genital lesions due to types 16 and 18 was 83% (95% CI, 51% to 96% [p < 0.001]).[72] Cervarix® immunogenicity has been evaluated in women up to age 55. Seroconversion rates for all subjects immunized was 100% after 18 months. The antibody levels of women 46–55 were slightly reduced, yet comparable, to those of younger women and remained 10 fold higher than the antibodies of women naturally infected at month 18.[73]
Figure 1.
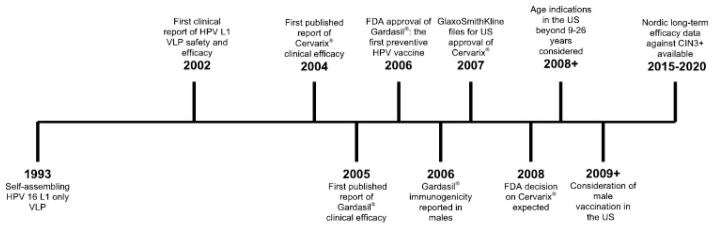
Past and future milestones of prophylactic HPV VLP vaccines.
Clinical studies have not been performed to evaluate the ability of VLP-based vaccines to protect immunosuppressed individuals from precancerous lesions. Such studies may provide valuable insight into the immunocorrelates of protection. GlaxoSmithKline is conducting a Phase 3 study in the US to directly evaluate the immunogenicity of Gardasil® and Cervarix® in women 18 to 45 years of age.[74] Whether a similar trial will be conducted in an immunosuppressed population is unclear.
The value of prophylactic vaccination of males with HPV VLPs requires careful consideration (Figure 1). Mexico and Australia have licensed VLP-based vaccination for both sexes, ahead of policy recommendations for males, which will be shaped by ongoing studies.[75] A model examining disease burden in females has suggested that vaccination of males is most cost effective when a lower proportion of females are immunized.[76] There is considerable male burden of HPV disease including genital warts, certain head and neck cancers, penile cancer and anal cancer.[77] HPV is highly prevalent in men-who-have-sex-with-men (MSM), and even more so in MSM infected with HIV.[78] HIV+ MSM are more likely to have high grade anal cytology that is hard to manage, and CD4+ T cell counts are inversely proportional the appearance of abnormal cytology.[79] Vaccination of boys with VLPs prior to sexual debut is likely important to reduce the incidence of HPV-associated cancer in men. Analysis of cost-effectiveness, immunogenicity, and efficacy of VLP vaccines in men should consider these potential benefits.
The future of prophylactic cervical cancer vaccines may include preparations containing L1 VLPs of many HPV genotypes. Simply increasing the number of VLPs in a vaccine preparation may result in formulation issues. Possibly, the inclusion of L2 mixed with, but not expressed within, L1 VLPs could reduce the number of VLP valencies needed to provide protection against all oncogenic types of HPV. Alternatively, L2 may be used alone, and Phase 1 trials of such a construct are planned at the University of Alabama at Birmingham.
Although many of the novel vaccines described in this review are reported to be immunogenic in humans, it may be necessary to continue developing novel adjuvants to establish clinical efficacy. In this regard, chimeric VLPs containing early viral proteins show significant promise. Inexpensive VLP and cellular therapeutic vaccines are also of great importance, as approximately 85% of cervical cancer deaths worldwide occur in developing countries.[80] Multiple bacterial and plant systems have been investigated and have produced immunogenic VLPs and E6 and E7 proteins, as well as mucosal immunity, which may facilitate local manufacture of low cost and efficacious cervical cancer vaccines.[81–86]
Acknowledgments
RBSR is supported by PHS grants (National Cancer Institute, SPORE in Cervical Cancer, P50 CA098252 and R01CA118790). RBSR is a paid consultant of Knobbe, Martens, Olson and Bear, LLC.
Footnotes
WKH has been Speaker: Merck & Co.,
Consultant: Nventa, MGI Pharma, Roche, Takeda Pharmaceuticals, GlaxoSmithKline
Research: GlaxoSmithKline, Merck & Co., MGI Pharma, Roche Molecular Systems, Takeda Pharmaceuticals.
Contributor Information
Warner K. Huh, Email: whuh@uab.edu, Division of Gynecologic Oncology, University of Alabama at Birmingham, 618 20th Street South, OHB Room 538, Birmingham, AL 35233, Phone: (205) 934-4986, Fax: (205) 975-6174.
Richard B.S. Roden, Email: roden@jhmi.edu, Departments of Pathology, Oncology, and Gynecology and Obstetrics, Johns Hopkins University, 1550 Orleans Street, Room 308 CRB2, Baltimore, MD 21231, Johns Hopkins University, College of Medicine, Department of Pathology, Phone: (410) 502-5161, Fax: (443) 287-4295.
References
- 1.Dunne EF, Unger ER, Sternberg M, McQuillan G, Swan DC, Patel SS, et al. Prevalence of HPV infection among females in the United States. Jama. 2007 Feb 28;297(8):813–9. doi: 10.1001/jama.297.8.813. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control. Genital HPV - CDC Fact Sheet. 2004 [cited; Available from: http://www.cdc.gov/std/HPV/STDFact-HPV.htm.
- 3.Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer. 2007 Aug 1;121(3):621–32. doi: 10.1002/ijc.22527. [DOI] [PubMed] [Google Scholar]
- 4.Saslow D, Castle PE, Cox JT, Davey DD, Einstein MH, Ferris DG, et al. American Cancer Society Guideline for human papillomavirus (HPV) vaccine use to prevent cervical cancer and its precursors. CA: a cancer journal for clinicians. 2007 Jan-Feb;57(1):7–28. doi: 10.3322/canjclin.57.1.7. [DOI] [PubMed] [Google Scholar]
- 5.Burd EM. Human papillomavirus and cervical cancer. Clinical microbiology reviews. 2003 Jan;16(1):1–17. doi: 10.1128/CMR.16.1.1-17.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Syrjanen SM, Syrjanen KJ. New concepts on the role of human papillomavirus in cell cycle regulation. Annals of medicine. 1999 Jun;31(3):175–87. doi: 10.3109/07853899909115976. [DOI] [PubMed] [Google Scholar]
- 7.Harper DM, Franco EL, Wheeler CM, Moscicki AB, Romanowski B, Roteli-Martins CM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet. 2006 Apr 15;367(9518):1247–55. doi: 10.1016/S0140-6736(06)68439-0. [DOI] [PubMed] [Google Scholar]
- 8.Bryan JT. Developing an HPV vaccine to prevent cervical cancer and genital warts. Vaccine. 2007 Apr 20;25(16):3001–6. doi: 10.1016/j.vaccine.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 9.Giannini SL, Hanon E, Moris P, Van Mechelen M, Morel S, Dessy F, et al. Enhanced humoral and memory B cellular immunity using HPV16/18 L1 VLP vaccine formulated with the MPL/aluminium salt combination (AS04) compared to aluminium salt only. Vaccine. 2006 Aug 14;24(33–34):5937–49. doi: 10.1016/j.vaccine.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Stanley M, Lowy DR, Frazer I. Chapter 12: Prophylactic HPV vaccines: Underlying mechanisms. Vaccine. 2006 Aug 21;24( Suppl 3):S106–13. doi: 10.1016/j.vaccine.2006.05.110. [DOI] [PubMed] [Google Scholar]
- 11.Suzich JA, Ghim SJ, Palmer-Hill FJ, White WI, Tamura JK, Bell JA, et al. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proceedings of the National Academy of Sciences of the United States of America. 1995 Dec 5;92(25):11553–7. doi: 10.1073/pnas.92.25.11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Breitburd F, Kirnbauer R, Hubbert NL, Nonnenmacher B, Trin-Dinh-Desmarquet C, Orth G, et al. Immunization with viruslike particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. Journal of virology. 1995 Jun;69(6):3959–63. doi: 10.1128/jvi.69.6.3959-3963.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.FUTURE, II. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. The New England journal of medicine. 2007 May 10;356(19):1915–27. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 14.Paavonen J, Jenkins D, Bosch FX, Naud P, Salmeron J, Wheeler CM, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007 Jun 30;369(9580):2161–70. doi: 10.1016/S0140-6736(07)60946-5. [DOI] [PubMed] [Google Scholar]
- 15.Villa LL, Ault KA, Giuliano AR, Costa RL, Petta CA, Andrade RP, et al. Immunologic responses following administration of a vaccine targeting human papillomavirus Types 6, 11, 16, and 18. Vaccine. 2006 Jul 7;24(27–28):5571–83. doi: 10.1016/j.vaccine.2006.04.068. [DOI] [PubMed] [Google Scholar]
- 16.Villa LL, Costa RL, Petta CA, Andrade RP, Ault KA, Giuliano AR, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. The lancet oncology. 2005 May;6(5):271–8. doi: 10.1016/S1470-2045(05)70101-7. [DOI] [PubMed] [Google Scholar]
- 17.Villa LL, Costa RL, Petta CA, Andrade RP, Paavonen J, Iversen OE, et al. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. British journal of cancer. 2006 Dec 4;95(11):1459–66. doi: 10.1038/sj.bjc.6603469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gall S, Teixeira J, Wheeler C, Naud P, Harper DM, Franco EL, Quint W, Zahaf T, Schuind A, Jenkins D, Dubin G on behalf of the HPV Vaccine Study Group. Substantial impact on precancerous lesions and HPV infections through 5.5 years in women vaccinated with the HPV-16/18 L1 VLP AS04 candidate vaccine. Proceedings of the AACR Annual Meeting; 2007; 2007. [cited April 18,2007]; Available from: http://www.aacr.org/home/scientists/meetings--workshops/annual-meeting-2007/publications.aspx. [Google Scholar]
- 19.Brown D. HPV type 6/11/16/18 vaccine: First analysis of cross-protection against persistent infection, cervical intraepithelial neoplasia (CIN), and adenocarcinoma in situ (AIS) caused by oncogenic HPV types in addition to 16/18. Poster presentation at the 47th interscience conference on antimicrobial agents and chemotherapy; Chicago, IL. September 17–20 2007. [Google Scholar]
- 20.Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005 Feb;14(2):467–75. doi: 10.1158/1055-9965.EPI-04-0551. [DOI] [PubMed] [Google Scholar]
- 21.Varnai AD, Bollmann M, Griefingholt H, Speich N, Schmitt C, Bollmann R, et al. HPV in anal squamous cell carcinoma and anal intraepithelial neoplasia (AIN). Impact of HPV analysis of anal lesions on diagnosis and prognosis. International journal of colorectal disease. 2006 Mar;21(2):135–42. doi: 10.1007/s00384-005-0777-7. [DOI] [PubMed] [Google Scholar]
- 22.Ferenczy A, Franco EL. Prophylactic human papillomavirus vaccines: potential for sea change. Expert review of vaccines. 2007 Aug;6(4):511–25. doi: 10.1586/14760584.6.4.511. [DOI] [PubMed] [Google Scholar]
- 23.Slupetzky K, Gambhira R, Culp TD, Shafti-Keramat S, Schellenbacher C, Christensen ND, et al. A papillomavirus-like particle (VLP) vaccine displaying HPV16 L2 epitopes induces cross-neutralizing antibodies to HPV11. Vaccine. 2007 Mar 1;25(11):2001–10. doi: 10.1016/j.vaccine.2006.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu Y, Wang Q, Han Y, Song G, Xu X. Type-specific and cross-reactive antibodies induced by human papillomavirus 31 L1/L2 virus-like particles. Journal of medical microbiology. 2007 Jul;56(Pt 7):907–13. doi: 10.1099/jmm.0.47073-0. [DOI] [PubMed] [Google Scholar]
- 25.Hildesheim A, Herrero R, Wacholder S, Rodriguez AC, Solomon D, Bratti MC, et al. Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infection: a randomized trial. Jama. 2007 Aug 15;298(7):743–53. doi: 10.1001/jama.298.7.743. [DOI] [PubMed] [Google Scholar]
- 26.Brinkman JA, Caffrey AS, Muderspach LI, Roman LD, Kast WM. The impact of anti HPV vaccination on cervical cancer incidence and HPV induced cervical lesions: consequences for clinical management. European journal of gynaecological oncology. 2005;26(2):129–42. [PubMed] [Google Scholar]
- 27.Bosch FX, Lorincz A, Munoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. Journal of clinical pathology. 2002 Apr;55(4):244–65. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wentzensen N, Vinokurova S, von Knebel Doeberitz M. Systematic review of genomic integration sites of human papillomavirus genomes in epithelial dysplasia and invasive cancer of the female lower genital tract. Cancer research. 2004 Jun 1;64(11):3878–84. doi: 10.1158/0008-5472.CAN-04-0009. [DOI] [PubMed] [Google Scholar]
- 29.Kobayashi A, Greenblatt RM, Anastos K, Minkoff H, Massad LS, Young M, et al. Functional attributes of mucosal immunity in cervical intraepithelial neoplasia and effects of HIV infection. Cancer research. 2004 Sep 15;64(18):6766–74. doi: 10.1158/0008-5472.CAN-04-1091. [DOI] [PubMed] [Google Scholar]
- 30.Busnach G, Piselli P, Arbustini E, Baccarani U, Burra P, Carrieri MP, et al. Immunosuppression and cancer: A comparison of risks in recipients of organ transplants and in HIV-positive individuals. Transplantation proceedings. 2006 Dec;38(10):3533–5. doi: 10.1016/j.transproceed.2006.10.144. [DOI] [PubMed] [Google Scholar]
- 31.Kadish AS, Timmins P, Wang Y, Ho GY, Burk RD, Ketz J, et al. Regression of cervical intraepithelial neoplasia and loss of human papillomavirus (HPV) infection is associated with cell-mediated immune responses to an HPV type 16 E7 peptide. Cancer Epidemiol Biomarkers Prev. 2002 May;11(5):483–8. [PubMed] [Google Scholar]
- 32.Nakagawa M, Stites DP, Farhat S, Judd A, Moscicki AB, Canchola AJ, et al. T-cell proliferative response to human papillomavirus type 16 peptides: relationship to cervical intraepithelial neoplasia. Clinical and diagnostic laboratory immunology. 1996 Mar;3(2):205–10. doi: 10.1128/cdli.3.2.205-210.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tigris Pharmaceuticals I. Research and Development. 2007 [cited October 29, 2007 ]; Available from: Available at: http://www.tigrispharma.com/a007.html.
- 34.Zwaveling S, Ferreira Mota SC, Nouta J, Johnson M, Lipford GB, Offringa R, et al. Established human papillomavirus type 16-expressing tumors are effectively eradicated following vaccination with long peptides. J Immunol. 2002 Jul 1;169(1):350–8. doi: 10.4049/jimmunol.169.1.350. [DOI] [PubMed] [Google Scholar]
- 35.Steller MA, Gurski KJ, Murakami M, Daniel RW, Shah KV, Celis E, et al. Cell-mediated immunological responses in cervical and vaginal cancer patients immunized with a lipidated epitope of human papillomavirus type 16 E7. Clin Cancer Res. 1998 Sep;4(9):2103–9. [PubMed] [Google Scholar]
- 36.Muderspach L, Wilczynski S, Roman L, Bade L, Felix J, Small LA, et al. A phase I trial of a human papillomavirus (HPV) peptide vaccine for women with high-grade cervical and vulvar intraepithelial neoplasia who are HPV 16 positive. Clin Cancer Res. 2000 Sep;6(9):3406–16. [PubMed] [Google Scholar]
- 37.Melief CJM. Immunotherapy of established papilloma virus-induced (pre-)malignant lesions in mice, rabbits and patients. Annals of Oncology. 2006;17(3):iii28. [Google Scholar]
- 38.Qian X, Lu Y, Liu Q, Chen K, Zhao Q, Song J. Prophylactic, therapeutic and anti-metastatic effects of an HPV-16mE6Delta/mE7/TBhsp70Delta fusion protein vaccine in an animal model. Immunology letters. 2006 Feb 15;102(2):191–201. doi: 10.1016/j.imlet.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 39.Einstein MH, Kadish AS, Burk RD, Kim MY, Wadler S, Streicher H, et al. Heat shock fusion protein-based immunotherapy for treatment of cervical intraepithelial neoplasia III. Gynecologic oncology. 2007 Jun 21; doi: 10.1016/j.ygyno.2007.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.ClinicalTrails.gov. Safety Study to Test the Safety of HspE7 and Poly-ICLC Given in Patients With Cervical Intraepithelial Neoplasia. NCT00493545 [cited July 9, 2007; Available from: http://www.clinicaltrials.gov/ct/show/NCT00493545?order=11.
- 41.Santin AD, Bellone S, Palmieri M, Ravaggi A, Romani C, Tassi R, et al. HPV16/18 E7-pulsed dendritic cell vaccination in cervical cancer patients with recurrent disease refractory to standard treatment modalities. Gynecologic oncology. 2006 Mar;100(3):469–78. doi: 10.1016/j.ygyno.2005.09.040. [DOI] [PubMed] [Google Scholar]
- 42.Clinical Trails.gov. Immunotherapy of Recurrent Cervical Cancers Using Dendritic Cells (DCs) NCT00155766 [cited July 9, 2007]; Available from: http://www.clinicaltrials.gov/ct/show/NCT00155766?order=2.
- 43.Qian J, Dong Y, Pang YY, Ibrahim R, Berzofsky JA, Schiller JT, et al. Combined prophylactic and therapeutic cancer vaccine: enhancing CTL responses to HPV16 E2 using a chimeric VLP in HLA-A2 mice. Int J Cancer. 2006 Jun 15;118(12):3022–9. doi: 10.1002/ijc.21781. [DOI] [PubMed] [Google Scholar]
- 44.Kaufmann AM, Nieland JD, Jochmus I, Baur S, Friese K, Gabelsberger J, et al. Vaccination trial with HPV16 L1E7 chimeric virus-like particles in women suffering from high grade cervical intraepithelial neoplasia (CIN 2/3) Int J Cancer. 2007 Aug 23; doi: 10.1002/ijc.23022. [DOI] [PubMed] [Google Scholar]
- 45.de Jong A, O’Neill T, Khan AY, Kwappenberg KM, Chisholm SE, Whittle NR, et al. Enhancement of human papillomavirus (HPV) type 16 E6 and E7-specific T-cell immunity in healthy volunteers through vaccination with TA-CIN, an HPV16 L2E7E6 fusion protein vaccine. Vaccine. 2002 Oct 4;20(29–30):3456–64. doi: 10.1016/s0264-410x(02)00350-x. [DOI] [PubMed] [Google Scholar]
- 46.Gambhira R, Gravitt PE, Bossis I, Stern PL, Viscidi RP, Roden RB. Vaccination of healthy volunteers with human papillomavirus type 16 L2E7E6 fusion protein induces serum antibody that neutralizes across papillomavirus species. Cancer research. 2006 Dec 1;66(23):11120–4. doi: 10.1158/0008-5472.CAN-06-2560. [DOI] [PubMed] [Google Scholar]
- 47.Fiander AN, Tristram AJ, Davidson EJ, Tomlinson AE, Man S, Baldwin PJ, et al. Prime-boost vaccination strategy in women with high-grade, noncervical anogenital intraepithelial neoplasia: clinical results from a multicenter phase II trial. Int J Gynecol Cancer. 2006 May-Jun;16(3):1075–81. doi: 10.1111/j.1525-1438.2006.00598.x. [DOI] [PubMed] [Google Scholar]
- 48.Davidson EJ, Faulkner RL, Sehr P, Pawlita M, Smyth LJ, Burt DJ, et al. Effect of TA-CIN (HPV 16 L2E6E7) booster immunisation in vulval intraepithelial neoplasia patients previously vaccinated with TA-HPV (vaccinia virus encoding HPV 16/18 E6E7) Vaccine. 2004 Jul 29;22(21–22):2722–9. doi: 10.1016/j.vaccine.2004.01.049. [DOI] [PubMed] [Google Scholar]
- 49.Smyth LJ, Van Poelgeest MI, Davidson EJ, Kwappenberg KM, Burt D, Sehr P, et al. Immunological responses in women with human papillomavirus type 16 (HPV-16)-associated anogenital intraepithelial neoplasia induced by heterologous prime-boost HPV-16 oncogene vaccination. Clin Cancer Res. 2004 May 1;10(9):2954–61. doi: 10.1158/1078-0432.ccr-03-0703. [DOI] [PubMed] [Google Scholar]
- 50.Ohlschlager P, Pes M, Osen W, Durst M, Schneider A, Gissmann L, et al. An improved rearranged Human Papillomavirus Type 16 E7 DNA vaccine candidate (HPV-16 E7SH) induces an E7 wildtype-specific T cell response. Vaccine. 2006 Apr 5;24(15):2880–93. doi: 10.1016/j.vaccine.2005.12.061. [DOI] [PubMed] [Google Scholar]
- 51.Brulet JM, Maudoux F, Thomas S, Thielemans K, Burny A, Leo O, et al. DNA vaccine encoding endosome-targeted human papillomavirus type 16 E7 protein generates CD4+ T cell-dependent protection. European journal of immunology. 2007 Feb;37(2):376–84. doi: 10.1002/eji.200636233. [DOI] [PubMed] [Google Scholar]
- 52.Kang TH, Lee JH, Bae HC, Noh KH, Kim JH, Song CK, et al. Enhancement of dendritic cell-based vaccine potency by targeting antigen to endosomal/lysosomal compartments. Immunology letters. 2006 Aug 15;106(2):126–34. doi: 10.1016/j.imlet.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 53.Hung CF, Tsai YC, He L, Wu TC. DNA vaccines encoding Ii-PADRE generates potent PADRE-specific CD4+ T-cell immune responses and enhances vaccine potency. Mol Ther. 2007 Jun;15(6):1211–9. doi: 10.1038/sj.mt.6300121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peng S, Tomson TT, Trimble C, He L, Hung CF, Wu TC. A combination of DNA vaccines targeting human papillomavirus type 16 E6 and E7 generates potent antitumor effects. Gene therapy. 2006 Feb;13(3):257–65. doi: 10.1038/sj.gt.3302646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hung CF, Yang M, Wu TC. Modifying professional antigen-presenting cells to enhance DNA vaccine potency. Methods in molecular medicine. 2006;127:199–220. doi: 10.1385/1-59745-168-1:199. [DOI] [PubMed] [Google Scholar]
- 56.Hsieh CY, Chen CA, Huang CY, Chang MC, Lee CN, Su YN, et al. IL-6-Encoding Tumor Antigen Generates Potent Cancer Immunotherapy Through Antigen Processing and Anti-apoptotic Pathways. Mol Ther. 2007 Jul 3; doi: 10.1038/sj.mt.6300243. [DOI] [PubMed] [Google Scholar]
- 57.Brinkman JA, Xu X, Kast WM. The efficacy of a DNA vaccine containing inserted and replicated regions of the E7 gene for treatment of HPV-16 induced tumors. Vaccine. 2007 Apr 30;25(17):3437–44. doi: 10.1016/j.vaccine.2006.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gasparic M, Rubio I, Thones N, Gissmann L, Muller M. Prophylactic DNA immunization against multiple papillomavirus types. Vaccine. 2007 Jun 6;25(23):4540–53. doi: 10.1016/j.vaccine.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 59.ClinicalTrials.gov. Vaccine Therapy in Preventing Cervical Cancer in Patients With Cervical Intraepithelial Neoplasia. NCT00121173 [cited July 9, 2007]; Available from: http://www.clinicaltrials.gov/ct/show/NCT00121173?order=4.
- 60.Kaufmann AM, Stern PL, Rankin EM, Sommer H, Nuessler V, Schneider A, et al. Safety and immunogenicity of TA-HPV, a recombinant vaccinia virus expressing modified human papillomavirus (HPV)-16 and HPV-18 E6 and E7 genes, in women with progressive cervical cancer. Clin Cancer Res. 2002 Dec;8(12):3676–85. [PubMed] [Google Scholar]
- 61.Transgene. Sustained Response at Month 12 for Transgene’s TG 4001 in HPV-induced Precancerous Lesions of the Cervix and Next Clinical Development Steps. 2006:3. [Google Scholar]
- 62.Corona Gutierrez CM, Tinoco A, Navarro T, Contreras ML, Cortes RR, Calzado P, et al. Therapeutic vaccination with MVA E2 can eliminate precancerous lesions (CIN 1, CIN 2, and CIN 3) associated with infection by oncogenic human papillomavirus. Human gene therapy. 2004 May;15(5):421–31. doi: 10.1089/10430340460745757. [DOI] [PubMed] [Google Scholar]
- 63.Garcia-Hernandez E, Gonzalez-Sanchez JL, Andrade-Manzano A, Contreras ML, Padilla S, Guzman CC, et al. Regression of papilloma high-grade lesions (CIN 2 and CIN 3) is stimulated by therapeutic vaccination with MVA E2 recombinant vaccine. Cancer gene therapy. 2006 Jun;13(6):592–7. doi: 10.1038/sj.cgt.7700937. [DOI] [PubMed] [Google Scholar]
- 64.Kim D, Elizaga M, Duerr A. HIV vaccine efficacy trials: towards the future of HIV prevention. Infectious disease clinics of North America. 2007 Mar;21(1):201–17. x. doi: 10.1016/j.idc.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 65.Jin HS, Park EK, Lee JM, NamKoong SE, Kim DG, Lee YJ, et al. Immunization with adenoviral vectors carrying recombinant IL-12 and E7 enhanced the antitumor immunity to human papillomavirus 16-associated tumor. Gynecologic oncology. 2005 May;97(2):559–67. doi: 10.1016/j.ygyno.2005.01.046. [DOI] [PubMed] [Google Scholar]
- 66.Baez-Astua A, Herraez-Hernandez E, Garbi N, Pasolli HA, Juarez V, Zur Hausen H, et al. Low-dose adenovirus vaccine encoding chimeric hepatitis B virus surface antigen-human papillomavirus type 16 E7 proteins induces enhanced E7-specific antibody and cytotoxic T-cell responses. Journal of virology. 2005 Oct;79(20):12807–17. doi: 10.1128/JVI.79.20.12807-12817.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gomez-Gutierrez JG, Elpek KG, Montes de Oca-Luna R, Shirwan H, Sam Zhou H, McMasters KM. Vaccination with an adenoviral vector expressing calreticulin-human papillomavirus 16 E7 fusion protein eradicates E7 expressing established tumors in mice. Cancer Immunol Immunother. 2007 Jul;56(7):997–1007. doi: 10.1007/s00262-006-0247-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Berg M, Gambhira R, Siracusa M, Hoiczyk E, Roden R, Ketner G. HPV16 L1 capsid protein expressed from viable adenovirus recombinants elicits neutralizing antibody in mice. Vaccine. 2007 Apr 30;25(17):3501–10. doi: 10.1016/j.vaccine.2006.06.080. [DOI] [PubMed] [Google Scholar]
- 69.Fraser C, Tomassini JE, Xi L, Golm G, Watson M, Giuliano AR, et al. Modeling the long-term antibody response of a human papillomavirus (HPV) virus-like particle (VLP) type 16 prophylactic vaccine. Vaccine. 2007 May 22;25(21):4324–33. doi: 10.1016/j.vaccine.2007.02.069. [DOI] [PubMed] [Google Scholar]
- 70.Viscidi RP, Schiffman M, Hildesheim A, Herrero R, Castle PE, Bratti MC, et al. Seroreactivity to human papillomavirus (HPV) types 16, 18, or 31 and risk of subsequent HPV infection: results from a population-based study in Costa Rica. Cancer Epidemiol Biomarkers Prev. 2004 Feb;13(2):324–7. doi: 10.1158/1055-9965.epi-03-0166. [DOI] [PubMed] [Google Scholar]
- 71.Lehtinen M, Herrero R, Mayaud P, Barnabas R, Dillner J, Paavonen J, et al. Chapter 28: Studies to assess the long-term efficacy and effectiveness of HPV vaccination in developed and developing countries. Vaccine. 2006 Aug 21;24( Suppl 3):S233–41. doi: 10.1016/j.vaccine.2006.05.109. [DOI] [PubMed] [Google Scholar]
- 72.Luna J, Saah A, Hood S, Bautista O, Barr E. Safety, efficacy, and immunogenicity of quadrivalent HPV vaccine (Gardasil) in women aged 24–45. 24th International Papillomavirus Congress; 2007 November 3–9; Beijing, China. 2007. [Google Scholar]
- 73.Schwarz T, Dubin G and the HPV Vaccine Study Investigators for Adult Women. Human papillomavirus (HPV) 16/18 I1 AS04 virus-like particle (VLP) cervical cancer vaccine is immunogenic and well tolerated 18 months after vaccination in women up to age 55 years. Journal of Clinical Oncology, 2007 ASCO Annual Meeting Proceedings Part I; 2007; p. 3007. [Google Scholar]
- 74.ClinicalTrials.Gov. Immunogenicity of GSK Bio’s HPV Vaccine (580299) Versus Merck’s Gardasil® in Healthy Females 18–45 Years of Age. [cited November 6, 2007]; Available from: http://www.clinicaltrials.gov/ct/show/NCT00423046?order=1.
- 75.Giuliano A. human papillomavirus vaccination in males. Gynecologic oncology. 2007;107:S24–S6. doi: 10.1016/j.ygyno.2007.07.075. [DOI] [PubMed] [Google Scholar]
- 76.Kim JJ, Andres-Beck B, Goldie SJ. The value of including boys in an HPV vaccination programme: a cost-effectiveness analysis in a low-resource setting. British journal of cancer. 2007 Oct 9; doi: 10.1038/sj.bjc.6604023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Elbasha EH, Dasbach EJ, Insinga RP. Model for assessing human papillomavirus vaccination strategies. Emerging infectious diseases. 2007 Jan;13(1):28–41. doi: 10.3201/eid1301.060438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Palefsky JM, Holly EA, Ralston ML, Jay N. Prevalence and risk factors for human papillomavirus infection of the anal canal in human immunodeficiency virus (HIV)-positive and HIV-negative homosexual men. The Journal of infectious diseases. 1998 Feb;177(2):361–7. doi: 10.1086/514194. [DOI] [PubMed] [Google Scholar]
- 79.Palefsky JM, Holly EA, Ralston ML, Arthur SP, Jay N, Berry JM, et al. Anal squamous intraepithelial lesions in HIV-positive and HIV-negative homosexual and bisexual men: prevalence and risk factors. J Acquir Immune Defic Syndr Hum Retrovirol. 1998 Apr 1;17(4):320–6. doi: 10.1097/00042560-199804010-00005. [DOI] [PubMed] [Google Scholar]
- 80.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA: a cancer journal for clinicians. 2005 Mar-Apr;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 81.Maclean J, Koekemoer M, Olivier AJ, Stewart D, Hitzeroth II, Rademacher T, et al. Optimization of human papillomavirus type 16 (HPV-16) L1 expression in plants: comparison of the suitability of different HPV-16 L1 gene variants and different cell-compartment localization. The Journal of general virology. 2007 May;88(Pt 5):1460–9. doi: 10.1099/vir.0.82718-0. [DOI] [PubMed] [Google Scholar]
- 82.Franconi R, Massa S, Illiano E, Mullar A, Cirilli A, Accardi L, et al. Exploiting the plant secretory pathway to improve the anticancer activity of a plant-derived HPV16 E7 vaccine. International journal of immunopathology and pharmacology. 2006 Jan-Mar;19(1):187–97. [PubMed] [Google Scholar]
- 83.Aires KA, Cianciarullo AM, Carneiro SM, Villa LL, Boccardo E, Perez-Martinez G, et al. Production of human papillomavirus type 16 L1 virus-like particles by recombinant Lactobacillus casei cells. Applied and environmental microbiology. 2006 Jan;72(1):745–52. doi: 10.1128/AEM.72.1.745-752.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cortes-Perez NG, Azevedo V, Alcocer-Gonzalez JM, Rodriguez-Padilla C, Tamez-Guerra RS, Corthier G, et al. Cell-surface display of E7 antigen from human papillomavirus type-16 in Lactococcus lactis and in Lactobacillus plantarum using a new cell-wall anchor from lactobacilli. Journal of drug targeting. 2005 Feb;13(2):89–98. doi: 10.1080/10611860400024219. [DOI] [PubMed] [Google Scholar]
- 85.Massa S, Franconi R, Brandi R, Muller A, Mett V, Yusibov V, et al. Anti-cancer activity of plant-produced HPV16 E7 vaccine. Vaccine. 2007 Apr 20;25(16):3018–21. doi: 10.1016/j.vaccine.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 86.Bermudez-Humaran LG, Cortes-Perez NG, Lefevre F, Guimaraes V, Rabot S, Alcocer-Gonzalez JM, et al. A novel mucosal vaccine based on live Lactococci expressing E7 antigen and IL-12 induces systemic and mucosal immune responses and protects mice against human papillomavirus type 16-induced tumors. J Immunol. 2005 Dec 1;175(11):7297–302. doi: 10.4049/jimmunol.175.11.7297. [DOI] [PubMed] [Google Scholar]


