Abstract
Asbestos exposure is known to cause lung cancer and mesothelioma and its health and economic impacts have been well documented. The exceptionally long latency periods of most asbestos-related diseases have hampered preventative and precautionary steps thus far. We aimed to summarize the state of knowledge on biomarkers of response to asbestos exposure. Asbestos is not present in human biological fluids; rather it is inhaled and trapped in lung tissue. Biomarkers of response, which reflect a change in biologic function in response to asbestos exposure, are analyzed. Several classes of molecules have been studied and evaluated for their potential utility as biomarkers of asbestos exposure. These studies range from small molecule oxidative stress biomarkers to proteins involved in immune responses.
Asbestos background
Asbestos is a class of silicate materials with a history of industrial uses due to its inert physicochemical properties such as fire- and heat-resistance and tensile strength, which makes it an ideal material for many commonly used products in both the construction industry and the home [1]. There are two major forms of asbestos: serpentine (chrysotile) and amphibole (which includes crocidolite amosite, tremolite, actinolite and anthophyllite) [2–4] (Figure 1). Though it was long thought to pose no risk to human health, asbestos is now known to cause a variety of adverse health effects and has been classified as a human carcinogen [5,6]. It has been clearly established in both animal models and human studies that asbestos fiber inhalation can lead to neoplastic diseases such as malignant mesothelioma (MM) and lung cancer [7,8], as well as pulmonary fibrosis (i.e., asbestosis). The toxic forms of chrysotile and crocidolite asbestos fibers are considered to be longer than 5 μm, with a thickness between 250 nm and 1 μm, and with an aspect ratio ≥ 3:1 as determined by transmission electron microscopy [9,10]. Fibers that are >10 μm in length and <250 nm in thickness are more potent than shorter and thicker fibers for both MM and lung cancer [10]. Despite widespread knowledge of the hazards of asbestos and bans on any use of asbestos in more than 50 countries, an estimated two million tons of asbestos continue to be used around the world each year.
Figure 1. . Classification of asbestos fibers.
Modified with permission from [4] © BMJ Publishing Group Ltd(1990).
Asbestos-related diseases
It has been clearly established in multiple models that asbestos fiber inhalation can lead to neoplastic diseases such as MM and lung cancer [7,8], as well as pulmonary fibrosis. MM is a highly heterogeneous and highly aggressive cancer of serosal surfaces, such as the pleura and the peritoneum [11]. The relative 5-year survival rate for mesothelioma compared with age-matched controls is between 5 and 10%. Current therapies, with the exception of surgery in very early disease states, are not curative [12]. The most recent statistics on MM mortality show that there were 2704 deaths in the USA in 2005 with little evidence that rates are declining [13]. Although asbestos use has been restricted in many western countries, it is still mined in Russia, China, Canada, Brazil, Kazakhstan and Zimbabwe [13,14]. It has been estimated approximately 125 million people are exposed to asbestos globally within their place of work, often in third world countries that do not have strictly regulated workplace safety controls. Thus, there will likely be an increase in MM cases in the third world, and in developed countries due to occupational exposure (e.g. plumbers, pipefitters, insulators, etc.) [15]. In addition, there are some nonoccupational environmental exposures. For example, there is an increased risk of asbestos-related diseases (ARDs) from old mining sites such as Libby Montana [16,17] or where asbestos-related industries once operated such as the BoRit site in Ambler, PA, USA [18]. Another major concern regarding environmental exposures includes areas within Europe and Australia that have large numbers of buildings where asbestos was used in construction materials and are thus likely to result in ongoing exposures [19–26].
The economic impact of asbestos is large and far reaching, from burdening health care systems to extraordinary costs associated with removal of environmental contaminants. One of the most challenging factors to address with ARDs is the considerable latency period between initial exposure (Figure 2) [27] and subsequent adverse health effects, as most clinical cases result from exposures decades (20–40 years) beforehand. For this reason it is imperative that markers of asbestos exposure be developed to facilitate detection of at-risk individuals prior to the onset of disease. Furthermore, individuals suspected of being exposed to asbestos would be able to confirm they are not likely to suffer from an ARD. Finally, a quantitative assay capable of assessing asbestos exposure would facilitate confirmation of the beneficial impacts resulting from environmental remediation of asbestos sites.
Figure 2. . Concentrations of asbestos fibers per milliliter of bronchoalveolar lavage fluid in the workplace-exposed subjects.
Data are subdivided on the basis of time spent between last exposure and bronchoscopy (geometric mean).
Reprinted with permission from [27] © Oxford University Press (2007).
Pleural changes resulting from asbestos exposure
Biologically significant asbestos exposure is associated with the presence of pleural changes (especially pleural plaques) visualized on chest x-ray (CXR), or more commonly by computerized tomography (CT) scan [28,29]. Pleural plaques are circumscribed areas of thickening of parietal or diaphragmatic pleura composed of avascular collagen connective tissue. Although they can be seen occasionally after pleural infections or pleural hemorrhage, they are most commonly the result of prior asbestos exposure, generally appearing some 20–40 years after first exposure (that is, after a ‘latent’ interval) and tend to calcify over time. Plaques tend to grow slowly over time, but they do not cause symptoms. It has been estimated that approximately 5–15% of those with occupational exposure to asbestos will have noncalcified pleural plaques 20 years after this exposure and about a third or more will have calcified plaques after 30 years [28]. A recent study of over 5000 French asbestos-exposed workers that used more sensitive CT screening found a pleural plaque prevalence of 20%. After adjustments for asbestos exposure and latency time, patients with plaques had a significantly elevated risk of mesothelioma [30]. Studies evaluating the association of pleural plaques and lung cancer risk have also provided conflicting evidence [31]. This means that there are significant limitations in using plaques as biomarkers of asbestos exposure. Since plaques evolve slowly over time and become large enough to see only many years after initial exposures, their reported prevalence increases with longer latency periods and at older ages. Furthermore, only a small percentage of subjects with pleural plaques develop mesothelioma. Thus, asbestos exposure history and imaging are useful but are not sensitive or specific enough to accurately identify those at greatest risk for developing malignant disease or asbestosis. This means that there is a real need for more specific and sensitive biomarkers of asbestos exposure that could permit personalization of risk assessment.
Asbestos exposure & oxidative stress
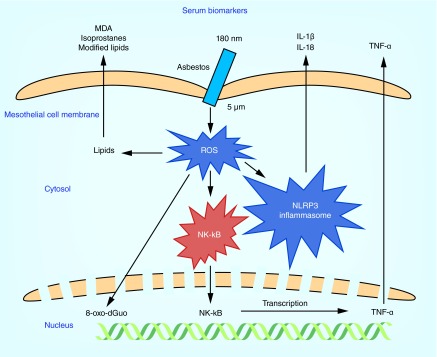
The most highly cited studies of asbestos exposure have tended to focus on biomarkers of oxidative stress [32,33], which cannot distinguish asbestos exposure from other causes of oxidative stress such as from cigarette smoking [34,35] or from diseases such as atherosclerosis [36]. However, there is substantial evidence that oxidative stress is involved in the etiology of ARDs such as pulmonary fibrosis [37] and mesothelioma [38]. One factor driving MM carcinogenesis is the DNA damage caused by asbestos fibers. The DNA damage could potentially be caused directly through mechanical interference of asbestos fibers with chromosome segregation during mitosis [39]. However, it is now largely believed that DNA damage is in fact caused by the oxidation that arises through sustained inflammation and subsequent production of reactive oxygen species (ROS) (Figure 3) [40].
Figure 3. . The effect of asbestos fibers on mesothelial cells.
Since only a small fraction of patients with significant asbestos exposure develop MM, the disease is now thought to progress through a combination of both genetic and environmental factors. For example, the Simian virus 40 T antigen has been detected in many mesotheliomas and has been implicated in the etiology of the disease [41]. Furthermore, some studies have shown that the cyclin-dependent kinase inhibitor 4a (P16INK4a) and ARF tumor suppressor (p14ARF) genes are frequently inactivated in mesothelioma and approximately 50% of MM cases contain missense or nonsense mutations in the neurofibromin type 2 gene. Mice lacking these genes are relatively normal but develop MM at an accelerated rate when exposed to asbestos by intraperitoneal injection. In addition, within the last few years, germ line and sporadic mutations of the tumor suppressor, breast cancer susceptibility (BRCA)-1 associated protein-1 (BAP-1) gene, which expresses a deubiquitinating enzyme, appear to predispose patients to DNA damage after environmental stress [2]. Inhaled asbestos fibers work their way into the lung and ultimately reach the pleural surface (Figure 4) [42] where they are engulfed by tissue phagocytes, primarily macrophages. This process stimulates intracellular ROS production and activates the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway, inducing the release of numerous cytokines (Figure 3). Recent studies suggest that asbestos also activates the nucleotide-binding oligomerization domain-like receptor family, pyrin domain containing 3 (NLRP3) inflammasome thus promoting the release of interleukin (IL)-1β and IL-18 [43]. Furthermore, genetic studies indicate that polymorphisms in genes within the NRLP3 family can both predispose and protect against fibrosis following asbestos exposure. Mice exposed to asbestos exhibit recruitment of activated macrophages to the mesothelium interacting with asbestos fibers. Macrophages are also thought to directly interact with and phagocytose asbestos. It is hypothesized that macrophages and mesothelial cells exposed to asbestos undergo frustrated phagocytosis of elongated fibers; this process is thought to cause chronic production of ROS and cytokines, which contribute to DNA damage and transformation of mesothelial cells [44].
Figure 4. . Electron microscopy of an ashed lung section after exposure to crocidolite and chrysotile.
The crocidolite fibers are at the upper left and the chrysotile fibers are at the lower right.
Reprinted with permission from [42] © BMJ Publishing Group Ltd.(1972).
The Carbone group has also suggested that high levels of the high-mobility group box 1 (HMGB1) protein are secreted after asbestos exposure and during the progression of MM. HMGB1 is a lysine-rich protein with an acidic carboxy-terminal region containing multiple aspartate and glutamate residues [45]. During tissue necrosis, such as the hepatic necrosis induced by high doses of acetaminophen, many of the lysine residues are acetylated, which facilitates secretion from tissues into the circulation [45]. A similar process after asbestos exposure would provide a useful biomarker (Table 1). HMGB1 amplifies the inflammatory response in general (by chemotaxing neutrophils and mast cells [46]), but also serves as an important protumor cytokine that enhances the growth, survival and invasiveness of the mesothelial cells [3]. MM tumor samples are associated with chronic inflammation including macrophage infiltration and inflammatory cytokine production. In addition to the cytokines discussed above, the activated macrophages contribute to tumorigenesis by forming harmful ROS and reactive nitrogen species that can in turn induce further DNA damage. This can lead to genomic instability; by increasing tissue proliferation, and inducing tissue remodeling and angiogenesis-promoting factors, as well as inducing extravasation of tumor cells from the microenvironment [47].
Table 1. . Response biomarkers of asbestos exposure.
| Asbestos biomarker | Abbreviation | Description | Bioanalytical technique | Ref. |
|---|---|---|---|---|
| 7,8-dihydro-8-oxo-2′-deoxyguanosine (8-hydroxy-2’-deoxyguanosine) | 8-oxo-dGuo | Oxidative stress DNA base adduct biomarker arises in urine from hMTH1-mediated hydrolysis of 8-oxo-dGuo-triphosphate. Alternatively, isolated from DNA or analyzed intact in the DNA of formalin-fixed cells | LC-ED | [48,49] |
| |
|
|
Fluorometric OxyDNA kit |
[50–52] |
| 8-iso-isoprostane PGF2α |
8-iso-PGF2α |
Oxidative stress lipid biomarker formed by ROS-mediated oxidation of esterified arachidonic acid appears as the free form in plasma, urine, an EBC after hydrolysis of esterified lipids |
Stable isotope dilution LC–MS |
[53] |
| Soluble mesothelin-related peptide or soluble mesothelin-related protein | SMRP | Mesothelin and SMRP are 40-kDa glycoproteins from proteolytic cleavage of the 69-kDa mesothelin precursor protein - both are analyzed | Mesomark ELISA kit | [54] |
| |
|
|
Sandwich ELISA with monoclonal antibodies |
[55] |
| Osteopontin |
None |
An integrin-binding protein involved in tumorigenesis, progression and metastasis |
ELISA kit |
[56] |
| Fibulin-3 |
None |
Fibulin-3 is protein that belongs to a family of extracellular proteins expressed in the basement membranes of blood vessels |
ELISA kit |
[57] |
| Fibronectin | None | Fibronectin is a glycoprotein involved in the extracellular matrix structure that plays a role in the generation of fibrotic tissue | Immunochemical assay with laser nephelometer detection | [58] |
| |
|
|
ELISA |
[59] |
| High mobility group box 1 |
HMGB1 |
HMGB1 is a chromatin protein. The unmodified protein has a nuclear location - lysine hyperacetylation causes translocation of HMGB1 into the cytosol |
ELISA kit |
[60] |
| Interleukin 6 and interleukin 8 |
IL-6 and IL-8 |
Members of a large family of cytokines that promote the development, differentiation and activation of lymphocytes and play an important role in the immune response |
Solid phase ELISA |
[61,62] |
| Regulated on activation normal T cell expressed protein | RANTES | A chemokine also known as chemokine (C–C motif) ligand 5 (CCL5) | Magnetic bead multiplex immunoassay | [63] |
EBC: Exhaled breath condensate; SMRP: Soluble mesothelin-related peptide.
Biomarkers of response to asbestos exposure
Biomarkers of exposure can be used to assess the amount of a chemical that is present within the body [64]. However, asbestos is not present in readily accessible biological fluids; it is inhaled and trapped in lung tissue for long periods of time. Although the asbestos fibers can appear in bronchoalveolar lavage (BAL) fluid, such measurements are more suitable for a qualitative/categorical approach to exposure assessment than a quantitative one [65]. This means that it is necessary to analyze biomarkers of response to asbestos rather than directly measuring asbestos exposure. Biomarkers of biological response can, in principle, provide more direct insight into the potential for adverse health effects than biomarkers of exposure. Before being implemented in a clinical setting, response biomarkers must first be fully characterized and validated in large sample sets. Candidate biomarkers often lack diagnostic utility because of poor sensitivity (false negatives) and/or inadequate specificity (false positives) derived from confounding exposures, nonspecific biomarkers or individual variability. To circumvent some of these issues, multiple biomarkers can be combined which may result in a more complex but thorough analysis. Additional issues may arise from variations in sample preparation and analytical methodology throughout sample collection, processing and analysis. For this reason robust and reproducible analytical techniques are critical to ensuring proper validation and utilization of a biomarker. Once fully validated and characterized, biomarkers can serve a critical role in characterizing levels of exposure, which would otherwise be unknown.
The primary route of exposure to asbestos is through inhalation. The fibers are able to travel deep into the respiratory system and penetrate pleural cells within the lining of the lung. This penetration can lead to innate immune responses and subsequent oxidative stress (Figure 3). Disrupted cellular processes can ultimately lead to the generation of fibrotic tissue and in some cases cancer. These pathogenic responses to asbestos exposure afford the use of resulting biochemical characteristics as biomarkers of exposure. For example, oxidative stress leads to modified lipids, proteins and DNA. These biochemical changes can be confounded by smoking cigarettes, which is known to induce oxidative stress, and these biomarkers are higher in tobacco smokers than nonsmokers [35]. In keeping with this concept, quantitative analysis of documented markers of oxidative stress may be useful for characterizing asbestos exposure across human populations, but may lack specificity without including other variables. In addition, several factors involved in immune response, cell proliferation and generation of fibrotic tissue also hold promise as useful biomarkers of asbestos exposure. The factors likely to impact disease risk include common environmental variables per se, functional polymorphisms in genes, [2,66] and differential expression of genes that interact with such variables [67,68].
Here we provide a review of biomarkers of response (including small molecules and proteins) that result from exposure of human populations to asbestos. An important point is that many studies have emphasized the need for biomarkers of MM and other ARDs such as lung cancer and pleural plaques [69,70]. However, in this review we have focused primarily on biomarkers of asbestos-exposed subgroups versus nonexposed individuals. Establishing biomarkers of response resulting from asbestos exposure will enable better classification of exposed populations, improving preventative measures, and help characterize previously unknown biochemical impacts of asbestos exposure.
DNA-adduct biomarkers
Urinary 8-oxo-7,8-dihydro-2’-deoxyguanosine (8-oxo-dGuo), often incorrectly referred to as 8-hydroxy-2’-deoxyguansoine, is a widely used nonspecific biomarker of oxidative stress. During oxidative stress, ROS can cause oxidative damage to dGuo to form 8-oxo-dGuo in DNA [71] and the trinucleotide pool. 8-oxo-dGuo arises from human Mut T homolog 1 (hMTH1)-mediated hydrolysis of 8-oxo-dGuo-triphosphate in the trinucleotide pool followed by excretion in the urine [35]. Three base excision repair enzymes, human MutY homolog (hMutY), hOGG and hOGG2 [72], are involved in the repair of 8-oxo-dGuo-derived lesions in DNA. However, the trinucleotide pool is considered to be the major source of urinary 8-oxo-dGuo. The gold standard for quantification of urinary 8-oxo-dGuo [73] involves the use of stable isotope dilution LC–MS coupled with the addition of [13C]-dGuo to monitor any artifactual formation.
There are several population studies that have examined levels of oxidative DNA base damage in workers exposed to asbestos with a large number of subjects and appropriate control groups (Table 1) [48,49]. Unfortunately, the LC-electrochemical detection (ED) methodology employed in the studies was not of very high quality because internal standards were not used and the assays were not validated by more specific LC–MS methodology [35]. Nevertheless, the older study, which examined levels of urinary 8-oxo-dGuo in asbestos workers, found a significant increase when compared with unexposed controls [48]. The more recent study, which was conducted in Japan [49], analyzed urinary 8-oxo-dGuo in construction workers who had been exposed to low levels of asbestos. The asbestos exposed group, which consisted of 48 construction workers with a history of suspected exposure to asbestos and CXR evidence of irregular opacities or pleural plaques, was compared with a control group of 41 unexposed healthy controls. This revealed that 8-oxo-dGuo levels in the asbestos exposed group were higher than those in the control group, although the levels did not reach statistical significance.
Additional studies have quantified 8-oxo-dGuo in the DNA of white blood cells (WBC) or circulating lymphocytes using a commercially available fluorometric OxyDNA kit (Table 1) [50–52]. This method for analyzing oxidative DNA damage is based upon the direct binding of a fluorescent probe to 8-oxoguanine moieties in DNA for formalin-fixed cells. Fluorescence is then quantified using flow cytometry. It is also necessary to remove all transition metal ions in buffers during formalin fixing in order to prevent additional artifactual DNA oxidation [71]. As no such precautions were used, the reported levels of 8-oxo-dGuo must be treated with some caution. The studies showed that asbestos-exposed subjects had significantly elevated 8-oxo-dGuo levels in WBCs and circulating lymphocytes from asbestos exposed populations compared with unexposed controls [50–52].
Another study conducted in a Chinese population measured levels of 8-oxo-dGuo in the DNA of peripheral blood leukocytes as a biomarker of asbestos exposure in a population occupationally exposed primarily to chrysotile asbestos [74]. Leukocyte DNA was extracted from 5 ml samples of peripheral blood and 8-oxo-dGuo levels were measured by LC after hydrolysis of the DNA. DNA isolation for the analysis 8-oxo-dGuo requires the use of chaotropic methods in order to prevent artifactual DNA oxidation [71]. It is also necessary to remove all transition metal ions from hydrolysis buffers in order to prevent additional artifactual DNA oxidation [71]. In this study, no attempt was made to prevent DNA oxidation during isolation and hydrolysis. Furthermore, the statistical power was very limited with only 19 controls and ten asbestos-exposed workers group-matched for age and sex. The geometric mean of 8-oxo-dGuo levels showed no significant difference between the control and asbestos exposed groups [74].
The largest studies to date were conducted in Germany by Marczynski et al. using a longitudinal design with annual measurements over 3 consecutive years [75,76]. In these studies, the ability of inhaled asbestos fibers to induce the formation of 8-oxo-dGuo in the DNA from WBCs of workers highly exposed was evaluated with 636 workers in the exposed group and 214 healthy unexposed individuals as the control group. The asbestos-exposed workers had an average exposure of around 19 years, so the levels of 8-oxo-dGuo were measured at steady state in hydrolyzed DNA using LC-ED. The study used WBCs isolated from 9 ml of whole blood but again there was no control for artifactual oxidation during DNA isolation and hydrolysis. The study reported 8-oxo-dGuo levels in asbestos-exposed workers that were significantly increased (p < 0.001) compared with that in the control group, with this significant difference identified in all 3 years of the study. Asbestos-exposed individuals displayed a mean value of 2.61 ±0.91 8-oxo-dGuo/105 dGuo (median 2.49; n = 496) in 1994–1995, 2.96 ±1.10 8-oxo-dGuo/105 dGuo (median 2.76; n = 437) in 1995–1996 and 2.55 ±0.56 8-oxo-dGuo/105 dGuo (median 2.53; n = 447) in 1996–1997 (Figure 5) [75]. The mean levels in the control group were also extremely high at 1.52 ±0.39 8-oxo-dGuo/105 dGuo (median 1.51; n = 214) [75], likely because there was no inhibition of DNA oxidation during isolation and hydrolysis. The studies indicated that levels of oxidative stress were between 1.7- and 2.0-times the level of oxidative damage relative to that found in control samples in all 3 years of the study [75,76]; however, the data must be treated with caution. Typical levels of 8-oxo-dGuo in cells where artifactual DNA oxidation is prevented are at least two orders of magnitude lower at 1.0 8-oxo-dGuo/107 dGuo [71]. Therefore, oxidative DNA damage could be higher in the WBCs of workers highly exposed to asbestos fibers but more rigorous assay methodology will be required for validation. If these findings are confirmed it would indicate that preventive and therapeutic approaches using antioxidants might be useful.
Figure 5. . 8-oxo-dGuo levels in the white blood cell DNA of asbestos-exposed workers over a period of three study years.
*p < 0.001, significantly different from nonasbestos-exposed controls.
Reprinted with permission from [75] © Elsevier (2000).
The most recent in vivo studies of 8-oxo-dGuo surveyed subjects occupationally exposed to asbestos in Italy [50,51]. The first study included 119 asbestos-exposed workers from the shipbuilding industry, and 54 aged matched controls unlikely to have been exposed to asbestos based on their job history. The second study examined 94 asbestos-exposed subjects and 54 controls. The studies quantified 8-oxo-dGuo in WBCs using the OxyDNA assay kit with no control of artifactual DNA oxidation [50,51]. Levels of WBC 8-oxo-dGuo were significantly elevated in subjects heavily exposed to asbestos but were identical with subjects with MM [50]. The area under the receiver operating characteristic curves (AUC) of 0.775 showed that WBC 8-oxo-dGuo levels poorly differentiated asbestos-exposed subjects from healthy controls [50].
Isoprostanes as biomarkers
Arachidonic acid is a major fatty acyl component of the lipidome that is present as the free fatty acid in plasma as well as esterified in sterol lipids and at the sn-2 position of glycerolipids and glycerophospholipids [77]. IsoPs are oxidation products of arachidonic acid, which were originally discovered as artifacts present in stored plasma samples [78]. Subsequently, we showed that they are also formed by ROS-mediated oxidation of esterified arachidonic acid, which appear in biofluids after hydrolysis of esterified lipids [79]. 8-iso-isoprostane PGF2 α (8-iso-PGF2 α also known as 8-epi-PGF2 α and iPF2 α-III) is the most widely analyzed isoP, with relatively few reports describing the detection of more than one isoP in a single LC–MS analysis [80]. Each class of isoP forms a specific product ion during LC–MS/MS analysis [81], making it possible to readily differentiate the four isoP classes [80]. IsoPs have been rigorously validated as reliable biomarkers of oxidative stress in two multilaboratory collaborative studies [82,83].
Very few studies have assessed isoP levels in asbestos exposed populations [53,84–87]. In one of the studies, 8-iso-PGF2α was analyzed by stable isotope dilution LC–MS in exhaled breath condensate (EBC) from healthy subjects who had been exposed to asbestos (Table 1) [53]. The limit of quantification for this method was 5 pg/ml and the mean levels were approximately ten-times higher. The study evaluated 92 former asbestos workers with mean age 68.8 ±1.7 years and mean duration of asbestos exposure of 24.1 ±2.0 years. The control group had 46 subjects with mean age 65.2 ±3.3 years. The mean level of 8-iso-PGF2α was higher in asbestos-exposed subjects (69.5 ±6.6 pg/ml; p = 0.0001) compared with the control group, where the concentration was 47.0 ±7.8 pg/ml. There was no correlation with asbestos fiber exposure years. However, the results support the hypothesis that oxidative stress will lead to increased 8-iso-PGF2 α in EBC, and there is a direct correlation between asbestos exposure and oxidative stress. Therefore, the analysis of isoPs in EBC is a promising noninvasive tool for assessing asbestos exposure but may also reveal any condition leading to oxidative stress. Thus, the measurements of isoPs could be coupled with more specific biomarkers of asbestos exposure. Strikingly, to date, there are no other known small molecule biomarkers available to distinguish between asbestos-exposed individuals and healthy controls. This lack of studies highlights the acute need for discovery of such biomarkers.
Protein biomarkers
Mesothelin, which was first identified on mesothelial cells, is known to be overexpressed in several types of cancer. The soluble form, which is known as soluble mesothelin-related protein or soluble mesothelin-related peptide (SMRP), has emerged as a potential biomarker for the early detection of asbestos-induced mesothelioma [88,89]. Multiple enzyme-linked immunosorbent assay (ELISA) kits have been developed to analyze serum SMRP including the Mesomark assay, which has been approved by the US FDA as a biomarker for mesothelioma (Table 1) [54]. A recent review reported a meta analysis of 30 publications to determine the sensitivity and specificity of SMRP as a biomarker of mesothelioma [90]. Studies on occupational exposure to asbestos in the Czech Republic revealed distinguishing serum levels of SMRP in which exposed subjects with benign disease had higher levels than normal subjects but lower levels than subjects with MM as determined by CXRs [91]. An additional study from Australia used a sandwich ELISA with two monoclonal antibodies (OV569 and 4H3) comparing a nonexposed control group of 28 controls with 40 asbestos exposed (Table 1) [55]. This study showed increased levels of SMRP in mesothelioma patients, but the small sample size did not permit adequate statistical power. There was no significant difference in the SMRP levels between asbestos exposed and controls.
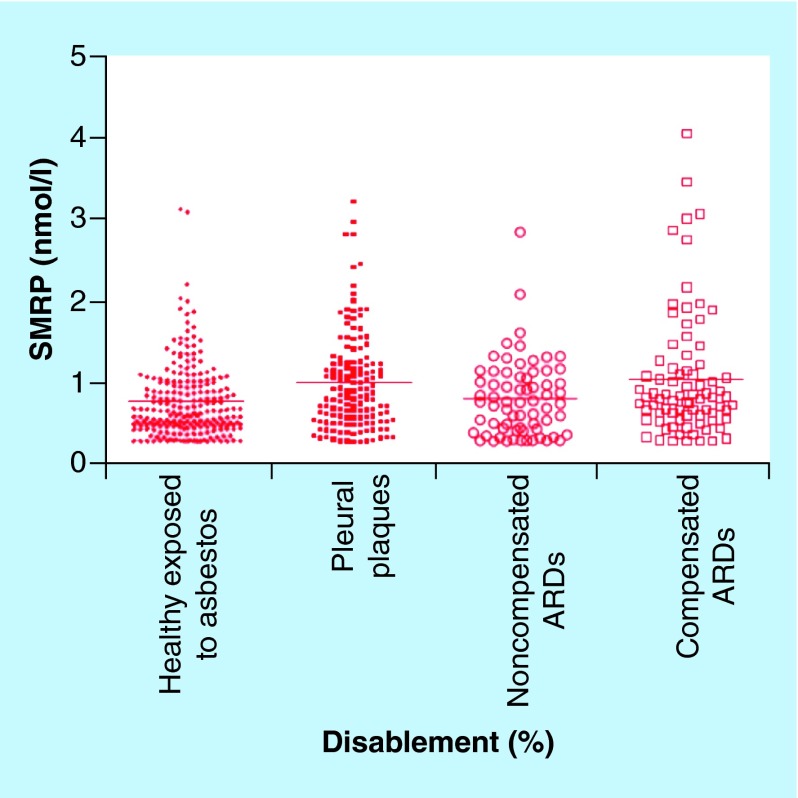
Similar ELISA-based studies utilizing plasma and serum have shown elevation of SMRP levels in MM subgroups with no statistical significance between exposed individuals and unexposed controls [51,92–94]. In contrast, analyses involving asbestos-exposed workers revealed no association between total exposure and serum SMRP levels [88,95]. Creaney et al. also reported steady serum concentrations of mesothelin in asbestos-exposed individuals over a time span of 4 years, suggesting their findings were not a result of sampling time in relation to exposure occurrence. In contrast to these reports, other studies found higher serum levels of SMRP in asbestos-exposed individuals than unexposed controls [96–98]. One of these studies [96] also examined the correlation between SMRP levels and frequency of micronuclei in blood. Measurement of micronuclei frequency in peripheral blood lymphocytes is extensively used to evaluate the presence of DNA damage in humans exposed to genotoxic agents and for the prediction of cancer risk. The micronuclei frequency test is easier to conduct than the chromosomal aberration test. It uses fluorescent in situ hybridization with probes targeted to the centromere region. A statistically significant positive correlation of the SMRP levels with the frequency of mononuclei in the mononucleated lymphocytes was observed. Additional evidence indicates serum SMRP levels may be useful for monitoring the progression of diseases resulting from asbestos exposure (Figure 6) [99]. Mean serum levels of SMRP differed between the four groups that were examined with the mean level in healthy subjects being lower than in subjects who were entitled to compensation for their ARD (Figure 6). The authors have suggested that asbestos biomarkers could potentially be used for monitoring disablement assessment in ARDs [99]. A potential issue with studies involving SMRP quantification is the confounding effects of sample storage, subject age, body mass index, glomerular filtration rate and smoking status within the cohorts [89,100–101].
Figure 6. . Serum soluble mesothelin-related peptide concentrations in asbestos-exposed and nonexposed subjects.
Categories from left to right: exposed to asbestos but apparently healthy; with pleural plaques; with ARDs (includes asbestosis, DPT and asbestosis/DPT) but not eligible for compensation due to their 0% disablement; and with compensated ARDs due to their 10–100% disablement. Horizontal scale bars denote mean concentrations. There was a significant difference between the groups (analysis of variance, p < 0.0001).
ARD: Asbestos-related disease; DPT: Diffuse pleural thickening; SMRP: Soluble mesothelin-related peptide.
Reproduced with permission from [99] © Elsevier (2012).
Osteopontin is a glycoprotein that is overexpressed in lung cancer and several other types of cancer [102]. It is an extracellular cell adhesion protein that plays a key role in cytokine mediated immune response. High levels of osteopontin are correlated with tumor progression and metastasis. In a rat model, osteopontin was upregulated in asbestos-induced tumors [103]. Compellingly, osteopontin levels in plasma or serum were able to differentiate between healthy subjects exposed to asbestos and mesothelioma patients [104]. Several human population studies looked at serum levels of osteopontin in asbestos-exposed subjects [56,92,95,104–106]. All studies employed a commercially available ELISA kit, which had a detection limit of 3 ng/ml (Table 1) [56]. The first population study [105], which was conducted in Michigan, examined 69 asbestos-exposed subjects and 45 healthy controls. The level of osteopontin was elevated in the asbestos group (30 ±3 ng/ml) compared with the unexposed group (20 ±4 ng/ml) but was of borderline statistical significant (p = 0.06). Creaney et al. [56] examined an Australian cohort to compare osteopontin levels in patients with different lung disorders, including ten healthy controls exposed to asbestos and ten nonexposed. Their findings agreed with the Michigan study in that osteopontin was not significantly different in the asbestos exposed population. Furthermore, even if serum and plasma levels of osteopontin were highly correlated with asbestos exposure [107], osteopontin can be hydrolyzed by thrombin and so the results might not be a true reflection of protein levels in the blood. A recent study conducted in Turkey had 120 healthy controls and 123 subjects exposed to asbestos [92]. This was the first study to examine a cohort of subjects exposed to naturally occurring surface asbestos. The subjects lived within 10 km of ophiolitic areas, which are known sources of naturally occurring asbestos. The study measured levels of mesothelin as well as osteopontin and concluded that both proteins were related to the severity of the inflammation observed in individual subjects. The difference in the levels of osteopontin was significant (p < 0.05) in controls versus asbestos-exposed individuals but was not useful to predict malignant transformations (Figure 7). Another recent study [95] analyzed mesothelin and osteopontin levels using ELISA kits in a very large number of asbestos-exposed workers (n = 1894) together with a smaller number of unexposed controls (n = 102). The levels of osteopontin were not significantly different between the two groups. This study also found no correlation between osteopontin and mesothelin levels with the duration of the asbestos exposure.
Figure 7. . The median and quartile serum osteopontin levels.
Samples were from mesothelioma patients, pleural plaque patients, healthy subjects exposed to asbestos and a control nonexposed group. Reprinted with permission from [92] © Springer(2013).
Fibulins are a group of secreted glycoproteins that act as connectors with components of the extracellular matrix and thus play an important role in the development of fibrotic tissue [108]. Fibulin-3 has recently emerged as a potential plasma protein biomarker of asbestos exposure with the capability of distinguishing between exposed and disease states within multiple cohorts using an ELISA kit (Table 1) [57]. Interestingly, Pass et al. also found that in a parallel analysis of matched samples fibulin-3 levels were lower in serum than in plasma [57]. The authors suggest this might be due to the presence of thrombin cleavage sites within fibulin-3, which has implications for other serum-based assays exploring the potential of protein biomarkers. Furthermore, the same study showed a poor correlation of fibulin-3 levels in plasma and effusions from matched samples, bringing to light a question of the validity of blood-based assays for fibulin-3 quantification. Additional studies have shown that fibulin-3 was not able to distinguish between patients with MM and asbestosis because serum levels were elevated in both groups [109]. However, it has been suggested that fibulin-3 is a better prognostic biomarker for MM than a diagnostic biomarker, because plasma fibulin-3 levels better predicted survival in MM patients [110]. Additional validation studies will be required to fully elucidate the utility of fibulin-3 as a dependable biomarker of asbestos exposure in human populations.
Fibronectin is a glycoprotein that is also involved in the extracellular matrix structure and therefore plays an important role in the generation of fibrotic tissue. For this reason there has been interest in fibronectin as a potential biomarker of asbestos exposure. Unfortunately, relatively few human studies have been performed to assess the utility of fibronectin for this role. In vitro experiments utilizing lung fibroblasts have shown that expression of the fibronectin gene increases in response to many of the cytokines known to be released in response to asbestos fibers. Bégin et al. reported increased fibronectin levels in BAL fluid (as determined immunochemically with a laser nephelometer instrument) from sheep exposed to asbestos fibers [58]. This same study found that humans exposed to asbestos who were suffering from ARDs also had elevated BAL fluid levels of fibronectin. Schwartz et al. performed a similar study involving 93 men who had been occupationally exposed to asbestos and found increased BAL fluid levels of fibronectin (as determined by ELISA) in patients with restricted lung function (Table 1) [59]. A similar finding was reported the following year by the same group with a different study population [111].
The HMGB1 protein has a known regulatory role in inflammatory immune responses and has received attention as a potential novel therapeutic target in MM [112]. Normally located in the nucleus, HMGB1 is acetylated and translocates during cell necrosis due to asbestos fibers into the cytosol and extracellular space, where it binds to and activates proinflammatory mediators. Specifically, activation of the NF-κB signaling pathway and release of tumor necrosis factor-alpha (TNFα) are downstream consequences of HMGB1 activity. Given the role it plays in inflammatory processes, HMGB1 may hold promise as a biomarker of cell transformative processes and thus hold utility as an indicator of asbestos exposure. Genetic profiling of mesothelial cells exposed to asbestos has shown upregulation of many genes targeted by HMGB1 [112]. Furthermore, exposing mice to asbestos increased serum levels of HMGB1, although they were only maintained as long as the exposure continued [112]. One possible explanation for this finding is that the mice were exposed to asbestos by intraperitoneal injection, whereas most human exposure are through inhalation, suggesting the route of exposure may have influences on the long-term biochemical impacts of asbestos fibers. One study found elevated serum levels of HMGB1, using an ELISA kit, in asbestos-exposed individuals compared with both smoking and nonsmoking controls (Table 1) [60], indicating serum HMGB1 could be exploited for assessing asbestos exposure in human populations. In agreement with this finding, serum levels of HMGB1 have also been reported to be elevated in MM patients using an HMBG1 ELISA kit [113–115].
TNFα can be produced by many different cell types and is heavily involved in the regulation of immune cells. Generated through an NF-κB dependent pathway, TNFα is likely to increase in response to oxidative insults (Figure 3). Typically upregulated after phagocytosis of fibers by macrophages, generation of TNFα may induce further binding of particles, thus exacerbating the effects of asbestos exposure. It has also been proposed that TNFα plays an important anticytotoxic role for human mesothelial cells and therefore results in a higher abundance of damaged cells that may go through a malignant transformation [116]. Although numerous cell-culture-based studies have confirmed the importance of TNFα in responding to asbestos fibers, it remains to be seen if TNFα levels hold promise as a marker of asbestos exposure in human studies.
The large family of cytokines, the ILs, promote the development, differentiation and activation of lymphocytes and therefore play an important role in the immune response. As such, levels of several ILs have been assessed as biomarkers of asbestos exposure. In particular, increased IL-6 and IL-8 production is associated with activation of the NRLP3 inflammasome in response to asbestos fibers [117]. Workers occupationally exposed to asbestos have increased serum levels of IL-6 and IL-8 when compared controls in two studies using a solid phase ELISA test (Table 1) [61,62]. In each of these studies, workers who were occupationally exposed to asbestos fibers within factories were compared with unexposed controls. Interestingly, each study employed two sets of controls both within each factory and within each town containing the factory. In all cases serum levels of IL-6 and IL-8 were significantly elevated in the asbestos exposed group over both sets of controls. In agreement with this finding, other work found serum levels of IL-6 to track with asbestos exposure in a cohort of workers differentially exposed to asbestos [50]. This particular study involved 119 subjects with a history of occupational exposure to asbestos that were compared with 54 age-matched controls. For IL-6 serum quantification the exposed population was divided into three groups based on cumulative asbestos exposure. The highest exposure group was found to have statistically increased levels of IL-6 while there was no statistical significance between the other two groups. In contrast to this finding, one comparison of healthy controls to exposed individuals with asbestosis found no difference in serum IL-8 levels [109]. It is noteworthy that IL polymorphisms do not associate with risk factors for diseases resulting from asbestos exposure [118].
The chemokine, regulated on activation normal T cell expressed and secreted (RANTES), also known as chemokine (C–C motif) ligand 5 (CCL5) has recently emerged as a potential biomarker of asbestos exposure. By studying Italian workers with a mean of 25 years of asbestos exposure one group found a significant elevation in serum levels of RANTES as determined by a magnetic bead multiplex immunoassay (Table 1) [63]. Importantly, RANTES levels were also able to distinguish between asbestos-exposed and MM subgroups. However, it remains to be seen whether this biomarker will continue to be valid in studies involving larger sample sizes and different ARDs.
Currently available biomarkers of response to asbestos exposure represent a good starting point for developing a specific and sensitive biomarker panel. The availability of such a panel would make it possible to identify individuals who have been exposed to asbestos and to distinguish them from subjects who are at risk for progression to mesothelioma. Availability of such a panel would have a significant impact on the health of asbestos-exposed individuals by providing early warning for monitoring the potential occurrence of ARDs. It would also provide significant evidence to regulatory authorities such as the US FDA that their remediation efforts have been successful, particularly when individual monitoring can be conducted on a regular basis.
Conclusion & future perspective
The detrimental health effects resulting from asbestos exposure are not likely to subside in the near future. Despite being banned in the USA, asbestos is still being used in other parts of the world with much less stringent controls on possible exposure of the workers [13]. In addition, the often decades-long latency periods of ARDs means that they will continue to manifest in the USA for years to come [7]. As such, a reliable biomarker panel capable of assessing levels of asbestos exposure would provide a useful clinical tool in screening, diagnosis, prevention, and alleviating concerns about possible exposure and ensuring effective removal of asbestos from the environment. The majority of response biomarker studies thus far have focused on small molecule biomarkers of oxidative stress, signaling factors for cell-mediated and humoral immune responses, and growth factors generated in response to inhalation of asbestos fibers. These studies have shed light on important factors involved with the pathogenesis of diseases resulting from asbestos exposure as well as guided future efforts for developing an effective biomarker panel for asbestos exposure. However, considering the lack of specificity for many of the biomarkers, it is likely that assessment of new classes of compounds in response to asbestos exposure will result in a synergistic effect on biomarker performance.
LC–MS-based methodology coupled with careful exclusion of artifact formation [35,71,80] could provide more definitive information on the role of oxidative DNA and lipid damage in response to asbestos exposure. One approach that could be applied to discovery of biomarkers of response resulting from asbestos exposure involves the implementation of untargeted serum metabolomics using ultraperformance LC coupled with high-resolution MS [119]. This could potentially lead to the discovery of a comprehensive panel of metabolomic biomarkers of response that would be capable of identifying individuals exposed to asbestos. The biomarker panel would also be useful for monitoring populations to ensure environmental sources of asbestos exposure have been adequately remediated. Stable isotope dilution LC–high-resolution MS could also be employed [73] to distinguish the multiple SMRPs that are present in serum (Table 1) as well as for defining the role of lysine acetylation in the secretion HMBG1 protein (Table 1). The resulting assays could potentially improve specificity in assessing biological responses to asbestos exposure as well as improving the early detection of mesothelioma. Finally, untargeted serum protein profiling, using either Slow Off-rate Modified Aptamers as employed for mesothelioma biomarker discovery [120] or LC–MS-based proteomics methodology as we described recently for biomarkers of preterm birth [121], could be employed to discover additional protein biomarkers of asbestos exposure. Linking more specific response biomarkers with existing radiographic markers could have a significant impact on our ability to diagnose and treat ARDs.
Key terms.
Serpentine asbestos: Curly class of fibers, chrysotile is the only member of the serpentine class.
Amphibole asbestos: Needle-like class of fibers, where crocidolite, amosite, tremolite, anthophyllite and actinolite are members of the amphibole class. Crocidolite, often known as blue asbestos, is considered to be one of the most toxic forms of asbestos due to its high iron content.
Malignant mesothelioma: Rare form of cancer that develops from cells of the mesothelium, the protective lining that covers many of the internal organs of the body. Mesothelioma is most commonly caused by exposure to asbestos. The most common anatomical site for mesothelioma is the pleura (the outer lining of the lungs and internal chest wall), but it can also arise in the peritoneum and the pericardium.
Asbestos-related diseases: Asbestos-related diseases include: malignant mesothelioma, lung cancer, pleural plaques, asbestosis and diffuse pleural thickening.
Oxidative stress: Reflects an imbalance between the formation of reactive oxygen species, reactive nitrogen species, or electrophilic reactive intermediates and a biological system's ability to readily detoxify the reactive species and electrophilic intermediates.
Prognostic biomarker: Indicator of the likely course of a disease in an untreated individual.
Executive summary.
Asbestos exposure is now known to cause lung cancer and mesothelioma.
Despite being banned in the US, asbestos is still used industrially in other parts of the world.
The detrimental health impacts resulting from asbestos exposure are not likely to subside in the near future.
The often decades-long latency periods of ARDs means health impacts will result for years to come.
A validated biomarker panel capable of assessing levels of asbestos exposure would provide a great service in preventative care.
Asbestos is not present in human biological fluids; this means that it is necessary to analyze biomarkers of effect, which reflect a change in biologic function in response to asbestos exposure.
Current biomarkers of effect, which include small molecule markers of oxidative stress and proteins involved in immune responses, are not specific for asbestos exposure
A validated panel of biomarkers of effect of asbestos exposure would alleviate concerns about exposure as well as identify subjects at risk for asbestos-related disease.
Footnotes
Financial & competing interests disclosure
This work was supported by National Institutes of Health grants P42ES023720, P30ES013508 and T32ES019851. C Mesaros is a Senior Research Investigator in Systems Pharmacology and Translational Therapeutics, A Worth is a Graduate Student in Pharmacology, M Christofidou-Solomidou is a Research Associate Professor of Medicine, A Vachani is an Assistant Professor of Medicine, SA Albelda is the William Maul Measey Professor of Medicine, IA Blair is the A N Richards Professor of Systems Pharmacology and Translational Therapeutics and Director of the Penn SRP Center. They are all employees of the University of Pennsylvania. N Snyder is an Assistant Professor in the A J Drexel Autism Institute at Drexel University. Studies described in the article were not influenced by the authors’ current appointments, although research in many of the areas is currently on-going. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Open Access
This work is licensed under the Creative Commons Attribution-NonCommercial 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/bync-nd/3.0/
References
Papers of special note have been highlighted as either: • of interest •• of considerable interest
- 1.Huncharek M. Asbestos and cancer: epidemiological and public health controversies. Cancer Invest. 1994;12(2):214–222. doi: 10.3109/07357909409024876. [DOI] [PubMed] [Google Scholar]
- 2.Testa JR, Cheung M, Pei J, Below JE, Tan Y, Sementino E, et al. Germline BAP1 mutations predispose to malignant mesothelioma. Nat. Genet. 2011;43(10):1022–1025. doi: 10.1038/ng.912. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Discussion on pre-disposition of patients to DNA damage and genetic mutations after environmental stress.
- 3.Carbone M, Yang H. Molecular pathways: targeting mechanisms of asbestos and erionite carcinogenesis in mesothelioma. Clin. Cancer Res. 2012;18(3):598–604. doi: 10.1158/1078-0432.CCR-11-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Excellent description mechanism of asbestos-induced oxidative stress.
- 4.Gibbs AR. Role of asbestos and other fibres in the development of diffuse malignant mesothelioma. Thorax. 1990;45(9):649–654. doi: 10.1136/thx.45.9.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.International Agency for Research on Cancer. Arsenic, metals, fibres, and dusts. IARC Monogr. Eval. Carcinog. Risks Hum. 2012;100(Pt C):11–465. [PMC free article] [PubMed] [Google Scholar]; • In-depth description of risks associated with asbestos exposure.
- 6.Cogliano VJ, Baan R, Straif K, et al. Preventable exposures associated with human cancers. J. Natl Cancer Inst. 2011;103(24):1827–1839. doi: 10.1093/jnci/djr483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carbone M, Ly BH, Dodson RF, et al. Malignant mesothelioma: facts, myths, and hypotheses. J. Cell. Physiol. 2012;227(1):44–58. doi: 10.1002/jcp.22724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neri M, Ugolini D, Boccia S, et al. Chemoprevention of asbestos-linked cancers: a systematic review. Anticancer Res. 2012;32(3):1005–1013. [PubMed] [Google Scholar]
- 9.Berman DW, Crump KS. Update of potency factors for asbestos-related lung cancer and mesothelioma. Crit. Rev. Toxicol. 2008;38(Suppl. 1):1–47. doi: 10.1080/10408440802276167. [DOI] [PubMed] [Google Scholar]
- 10.Stayner L, Kuempel E, Gilbert S, Hein M, Dement J. An epidemiological study of the role of chrysotile asbestos fibre dimensions in determining respiratory disease risk in exposed workers. Occup. Environ. Med. 2008;65(9):613–619. doi: 10.1136/oem.2007.035584. [DOI] [PubMed] [Google Scholar]
- 11.Montjoy C, Parker J, Petsonk L, Luis T, Fallon K. Mesothelioma review. W. V. Med. J. 2009;105(3):13–16. [PubMed] [Google Scholar]
- 12.Sterman DH, Albelda SM. Advances in the diagnosis, evaluation, and management of malignant pleural mesothelioma. Respirology. 2005;10(3):266–283. doi: 10.1111/j.1440-1843.2005.00714.x. [DOI] [PubMed] [Google Scholar]
- 13.Linton A, Vardy J, Clarke S, van ZN. The ticking time-bomb of asbestos: its insidious role in the development of malignant mesothelioma. Crit. Rev. Oncol. Hematol. 2012;84(2):200–212. doi: 10.1016/j.critrevonc.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Frank AL, Joshi TK. The global spread of asbestos. Ann. Glob. Health. 2014;80(4):257–262. doi: 10.1016/j.aogh.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 15.Stayner L, Welch LS, Lemen R. The worldwide pandemic of asbestos-related diseases. Annu. Rev. Public Health. 2013;34:205–216. doi: 10.1146/annurev-publhealth-031811-124704. [DOI] [PubMed] [Google Scholar]; •• Excellent overview of issues related to worldwide asbestos exposure.
- 16.Peipins LA, Lewin M, Campolucci S, et al. Radiographic abnormalities and exposure to asbestos-contaminated vermiculite in the community of Libby, Montana, USA. Environ. Health Perspect. 2003;111(14):1753–1759. doi: 10.1289/ehp.6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noonan CW. Exposure matrix development for the Libby cohort. Inhal. Toxicol. 2006;18(12):963–967. doi: 10.1080/08958370600835021. [DOI] [PubMed] [Google Scholar]
- 18.BoRit asbestos superfund site community advisory group. www.boritcag.org
- 19.Rushton L, Hutchings SJ, Fortunato L, et al. Occupational cancer burden in Great Britain. Br. J. Cancer. 2012;107(Suppl. 1):S3–S7. doi: 10.1038/bjc.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paglietti F, Malinconico S, Di Molfetta V, Giangrasso M. Guidelines for asbestos remediation at Italian superfund sites. J. Environ. Sci. Health C. Environ. Carcinog. Ecotoxicol. Rev. 2012;30(3):253–286. doi: 10.1080/10590501.2012.705161. [DOI] [PubMed] [Google Scholar]
- 21.Szeszenia-Dabrowska N, Swiatkowska B, Szubert Z, Wilczynska U. Asbestos in Poland: occupational health problems. Int. J. Occup. Med. Environ. Health. 2011;24(2):142–152. doi: 10.2478/s13382-011-0020-4. [DOI] [PubMed] [Google Scholar]
- 22.Offermans NS, Vermeulen R, Burdorf A, et al. Occupational asbestos exposure and risk of pleural mesothelioma, lung cancer, and laryngeal cancer in the prospective Netherlands cohort study. J. Occup. Environ. Med. 2014;56(1):6–19. doi: 10.1097/JOM.0000000000000060. [DOI] [PubMed] [Google Scholar]
- 23.Kameda T, Takahashi K, Kim R, et al. Asbestos: use, bans and disease burden in Europe. Bull. World Health Organ. 2014;92(11):790–797. doi: 10.2471/BLT.13.132118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacTiernan A, Fritschi L, Slevin T, Jalleh G, Donovan R, Heyworth J. Public perceptions of cancer risk factors: a Western Australian study. Health Promot. J. Austr. 2014;25(2):90–96. doi: 10.1071/HE13081. [DOI] [PubMed] [Google Scholar]
- 25.Reid A, de Klerk NH, Magnani C, et al. Mesothelioma risk after 40 years since first exposure to asbestos: a pooled analysis. Thorax. 2014;69(9):843–850. doi: 10.1136/thoraxjnl-2013-204161. [DOI] [PubMed] [Google Scholar]
- 26.Van den Borre L, Deboosere P. Asbestos in Belgium: an underestimated health risk. The evolution of mesothelioma mortality rates (1969–2009) Int. J. Occup. Environ. Health. 2014;20(2):134–140. doi: 10.1179/2049396714Y.0000000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sartorelli P, Scancarello G, Romeo R, et al. Asbestos exposure assessment by mineralogical analysis of bronchoalveolar lavage fluid. J. Occup. Environ. Med. 2001;43(10):872–881. doi: 10.1097/00043764-200110000-00006. [DOI] [PubMed] [Google Scholar]
- 28.The Industrial Injuries Advisory Council. Pleural Plaques. 2009;23:1–60. Postion Paper. [Google Scholar]; • Comprehensive report on asbestos-induced pleural plaques in the UK.
- 29.American Thoracic Society. Diagnosis and initial management of nonmalignant diseases related to asbestos. Am. J. Respir. Crit. Care Med. 2004;170(6):691–715. doi: 10.1164/rccm.200310-1436ST. [DOI] [PubMed] [Google Scholar]; •• Excellent overview of diagnosis of asbestos-related diseases (ARDs).
- 30.Pairon JC, Laurent F, Rinaldo M, et al. Pleural plaques and the risk of pleural mesothelioma. J. Natl Cancer Inst. 2013;105(4):293–301. doi: 10.1093/jnci/djs513. [DOI] [PubMed] [Google Scholar]; • Very informative paper describing the relationship between asbestos exposure, pleural plaques, and mesothelioma
- 31.Cullen MR, Barnett MJ, Balmes JR, et al. Predictors of lung cancer among asbestos-exposed men in the {beta}-carotene and retinol efficacy trial. Am. J. Epidemiol. 2005;161(3):260–270. doi: 10.1093/aje/kwi034. [DOI] [PubMed] [Google Scholar]
- 32.Pilger A, Rudiger HW. 8-Hydroxy-2’-deoxyguanosine as a marker of oxidative DNA damage related to occupational and environmental exposures. Int. Arch. Occup. Environ. Health. 2006;80(1):1–15. doi: 10.1007/s00420-006-0106-7. [DOI] [PubMed] [Google Scholar]; • Discussion of 8-oxo-dGuo as a biomarker of oxidative stress.
- 33.Valavanidis A, Fiotakis K, Vlachogianni T. Airborne particulate matter and human health: toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J. Environ. Sci. Health C. Environ. Carcinog. Ecotoxicol. Rev. 2008;26(4):339–362. doi: 10.1080/10590500802494538. [DOI] [PubMed] [Google Scholar]
- 34.Valavanidis A, Vlachogianni T, Fiotakis C. 8-hydroxy-2’-deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health C. Environ. Carcinog. Ecotoxicol. Rev. 2009;27(2):120–139. doi: 10.1080/10590500902885684. [DOI] [PubMed] [Google Scholar]
- 35.Mesaros C, Arora JS, Wholer A, Vachani A, Blair IA. 8-Oxo-2’-deoxyguanosine as a biomarker of tobacco-smoking-induced oxidative stress. Free Radic. Biol. Med. 2012;53(3):610–617. doi: 10.1016/j.freeradbiomed.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Rigorous bioanalytical method for the analysis of urinary 8-oxo-dGuo.
- 36.Serban C, Dragan S. The relationship between inflammatory and oxidative stress biomarkers, atherosclerosis and rheumatic diseases. Curr. Pharm. Des. 2014;20(4):585–600. doi: 10.2174/138161282004140213145806. [DOI] [PubMed] [Google Scholar]
- 37.Cheresh P, Morales-Nebreda L, Kim SJ, et al. Asbestos-induced pulmonary fibrosis is augmented in 8-oxoguanine DNA glycosylase knockout mice. Am. J. Respir. Cell Mol. Biol. 2015;52(1):25–36. doi: 10.1165/rcmb.2014-0038OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sezgi C, Taylan M, Sen HS, et al. Oxidative status and acute phase reactants in patients with environmental asbestos exposure and mesothelioma. ScientificWorldJournal. 2014:902748. doi: 10.1155/2014/902748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lamote K, Nackaerts K, van Meerbeeck JP. Strengths, weaknesses, and opportunities of diagnostic breathomics in pleural mesothelioma-a hypothesis. Cancer Epidemiol. Biomarkers Prev. 2014;23(6):898–908. doi: 10.1158/1055-9965.EPI-13-0737. [DOI] [PubMed] [Google Scholar]
- 40.Mossman BT, Shukla A, Heintz NH, Verschraegen CF, Thomas A, Hassan R. New insights into understanding the mechanisms, pathogenesis, and management of malignant mesotheliomas. Am. J. Pathol. 2013;182(4):1065–1077. doi: 10.1016/j.ajpath.2012.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Procopio A, Marinacci R, Marinetti MR, et al. SV40 expression in human neoplastic and non-neoplastic tissues: perspectives on diagnosis, prognosis and therapy of human malignant mesothelioma. Dev. Biol. Stand. 1998;94:361–367. [PubMed] [Google Scholar]
- 42.Pooley FD. Electron microscope characteristics of inhaled chrysotile asbestos fibre. Br. J. Ind. Med. 1972;29(2):146–153. doi: 10.1136/oem.29.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hillegass JM, Miller JM, Macpherson MB, et al. Asbestos and erionite prime and activate the NLRP3 inflammasome that stimulates autocrine cytokine release in human mesothelial cells. Part. Fibre Toxicol. 2013;10:39. doi: 10.1186/1743-8977-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramos-Nino ME, Testa JR, Altomare DA, et al. Cellular and molecular parameters of mesothelioma. J. Cell. Biochem. 2006;98(4):723–734. doi: 10.1002/jcb.20828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Antoine DJ, Williams DP, Kipar A, et al. High-mobility group box-1 protein and keratin-18, circulating serum proteins informative of acetaminophen-induced necrosis and apoptosis in vivo . Toxicol. Sci. 2009;112(2):521–531. doi: 10.1093/toxsci/kfp235. [DOI] [PubMed] [Google Scholar]; •• Description of how acetylation of HMGB1 results in secretion of the protein.
- 46.Hold GL, El-Omar EM. Genetic aspects of inflammation and cancer. Biochem. J. 2008;410(2):225–235. doi: 10.1042/BJ20071341. [DOI] [PubMed] [Google Scholar]
- 47.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30(7):1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 48.Tagesson C, Chabiuk D, Axelson O, Baranski B, Palus J, Wyszynska K. Increased urinary excretion of the oxidative DNA adduct, 8-hydroxydeoxyguanosine, as a possible early indicator of occupational cancer hazards in the asbestos, rubber, and azo-dye industries. Pol. J. Occup. Med. Environ. Health. 1993;6(4):357–368. [PubMed] [Google Scholar]
- 49.Yoshida R, Ogawa Y, Shioji I, et al. Urinary 8-oxo-7, 8-dihydro-2’-deoxyguanosine and biopyrrins levels among construction workers with asbestos exposure history. Ind. Health. 2001;39(2):186–188. doi: 10.2486/indhealth.39.186. [DOI] [PubMed] [Google Scholar]
- 50.Amati M, Tomasetti M, Mariotti L, Tarquini LM, Valentino M, Santarelli L. Assessment of biomarkers in asbestos-exposed workers as indicators of cancer risk. Mutat. Res. 2008;655(1–2):52–58. doi: 10.1016/j.mrgentox.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 51.Amati M, Tomasetti M, Scartozzi M, et al. Profiling tumor-associated markers for early detection of malignant mesothelioma: an epidemiologic study. Cancer Epidemiol. Biomarkers Prev. 2008;17(1):163–170. doi: 10.1158/1055-9965.EPI-07-0607. [DOI] [PubMed] [Google Scholar]
- 52.Tomasetti M, Amati M, Nocchi L, et al. Asbestos exposure affects poly(ADP-ribose) polymerase-1 activity: role in asbestos-induced carcinogenesis. Mutagenesis. 2011;26(5):585–591. doi: 10.1093/mutage/ger020. [DOI] [PubMed] [Google Scholar]
- 53.Pelclova D, Fenclova Z, Kacer P, Kuzma M, Navratil T, Lebedova J. Increased 8-isoprostane, a marker of oxidative stress in exhaled breath condensate in subjects with asbestos exposure. Ind. Health. 2008;46(5):484–489. doi: 10.2486/indhealth.46.484. [DOI] [PubMed] [Google Scholar]
- 54.Beyer HL, Geschwindt RD, Glover CL, et al. MESOMARK: a potential test for malignant pleural mesothelioma. Clin. Chem. 2007;53(4):666–672. doi: 10.1373/clinchem.2006.079327. [DOI] [PubMed] [Google Scholar]
- 55.Robinson BW, Creaney J, Lake R, et al. Mesothelin-family proteins and diagnosis of mesothelioma. Lancet. 2003;362(9396):1612–1616. doi: 10.1016/S0140-6736(03)14794-0. [DOI] [PubMed] [Google Scholar]
- 56.Creaney J, Yeoman D, Demelker Y, et al. Comparison of osteopontin, megakaryocyte potentiating factor, and mesothelin proteins as markers in the serum of patients with malignant mesothelioma. J. Thorac. Oncol. 2008;3(8):851–857. doi: 10.1097/JTO.0b013e318180477b. [DOI] [PubMed] [Google Scholar]
- 57.Pass HI, Levin SM, Harbut MR, et al. Fibulin-3 as a blood and effusion biomarker for pleural mesothelioma. N. Engl. J. Med. 2012;367(15):1417–1427. doi: 10.1056/NEJMoa1115050. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Landmark paper describing the use of ELISA-based bioanalytical methodology for analysis of Fibulin-3 as a biomarker or ARDs.
- 58.Begin R, Martel M, Desmarais Y, et al. Fibronectin and procollagen 3 levels in bronchoalveolar lavage of asbestos-exposed human subjects and sheep. Chest. 1986;89(2):237–243. doi: 10.1378/chest.89.2.237. [DOI] [PubMed] [Google Scholar]
- 59.Schwartz DA, Galvin JR, Frees KL, et al. Clinical relevance of cellular mediators of inflammation in workers exposed to asbestos. Am. Rev. Respir. Dis. 1993;148(1):68–74. doi: 10.1164/ajrccm/148.1.68. [DOI] [PubMed] [Google Scholar]
- 60.Yang H, Rivera Z, Jube S, et al. Programmed necrosis induced by asbestos in human mesothelial cells causes high-mobility group box 1 protein release and resultant inflammation. Proc. Natl Acad. Sci. USA. 2010;107(28):12611–12616. doi: 10.1073/pnas.1006542107. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Excellent paper describing the potential utility of ELISA-based bioanalytical methodology for analysis of HMGB1 as a response biomarker of asbestos exposure.
- 61.Ilavska S, Jahnova E, Tulinska J, et al. Immunological monitoring in workers occupationally exposed to asbestos. Toxicology. 2005;206(2):299–308. doi: 10.1016/j.tox.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 62.Tulinska J, Jahnova E, Dusinska M, et al. Immunomodulatory effects of mineral fibres in occupationally exposed workers. Mutat. Res. 2004;553(1–2):111–124. doi: 10.1016/j.mrfmmm.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 63.Comar M, Zanotta N, Bonotti A, et al. Increased levels of C-C chemokine RANTES in asbestos exposed workers and in malignant mesothelioma patients from an hyperendemic area. PLoS ONE. 2014;9(8):e104848. doi: 10.1371/journal.pone.0104848. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Interesting paper on the use ELISA-based bioanalytical methodology for analysis of regulated on activation normal T cell expressed and secreted (RANTES) as a biomarker of asbestos exposure.
- 64.US EPA. Pesticides: Science and Policy. www.epa.gov/pesticides/science/biomarker.html
- 65.Sartorelli P, Romeo R, Scancarello G, Montomoli L, Muzzupappa C, Barabesi L. Measurement of asbestos fibre concentrations in fluid of repeated bro-choalveolar lavages of exposed workers. Ann. Occup. Hyg. 2007;51(5):495–500. doi: 10.1093/annhyg/mem014. [DOI] [PubMed] [Google Scholar]
- 66.Altomare DA, Menges CW, Pei J, et al. Activated TNF-alpha/NF-kappaB signaling via down-regulation of Fas-associated factor 1 in asbestos-induced mesotheliomas from Arf knockout mice. Proc. Natl Acad. Sci. USA. 2009;106(9):3420–3425. doi: 10.1073/pnas.0808816106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gustafson AM, Soldi R, Anderlind C, et al. Airway PI3K pathway activation is an early and reversible event in lung cancer development. Sci. Transl. Med. 2010;2(26):26ra25. doi: 10.1126/scitranslmed.3000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hillegass JM, Shukla A, Lathrop SA, et al. Inflammation precedes the development of human malignant mesotheliomas in a SCID mouse xenograft model. Ann. NY Acad. Sci. 2010;1203:7–14. doi: 10.1111/j.1749-6632.2010.05554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lotti M, Bergamo L, Murer B. Occupational toxicology of asbestos-related malignancies. Clin. Toxicol. (Phila) 2010;48(6):485–496. doi: 10.3109/15563650.2010.506876. [DOI] [PubMed] [Google Scholar]
- 70.Myers R. Asbestos-related pleural disease. Curr. Opin. Pulm. Med. 2012;18(4):377–381. doi: 10.1097/MCP.0b013e328354acfe. [DOI] [PubMed] [Google Scholar]
- 71.Mangal D, Vudathala D, Park JH, Lee SH, Penning TM, Blair IA. Analysis of 7,8-dihydro-8-oxo-2’-deoxyguanosine in cellular DNA during oxidative stress. Chem. Res. Toxicol. 2009;22(5):788–797. doi: 10.1021/tx800343c. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Techniques for preventing oxidation of DNA in bioanalytical methods for 8-oxo-dGuo.
- 72.Weiss JM, Goode EL, Ladiges WC, Ulrich CM. Polymorphic variation in hOGG1 and risk of cancer: a review of the functional and epidemiologic literature. Mol. Carcinog. 2005;42(3):127–141. doi: 10.1002/mc.20067. [DOI] [PubMed] [Google Scholar]
- 73.Ciccimaro E, Blair IA. Stable-isotope dilution LC-MS for quantitative biomarker analysis. Bioanalysis. 2010;2(2):311–341. doi: 10.4155/bio.09.185. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Comprehensive overview describing the important role that stable isotope analog internal standards can play in bioanalytical methods for small molecule and protein biomarkers.
- 74.Takahashi K, Pan G, Kasai H, et al. Relationship between Asbestos Exposures and 8-Hydroxydeoxyguanosine Levels in Leukocytic DNA of Workers at a Chinese Asbestos-material Plant. Int. J. Occup. Environ. Health. 1997;3(2):111–119. doi: 10.1179/107735297800407767. [DOI] [PubMed] [Google Scholar]
- 75.Marczynski B, Rozynek P, Kraus T, Schlosser S, Raithel HJ, Baur X. Levels of 8-hydroxy-2’-deoxyguanosine in DNA of white blood cells from workers highly exposed to asbestos in Germany. Mutat. Res. 2000;468(2):195–202. doi: 10.1016/s1383-5718(00)00053-x. [DOI] [PubMed] [Google Scholar]
- 76.Marczynski B, Kraus T, Rozynek P, Raithel HJ, Baur X. Association between 8-hydroxy-2’-deoxyguanosine levels in DNA of workers highly exposed to asbestos and their clinical data, occupational and non-occupational confounding factors, and cancer. Mutat. Res. 2000;468(2):203–212. doi: 10.1016/s1383-5718(00)00054-1. [DOI] [PubMed] [Google Scholar]
- 77.Roberts LJ, Morrow JD. Measurement of F(2)-isoprostanes as an index of oxidative stress in vivo . Free Radic. Biol. Med. 2000;28(4):505–513. doi: 10.1016/s0891-5849(99)00264-6. [DOI] [PubMed] [Google Scholar]; •• Excellent overview of isoprostanes as biomarkers of oxidative stress.
- 78.Morrow JD, Harris TM, Roberts LJ. Noncyclooxygenase oxidative formation of a series of novel prostaglandins: analytical ramifications for measurement of eicosanoids. Anal. Biochem. 1990;184(1):1–10. doi: 10.1016/0003-2697(90)90002-q. [DOI] [PubMed] [Google Scholar]; • Original description of isoprostanes.
- 79.Morrow JD, Awad JA, Boss HJ, Blair IA, Roberts LJ. Non-cyclooxygenase-derived prostanoids (F2-isoprostanes) are formed in situ on phospholipids. Proc. Natl Acad. Sci. USA. 1992;89(22):10721–10725. doi: 10.1073/pnas.89.22.10721. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Original description of the formation of esterified isoprostanes.
- 80.Mesaros C, Lee SH, Blair IA. Targeted quantitative analysis of eicosanoid lipids in biological samples using liquid chromatography-tandem mass spectrometry. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 2009;877(26):2736–2745. doi: 10.1016/j.jchromb.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Overview of bioanalytical methods for oxidized lipids and isoprostanes.
- 81.Li H, Lawson JA, Reilly M, et al. Quantitative high performance liquid chromatography/tandem mass spectrometric analysis of the four classes of F(2)-isoprostanes in human urine. Proc. Natl Acad. Sci. USA. 1999;96(23):13381–13386. doi: 10.1073/pnas.96.23.13381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kadiiska MB, Gladen BC, Baird DD, et al. Biomarkers of oxidative stress study III. Effects of the nonsteroidal anti-inflammatory agents indomethacin and meclofenamic acid on measurements of oxidative products of lipids in CCl4 poisoning. Free Radic. Biol. Med. 2005;38(6):711–718. doi: 10.1016/j.freeradbiomed.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 83.Kadiiska MB, Gladen BC, Baird DD, et al. Biomarkers of oxidative stress study II: are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radic. Biol. Med. 2005;38(6):698–710. doi: 10.1016/j.freeradbiomed.2004.09.017. [DOI] [PubMed] [Google Scholar]; • Bioanalytical validation of isoprostanes as oxidative stress biomarkers.
- 84.Pelclova D, Fenclova Z, Syslova K, et al. Oxidative stress markers in exhaled breath condensate in lung fibroses are not significantly affected by systemic diseases. Ind. Health. 2011;49(6):746–754. doi: 10.2486/indhealth.ms1237. [DOI] [PubMed] [Google Scholar]
- 85.Lehtimaki L, Oksa P, Jarvenpaa R, et al. Pulmonary inflammation in asbestos-exposed subjects with borderline parenchymal changes on HRCT. Respir. Med. 2010;104(7):1042–1049. doi: 10.1016/j.rmed.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 86.Lehtonen H, Oksa P, Lehtimaki L, et al. Increased alveolar nitric oxide concentration and high levels of leukotriene B(4) and 8-isoprostane in exhaled breath condensate in patients with asbestosis. Thorax. 2007;62(7):602–607. doi: 10.1136/thx.2006.067868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chow S, Campbell C, Sandrini A, Thomas PS, Johnson AR, Yates DH. Exhaled breath condensate biomarkers in asbestos-related lung disorders. Respir. Med. 2009;103(8):1091–1097. doi: 10.1016/j.rmed.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 88.Creaney J, Olsen NJ, Brims F, et al. Serum mesothelin for early detection of asbestos-induced cancer malignant mesothelioma. Cancer Epidemiol. Biomarkers Prev. 2010;19(9):2238–2246. doi: 10.1158/1055-9965.EPI-10-0346. [DOI] [PubMed] [Google Scholar]
- 89.Park EK, Thomas PS, Creaney J, Johnson AR, Robinson BW, Yates DH. Factors affecting soluble mesothelin related protein levels in an asbestos-exposed population. Clin. Chem. Lab. Med. 2010;48(6):869–874. doi: 10.1515/CCLM.2010.165. [DOI] [PubMed] [Google Scholar]
- 90.Cui A, Jin XG, Zhai K, Tong ZH, Shi HZ. Diagnostic values of soluble mesothelin-related peptides for malignant pleural mesothelioma: updated meta-analysis. BMJ Open. 2014;4(2):e004145. doi: 10.1136/bmjopen-2013-004145. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Comprehensive review and meta-analysis of soluble mesothelin-related protein (SMRP) biomarkers.
- 91.Jakubec P, Pelclova D, Smolkova P, Kolek V, Nakladalova M. Significance of serum mesothelin in an asbestos-exposed population in the Czech Republic. Biomed. Pap. Med Fac. Univ Palacky. Olomouc. Czech Repub. 2014 doi: 10.5507/bp.2014.015. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 92.Bayram M, Dongel I, Akbas A, Benli I, Akkoyunlu ME, Bakan ND. Serum biomarkers in patients with mesothelioma and pleural plaques and healthy subjects exposed to naturally occurring asbestos. Lung. 2014;192(1):197–203. doi: 10.1007/s00408-013-9526-9. [DOI] [PubMed] [Google Scholar]
- 93.Hollevoet K, Nackaerts K, Thimpont J, et al. Diagnostic performance of soluble mesothelin and megakaryocyte potentiating factor in mesothelioma. Am. J. Respir. Crit. Care Med. 2010;181(6):620–625. doi: 10.1164/rccm.200907-1020OC. [DOI] [PubMed] [Google Scholar]
- 94.Iwahori K, Osaki T, Serada S, et al. Megakaryocyte potentiating factor as a tumor marker of malignant pleural mesothelioma: evaluation in comparison with mesothelin. Lung Cancer. 2008;62(1):45–54. doi: 10.1016/j.lungcan.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 95.Felten MK, Khatab K, Knoll L, Schettgen T, Muller-Berndorff H, Kraus T. Changes of mesothelin and osteopontin levels over time in formerly asbestos-exposed power industry workers. Int. Arch. Occup. Environ. Health. 2014;87(2):195–204. doi: 10.1007/s00420-013-0853-1. [DOI] [PubMed] [Google Scholar]
- 96.Marini V, Michelazzi L, Cioe A, Fucile C, Spigno F, Robbiano L. Exposure to asbestos: correlation between blood levels of mesothelin and frequency of micronuclei in peripheral blood lymphocytes. Mutat. Res. 2011;721(1):114–117. doi: 10.1016/j.mrgentox.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 97.Rodriguez Portal JA, Rodriguez BE, Rodriguez RD, et al. Serum levels of soluble mesothelin-related peptides in malignant and nonmalignant asbestos-related pleural disease: relation with past asbestos exposure. Cancer Epidemiol. Biomarkers Prev. 2009;18(2):646–650. doi: 10.1158/1055-9965.EPI-08-0422. [DOI] [PubMed] [Google Scholar]
- 98.Pass HI, Wali A, Tang N, et al. Soluble mesothelin-related peptide level elevation in mesothelioma serum and pleural effusions. Ann. Thorac. Surg. 2008;85(1):265–272. doi: 10.1016/j.athoracsur.2007.07.042. [DOI] [PubMed] [Google Scholar]
- 99.Park EK, Yates DH, Creaney J, Thomas PS, Robinson BW, Johnson AR. Association of biomarker levels with severity of asbestos-related diseases. Saf. Health Work. 2012;3(1):17–21. doi: 10.5491/SHAW.2012.3.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Park EK, Wilson D, Yates DH. A predictive equation to adjust for clinical variables in soluble mesothelin-related protein (SMRP) levels. Clin. Chem. Lab. Med. 2012;50(12):2199–2204. doi: 10.1515/cclm-2012-0314. [DOI] [PubMed] [Google Scholar]
- 101.Weber DG, Johnen G, Taeger D, Weber A, Gross IM, Pesch B, et al. Assessment of confounding factors affecting the tumor markers SMRP, CA125, and CYFRA21–1 in Serum. Biomarker Insights. 2010;5:1–8. doi: 10.4137/bmi.s3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Denhardt DT, Chambers AF. Overcoming obstacles to metastasis–defenses against host defenses: osteopontin (OPN) as a shield against attack by cytotoxic host cells. J. Cell. Biochem. 1994;56(1):48–51. doi: 10.1002/jcb.240560109. [DOI] [PubMed] [Google Scholar]
- 103.Sandhu H, Dehnen W, Roller M, Abel J, Unfried K. mRNA expression patterns in different stages of asbestos-induced carcinogenesis in rats. Carcinogenesis. 2000;21(5):1023–1029. doi: 10.1093/carcin/21.5.1023. [DOI] [PubMed] [Google Scholar]
- 104.Grigoriu BD, Scherpereel A, Devos P, et al. Utility of osteopontin and serum mesothelin in malignant pleural mesothelioma diagnosis and prognosis assessment. Clin. Cancer Res. 2007;13(10):2928–2935. doi: 10.1158/1078-0432.CCR-06-2144. [DOI] [PubMed] [Google Scholar]
- 105.Pass HI, Lott D, Lonardo F, et al. Asbestos exposure, pleural mesothelioma, and serum osteopontin levels. N. Engl. J. Med. 2005;353(15):1564–1573. doi: 10.1056/NEJMoa051185. [DOI] [PubMed] [Google Scholar]
- 106.Park EK, Yates DH, Creaney J, Thomas PS, Robinson BW, Johnson AR. Association of biomarker levels with severity of asbestos-related diseases. Saf. Health Work. 2012;3(1):17–21. doi: 10.5491/SHAW.2012.3.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Park EK, Thomas PS, Johnson AR, Yates DH. Osteopontin levels in an asbestos-exposed population. Clin. Cancer Res. 2009;15(4):1362–1366. doi: 10.1158/1078-0432.CCR-08-0360. [DOI] [PubMed] [Google Scholar]
- 108.Argraves WS, Greene LM, Cooley MA, Gallagher WM. Fibulins: physiological and disease perspectives. EMBO Rep. 2003;4(12):1127–1131. doi: 10.1038/sj.embor.7400033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Corradi M, Goldoni M, Alinovi R, et al. YKL-40 and mesothelin in the blood of patients with malignant mesothelioma, lung cancer and asbestosis. Anticancer Res. 2013;33(12):5517–5524. [PubMed] [Google Scholar]
- 110.Creaney J, Dick IM, Meniawy TM, et al. Comparison of fibulin-3 and mesothelin as markers in malignant mesothelioma. Thorax. 2014;69(10):895–902. doi: 10.1136/thoraxjnl-2014-205205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schwartz DA, Davis CS, Merchant JA, et al. Longitudinal changes in lung function among asbestos-exposed workers. Am. J. Respir. Crit. Care Med. 1994;150(5 Pt 1):1243–1249. doi: 10.1164/ajrccm.150.5.7952547. [DOI] [PubMed] [Google Scholar]
- 112.Qi F, Okimoto G, Jube S, et al. Continuous exposure to chrysotile asbestos can cause transformation of human mesothelial cells via HMGB1 and TNF-alpha signaling. Am. J. Pathol. 2013;183(5):1654–1666. doi: 10.1016/j.ajpath.2013.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jube S, Rivera ZS, Bianchi ME, et al. Cancer cell secretion of the DAMP protein HMGB1 supports progression in malignant mesothelioma. Cancer Res. 2012;72(13):3290–3301. doi: 10.1158/0008-5472.CAN-11-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tabata C, Kanemura S, Tabata R, et al. Serum HMGB1 as a diagnostic marker for malignant peritoneal mesothelioma. J. Clin. Gastroenterol. 2013;47(8):684–688. doi: 10.1097/MCG.0b013e318297fa65. [DOI] [PubMed] [Google Scholar]
- 115.Tabata C, Shibata E, Tabata R, et al. Serum HMGB1 as a prognostic marker for malignant pleural mesothelioma. BMC Cancer. 2013;13:205–211. doi: 10.1186/1471-2407-13-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yang H, Bocchetta M, Kroczynska B, et al. TNF-alpha inhibits asbestos-induced cytotoxicity via a NF-kappaB-dependent pathway, a possible mechanism for asbestos-induced oncogenesis. Proc. Natl Acad. Sci. USA. 2006;103(27):10397–10402. doi: 10.1073/pnas.0604008103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dostert C, Petrilli V, Van BR, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320(5876):674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Overview of asbestos-mediated activation of the Nalp3 inflammasome.
- 118.Helmig S, Grossmann M, Wubbeling J, Schneider J. Interleukin gene polymorphisms in pneumoconiosis. Int. J. Mol. Med. 2012;30(2):401–408. doi: 10.3892/ijmm.2012.996. [DOI] [PubMed] [Google Scholar]
- 119.Snyder NW, Khezam M, Mesaros CA, Worth A, Blair IA. Untargeted metabolomics from biological sources using ultraperformance liquid chromatography-high resolution mass spectrometry (UPLC-HRMS) J. Vis. Exp. 2013;(75):e50433. doi: 10.3791/50433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ostroff RM, Mehan MR, Stewart A, et al. Early detection of malignant pleural mesothelioma in asbestos-exposed individuals with a noninvasive proteomics-based surveillance tool. PLoS ONE. 2012;7(10):e46091. doi: 10.1371/journal.pone.0046091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Parry S, Zhang H, Biggio J, et al. Maternal serum serpin B7 is associated with early spontaneous preterm birth. Am. J. Obstet. Gynecol. 2014;211(6):678, E1–E12. doi: 10.1016/j.ajog.2014.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]