Summary
Resveratrol, a natural polyphenol found in grapes, berries and other plants, has been proposed as an ideal chemopreventative agent due to its plethora of health promoting activities. However, despite its lofty promise as a cancer prevention agent its success in human clinical trials has been limited due to its poor bioavailability. Thus, interest in other natural polyphenols is intensifying including the naturally occurring dimethylated analog of resveratrol, pterostilbene. The UDP-glucuronosyltransferase (UGT) family of enzymes plays a vital role in the metabolism of both resveratrol and pterostilbene. The current study sought to elucidate the UGT family members responsible for the metabolism of pterostilbene and to examine gender differences in the glucuronidation of resveratrol and pterostilbene. We demonstrate that UGT1A1 and UGT1A3 are mainly responsible for pterostilbene glucuronidation although UGT1A8, UGT1A9 and UGT1A10 also had detectable activity. Intriguingly, UGT1A1 exhibits the highest activity against both resveratrol and pterostilbene despite altered hydroxyl group specificity. Using pooled human liver microsomes, enzyme kinetics were determined for pterostilbene and resveratrol glucuronides. In all cases females were more efficient than males, indicating potential gender differences in stilbene metabolism. Importantly, the glucuronidation of pterostilbene is much less efficient than that of resveratrol, indicating that pterostilbene will have dramatically decreased metabolism in humans.
Keywords: resveratrol, pterostilbene, UDP-glucuronosyltransferase, glucuronidation, gender
Introduction
Resveratrol (trans-3,5,4′-trihydroxystilbene), a natural polyphenol present in grapes, berries and other plants, exerts several beneficial effects including antioxidant, anti-inflammatory, anti-aging and anti-carcinogenic.1,2) Thus, resveratrol has been heralded as a dietary supplement that can combat several chronic diseases in humans. Two major ailments resveratrol is predicted to prevent are cardiovascular disease and cancer. In fact, resveratrol’s notoriety skyrocketed with the report that it could prevent all three phases of tumor development: initiation, promotion and progression.3) In the fifteen years since that seminal publication, intense interest in resveratrol’s anti-cancer abilities have led to a myriad of reports demonstrating the ability of resveratrol to combat and prevent several types of human cancers in vitro and in vivo including breast, prostate, colon, bladder, endometrial, and skin, to name a few.2,4) However, its use in humans as a chemopreventative agent seems to be unlikely at the present time due to its poor bioavailability.4,5) Resveratrol is well tolerated in humans, but is readily metabolized, leading to a short half-life which hinders its effectiveness as a chemopreventitive agent.6) Therefore, understanding how the human body metabolizes resveratrol is important.
One major pathway of metabolism for resveratrol is glucuronidation.7) Two resveratrol glucuronides have been reported, resveratrol-3-O-glucuronide and resveratrol-4′-O-glucuronide.7,8) The UDP-glucuronosyltransferase (UGT) family of enzymes catalyzes the glucuronidation of resveratrol by conjugating a glucuronic acid moiety to either the 3 or 4′ hydroxyl, thereby altering the biological properties of resveratrol and facilitating its elimination from the body. There are 19 functional human UGTs classified into three subfamilies based upon structural and amino acid sequence homology, UGT1A, UGT2A and UGT2B.9,10)
The UGTs are membrane-bound enzymes largely localized to the endoplasmic reticulum.10,11) Substrate specificity varies greatly between family members, with broad overlap, and their substrate specificity can be altered by posttranslational modifications such as phosphorylation.12) Although UGTs are primarily expressed in the liver, they also play vital roles in other tissues in the body. For example, UGT2B15 and UGT2B17 are expressed in the prostate where they regulate local androgen levels through glucuronidation13) and UGT1A10 and UGT2B7 are expressed in the breast where they regulate estrogens.14)
Previous reports have identified that human liver microsomes (HLM), which contain high concentrations of most UGT family members, preferentially form the 3-O-glucuronide (~2.5 fold more activity) as compared to the 4′-O-glucuronide.8) Further, UGT1A1 is mostly responsible for the observed 3-O-glucuronidation, while UGT1A9 is primarily responsible for 4′-O-glucuronidation. Several other UGTs also have detectable activity against resveratrol (at one or both positions) including UGT1A3, UGT1A6, UGT1A7, UGT1A8 and UGT1A10.7,8) Another recent report demonstrated interesting variation in resveratrol glucuronidation across species (human, mouse, rat and dog were examined).15) However, human gender differences in the glucuronidation of resveratrol have not been reported previously.
There are several ongoing investigative avenues being pursued to overcome resveratrol’s poor bioavailability in humans. Human trials looking at different dosing regiments of resveratrol supplementation, while varying the source and delivery method of resveratrol, have produced some promising results.16) Other researchers are devising targeted delivery methodologies,17) while still others have turned to other natural polyphenols with similar anti-cancer properties.18)
Pterostilbene [trans-3,5-dimethoxy-4′-hydroxystilbene] is another natural product found in grapes and berries. It is a naturally occurring dimethylated analog of resveratrol, but is predicted to have a longer half-life due to the 3 position being methylated and, therefore, being unable to be glucuronidated at this position.19) Pterostilbene has been shown to be equally or significantly more potent than resveratrol in several biological assays in mice including inhibition of skin,20) colon,21) and liver22) cancer. Although pterostilbene-4′-O-glucuronide has been characterized as a major metabolite for pterostilbene,23) the UGTs responsible for its glucuronidation have not been examined previously, nor have gender differences been assessed.
The current report seeks to elucidate the human UGTs responsible for pterostilbene glucuronidation and to evaluate possible gender differences in the glucuronidation of pterostilbene and resveratrol.
Methods
Reagents
UDPGA, β-glucuronidase and alamethicin were purchased from Sigma (St. Louis, MO). Resvertrol-3-O-glucuronide and resveratrol-4′-O-glucuronide standards were purchased from Cayman Chemical (Ann Arbor, MI). Resveratrol and pterostilbene were obtained from ChromaDex, Inc. (Irvine, CA). Supersomes expressing individual UGT family members and the UGT1A antisera were purchased from BD Biosciences (Woburn, MA). Human liver microsomes (HLM) were purchased from XenoTech (Lenexa, KS). Both male and female pooled human liver microsomes consisted of the microsomal liver fraction of 10 individual human donors. Male HLM consisted of 7 Caucasian and 3 Hispanic donors while the female HLM consisted of 9 Caucasian and 1 African American donors. HPLC grade methanol and water were purchased from Fisher Scientific (Pittsburgh, PA).
Western blot
Relative levels of total UGT1A protein in human liver microsomes were measured by 10% SDS-PAGE (with 4% stacking gel) followed by Western blot analysis using the anti-UGT1A antibody (1:5,000 dilution as per the manufacturer’s instructions). Proteins were detected by chemiluminescence using the SuperSignal West Dura Extended Duration Substrate (Pierce Biotechnology, Inc., Rockford, IL). Secondary antibodies supplied with the Dura ECL kit (anti-rabbit) were used at 1:3,000. Relative UGT1A protein levels were quantified against a known amount of human UGT1A protein (supplied in the Western blotting kit provided by Gentest, Tewksbury, MA) by densitometric analysis of X-ray film exposures.
Glucuronidation assays
The rate of glucuronidation by was determined essentially as previously described.11,24,25) For determination of UGT family members with activity against pterostilbene, 100 μg of Supersomes overexpressing the indicated UGT was incubated on ice with alamethicin (50 μg/mg protein) for 10 min. The final incubation reaction was carried out (100 μL final volume) in 50 mM Tris-HCl (pH 7.5), 10 mM MgCl2, 4 mM UDPGA, and 500 μM pterostilbene at 37°C for 60 min. Reactions were quenched by addition of 100 μL of cold methanol and centrifuged at 13,000 rpm, and supernatants were analyzed by HPLC (Alliance, Waters), equipped with a UV detector operated at 308 nm for both resveratrol and pterostilbene, using an HPLC Atlantis T3 C18 5 μm 6 × 250 mm column (Waters, Milford, MA). The following gradient conditions were utilized: 90% buffer A (20 mM ammonium acetate pH 5.0) for 4 min, followed by a linear gradient up to 80% buffer B (100% methanol) over 20 min at a flow rate of 1.0 mL/min, then 95% buffer B for 4 min and, finally, initial conditions for 2 min. As a control, this UGT screen was repeated with 500 μM resveratrol exactly as described for pterostilbene.
For all glucuronidation rate determinations, substrate concentrations, Supersome levels, HLM levels and incubation times for individual assays were chosen to maximize detection within a linear range of uptake and were similar to established protocols.11,26) For kinetic analysis using HLM (either male or female) of O-glucuronidation against resveratrol or pterostilbene, 20 μg protein was used in each reaction as described above. For kinetic analysis of resveratrol-4′-O-glucuronide formation, a concentration range of 3.9–250 μM resveratrol and the incubation time was 60 min were used. For kinetic analysis of resveratrol-3-O-glucuronide formation, a concentration range of 50–800 μM resveratrol and the incubation time was 60 min were employed. For kinetic analysis of pterostilbene-4′-O-glucuronide formation, a concentration range of 3.9–250 μM pterostilbene and the incubation time of 60 min were used. The chosen substrate concentration ranges encompassed the KM for all conditions tested and Michaelis-Menten kinietics was observed in all cases. The putative glucuronide peaks were confirmed first using β-glucuronidase as previously described.11,26) The amount of glucuronide was determined by the area under the curve of glucuronide peak relative to the area under the curve of substrate peak. Experiments were always performed in triplicate as independent assays. GraphPad Prism 5 software (GraphPad Software Inc., San Diego, CA) was employed to calculate kinetic values and standard errors.
Results
Characterization of resveratrol and pterostilbene glucuronides
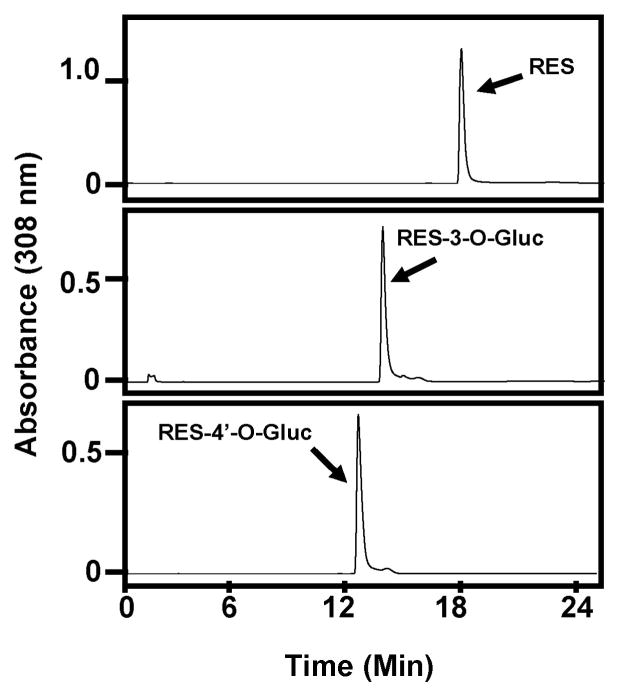
To elucidate which UGTs are responsible for pterostilbene glucuronidation and to examine possible gender differences in the glucuronidation of selected polyphenols, it was necessary to first characterize the parent compounds and glucuronides by HPLC. First, gradient conditions were optimized so one HPLC method would separate out all informative peaks (resveratrol, pterostilbene and metabolites) and those conditions are described in Methods. A chromatogram of resveratrol injected alone is shown in Figure 1 (top panel). Resveratrol eluted at around 18 min. A chromatogram of resveratrol-3-O-glucuronide (RES-3-O-Gluc) standard injected alone is depicted in Figure 1 (middle panel) and eluted at about 14 min. Lastly, resveratrol-4′-O-glucuronide (RES-4′-O-Gluc) standard was injected alone and eluted at about 13 min (Fig. 1; lower panel).
Fig. 1. Characterization of resveratrol and resveratrol-glucuronide standards by HPLC.
Representative HPLC chromatograms for: 1) resveratrol alone (top panel), 2) RES-3-O-Gluc standard (middle panel), 3) RES-4′-O-Gluc standard (bottom panel).
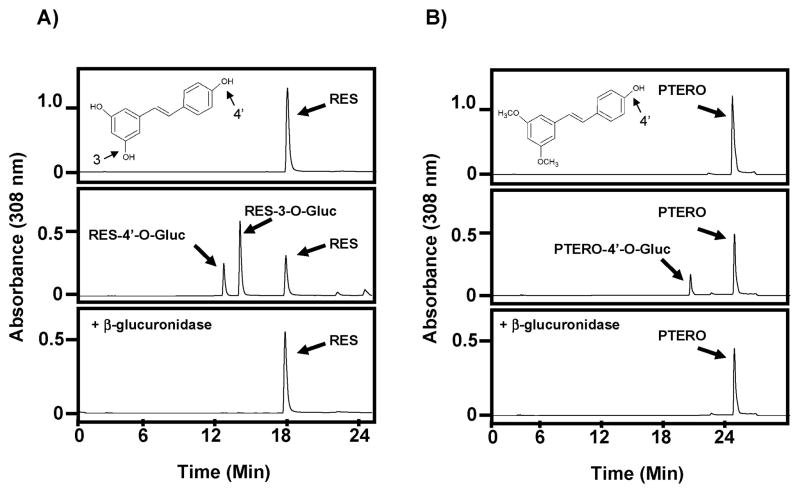
A glucuronidation assay was then performed with 100 μg of female human liver microsomes (HLM) incubated with 500 μM resveratrol for 1 h as described in Methods. The resulting chromatogram is displayed in Figure 2A (middle panel). Two putative resveratrol glucuronides are easily distinguishable from the parent compound. The first peak is the RES-4′-O-Gluc; the second, larger peak is the RES-3-O-Gluc and the third peak is the unconjugated resveratrol (RES). The identities of these peaks are consistent with previous reports8,15) and with the synthesized standards displayed in Figure 1. The chemical structure of resveratrol is also shown with 3- and 4′-hydroxyl groups identified along with a repeat of RES alone as a control (Fig. 2A; top panel). To further confirm that the two new peaks were in fact glucuronides, a β-glucuronidase assay was performed on one half of the reaction used to generate the chromatogram in the middle panel of Figure 2A as described in Methods. As shown in Figure 2A (lower panel) the two resveratrol glucuronides were cleaved by β-glucuronidase leaving only the parent compound and confirming the identities of the two new peaks as glucuronide conjugates.
Fig. 2. Characterization of resveratrol and pterostilbene glucuronides.
(A) Representative HPLC chromatograms for: 1) resveratrol alone (top panel), 2) glucuronidation assay using female HLMs and resveratrol for 60 min (middle panel), and 3) β-glucuronidase assay of one-half of the reaction from middle panel (bottom panel). (B) Representative HPLC chromatograms for: 1) pterostilbene alone (top panel), 2) glucuronidation assay using female HLMs and pterostilbene for 60 min (middle panel), and 3) β-glucuronidase assay of one-half of the reaction from middle panel (bottom panel). All peaks are labeled and the chemical structures of resveratrol (A) and pterostilbene (B) are inset in their respective top panels with reactive hydroxyl groups indicated. RES, resveratrol; PTERO, pterostilbene.
A similar set of experiments was then performed for pterostilbene. Figure 2B (top panel) depicts the chromatogram of pterostilbene injected alone. A single peak eluted at about 25 min. Glucuronidation assay utilizing female HLM performed identically to the resveratrol experiment showed the appearance of one metabolite peak eluting at about 20 min. This peak is labeled as pterostilbene-4′-O-glucuronide (PTERO-4′-O-Gluc) since pterostilbene can only be glucuronidated at the 4′ position because the 3 position is methylated (see inset on Fig. 2B; top panel). Further, this peak was confirmed to be a glucuronide since it disappeared after addition of β-glucuronidase as shown in the bottom panel of Figure 2B. This is the first demonstration of pterostilbene-glucuronidation from a human source, either in vitro or in vivo, although this data is consistent with previous reports in mice27) and rats.23)
Individual human UGTs responsible for glucuronidation of pterostilbene
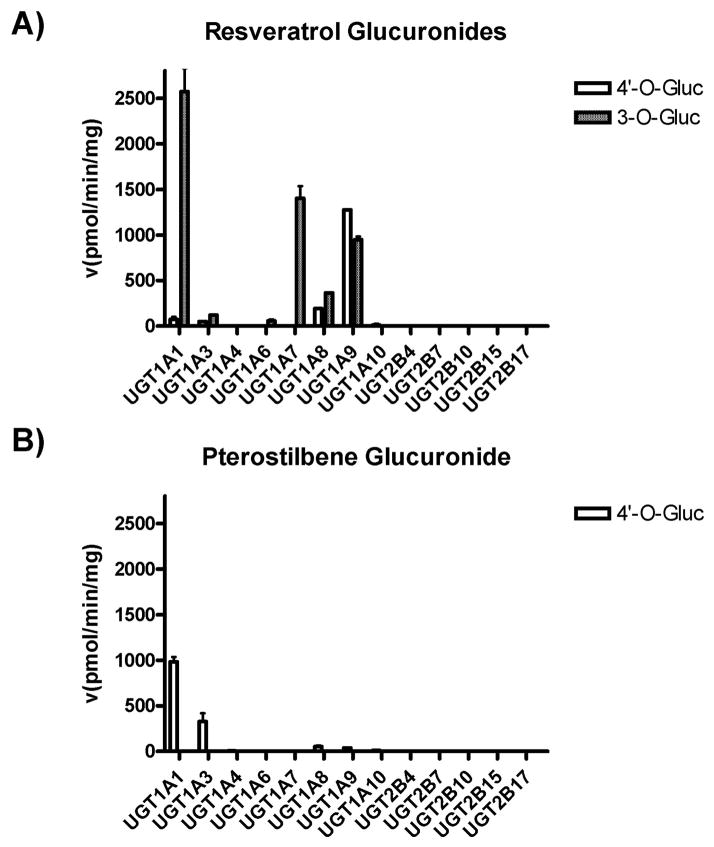
Human, recombinant, over-expressed UGT family members were used to screen for glucuronidation activity against pterostilbene. This screen was patterned after a previous report that screened for UGT activity against resveratrol.7) Those reported experiments were repeated here as a control. As shown in Figure 3A, UGT1A1 is the primary UGT responsible for resveratrol-3-O-glucuronidation. UGT1A7, UGT1A9 and UG-T1A8 also have considerable activity against resveratrol at the 3 hydroxyl position (Fig. 3A). For the resveratrol-4′-O-glucuronide, UGT1A9 clearly shows the highest activity, with UGT1A1, UGT1A3, UGT1A8 and UGT1A10 all showing minimal activity (Fig. 3A). These data are consistent with the previous publication.7) Next, we performed the novel identification of human UGTs responsible for pterostilbene glucuronidation. Interestingly, UGT1A1 demonstrated the highest activity against pterostilbene, yielding the pterostilbene-4′-O-glucuronide (Fig. 3B). UGT1A3 also demonstrated significant activity against pterostilbene, while UGT1A8, UGT1A9 and UGT1A10 all showed minimal activity (Fig. 3B). No other human UGTs examined had activity against pterostilbene. Overall, considerably more UGT activity was observed against resveratrol than against pterostilbene.
Fig. 3. Screen for human UGTs active against pterostilbene.
Supersomes over-expressing individual, recombinant, human UGTs were used to screen for glucuronidation activity against resveratrol (A) and pterostilbene (B). 4′-O-Glucuronides are shown in open boxes and 3-O-glucuronides are shown in shaded boxes. All reactions were performed for 2 h using 25 μg of the indicated UGT with 500 μM of substrate. All data shown are the composite of at least three independent experiments with standard error.
Kinetic evaluation of human UGT1A1 and UGT1A3 against pterostilbene
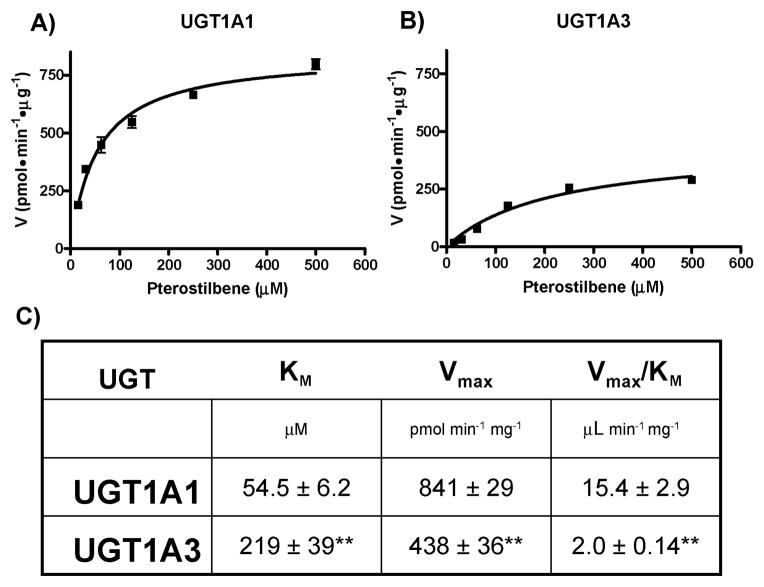
Kinetic glucuronidation analysis was then carried out for the two UGTs that demonstrated significant activity against pterostilbene, UGT1A1 and UGT1A3. Kinetic analysis was carried out as described in Methods using Supersomes overexpressing recombinant UGT1A1 (Fig. 4A) or UGT1A3 (Fig. 4B). Both curves followed Michaelis-Menten kinetics over the range examined and are composites of three independent experiments. Kinetic values for both curves are reported in Figure 4C including the Michaelis constant (KM), maximum rate (Vmax) and catalytic efficiency (Vmax/KM). The KM and Vmax values for UGT1A1 (54.5 ± 6.2 μM and 841 ± 29 pmol·min−1·mg−1, respectively) were significantly (p < 0.01) higher than the corresponding values for UGT1A3 (KM = 219 ± 39 μM and Vmax = 438 ± 36 pmol·min−1·mg−1). Accordingly, the catalytic efficiency of UG-T1A1 was ~8-fold higher than UGT1A3.
Fig. 4. Kinetic analysis of pterostilbene-glucuronide formation by UGT1A1 and UGT1A3.
Kinetic curves of the rate of pterostilbene-glucuronide formation by UGT1A1 (A) and UGT1A3 (B). Plots were generated by GraphPad Prism 5 software and calculated kinetic values are shown in (C). ** indicates significance of p < 0.01 as compared to the same kinetic parameter above.
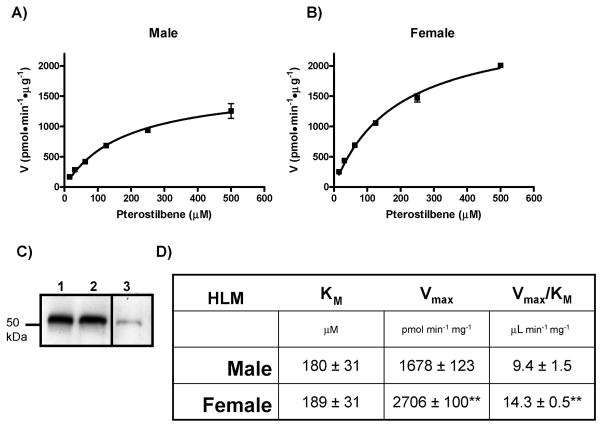
Gender differences in pterostilbene glucuronidation
To ascertain if gender differences exist in the glucuronidation of pterostilbene, pooled human liver microsomes (HLM) from either ten male donors or ten female donors were used. By pooling samples from ten different subjects inter-individual variation in UGT expression and activity can be averaged out. Kinetic analysis of the glucuronidation of pterostilbene was carried out as described in Methods using either male (Fig. 5A) or female (Fig. 5B) HLMs. Both curves followed Michaelis-Menten kinetics and are composites of three independent experiments. Kinetic values for both curves are reported in Figure 5D including the KM, Vmax and Vmax/ KM. There was no observable difference in KM between male HLM (180 ± 31 μM) and female HLM (189 ± 31 μM). However, there was a significant (p < 0.01) increase in the Vmax for female HLM (2,706 ± 100 pmol·min−1·mg−1) as compared with male HLM (1,678 ± 123 pmol·min−1·mg−1). These data corresponded to a significant increase of approximately 50% in catalytic efficiency for female HLMs (Fig. 5D) indicating that women are more efficient at metabolizing pterostilbene than men. To rule out that the observed differences were due to differences in UGT1A expression, a total UGT1A Western blot was performed on 20 μg of male and female HLMs. Figure 5C clearly shows that UGT1A protein is expressed in equivalent amounts between male (lane 1) and female (lane 2) HLM. Unfortunately, there are no commercial antibodies available capable of distinguishing all the individual UGT1A family members.
Fig. 5. Gender differences in the glucuronidation of pterostilbene.
Kinetic curves of the rate of pterostilbene-glucuronide formation by Male (A) and Female (B) HLMs. (C) Western blot showing relative UGT1A protein levels in male (lane 1) and female (lane 2) HLMs. UGT1A positive control that came with antibody is shown in lane 3. Kinetic plots were generated by GraphPad Prism 5 software and calculated kinetic values are shown in (D). ** indicates significance of p < 0.01 as compared to the same kinetic parameter above.
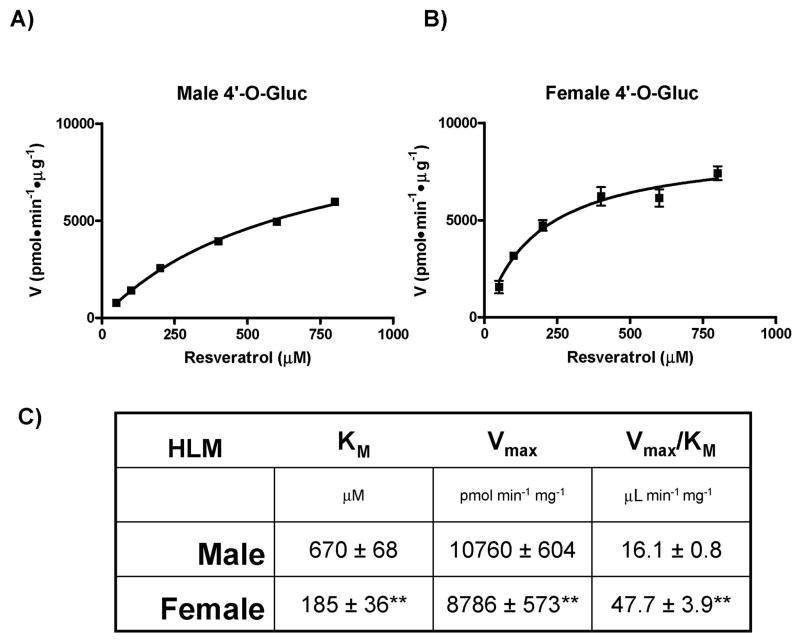
Gender differences in resveratrol glucuronidation
With our observation that pterostilbene-glucuronidation exhibited gender differences, we next evaluated resveratrol for potential gender differences in its metabolism. Although resveratrol glucuronidation by human UGTs has been addressed in several previous reports as well as compared to UGTs from several others species, human gender differences have never been reported.7,8,15) Thus, we tested the glucuronidation of resveratrol by male and female HLMs in a similar manner to that described above. The only difference is that two glucuronides of resveratrol are possible, resveratrol-3-O-glucuronide (RES-3-O-Gluc) and resveratrol-4′-O-glucuronide (RES-4′O-Gluc), and the enzyme kinetics need to be performed for formation of each glucuronide separately. First, the kinetics of RES-4′-O-Gluc formation was examined using male and female HLMs (Fig. 6). Once again a clear difference in glucuronidation activity was observed between genders. Specifically, the catalytic efficiency of female HLMs was ~3-fold higher than male HLMs (Fig. 6) due to significant differences (p < 0.01) in both KM and Vmax between genders. The kinetic curves for male HLMs (Fig. 6A) and female HLMs (Fig. 6B) did follow Michaelis-Menten kinetics over the range examined and are the composite of three independent experiments. These results show that the formation of RES-4′-O-Gluc displays gender differences similar to PTERO-4′-O-Gluc formation. Next, we analyzed RES-3-O-Gluc formation for human gender differences.
Fig. 6. Gender differences in the glucuronidation of resveratrol at the 4′-hydroxyl position.
Kinetic curves depicting the rate of resveratrol-4′-O-glucuronide formation by Male (A) and Female (B) HLMs. Plots were generated by GraphPad Prism 5 software and calculated kinetic values are shown in (C). ** indicates significance of p < 0.01 as compared to the same kinetic parameter above.
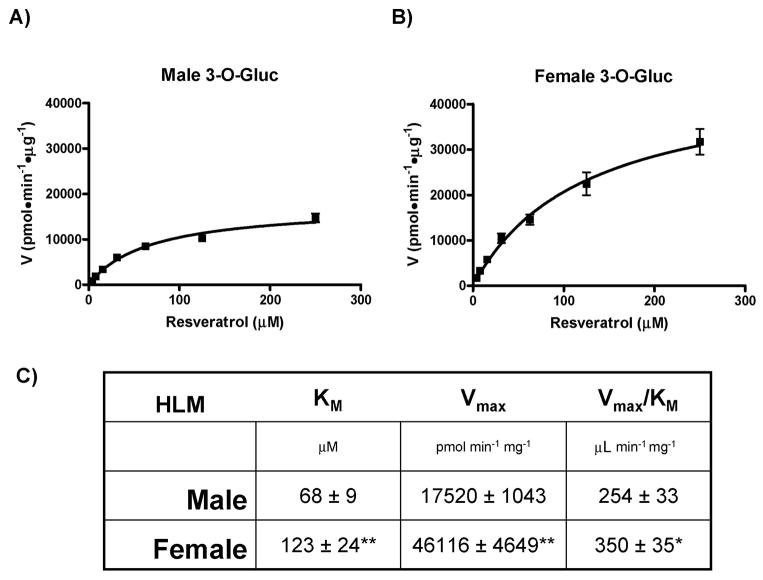
As shown in Figure 7, female HLMs are significantly (p < 0.0253) more efficient at generating the RES-3-O-Gluc than male HLMs (Vmax/KM of 350 ± 35 versus 254 ± 33, respectively). Once again, the kinetic curves for male HLM (Fig. 7A) and female HLMs (Fig. 7B) shown are composites of three independent experiments and displayed Michaelis-Menten kinetics. Significant (p < 0.01) differences in both KM and Vmax were seen between genders (Fig. 7C). Taken together, this is the first demonstration that human gender differences exist in the glucuronidation of both pterostilbene and resveratrol.
Fig. 7. Gender differences in the glucuronidation of resveratrol at the 3-hydroxyl position.
Kinetic curves of the rate of resveratrol-3-O-glucuronide formation by Male (A) and Female (B) HLMs. Plots were generated by GraphPad Prism 5 software and calculated kinetic values are shown in (C). Significance value of p < 0.01 (**) or p < 0.05 (*) is shown compared to the same kinetic parameter above.
Discussion
Resveratrol and pterostilbene hold great promise as dietary supplements that can combat and prevent chronic human diseases including cancer. Both compounds are found naturally in grapes, berries, peanuts and other plants. As individual compounds they have both shown prowess in the prevention of cancer in mouse models.4,18) To date, human clinical trials looking at cancer prevention by resveratrol have failed to live up to the promise of the rodent models most likely due to its poor bioavailability in humans.16) Therefore, understanding the metabolism of resveratrol is vital to its moving forward in the clinic. By extension, understanding the metabolism of pterostilbene will be important in its journey to the clinic to ensure it will not also be hampered by poor bioavailability. One major elimination pathway for both of these compounds from the human body is glucuronidation. This investigation had two main goals: first, to elucidate the human UGTs responsible for pterostilbene glucuronidation and, second, to explore possible gender differences in the glucuronidation of these polyphenols.
We screened human UGTs, except UGT2A enzymes since they are exclusively localized to the nasal cavity,10) for activity against pterostilbene. Human UGT1A1 and UGT1A3 were identified as the two UGTs primarily responsible for the glucuronidation of pterostilbene. UGT1A8, UGT1A9 and UGT1A10 also had discernable activity, but at a significantly lower rate than UGT1A1 and UGT1A3 (Fig. 3). A similar screen was performed for glucuronidation of resveratrol as a control. Our results mirrored a previous report for resveratrol glucuronidation.7) Intriguingly, both UGT1A1 and UGT1A3 preferentially attacked the 3-hydroxyl of resveratrol, yet have the highest activity against pterostilbene, which can only be attacked at the 4′-hydroxyl group (Fig. 3). Conversely, UGT1A9, which has the most glucuronidation activity against the 4′-hydroxyl of resveratrol, had minimal activity against the 4′-hydroxyl group of pterostilbene (Fig. 3). These results speak to both the elastic and finicky nature of UGT substrate affinity. Why does UGT1A1 have the most activity against pterostilbene when it can only attack at the opposite side of the molecule as compared to resveratrol? Why do distal methylations render UGT1A9 ineffective against pterostilbene despite the 4′-hydroxyl group remaining unchanged? Answers to these questions will hopefully be available as UGT/substrate prediction modeling continues to mature.28,29)
Another major route of polyphenol elimination from the human body is sulfonation.30) A previous report examining resveratrol sulfonation in detail demonstrated that the preferred hydroxyl group for conjugation of the sulfo group is the 3-hydroxyl position. In fact, this preference is very striking for three of the four human sulfotransferases (SULTs) that demonstrated activity against resveratrol. Only SULT1E1 demonstrated some specificity for the 4′-hydroxyl group.30) Furthermore, sulfonation at the 4′-OH of resveratrol is much less efficient than at the 3-OH. These results mirror the glucuronidation specificity for resveratrol. Thus, we hypothesize that since pterostilbene only has an available 4′-OH (as the 3 position is methylated), pterostilbene will be a poorer substrate for SULTs than resveratrol in the human body. Our demonstration that pterostilbene is glucuronidated at a significantly lower rate than resveratrol is extremely encouraging in suggesting that pterostilbene will achieve greater bioavailability in humans than resveratrol. Of course this hypothesis will have to be tested in humans.
Gender differences in resveratrol and pterostilbene glucuronidation were addressed here for the first time. Pooled HLMs from either male (n = 10) or female (n = 10) donors were used to examine potential differences in glucuronidation profiles. Our data clearly show that female HLMs are more efficient than male HLMs in the glucuronidation of both pterostilbene and resveratrol. For pterostilbene, kinetic data revealed that although male and female KMs are similar, female HLMs had a significantly higher Vmax indicating they are capable of much higher catalytic turnover than male HLMs (Fig. 5). Since UGT1A1 has the most activity against pterostilbene and has the highest expression of any UGT in human livers,31) we hypothesize that the observed gender difference is most likely attributable to varying UGT1A1 expression levels between the two HLM samples despite there being no difference in total UGT1A expression (Fig. 5C). Furthermore, the similarity in KMs between genders for pterostilbene glucuronidation is intriguing and may be indicative of a single UGT (UGT1A1) being responsible for pterostilbene glucuronidation. However, further studies are required to validate this theory.
Similar differences were seen in RES-3-O-Gluc formation where female HLMs exhibited a dramatically increased Vmax (although the KM was higher as well) resulting in a higher Vmax/KM as compared to male HLMs (Fig. 7). A similar trend was also observed for RES-4′-O-Gluc formation where the female Vmax/KM was significantly higher than for male HLMs, but in this case it was a dramatically decreased KM value driving this efficiency (Fig. 6). The variation in KM values may indicate that several UGTs are playing a role in the glucuronidation of resveratrol. This situation is clearly different than for pterostilbene. However, it is very interesting that in all cases examined, females were more efficient than males in the metabolism of these promising prevention agents. These results entreat the incorporation of gender into future studies of resveratrol and pterostilbene metabolization. Furthermore, this conclusion is consistent with other reports of gender differences in the glucuronidation of aspirin32) and emodin.33)
One limitation to our study is that inter-individual differences in stilbene glucuronidation were not examined. We chose to examine pooled HLM samples (n = 10 male or female donors) to try and minimize outliers and obtain initial results on gender differences. This approach did not allow individual livers to be examined and patient histories were not available. This data would be desirable in future studies as environmental factors such as smoking and current medications can influence UGT expression levels. Genetic differences in UGT genes, mainly in the form of single nucleotide polymorphisms, can also influence UGT expression and activity levels. Once again, individual livers would be needed to assess potential genetic differences.
Overall, our results clearly show that resveratrol is a much better UGT substrate than pterostilbene. This preference is demonstrated clearly in Figure 2 (compare middle panels) where glucuronidation assays were performed under identical conditions using female HLMs for 1 h. The difference is self evident, where approximately 68% of the resveratrol has already been glucuronidated (Fig. 2A; middle panel) while only 22% of the pterostilbene has been metabolized (Fig. 2B; middle panel) in this head-to-head comparison. The UGT screen supports this conclusion further since more UGTs have activity against resveratrol as compared to pterostilbene and the glucuronidation rates are significantly higher for resveratrol (Fig. 3). The kinetic data also support this claim with significantly higher Vmax/KM values seen against resveratrol as opposed to pterostilbene (Figs. 5–7). Lastly, these data are logical, considering that resveratrol can be glucuronidated at two sites as compared to pterostilbene’s single site and the preferred glucuronidation site on resveratrol (the 3-hydroxyl group) cannot be glucuronidated on pterostilbene. Taken together, pterostilbene is predicted to have a significantly higher half-life in humans, which may translate to greater bioavailability. While this will have to be confirmed in humans, it does correlate nicely with published data from rats in which pterostilbene showed 95% bioavailability and a half-life of 105 min contrasting with resveratrol’s 20% bioavailiblity and 14 min half-life.18)
In summary, we have identified the UGTs responsible for the glucuronidation of pterostilbene. Surprisingly, UGT1A1 has the most activity against pterostilbene and resveratrol despite having to re-orient its attack to another hydroxyl position. Evidence of a potential gender difference in the glucuronidation of resveratrol and pterostilbene is also presented. We hypothesize this UGT activity difference is due to gender-specific UGT expression profiles since the UGT expression is known to be regulated by sex hormones34–37) and we have ruled out differences in overall UGT1A protein expression (Fig. 5C). Furthermore, the novel results presented here predict that pterostilbene will have greater bioavailability in humans than resveratrol and clearly warrant human pharmacokinetic studies to directly test this prediction. If pterostilbene is shown to have much higher bioavailability in humans it may prove a superior alternative to resveratrol in the prevention of human chronic diseases.
Acknowledgments
This work was supported by a grant from the Waltmar Foundation to RWD as well as grants from the National Institutes of Health: National Cancer Institute [P30CA62330] to FLM and National Institute of Environmental Health Sciences [R03ES019668] to RWD. NIH training grant [T32CA009054] also provided salary support for RWD.
The authors would like to thank Drs. Sun Yang, Feng Liu and Lifang Xie for scientific discussions. We would also like to thank ChromaDex, Inc. for their generous gift of pure pterostilbene and resveratrol and, in particular, Dr. Jeremy Bartos for his scientific insight and support.
References
- 1.Park K, Lee JH. Protective effects of resveratrol on UVB-irradiated HaCaT cells through attenuation of the caspase pathway. Oncol Rep. 2008;19:413–417. [PubMed] [Google Scholar]
- 2.Yu W, Fu YC, Wang W. Cellular and molecular effects of resveratrol in health and disease. J Cell Biochem. 2012;113:752–759. doi: 10.1002/jcb.23431. [DOI] [PubMed] [Google Scholar]
- 3.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 4.Ndiaye M, Philippe C, Mukhtar H, Ahmad N. The grape antioxidant resveratrol for skin disorders: Promise, prospects, and challenges. Arch Biochem Biophys. 2011;508:164–170. doi: 10.1016/j.abb.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roupe KA, Remsberg CM, Yanez JA, Davies NM. Pharmacometrics of stilbenes: seguing towards the clinic. Curr Clin Pharmacol. 2006;1:81–101. doi: 10.2174/157488406775268246. [DOI] [PubMed] [Google Scholar]
- 6.Cottart CH, Nivet-Antoine V, Laguillier-Morizot C, Beaudeux JL. Resveratrol bioavailability and toxicity in humans. Mol Nutr Food Res. 2010;54:7–16. doi: 10.1002/mnfr.200900437. [DOI] [PubMed] [Google Scholar]
- 7.Brill SS, Furimsky AM, Ho MN, Furniss MJ, Li Y, Green AG, Bradford WW, Green CE, Kapetanovic IM, et al. Glucuronidation of transresveratrol by human liver and intestinal microsomes and UGT isoforms. J Pharm Pharmacol. 2006;58:469–479. doi: 10.1211/jpp.58.4.0006. [DOI] [PubMed] [Google Scholar]
- 8.Aumont V, Krisa S, Battaglia E, Netter P, Richard T, Merillon JM, Magdalou J, Sabolovic N. Regioselective and stereospecific glucuronidation of trans- and cis-resveratrol in human. Arch Biochem Biophys. 2001;393:281–289. doi: 10.1006/abbi.2001.2496. [DOI] [PubMed] [Google Scholar]
- 9.Tukey RH, Strassburg CP. Human UDP-glucuronosyltransferases: metabolism, expression, and disease. Annu Rev Pharmacol Toxicol. 2000;40:581–616. doi: 10.1146/annurev.pharmtox.40.1.581. [DOI] [PubMed] [Google Scholar]
- 10.Nagar S, Remmel RP. Uridine diphosphoglucuronosyltransferase pharmacogenetics and cancer. Oncogene. 2006;25:1659–1672. doi: 10.1038/sj.onc.1209375. [DOI] [PubMed] [Google Scholar]
- 11.Dellinger RW, Chen G, Blevins-Primeau AS, Krzeminski J, Amin S, Lazarus P. Glucuronidation of PhIP and N-OH-PhIP by UDP-glucuronosyltransferase 1A10. Carcinogenesis. 2007;28:2412–2418. doi: 10.1093/carcin/bgm164. [DOI] [PubMed] [Google Scholar]
- 12.Basu NK, Kovarova M, Garza A, Kubota S, Saha T, Mitra PS, Banerjee R, Rivera J, Owens IS. Phosphorylation of a UDP-glucuronosyltransferase regulates substrate specificity. Proc Natl Acad Sci USA. 2005;102:6285–6290. [Google Scholar]
- 13.Chouinard S, Yueh MF, Tukey RH, Giton F, Fiet J, Pelletier G, Barbier O, Belanger A. Inactivation by UDP-glucuronosyltransferase enzymes: the end of androgen signaling. J Steroid Biochem Mol Biol. 2008;109:247–253. doi: 10.1016/j.jsbmb.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 14.Starlard-Davenport A, Lyn-Cook B, Radominska-Pandya A. Identification of UDP-glucuronosyltransferase 1A10 in non-malignant and malignant human breast tissues. Steroids. 2008;73:611–620. doi: 10.1016/j.steroids.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maier-Salamon A, Bohmdorfer M, Thalhammer T, Szekeres T, Jaeger W. Hepatic glucuronidation of resveratrol: interspecies comparison of enzyme kinetic profiles in human, mouse, rat, and dog. Drug Metab Pharmacokinet. 2011;26:364–373. doi: 10.2133/dmpk.dmpk-11-rg-006. [DOI] [PubMed] [Google Scholar]
- 16.Smoliga JM, Baur JA, Hausenblas HA. Resveratrol and health—a comprehensive review of human clinical trials. Mol Nutr Food Res. 2011;55:1129–1141. doi: 10.1002/mnfr.201100143. [DOI] [PubMed] [Google Scholar]
- 17.Gokce EH, Korkmaz E, Dellera E, Sandri G, Bonferoni MC, Ozer O. Resveratrol-loaded solid lipid nanoparticles versus nano-structured lipid carriers: evaluation of antioxidant potential for dermal applications. Int J Nanomedicine. 2012;7:1841–1850. doi: 10.2147/IJN.S29710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCormack D, McFadden D. Pterostilbene and cancer: current review. J Surg Res. 2012;173:e53–e61. doi: 10.1016/j.jss.2011.09.054. [DOI] [PubMed] [Google Scholar]
- 19.Hougee S, Faber J, Sanders A, de Jong RB, van den Berg WB, Garssen J, Hoijer MA, Smit HF. Selective COX-2 inhibition by a Pterocarpus marsupium extract characterized by pterostilbene, and its activity in healthy human volunteers. Planta Med. 2005;71:387–392. doi: 10.1055/s-2005-864130. [DOI] [PubMed] [Google Scholar]
- 20.Tsai ML, Lai CS, Chang YH, Chen WJ, Ho CT, Pan MH. Pterostilbene, a natural analogue of resveratrol, potently inhibits 7,12-dimethylbenz[a]anthracene (DMBA)/12-O-tetradecanoylphorbol-13-acetate (TPA)-induced mouse skin carcinogenesis. Food Funct. 2012;3:1185–1194. doi: 10.1039/c2fo30105a. [DOI] [PubMed] [Google Scholar]
- 21.Chiou YS, Tsai ML, Nagabhushanam K, Wang YJ, Wu CH, Ho CT, Pan MH. Pterostilbene is more potent than resveratrol in preventing azoxymethane (AOM)-induced colon tumorigenesis via activation of the NF-E2-related factor 2 (Nrf2)-mediated antioxidant signaling pathway. J Agric Food Chem. 2011;59:2725–2733. doi: 10.1021/jf2000103. [DOI] [PubMed] [Google Scholar]
- 22.Pan MH, Chiou YS, Chen WJ, Wang JM, Badmaev V, Ho CT. Pterostilbene inhibited tumor invasion via suppressing multiple signal transduction pathways in human hepatocellular carcinoma cells. Carcinogenesis. 2009;30:1234–1242. doi: 10.1093/carcin/bgp121. [DOI] [PubMed] [Google Scholar]
- 23.Kapetanovic IM, Muzzio M, Huang Z, Thompson TN, McCormick DL. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemother Pharmacol. 2011;68:593–601. doi: 10.1007/s00280-010-1525-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olson KC, Dellinger RW, Zhong Q, Sun D, Amin S, Spratt TE, Lazarus P. Functional characterization of low-prevalence missense polymorphisms in the UDP-glucuronosyltransferase 1A9 gene. Drug Metab Dispos. 2009;37:1999–2007. doi: 10.1124/dmd.108.024596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dellinger RW, Fang JL, Chen G, Weinberg R, Lazarus P. Importance of UDP-glucuronosyltransferase 1A10 (UGT1A10) in the detoxification of polycyclic aromatic hydrocarbons: decreased glucuronidative activity of the UGT1A10139Lys isoform. Drug Metab Dispos. 2006;34 :943–949. doi: 10.1124/dmd.105.009100. [DOI] [PubMed] [Google Scholar]
- 26.Sun D, Sharma AK, Dellinger RW, Blevins-Primeau AS, Balliet RM, Chen G, Boyiri T, Amin S, Lazarus P. Glucuronidation of active tamoxifen metabolites by the human UDP glucuronosyltransferases. Drug Metab Dispos. 2007;35:2006–2014. doi: 10.1124/dmd.107.017145. [DOI] [PubMed] [Google Scholar]
- 27.Shao X, Chen X, Badmaev V, Ho CT, Sang S. Structural identification of mouse urinary metabolites of pterostilbene using liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2010;24:1770–1778. doi: 10.1002/rcm.4579. [DOI] [PubMed] [Google Scholar]
- 28.Miley MJ, Zielinska AK, Keenan JE, Bratton SM, Radominska-Pandya A, Redinbo MR. Crystal structure of the cofactor-binding domain of the human phase II drug-metabolism enzyme UDP-glucuronosyltransferase 2B7. J Mol Biol. 2007;369:498–511. doi: 10.1016/j.jmb.2007.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dong D, Ako R, Hu M, Wu B. Understanding substrate selectivity of human UDP-glucuronosyltransferases through QSAR modeling and analysis of homologous enzymes. Xenobiotica. 2012;42:808–820. doi: 10.3109/00498254.2012.663515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miksits M, Maier-Salamon A, Aust S, Thalhammer T, Reznicek G, Kunert O, Haslinger E, Szekeres T, Jaeger W. Sulfation of resveratrol in human liver: evidence of a major role for the sulfotransferases SULT1A1 and SULT1E1. Xenobiotica. 2005;35:1101–1119. doi: 10.1080/00498250500354253. [DOI] [PubMed] [Google Scholar]
- 31.Ohno S, Nakajin S. Determination of mRNA expression of human UDP-glucuronosyltransferases and application for localization in various human tissues by real-time reverse transcriptase-polymerase chain reaction. Drug Metab Dispos. 2009;37:32–40. doi: 10.1124/dmd.108.023598. [DOI] [PubMed] [Google Scholar]
- 32.Navarro SL, Saracino MR, Makar KW, Thomas SS, Li L, Zheng Y, Levy L, Schwarz Y, Bigler J, et al. Determinants of aspirin metabolism in healthy men and women: effects of dietary inducers of UDP-glucuronosyltransferases. J Nutrigenet Nutrigenomics. 2011;4:110–118. doi: 10.1159/000327782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu W, Tang L, Ye L, Cai Z, Xia B, Zhang J, Hu M, Liu Z. Species and gender differences affect the metabolism of emodin via glucuronidation. AAPS J. 2010;12:424–436. doi: 10.1208/s12248-010-9200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen H, Yang K, Choi S, Fischer JH, Jeong H. Up-regulation of UDP-glucuronosyltransferase (UGT) 1A4 by 17beta-estradiol: a potential mechanism of increased lamotrigine elimination in pregnancy. Drug Metab Dispos. 2009;37:1841–1847. doi: 10.1124/dmd.109.026609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeong H, Choi S, Song JW, Chen H, Fischer JH. Regulation of UDP-glucuronosyltransferase (UGT) 1A1 by progesterone and its impact on labetalol elimination. Xenobiotica. 2008;38:62–75. doi: 10.1080/00498250701744633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Starlard-Davenport A, Lyn-Cook B, Radominska-Pandya A. Novel identification of UDP-glucuronosyltransferase 1A10 as an estrogen-regulated target gene. Steroids. 2008;73:139–147. doi: 10.1016/j.steroids.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harrington WR, Sengupta S, Katzenellenbogen BS. Estrogen regulation of the glucuronidation enzyme UGT2B15 in estrogen receptor-positive breast cancer cells. Endocrinology. 2006;147:3843–3850. doi: 10.1210/en.2006-0358. [DOI] [PubMed] [Google Scholar]