Abstract
The insulin-like growth factor (IGF) axis plays an essential role in the development of the mammary gland. High circulating levels of IGF-I and of its major binding protein IGFBP3 have been related with increased mammographic density in Caucasian premenopausal women. Some common single nucleotide polymorphisms (SNPs) in genes of the IGF pathway have also been suggested to play a role in mammographic density. We conducted a cross-sectional study nested within the large Mexican ESMaestras cohort, to investigate the relation between circulating levels of IGF-I, IGFBP-3, the IGF-I/IGFBP-3 ratio, five common SNPs in the IGF-1, IGFBP-3 and IGF-1R genes, and mammographic density in 593 premenopausal Mexican women. Mean age at mammogram was 43.1 (standard deviation–SD=3.7) years, and average body mass index (BMI) at recruitment was 28.5 kg/m2. Mean percent mammographic density was 36.5% (SD: 17.1), with mean dense tissue area of 48.3 (SD: 33.3) cm2. Mean IGF-I and IGFBP-3 concentrations were 15.33 (SD: 5.52) nmol/l and 114.96 (SD: 21.34) nmol/l, respectively. No significant associations were seen between percent density and biomarker concentrations but women with higher IGF-I and IGF-I/IGFBP-3 concentrations had lower absolute dense (ptrend =0.03 and 0.09, respectively) and non-dense tissue areas (ptrend <0.001 for both parameters). However, these associations were null after adjustment by BMI. SNPs in specific genes were associated with circulating levels of growth factors, but not with mammographic density features. These results do not support the hypothesis of a strong association between circulating levels of growth hormones and mammographic density in Mexican premenopausal women.
Keywords: IGF, mammographic density, genetic polymorphisms, Mexican premenopausal women
Introduction
Mammographic density is among the strongest predictors of breast cancer, with women having more than 75% of dense tissue showing between 4 to 6 times the risk of breast cancer compared to women with little dense tissue (1). Mammographic density is influenced by age, body mass index (BMI) and by several reproductive and lifestyle factors (such as parity, menopause, hormone use), which also are associated with breast cancer risk (2;3). Overall, however, these factors explain only between 20 and 30% of inter-women variability in breast density, while a larger proportion (up to 60% in homozygous twins) appears to be associated with genetic factors (3–5). Identifying genetic variants associated with mammographic density may help better understand the strong association between mammographic density and breast cancer risk.
Endogenous hormones play an essential role in the development of the mammary gland. Insulin-like growth factor-I (IGF-I) is a polypeptide hormone with mitogenic and anti-apoptotic properties, and co-regulates the proliferation of many different types of cells, including breast epithelium (6;7). Most of circulating IGF-I present is synthesized by the liver, and about 90% of it is bound to IGF-binding protein 3 (IGFBP-3), the most abundant IGF-binding protein present in blood. In addition to regulating IGF-I bio-availability, IGFBP-3 has independent anti-mitogenic and anti-proliferative properties on several cell types, including breast epithelium (8). To exert their biological functions, both IGF-I and IGFBP-3 bind to receptors, and although several receptors have been identified for IGFs, it appears that most of the effects are mediated through the IGF-I receptor (IGF-IR) (6).
High circulating levels of IGF-I have been associated with increased breast cancer risk (9), but results on the associations of this hormone with mammographic density are inconsistent (10–15). Results from large international studies indicate that some common genetic variations in IGF-1 and IGFBP-3 in Caucasian women are associated with circulating levels of the IGF-I and IGFBP-3 (16;17), but not with breast cancer risk (17–19). Conversely, in women from other ethnic groups, some IGF-1 polymorphisms were significantly associated with breast cancer risk (20), especially in young women (21). Results from large cohort studies, including mainly Caucasian women, suggested that some common genetic variations in growth hormone pathways (especially for the minor allele for rs6220 in the IGF-I pathway) are associated with higher mammographic density (10;22–24), although sometimes modestly (23;24). It has also been suggested that associations may differ among different racial/ethnic groups (24).
The purpose of this study was to evaluate the relation between five candidate IGF-I pathway single nucleotide polymorphisms (SNPs), circulating concentrations of IGF-I and IGFBP-3 and mammographic density a sample of Mexican pre-menopausal women who are part of the large ESMaestras cohort (25).
Material and Methods
Study population and blood collection
The ESMaestras cohort is a cohort of 115,345 female teachers from 12 Mexican states that was established in 2006–08 to identify risk factors related to cancer and other chronic diseases among Mexican women (25). Participants responded to questionnaires on demographics, socio-economic status, reproductive history and use of oral contraceptives, menopausal hormone therapy, medical conditions, anthropometry, lifestyle (including a food frequency questionnaire), physical activity, smoking habits and early-life risk factors. In 2007, a subsample of 2,084 ESMaestras teachers from two Mexican states (Jalisco and Veracruz) participated in a clinical evaluation that included an interview, anthropometric measurements, a mammogram, and the collection of biological specimens. Fasting blood samples (approximately 25 ml) were obtained by venepuncture by trained nurses and in 5 different tubes, two of them containing disodium ethylene diamine tetraacetic acid (EDTA). In no more than 30 minutes after blood draw, plasma, serum, erythrocytes and buffy coat were separated by centrifugation at 2500 rpm during 10 minutes in a refrigerated centrifuge (4°C), and aliquoted into several cryotubes at the field work site. Samples were frozen and kept in ultra low freezers at −70°C at the National Institute of Public Health (INSP) until shipment to IARC where they were stored at −80 °C in ultra-low freezer until hormone analyses were performed.
All participants gave informed consent for future use of biological specimens and questionnaire data. The study was approved by the Institutional Review Board at INSP and by the IARC Ethics Committee.
Selection of subjects
Among the 2,084 ESMaestras teachers who participated in the clinical sub-cohort, we excluded 230 women who had insufficient information on metabolic syndrome components (because of a parallel study on metabolic syndrome in the same population (26)), 67 who had an unknown menopausal status and 624 who were postmenopausal at the time of their mammogram (women were considered as pre-menopausal if they had menstruated at least once over the 12 months prior to the visit, and were considered as postmenopausal if they had no menstruation over the last 12 months prior to the visit, and those with surgical menopause who reported bilateral oophorectomy or those who did not know the type of surgery but who were over 48 years, given that mean age at menopause in Mexican women is 48 years (27)). We then stratified women by 4 breast density categories: <10%, 10 to <25%, 25 to <50% and >=50% (28). Women were randomly selected from each group proportionally to its size. Thirty-five were selected for the first group, 158 for the second, 247 for the third and 160 for the fourth group. Out of these 600, 7 declared to be older than 55 and be premenopausal, and were therefore excluded from the analyses. Our final study population was composed of 593 women.
Mammographic density
Measurement of mammographic density was performed and validated as previously described (26). Briefly, a radiology technician performed mammography using the Giotto Image M (Internazionale Medico Scientifica, Bologna, Italy) in Jalisco and the Hologic Lorad M-III (Hologic, Bedford, MA) in Veracruz. Mammograms were developed using the Agfa CP1000 (Agfa-Gevaert Group, Belgium) developer. Cranio-caudal (CC) views were taken on each breast. An Astra 2400S scanner (Umax, Fremont, CA) was used to digitize the analogue films. A single observer measured mammographic density on the left CC view using Mamgr, a computer-assisted program developed at the Faculty of Epidemiology and Population Health, London School of Hygiene and Tropical Medicine (29). This is a quantitative interactive-threshold method based on Byng et al. (30) and Ursin et al. (31), in which an observer selects one threshold grey level to identify pixels within the breast area on the digitized image and another to distinguish dense pixels from lucent ones within the breast gland. Percent mammographic density is automatically calculated as the percent of “dense” pixels within the breast area. Non-dense area was calculated by subtracting the dense area from the total breast area. Absolute dense and non-dense area values are converted to cm2 according to the pixel size used in the digitisation. The intra-observer intra-class correlation in 108 duplicate mammograms was 0.84.
Laboratory assays
Hormone analyses
All laboratory analyses were performed on never-thawed serum samples continuously stored at −80C. Serum IGF-I and IGFBP3 concentrations were measured by immunoradiometric assays by Beckmann Coulter (Marseille, France) at the laboratory of hormone analyses, Biomarkers Group, International Agency for Research on Cancer (Lyon, France). Samples were batched by and randomly ordered within states of recruitment (Jalisco/Veracruz). The intra-assay and inter-assay coefficients of variations were 0.8% and 4.2%, respectively, for a concentration of 19.5 nmol/l for IGF-I, and 1.3% and 3.0%, respectively, for a concentration of 125 nmol/l for IGFBP-3.
DNA extraction
Genomic DNA from participants was extracted from a 0.5 ml aliquot of buffy coat, which had been kept frozen since blood collection and processing (32). All DNAs were extracted at IARC by use of the Gentra Autopure LS DNA preparation platform (Gentra Systems, Minneapolis, USA).
SNPs selection and genotyping
Five candidate SNPs which were observed in previous studies to be associated with IGF-I or IGFBP-3 levels, mammographic density, or breast cancer risk, especially in premenopausal women, were selected for genotyping: rs1549593 and rs1520220, in intron 3 of IGF-1, rs6220 in the 3′UTR of IGF-1, rs2854744 upstream of IGFBP-3 (promoter region), and rs2229765, a synonymous SNP (p.Glu1043Glu) in IGFR.
To assess these five candidate SNPs, genotyping was performed on 10 ng of genomic DNA using TaqMan pre-designed SNP genotyping assays (Applied Biosystems, Foster City, CA, USA). The fluorescence reading and allelic discrimination analyses were performed with the Applied Biosystems ABI PRISM 7900HT Sequence detection system.
DNA from study participants was randomized on plates and all samples were analysed simultaneously. For quality control purposes, duplicates of 10% of the samples were interspersed throughout the plates.
Statistical analysis
The age of the subjects was calculated based on the date of birth and the date of the clinical visit. BMI was defined as measured weight (kg) divided by measured height squared (m2). Means and standard deviations, or percentages (where appropriate), of selected baseline characteristics and biomarkers were estimated. Correlations between hormone concentrations, mammographic density measures, age at recruitment and anthropometry were calculated as Spearman’s partial correlation coefficients, adjusting for age, batch of laboratory analyses, and region, as appropriate. Multivariate regression analyses were performed to compare means of mammographic characteristics by quartiles of IGF-I, IGFBP-3 and the ratio IGF-I/IGFBP3. Covariates included in the analyses included BMI (continuous), age, batch of analyses, state (Jalisco and Veracruz), family history of breast cancer (yes/no), benign breast disease (yes/no), age at menarche (<12, 12, 13, 14+, unknown), oral contraceptive use (never used, used for less than 5 years, used for more than 5 years, ever unknown duration, unknown), number of full term pregnancies (nulliparous, 1, 2, 3, 4+, missing), age at first full term pregnancy (nulliparous, <20 years, 20–25 years, 25–30 years, >30 years, missing), alcohol intake (0, <0.1 drinks/day, 0.1–0.2 drinks/day, >0.2 drinks/day, missing), smoking status (never, past, current, missing), physical activity (as a continuous variable expressed as MetS/h) and social economic status (low, medium, high, missing. These categories were based on questionnaire data about having a telephone, a mobile telephone, a car, a computer, a vacuum cleaner, a microwave oven and internet access, and were classified as follows: low social economic status: ≤3 items, medium: SES 4–5 items, high SES: 6+ items). We tested for trends across categories of variables by assigning equally spaced scores to the categories (p-trend). As BMI may be a mediator of the IGF-mammographic density associations, all analyses were conducted with and without further adjustment for BMI. Women with missing BMI values (n=19) were excluded from the BMI-specific analyses. To further evaluate possible effect modification of body size on the association between hormones and measures of mammographic density, we explored these associations separately in obese women (BMI ≥30 kg/m2, n=198), using multivariate regression analyses through tertiles of exposure.
SNPs analysis
For each of the genotyped SNPs, the average call rate was 90.2 % (range 87.5- 93.9) and quality control analysis showed a concordance rate >99% between duplicate samples. Allele and genotype frequencies were calculated and Hardy-Weinberg equilibrium (HWE) was tested in the studied sample set for each SNP. The five SNPs were in HWE among the subjects analysed.
Genotype-trend regression models were used to assess whether genotypes were associated with mammographic features, and with hormone levels. For each SNP, we estimated the effects of heterozygote and rare-allele homozygote genotype relative to the common homozygote genotype as this approach does not imply any assumptions regarding the structure of associations across genotypes. Covariates included in the analyses were age, batch (where applicable), BMI and state. All analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC).
Results
Selected characteristics of the study population are presented in Table 1. Mean age at mammogram was 43.1 (standard deviation–SD=3.7) years, and average BMI at the clinical assessment was 28.5 kg/m2. Mean percentage mammographic density was 36.5 (SD: 17.1), with mean dense tissue area of 48.3 (SD: 33.3) cm2. Mean IGF-I and IGFBP-3 concentrations were, respectively, 15.33 (SD: 5.52) nmol/l, and 114.96 (SD: 21.34) nmol/l. Smoking status was unknown for 65 subjects, while 378 women were never smokers, 59 were current smokers and 91 previous smokers (results not shown). Habits for alcohol drinking were unknown for 46 subjects, while 170 women were teetotallers, 267 women drank less than 0.1 drink/day, 77 between 0.1 and 0.2 drinks/day, and 33 equal or more than 0.2 drinks/day (data not shown). Only 15 women declared to take hormone replacement therapy at the time of the clinical assessment.
Table 1.
Selected characteristics of the 593 premenopausal women included in the study.
| Mean (± standard deviation) | All |
|---|---|
| Age at screening (years) | 43.1 (3.7) |
| Age at menarche (years) | 12.6 (1.5) |
| Age at first birth* (years) | 24.8 (4.5) |
| Number of full term pregnancies* | 2.4 (1.0) |
| Duration of breast feeding* (months) | 15.8 (14.3) |
| BMI# (kg/m2) | 28.5 (5.3) |
| Frequency (%) | |
| Parous | 88.6 |
| Family history of breast cancer | 4.7 |
| History of benign breast disease | 14.7 |
| Ever hormone use | 51.8 |
| Mean (± standard deviation) | |
| Density (%) | 36.52 (17.05) |
| Dense tissue area (cm2) | 48.34 (33.25) |
| Non-dense tissue area (cm2) | 82.33 (38.80) |
| IGF-I (nmol/l) | 15.33 (5.52) |
| IGFBP-3 (nmol/l) | 114.96 (21.34) |
| IGF-I/IGFBP-3 | 0.13 (0.04) |
among parous women
N=19 missing
In our study population, IGF-I concentrations were positively correlated with IGFBP-3 concentrations and with the ratio IGF-I/IGFBP-3 (Spearman’s r = 0.64 and 0.86, respectively, p<0.01; data not shown). Concentrations of IGF-I, IGFBP-3 and the ratio of IGF-I/IGFBP-3 were negatively correlated with age at the clinical visit (Spearman’s r = −0.22, p<0.001, r = −0.13, p=0.002 and r = −0.20, p<0.001, respectively), while only IGF-I and IGF-I/IGFBP-3 (but not IGFBP-3) were significantly, and negatively, correlated with BMI (Spearman’s r = −0.32, p<0.001 and r= −0.34, p<0.001, respectively). BMI was negatively correlated with percentage of breast density (Spearman’s: −0.17, p<0.001), and positively correlated with absolute dense area (Spearman’s r = 0.18, p<0.001) and, more strongly so, with absolute non-dense area (Spearman’s r = 0.61, p<0.001).
Means of mammographic characteristics by quartiles of biomarkers are shown in Table 2. No statistically significant relations were seen between increasing biomarker concentrations and percentage mammographic density. Women with higher IGF-I and IGF-I/IGFBP-3 concentrations had lower absolute dense and non-dense tissue areas (for dense tissue area: 40.08 (29.5–52.2) vs 50.0 (38.9–61.0) ptrend=0.03; and 42.8 (31.4–54.2) vs 48.3 (37.4–59.3) ptrend = 0.09 (non-significant) highest vs lowest IGF-I and IGF-I/IGFBP-3 ratio quartile, respectively. For non-dense tissue area: 72.2 (59.0–85.4) vs 90.4 (77.5–103.2) ptrend<0.0001; and 68.8 (55.7–82.0) vs 89.8 (77.1–102.4) ptrend < 0.0001 highest vs lowest IGF-I and IGF-I/IGFBP-3 ratio quartile, respectively). However, associations were attenuated and no longer statistically significant after adjustment by BMI (Table 2). When exploring the associations between mammographic characteristics and biomarker concentrations separately in obese women (BMI≥30, n=198), absolute non-dense tissue area was significantly lower among women with higher IGF-I/IGFBP-3 ratio levels (highest vs lowest tertile of absolute non-dense tissue area= 103.7 (75.9–131.5) vs 126.9 (98.7–155.0), ptrend: 0.01; data not shown). No significant relations were observed between other mammographic characteristics and hormone concentrations in obese women (data not shown).
Table 2.
Adjusted means (95% confidence intervals) of mammographic characteristics by quartiles of IGF-I, IGFBP-3 and their ratio1
| IGF-I (nmol/l) | |||||
|---|---|---|---|---|---|
| < 11.5 | 11.5–15.4 | 15.4–18.8 | > 18.8 | ||
| Number of subjects | 148 | 149 | 147 | 149 | p trend |
| Percent mammographic density | 37.3 (31.7–42.8) | 35.3 (30.0–40.6) | 37.5 (32.0–42.9) | 36.9 (31.2–42.6) | 0.85 |
| Further adjusted2 | 37.9 (32.1–43.6) | 36.3 (30.8–41.7) | 36.3 (30.6–42.0) | 35.4 (29.5–41.3) | 0.29 |
| Absolute dense tissue area (cm2) | 50.0 (38.9–61.0) | 43.6 (33.0–54.1) | 42.3 (31.4–53.2) | 40.8 (29.5–52.2) | 0.03 |
| Further adjusted2 | 46.5 (35.1–57.9) | 42.2 (31.4–53.1) | 43.6 (32.3–54.9) | 43.4 (31.6–55.1) | 0.56 |
| Absolute non dense tissue area (cm2) | 90.4 (77.5–103.2) | 85.6 (73.3–97.9) | 76.6 (63.9–97.9) | 72.2 (59.0–85.4) | <.0001 |
| Further adjusted2 | 82.3 (71.4–93.2) | 79.6 (69.2–89.9) | 83.3 (72.5–94.1) | 82.6 (71.4–93.8) | 0.73 |
|
| |||||
| IGFBP-3 (nmol/l) | |||||
|
| |||||
| < 101.9 | 101.9–116.8 | 116.8–129.4 | > 129.4 | ||
| Number of subjects | 148 | 148 | 149 | 148 | |
| Percent mammographic density | 36.1 (30.5–41.6) | 37.5 (32.0–43.0) | 37.0 (31.6–42.4) | 35.7 (30.3–41.1) | 0.81 |
| Further adjusted2 | 36.6 (30.9–42.3) | 37.3 (31.7–42.9) | 37.1 (31.5–42.6) | 35.3 (29.7–40.9) | 0.52 |
| Absolute dense tissue area (cm2) | 46.4 (35.3–57.5) | 44.7 (33.7–55.7) | 43.7 (32.8–54.6) | 43.3 (32.5–54.1) | 0.42 |
| Further adjusted2 | 44.5 (33.1–55.8) | 43.9 (32.7–55.1) | 43.1 (31.9–54.2) | 43.5 (32.3–54.7) | 0.77 |
| Absolute non dense tissue area (cm2) | 87.8 (74.7–100.8) | 81.1 (68.2–94.1) | 82.3 (69.5–95.0) | 80.7 (68.0–93.4) | 0.18 |
| Further adjusted2 | 82.2 (71.3–93.0) | 80.6 (69.9–91.4) | 80.8 (70.2–91.4) | 82.5 (71.9–93.2) | 0.91 |
|
| |||||
| Ratio IGF-I/BP-3 | |||||
|
| |||||
| < 0.11 | 0.11–0.13 | 0.13–0.16 | > 0.16 | ||
| Number of subjects | 148 | 148 | 149 | 148 | |
| Percent mammographic density | 36.7 (31.3–42.2) | 35.8 (30.4–41.1) | 36.6 (31.1–42.1) | 38.6 (32.9–44.3) | 0.31 |
| Further adjusted2 | 37.4 (31.7–43.1) | 36.2 (30.7–41.8) | 35.8 (30.2–41.4) | 37.1 (31.2–43.0) | 0.87 |
| Absolute dense tissue area (cm2) | 48.3 (37.4–59.3) | 45.3 (34.7–56.0) | 39.6 (28.7–50.6) | 42.8 (31.4–54.2) | 0.09 |
| Further adjusted2 | 45.1 (33.8–56.4) | 44.3 (33.2–55.3) | 40.7 (29.6–51.9) | 45.8 (34.1–57.5) | 0.94 |
| Absolute non dense tissue area (cm2) | 89.8 (77.1–102.4) | 85.7 (73.4–98.0) | 77.0 (64.4–89.6) | 68.8 (55.7–82.0) | <.0001 |
| Further adjusted2 | 82.0 (71.2–92.8) | 81.5 (70.9–92.0) | 81.8 (71.1–92.5) | 79.8 (68.6–91.0) | 0.62 |
Adjusted for age, batch, state, family history of breast cancer (yes/no), benign breast disease (yes/no), age at menarche (<12, 12, 13, 14+, unknown), oral contraceptive use (never, ever <5 years, ever 5+ years, ever unknown duration, unknown), number of full term pregnancies (0, 1, 2, 3, 4+, missing), age at first full term pregnancy (nulliparous, <20, [20–25[, [25–30[, 30+, missing), alcohol intake (0, <0.1, [0.1–0.2[, 0.2+, missing), smoking status (never, past, current, missing) and social economic status (low, medium, high, missing)
Model 2 is Model 1 plus additional adjustment for BMI
Allele and genotype frequencies in the IGF-1, IGFBP-3 and IGFR genes are shown in Table 3, together with single SNP associations with hormone levels and mammographic density characteristics. In IGF-1, the number of copies of the minor allele (T) of rs1549593 was inversely associated with IGF-I and IGF-I/IGFBP-3 ratio (ptrend= 0.01 and 0.02, respectively). In contrast, there were no associations between the rs1520220 or the rs6220 and circulating hormone levels. In IGFBP-3, the number of copies of the minor allele (T) of rs2854744 was associated with increased levels of IGF-I and IGFBP-3 concentrations, and decreased levels of the IGF-I/IGFBP-3 ratio (ptrend: 0.03, <0.0001, and 0.02, respectively). The examined SNP in IGFR (rs2229765) was not related to hormone concentrations. No significant associations were observed between the studied SNPs and mammographic density measures.
Table 3.
Common genetic variations in IGF-1, IGFBP-3 and IGFR and concentrations of IGF-I, IGFBP-3 and their ratio, and mammographic density
| IGF-Ia (nmol/l) | IGFBP-3a (nmol/l) | Ratioa | %densityb | Absolute dense areab | Non-dense areab | |||
|---|---|---|---|---|---|---|---|---|
| IGF-1 | ||||||||
| rs1549593 | Minor frequency allele Major frequency allele |
A1/A1 A1/A2 A2/A1 |
||||||
|
| ||||||||
| 0.GG | G = 0.80 T = 0.20 |
333 | 15.91 (15.35–16.48) | 116.7 (114.4–119.0) | 0.13 (0.13–0.14) | 36.60 (34.87–38.33) | 48.67 (45.196–52.15) | 83.29 (79.99–86.59) |
| 1.GT | 169 | 14.35 (13.74–15.32) | 113.5 (110.3–116.8) | 0.12 (0.12–0.13) | 35.68 (33.25–38.10) | 46.07 (41.18–50.96) | 82.27 (77.64–86.91) | |
| 2.TT | 18 | 15.17 (12.78–17.56) | 112.4 (102.6–122.3) | 0.13 (0.12–0.15) | 40.76 (33.31–48.20) | 59.16 (44.19–74.14) | 80.15 (65.95–94.35) | |
| ptrend# | 0.01 | 0.09 | 0.02 | 0.90 | 0.93 | 0.62 | ||
|
| ||||||||
| rs1520220 | Frequency allele 1 Frequency allele 2 |
A1/A1 A1/A2 A2/A1 |
||||||
|
| ||||||||
| 0.CC | C = 0.76 G = 0.24 |
287 | 15.06 (14.44–15.68) | 114.7 (112.2–117.3) | 0.13 (0.13–0.13) | 36.31 (34.43–38.19) | 47.27 (43.50–51.04) | 82.76 (79.18–86.33) |
| 1.CG | 179 | 15.55 (14.78–16.32) | 115.5 (112.3–118.6) | 0.13 (0.13–0.1) | 36.46 (34.10–38.81) | 50.30 (45.59–55.02) | 81.96 (77.48–86.43) | |
| 2.GG | 37 | 16.50 (14.80–18.20) | 122.5 (115.6–129.5) | 0.13 (0.12–0.15) | 34.70 (29.47–39.93) | 40.22 (29.73–50.71) | 81.67 (71.72–91.62) | |
| ptrend# | 0.10 | 0.10 | 0.28 | 0.74 | 0.81 | 0.76 | ||
|
| ||||||||
| rs6220 | Frequency allele 1 Frequency allele 2 |
A1/A1 A1/A2 A2/A1 |
||||||
|
| ||||||||
| 0.AA | A = 0.71 G = 0.29 |
253 | 15.11 (14.46–15.76) | 115.5 (112.8–118.1) | 0.13 (0.13–0.13) | 36.23 (34.25–38.22) | 47.12 (43.12–51.12) | 83.02 (79.23–86.81) |
| 1.AG | 197 | 15.37 (14.63–16.11) | 114.4 (111.4–117.4) | 0.13 (0.13–0.14) | 36.59 (34.34–38.83) | 50.05 (45.53–54.56) | 80.88 (76.60–85.16) | |
| 2.GG | 50 | 16.16 (14.69–17.62) | 121.1 (115.2–127.1) | 0.13 (0.12–0.14) | 35.77 (31.28–40.26) | 42.74 (33.70–51.78) | 84.37 (75.80–92.94) | |
| ptrend# | 0.22 | 0.33 | 0.39 | 0.99 | 0.55 | 0.86 | ||
| IGFBP–3 | ||||||||
|
| ||||||||
| rs2854744 | Frequency allele 1 Frequency allele 2 |
A1/A1 A1/A2 A2/A1 |
||||||
|
| ||||||||
| 0.GG | G = 0.72 T = 0.28 |
280 | 15.04 (14.42–15.66) | 109.6 (107.3–112.0) | 0.14 (0.13–0.14) | 37.08 (35.20–38.97) | 48.88 (45.13–52.62) | 80.91 (77.33–84.49) |
| 1.GT | 214 | 15.65 (14.94–16.36) | 120.9 (118.2–123.6) | 0.13 (0.12–0.13) | 35.49 (33.32–37.65) | 47.57 (43.26–51.88) | 84.47 (80.35–88.58) | |
| 2.TT | 43 | 16.84 (15.29–18.40) | 131.4 (125.4–137.4) | 0.13 (0.12–0.14) | 36.16 (31.31–41.01) | 46.28 (36.65–55.91) | 83.82 (74.62–93.02) | |
| ptrend# | 0.03 | <.0001 | 0.02 | 0.38 | 0.55 | 0.26 | ||
| IGFR | ||||||||
|
| ||||||||
| rs2229765 | Frequency allele 1 Frequency allele 2 |
A1/A1 A1/A2 A2/A1 |
||||||
|
| ||||||||
| 0.GG | G = 0.67 A = 0.33 |
231 | 15.54 (14.86–16.23) | 116.0 (113.1–118.8) | 0.13 (0.13–0.14) | 35.83 (33.74–37.91) | 47.16 (43.01–51.31) | 82.28 (78.32–86.24) |
| 1.GA | 238 | 14.87 (14.19–15.55) | 114.6 (111.9–117.4) | 0.13 (0.12–0.13) | 36.66 (34.60–38.71) | 47.73 (43.64–51.83) | 82.36 (78.45–86.27) | |
| 2.AA | 53 | 16.48 (15.07–17.88) | 120.7 (115.0–126.5) | 0.13 (0.12–0.14) | 34.20 (29.82–38.58) | 45.17 (36.44–53.90) | 82.39 (74.06–90.72) | |
| ptrend# | 0.86 | 0.47 | 0.73 | 0.85 | 0.85 | 0.60 | ||
Means and (95% confidence intervals), adjusted by age, state, batch of laboratory analyses and BMI
Means and (95% confidence intervals), adjusted by age, state and BMI
p-value for a linear trend in hormone/density levels across genotype categories estimated by assigning scores equal to the number of minor alleles to the categories (0, 1 or 2 alleles).
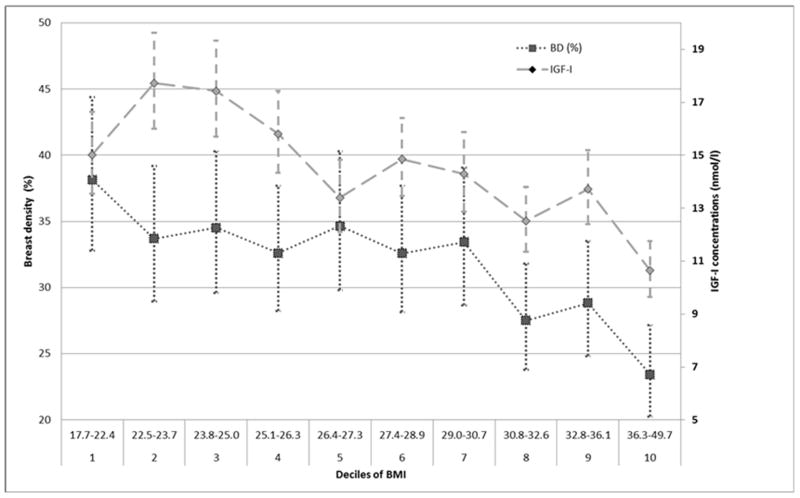
Mean IGF-I concentrations and mean percent breast density values (with 95% confidence intervals) by deciles of BMI are shown in Figure 1. The lowest IGF-I concentrations (mean: 10.7 nmol) were observed in women with the highest BMI (>36.3 kg/m2), who, in turn, had also the smallest percent of breast density (mean: 23%).
Fig 1.
Adjusteda mean IGF-I circulating levels and mean percent breast density (with 95% confidence intervals) in 593 premenopausal Mexican women by deciles of BMI
aAdjusted by age, batch (for IGF-I) and state
Discussion
In the present study, we observed that premenopausal Mexican women with higher IGF-I levels had lower absolute dense and non-dense tissue areas. However, these associations were attenuated after adjustment for BMI. In the same population, one SNP in IGF-1 and one SNP in IGFBP-3, were significantly associated with circulating hormone levels, but not with mammographic density. When exploring the association between IGF-I concentrations and mammographic density by adiposity, the lowest IGF-I concentrations, as well as the lowest percent breast density, were observed in women in the highest decile of BMI.
Previously published cross-sectional studies reporting on the associations between measures of mammographic density and circulating growth factors in pre-menopausal women, mainly focussed on percent mammographic density. They have mostly shown positive associations between percent mammographic density, IGF-I concentrations and the IGF-I/IGFBP-3 ratio (10;11;14;33), mainly independently of BMI, while reported no significant associations with IGFBP-3 concentrations. However, a study within the Guernsey cohort (15), and a recent study undertaken within the Nurses’ Health Study (12), showed no associations at all between circulating growth factors and percent density. The results from our study are consistent with this lack of association. Nevertheless, our population is of Mexican origin, whereas the populations from the previously mentioned studies are composed mainly of Caucasian women, therefore comparison of results should be taken cautiously.
Only a few cross-sectional studies presented data specifically on the associations between blood levels of IGF-I, IGFBP-3 and their ratio, with absolute dense and absolute non-dense areas in pre-menopausal women (11;14;15;23): two studies (11;15) showed a direct association between increasing absolute dense area and increasing IGF-I concentrations, one study (14) reported a positive association with the IGF-I/IGFBP-3 ratio, one study (15) showed positive associations with both IGF-I and IGFBP-3 concentrations, which however lost statistical significance after adjustment for BMI, and two studies reported no significant associations (12;23). In our study, we observed an inverse association between absolute dense and non-dense areas with increasing concentrations of IGF-I and the IGF-I/IGFBP-3 ratio, as women who had lower absolute tissue areas had higher hormone concentrations. However, these associations were attenuated and null after adjustment for BMI, suggesting that overall adiposity is an important confounder (or mediator) in these associations.
Indeed, it is extremely important to consider adiposity when exploring the associations between growth factors and mammographic density (2;34). In Caucasian populations, increased BMI is generally associated with a moderate decrease in IGF-I concentrations, although this association appears to be non-linear (35;36). Women with very high BMI have higher free IGF-I concentrations, which suppress growth hormone secretion, and therefore IGF-I production in the liver, through a negative feedback control (37). As high BMI inversely correlates with percent mammographic density, a positive correlation between IGF-I and mammographic density may be observed, which is however mainly obesity-driven. The importance of adiposity on the IGF-breast density association is even more evident for absolute non-dense area, which primarily represents the amount of adipose tissue that is present in the breast (2). Although absolute dense area is less associated with adiposity than the non-dense area, it is interesting to notice that in our study the association between endogenous hormones and absolute dense area was also substantially attenuated after adjustment for overall adiposity.
In our study, IGF-I concentrations were linearly inversely related to BMI. These results contrast somewhat with those observed in Caucasian women, where results from cross-sectional studies indicate a non-linear, inverse U-shape correlation between adiposity and growth factor concentrations, with the lowest IGF-I concentrations observed in women with BMI<20 as well as in women with BMI > 30 (9;35;36). This difference in results confirms previous observations from the Multi Ethnic Cohort (38) where discrepancies in the strength and in the shape of associations between circulating IGF-I concentrations and BMI have been observed in women from different ethnicities. Nevertheless, it should also be noted that, in our population, mean BMI was about 28, while mean BMI in most of the studies reported on Caucasian women was about 25 or lower (35).
Results from the large Breast and Prostate Cancer Cohort Consortium (BPC3) (16;19), reporting associations between 18 genes and more than 300 SNPs in the IGF signalling pathway from more than 5,000 Caucasian women (mainly post-menopausal), showed that the rs1520220 polymorphism in the IGF-1 and the rs2854744 polymorphism in the IGFBP-3 were associated with an increase in circulating hormone levels, while no significant relation was observed for rs1549693 polymorphisms. Similar to the BPC3 study, we observed a strong association between rs2854744 and circulating levels of IGFBP-3. But, in contrast to the BCP3 study, IGF-1 rs1520220 was not associated with circulating IGF-I levels; however we found levels to be associated with rs1549693 polymorphism. Overall, these observations may suggest that associations between common genetic variants in IGFBP-3 pathway and IGFBP-3 circulating levels seem to be independent from ethnicity, while variants in IGF1 seem to differ in different ethnicities, supporting previous results (although on different SNPs) comparing Caucasian and African American pre-menopausal women (39). Inconsistency in the associations among specific SNPs in the IGF-1 and circulating hormone levels may also suggest that the SNPs that have been genotyped are not tagging the sequence variant(s) having a direct/functional impact on the circulating level of the studied hormones.
Few studies have explored the association between circulating IGF-I concentrations and polymorphisms in IGF-1 rs6220, with some studies undertaken in Caucasian premenopausal women, indicating a strong association (40), while others (including pre and post-menopausal women) showing no association. The results from our study are not supportive of an association between this SNP and biomarker levels in our Mexican population.
Despite the significant associations observed between some genetic polymorphisms in IGF-1 and IGFBP3, and circulating IGF-I and IGFBP-3 levels, no associations could be seen between these polymorphisms and any of the mammographic density measures. This confirms observations in previous studies (5;24). The influence of these variants, when tested individually, on circulating levels might be small, and have therefore very little influence on the association with mammographic density measures.
Our study has several strengths: to our knowledge, this is the first study to examine the association between IGF-I and IGFBP-3 concentrations, SNPs and mammographic density in a large sample of premenopausal Mexican women. Because of detailed questionnaire information and anthropometric measurements, we were able to adjust for several potential confounders. The large sample size allowed the stratification of analyses by BMI. Biological samples were collected according to standard operating procedures and have been stored at −70C. Hormone levels were measured on never-thawed aliquots. Our study has also some weaknesses: we only had one biological sample collected per subject, and no information was collected on phase of the menstrual cycle at blood donation/mammography. However, previous studies have shown high intra-class correlations between IGF-I and IGFBP-3 concentrations overtime in premenopausal women (35), and indicate that variations of mammographic density (as well as IGF-I concentrations) through the menstrual cycle are modest (41).
In conclusion, the results of our study do not support the hypothesis of a strong association between circulating levels of IGF-I and IGFBP-3 and mammographic density in a sample of Mexican premenopausal women. The relatively strong associations observed between IGF-I levels and absolute dense and non-dense areas seemed to be mainly driven by body fatness. Polymorphisms in specific genes were found to be associated with circulating levels of insulin-like growth factors, but not with mammographic density measures. Further research is needed to better understand the potential interaction between body fatness, genetic factors and mammographic density, especially in non-Caucasian populations.
Novelty and impact of the work.
This study is the first to evaluate the association between IGF-1 pathway SNPs, circulating concentrations of IGF-I and IGFBP-3 and mammographic density in a large sub-sample of Mexican premenopausal women. Although our results are not supportive of a strong association between concentrations of growth factors and mammographic density in this population, they are important to better understand the relations among breast cancer risk factors in a population with high breast cancer rates in young women.
Acknowledgments
Funding: Research relating to this manuscript was funded by the American Institute for Cancer Research (AICR) (Grant # 10A035), Consejo Nacional de Ciencia y Tecnologia (CONACYT) (Grant # 115312). Ministry of Health of Mexico, AVON, Banorte. Dr. Rice is supported by NIH grant number T32 CA090012.
The Authors would like to thank Ms Beatrice Vozar for her help with the handling of the biological samples, Ms Amélie Chabrier for her technical expertise regarding Taqman genotyping, the ESMaestras women for their participating in the study, and the American Institute for Cancer Research, Conacyt, (Consejo Nacional de Ciencia y Technología, México) and the Ministry of Health of Mexico, AVON, Banorte, for support. The work by Prof dos-Santos-Silva was partly undertaken during the tenure of a Senior Visiting Scientist Award by the International Agency for Research on Cancer. Dr. Rice is supported by NIH grant number T32 CA090012.
Footnotes
Authors contributions:
Conceived and designed the project: RI, TMG, LM, LRR
Performed and supervised laboratory and statistical analyses: BC, HM, LF, RS, RI
Critically participated in the interpretation of results and in manuscript revision: RS, BC, HM, LF, dSSI, MSR, LM, LRR, TMG, RI
Conflict of Interest: None disclosed
References
- 1.McCormack VA, dos-Santos-Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15(6):1159–69. doi: 10.1158/1055-9965.EPI-06-0034. [DOI] [PubMed] [Google Scholar]
- 2.Becker S, Kaaks R. Exogenous and endogenous hormones, mammographic density and breast cancer risk: can mammographic density be considered an intermediate marker of risk? Recent Results Cancer Res. 2009;181:135–57. doi: 10.1007/978-3-540-69297-3_14. [DOI] [PubMed] [Google Scholar]
- 3.Stone J, Dite GS, Gunasekara A, English DR, McCredie MR, Giles GG, Cawson JN, Hegele RA, Chiarelli AM, Yaffe MJ, Boyd NF, Hopper JL. The heritability of mammographically dense and nondense breast tissue. Cancer Epidemiol Biomarkers Prev. 2006;15(4):612–7. doi: 10.1158/1055-9965.EPI-05-0127. [DOI] [PubMed] [Google Scholar]
- 4.Boyd NF, Dite GS, Stone J, Gunasekara A, English DR, McCredie MR, Giles GG, Tritchler D, Chiarelli A, Yaffe MJ, Hopper JL. Heritability of mammographic density, a risk factor for breast cancer. N Engl J Med. 2002;347(12):886–94. doi: 10.1056/NEJMoa013390. [DOI] [PubMed] [Google Scholar]
- 5.Biong M, Gram IT, Brill I, Johansen F, Solvang HK, Alnaes GI, Fagerheim T, Bremnes Y, Chanock SJ, Burdett L, Yeager M, Ursin G, et al. Genotypes and haplotypes in the insulin-like growth factors, their receptors and binding proteins in relation to plasma metabolic levels and mammographic density. BMC Med Genomics. 2010;3:9. doi: 10.1186/1755-8794-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sachdev D, Yee D. The IGF system and breast cancer. Endocr Relat Cancer. 2001;8(3):197–209. doi: 10.1677/erc.0.0080197. [DOI] [PubMed] [Google Scholar]
- 7.Ellis MJ, Singer C, Hornby A, Rasmussen A, Cullen KJ. Insulin-like growth factor mediated stromal-epithelial interactions in human breast cancer. Breast Cancer Res Treat. 1994;31(2–3):249–61. doi: 10.1007/BF00666158. [DOI] [PubMed] [Google Scholar]
- 8.Wood TL, Yee D. Introduction: IGFs and IGFBPs in the normal mammary gland and in breast cancer. J Mammary Gland Biol Neoplasia. 2000;5(1):1–5. doi: 10.1023/a:1009580913795. [DOI] [PubMed] [Google Scholar]
- 9.Key TJ, Appleby PN, Reeves GK, Roddam AW. Insulin-like growth factor 1 (IGF1), IGF binding protein 3 (IGFBP3), and breast cancer risk: pooled individual data analysis of 17 prospective studies. Lancet Oncol. 2010;11(6):530–42. doi: 10.1016/S1470-2045(10)70095-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diorio C, Pollak M, Byrne C, Masse B, Hebert-Croteau N, Yaffe M, Cote G, Berube S, Morin C, Brisson J. Insulin-like growth factor-I, IGF-binding protein-3, and mammographic breast density. Cancer Epidemiol Biomarkers Prev. 2005 May;14(5):1065–73. doi: 10.1158/1055-9965.EPI-04-0706. [DOI] [PubMed] [Google Scholar]
- 11.Boyd NF, Martin LJ, Yaffe MJ, Minkin S. Mammographic density: a hormonally responsive risk factor for breast cancer. J Br Menopause Soc. 2006 Dec;12(4):186–93. doi: 10.1258/136218006779160436. [DOI] [PubMed] [Google Scholar]
- 12.Rice MS, Tworoger SS, Rosner BA, Pollak MN, Hankinson SE, Tamimi RM. Insulin-like growth factor-1, insulin-like growth factor-binding protein-3, growth hormone, and mammographic density in the Nurses’ Health Studies. Breast Cancer Res Treat. 2012;136(3):805–12. doi: 10.1007/s10549-012-2303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker K, Fletcher O, Johnson N, Coupland B, McCormack VA, Folkerd E, Gibson L, Hillier SG, Holly JM, Moss S, Dowsett M, Peto J, et al. Premenopausal mammographic density in relation to cyclic variations in endogenous sex hormone levels, prolactin, and insulin-like growth factors. Cancer Res. 2009;69(16):6490–9. doi: 10.1158/0008-5472.CAN-09-0280. [DOI] [PubMed] [Google Scholar]
- 14.Maskarinec G, Williams AE, Kaaks R. A cross-sectional investigation of breast density and insulin-like growth factor I. Int J Cancer. 2003;107(6):991–6. doi: 10.1002/ijc.11505. [DOI] [PubMed] [Google Scholar]
- 15.dos-Santos-Silva I, Johnson N, De Stavola B, Torres-Mejia G, Fletcher O, Allen DS, Allen NE, Key TJ, Fentiman IS, Holly JM, Peto J. The insulin-like growth factor system and mammographic features in premenopausal and postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2006;15(3):449–55. doi: 10.1158/1055-9965.EPI-05-0555. [DOI] [PubMed] [Google Scholar]
- 16.Patel AV, Cheng I, Canzian F, Le ML, Thun MJ, Berg CD, Buring J, Calle EE, Chanock S, Clavel-Chapelon F, Cox DG, Dorronsoro M, et al. IGF-1, IGFBP-1, and IGFBP-3 polymorphisms predict circulating IGF levels but not breast cancer risk: findings from the Breast and Prostate Cancer Cohort Consortium (BPC3) PLoS One. 2008;3(7):e2578. doi: 10.1371/journal.pone.0002578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen W, Wang S, Tian T, Bai J, Hu Z, Xu Y, Dong J, Chen F, Wang X, Shen H. Phenotypes and genotypes of insulin-like growth factor 1, IGF-binding protein-3 and cancer risk: evidence from 96 studies. Eur J Hum Genet. 2009;17(12):1668–75. doi: 10.1038/ejhg.2009.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Canzian F, Cox DG, Setiawan VW, Stram DO, Ziegler RG, Dossus L, Beckmann L, Blanche H, Barricarte A, Berg CD, Bingham S, Buring J, et al. Comprehensive analysis of common genetic variation in 61 genes related to steroid hormone and insulin-like growth factor-I metabolism and breast cancer risk in the NCI breast and prostate cancer cohort consortium. Hum Mol Genet. 2010;19(19):3873–84. doi: 10.1093/hmg/ddq291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gu F, Schumacher FR, Canzian F, Allen NE, Albanes D, Berg CD, Berndt SI, Boeing H, Bueno-de-Mesquita HB, Buring JE, Chabbert-Buffet N, Chanock SJ, et al. Eighteen insulin-like growth factor pathway genes, circulating levels of IGF-I and its binding protein, and risk of prostate and breast cancer. Cancer Epidemiol Biomarkers Prev. 2010;19(11):2877–87. doi: 10.1158/1055-9965.EPI-10-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarkissyan M, Mishra DK, Wu Y, Shang X, Sarkissyan S, Vadgama JV. IGF gene polymorphisms and breast cancer in African-American and Hispanic women. Int J Oncol. 2011;38(6):1663–73. doi: 10.3892/ijo.2011.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khoury-Shakour S, Lejbkowicz F, Barnett-Griness O, Tamir A, Pinchev M, Rennert G. Genetic variation in IGF-1 and breast cancer risk in Ashkenazi carriers and noncarriers of BRCA1/2 mutations. Eur J Cancer Prev. 2009;18(5):361–7. doi: 10.1097/CEJ.0b013e32832e0942. [DOI] [PubMed] [Google Scholar]
- 22.Tamimi RM, Cox DG, Kraft P, Pollak MN, Haiman CA, Cheng I, Freedman ML, Hankinson SE, Hunter DJ, Colditz GA. Common genetic variation in IGF1, IGFBP-1, and IGFBP-3 in relation to mammographic density: a cross-sectional study. Breast Cancer Res. 2007;9(1):R18. doi: 10.1186/bcr1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verheus M, Peeters PH, Kaaks R, van Noord PA, Grobbee DE, van Gils CH. Premenopausal insulin-like growth factor-I serum levels and changes in breast density over menopause. Cancer Epidemiol Biomarkers Prev. 2007;16(3):451–7. doi: 10.1158/1055-9965.EPI-06-0642. [DOI] [PubMed] [Google Scholar]
- 24.Verheus M, Maskarinec G, Woolcott CG, Haiman CA, Marchand LL, Henderson BE, Cheng I, Kolonel LN. IGF1, IGFBP1 and IGFBP3 genes and mammographic density: The Multiethnic Cohort. Int J Cancer. 2010;127(5):1115–23. doi: 10.1002/ijc.25142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romieu I, Escamilla-Nunez MC, Sanchez-Zamorano LM, Lopez-Ridaura R, Torres-Mejia G, Yunes EM, Lajous M, Rivera-Dommarco JA, Lazcano-Ponce E. The association between body shape silhouette and dietary pattern among Mexican women. Public Health Nutr. 2012;15(1):116–25. doi: 10.1017/S1368980011001182. [DOI] [PubMed] [Google Scholar]
- 26.Rice MS, Bertrand KA, Lajous M, Tamimi RM, Torres-Mejia G, Biessy C, Lopez-Ridaura R, Romieu I. Body size throughout the life course and mammographic density in Mexican women. Breast Cancer Res Treat. 2013;138(2):601–10. doi: 10.1007/s10549-013-2463-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Angeles-Llerenas A, Ortega-Olvera C, Perez-Rodriguez E, Esparza-Cano JP, Lazcano-Ponce E, Romieu I, Torres-Mejia G. Moderate physical activity and breast cancer risk: the effect of menopausal status. Cancer Causes Control. 2010;21(4):577–86. doi: 10.1007/s10552-009-9487-8. [DOI] [PubMed] [Google Scholar]
- 28.Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, Jong RA, Hislop G, Chiarelli A, Minkin S, Yaffe MJ. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356(3):227–36. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- 29.Torres-Mejia G, De Stavola B, Allen DS, Perez-Gavilan JJ, Ferreira JM, Fentiman IS, dos-Santos-Silva I. Mammographic features and subsequent risk of breast cancer: a comparison of qualitative and quantitative evaluations in the Guernsey prospective studies. Cancer Epidemiol Biomarkers Prev. 2005;14(5):1052–9. doi: 10.1158/1055-9965.EPI-04-0717. [DOI] [PubMed] [Google Scholar]
- 30.Byng JW, Boyd NF, Fishell E, Jong RA, Yaffe MJ. The quantitative analysis of mammographic densities. Phys Med Biol. 1994;39(10):1629–38. doi: 10.1088/0031-9155/39/10/008. [DOI] [PubMed] [Google Scholar]
- 31.Ursin G, Astrahan MA, Salane M, Parisky YR, Pearce JG, Daniels JR, Pike MC, Spicer DV. The detection of changes in mammographic densities. Cancer Epidemiol Biomarkers Prev. 1998;7(1):43–7. [PubMed] [Google Scholar]
- 32.Caboux E, Lallemand C, Ferro G, Hemon B, Mendy M, Biessy C, Sims M, Wareham N, Britten A, Boland A, Hutchinson A, Siddiq A, et al. Sources of pre-analytical variations in yield of DNA extracted from blood samples: analysis of 50,000 DNA samples in EPIC. PLoS One. 2012;7(7):e39821. doi: 10.1371/journal.pone.0039821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Byrne C, Colditz GA, Willett WC, Speizer FE, Pollak M, Hankinson SE. Plasma insulin-like growth factor (IGF) I, IGF-binding protein 3, and mammographic density. Cancer Res. 2000;60(14):3744–8. [PubMed] [Google Scholar]
- 34.Kaaks R. Insulin-like growth factor-I and mammographic breast density. Cancer Epidemiol Biomarkers Prev. 2005;14(12):3019. doi: 10.1158/1055-9965.EPI-05-0607. [DOI] [PubMed] [Google Scholar]
- 35.Gram IT, Norat T, Rinaldi S, Dossus L, Lukanova A, Tehard B, Clavel-Chapelon F, van Gils CH, van Noord PA, Peeters PH, Bueno-de-Mesquita HB, Nagel G, et al. Body mass index, waist circumference and waist-hip ratio and serum levels of IGF-I and IGFBP-3 in European women. Int J Obes (Lond) 2006;30(11):1623–31. doi: 10.1038/sj.ijo.0803324. [DOI] [PubMed] [Google Scholar]
- 36.Crowe FL, Key TJ, Allen NE, Appleby PN, Overvad K, Gronbaek H, Tjonneland A, Halkjaer J, Dossus L, Boeing H, Kroger J, Trichopoulou A, et al. A cross-sectional analysis of the associations between adult height, BMI and serum concentrations of IGF-I and IGFBP-1 -2 and -3 in the European Prospective Investigation into Cancer and Nutrition (EPIC) Ann Hum Biol. 2011;38(2):194–202. doi: 10.3109/03014460.2010.507221. [DOI] [PubMed] [Google Scholar]
- 37.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4(8):579–91. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 38.Faupel-Badger JM, Berrigan D, Ballard-Barbash R, Potischman N. Anthropometric correlates of insulin-like growth factor 1 (IGF-1) and IGF binding protein-3 (IGFBP-3) levels by race/ethnicity and gender. Ann Epidemiol. 2009;19(12):841–9. doi: 10.1016/j.annepidem.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.D’Aloisio AA, Schroeder JC, North KE, Poole C, West SL, Travlos GS, Baird DD. IGF-I and IGFBP-3 polymorphisms in relation to circulating levels among African American and Caucasian women. Cancer Epidemiol Biomarkers Prev. 2009;18(3):954–66. doi: 10.1158/1055-9965.EPI-08-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verheus M, McKay JD, Kaaks R, Canzian F, Biessy C, Johansson M, Grobbee DE, Peeters PH, van Gils CH. Common genetic variation in the IGF-1 gene, serum IGF-I levels and breast density. Breast Cancer Res Treat. 2008;112(1):109–22. doi: 10.1007/s10549-007-9827-x. [DOI] [PubMed] [Google Scholar]
- 41.Soderqvist G, Isaksson E, Von SB, Carlstrom K, Tani E, Skoog L. Proliferation of breast epithelial cells in healthy women during the menstrual cycle. Am J Obstet Gynecol. 1997;176(1 Pt 1):123–8. doi: 10.1016/s0002-9378(97)80024-5. [DOI] [PubMed] [Google Scholar]