Abstract
This Concept article surveys methods for attaching single polymer molecules on solid substrates. A general approach to single polymer immobilization based on the photochemistry of perfluorophenylazides is elaborated.
Keywords: atomic force microscopy, cross-linking, immobilization, photochemistry, polymers
Introduction
Materials based on synthetic polymers have gained increasing popularity since the last century, serving as structural frameworks and providing mechanical functions. With the rapid development of nano-, bio-, and information technologies, the demand for new materials with novel properties and functionalities has reached an unprecedented level. These include materials that are multifunctional and materials that possess tuneable properties in response to external stimuli. Polymers as soft materials are intrinsically dynamic making them the ideal choice in many of these applications.[1, 2] Polymers are molecules prepared by linking many small repeat units. Through the control over the chemical nature, the number of repeat units, and the manner that these repeat units are linked together, polymers with diverse structures, chemical functionalities, and molecular architectures can be obtained. This has been further enhanced by the recent development of new and powerful synthetic methodologies that allow the preparation of polymers with well-defined structures as well as dimensions.[3–6] Because the structure directly affects the final properties of the material, a significant outcome of the structural diversity of polymers is the wide range of associated properties that are tuneable and can be tailored by rational design.
Extensive theoretical work and experimental data accumulated over the past several decades have greatly improved the understanding of the relationship between molecular level structures of polymers and their ultimate properties. One issue associated with synthetic polymers is that they are inherently heterogeneous. Even the most well-controlled polymerization techniques yield polymers of various molecular weights or contour lengths. The conventional ensemble experiments thus average over the entire population of polymers. The length-dependent deviation is concealed and details of each individual polymer molecules are hidden.
With the rapid development of single-molecule imaging and characterization techniques, polymer structures, conformation, and dynamics can now be studied at an unprecedented level of detail.[7–9] Among these techniques are single molecule fluorescence microscopy, scanning force microscopy, laser scanning confocal microscopy (LSCM), scanning near-field optical microscopy (NSOM), and optical tweezers. Atomic force microscopy (AFM) in particular is highly versatile for imaging, manipulating, and stretching single polymer molecules in a non-invasive manner and under mild conditions. These powerful tools reveal the configuration of single polymer molecules, measure intra- and intermolecular interactions, and uncover a series of single molecule properties of conformation,[10, 11] bond strength,[12] end-to-end distance and contour length,[13, 14] radius of gyration, elastic modulus and friction.[15] Such information provides the fundamental understanding of molecular phenomena enabling the evaluation of polymer properties at the molecular length scale. These studies are furthermore essential for the design of single polymer reactions, and in the development of single polymer-based advanced nanomaterials and nanodevices.[16–18]
Many of the single molecule studies require samples prepared on a suitable substrate. Single polymers tethered on a solid substrate have unique properties that differ from either the bulk polymers or single polymer molecules in solution. Polymer single molecules in a dilute solution move rapidly and measured properties give averaged values. Spatially- separated polymer chains tethered on a surface, on the other hand, are individual entities free from intermolecular interactions. The measurements can therefore reflect the true properties of a single polymer molecule.
Important to single polymer research is developing surface chemistry and immobilization methods that allow the attachment of polymer single molecules in a well-defined manner and on the desired substrate. Methods that are simple, reproducible and versatile are especially valuable. This Concept article discusses surface immobilization chemistries for tethering single polymer molecules on solid substrates. A general approach based on the photochemistry of perfluorophenylazides for the covalent immobilization of single polymers will be elaborated.
Immobilization of Polymers
Polymers can be anchored to surfaces by physisorption or chemisorption. Physisorbed polymers can be prepared by a simple solution casting procedure. In this case, a polymer solution is placed on a substrate by dipping, spraying, or spin coating. After the solvent is evaporated, polymer molecules are left on the substrate. Because the molecules are held on the substrate surface by weak van der Waals forces, they can be easily removed by rinsing with the solvent. Chemisorbed molecules are firmly bound to the surface and are therefore more robust towards environmental and processing conditions. Chemisorption can be generally accomplished by either a graft-to or a graft-from approach. The graft-to approach employs polymers that possess substrate-specific functional groups. The reaction between the functional group and the substrate drives the chemisorption of the polymer to the substrate (Figure 1a). Frequently the polymers are end-functionalized and are attached to the substrate via a coupling reaction of, for example, thiols/disulfides to metallic surfaces such as gold or silver, or silanes to oxides. In the graft-from approach, the polymer is generated in situ by chain growth polymerization. A common practice is to covalently immobilize a polymerization initiator on the substrate and subsequently grow polymer chains from the substrate by the addition of monomers (Figure 1b). Polymers prepared by the graft-from technique have a higher packing density, the driving force being the covalent bond formation that compensates for the entropy loss resulting from the chain extension away from the surface.
Figure 1.
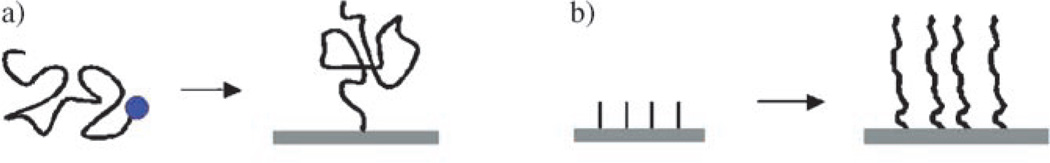
Chemisorbed polymers prepared by a) graft-to, or b) graft-from approach.
Immobilization of Single Polymer Molecules
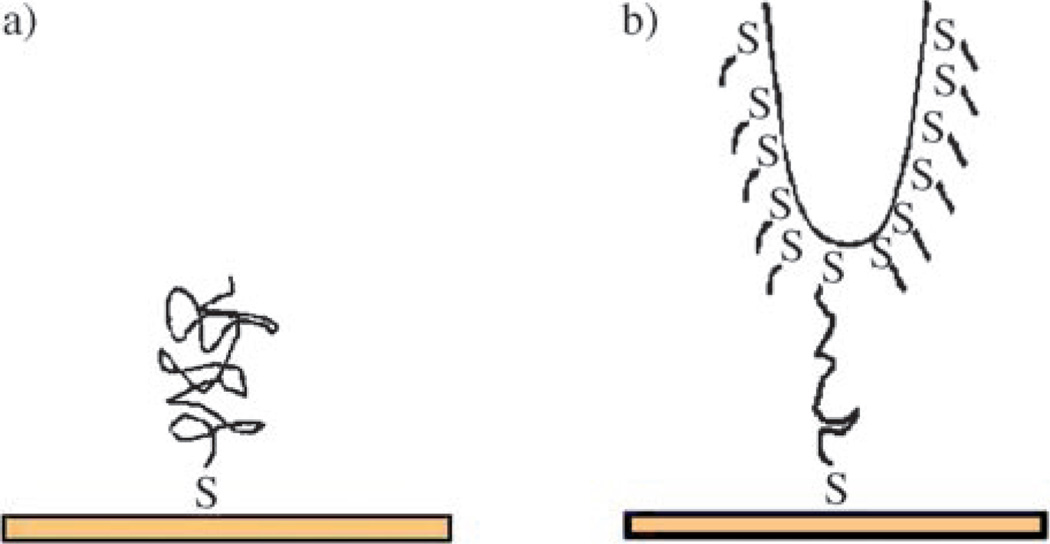
Single polymer molecules can be tethered to a substrate following similar approaches for the immobilization of polymers. Directly casting a polymer solution deposits the polymer on a substrate by physisorption. Single polymer molecules are frequently obtained simply by using a very dilution polymer solution. Below the critical chain overlap concentration, the intermolecular overlapping is minimized and isolated individual polymer molecules can be obtained. Because the adsorption force between the polymer and the substrate is the weak van der Waals interactions, the polymer molecules are found to have high in-plane lateral mobility.[14] When the rate of the polymer chain movement exceeds the scanning speed of the instrument, visualization of the molecules is difficult and the imaging resolution may suffer. Also problematic is the in situ experiment under fluidic conditions where the solvent may easily remove the weakly attached molecules from the substrate. Studies with physisorbed single polymers are therefore mostly conducted under ambient conditions. By varying the environment parameters such as temperature,[19] humidity,[20] and solvent vapour,[21] the corresponding changes in polymer chain conformation, swelling, segment movement can be directly visualized. The work by Kumaki and co-workers is a fine example where the lateral chain diffusion force, the interfacial interactions, and the AFM tip effect were fine-tuned to capture the molecular movement of single polymer molecules. The authors were able to observe that when isotactic poly-(methyl methacrylate) was exposed to humid air, the polymer travelled on the mica surface in a caterpillar-like motions along the direction of the chain axis.[22]
Chemisorbed single polymers are often prepared by the graft-to approach that involves coupling a functionalized polymer to the substrate. By covalently attaching the molecule to the substrate, the chain mobility is restricted and so is the chain movement through lateral diffusion. However, segment mobility is still present and the molecules retain their conformational freedom. Because the chains are covalently tethered to the substrate, it is possible to manipulate the molecules both in the dry state and under fluidic conditions. A special situation is polyelectrolytes that are adsorbed to charged substrates by way of electrostatic interactions. In this case, the substrate can be mica, which is negatively charged at neutral pH or in water, and therefore attracts positively charged polymers. Other substrates can also be used when chemically modified to introduce charged groups on the surface. The conformation of the adsorbed polyelectrolytes varies depending on the charge density of the substrate. At a higher surface charge density, adsorbed polymers adopt a relatively flat pancake conformation,[23, 24] whereas more extended wormlike chains are obtained on substrates of lower charge densities.[25] An interesting observation is that these polyelectrolyte molecules could no longer be re-conformed after deposition most likely due to the multiple charge interactions leading to reduced lateral chain mobility.
The chain mobility can be further reduced by covalently tethering polymers on the substrate surface. Frequently the polymer is attached at its end thus giving the precise location of the attachment point on the polymer. Conventional coupling chemistries can be adopted to immobilize single polymers, for example, the immobilization of carboxyl-containing polysaccharides on amine-functionalized glass slides through amide formation,[14] poly(vinyl amine) on epoxyfunctionalized surfaces giving β-hydroxyalkylamine,[26] chloro-terminated poly(dimethylsiloxane) on silicon oxide surface by way of a base-catalyzed nucleophilic substitution reaction.[13] The reactions of silanes with oxides or thiols/disulfides with gold or silver (Figure 2a) are also frequently used to prepare covalently immobilized single polymers on these popular substrates. Thiol-functionalized polymers can be synthesized by the photoiniferter-mediated living polymerization with thiocarbamate as the initiator.[27] The thiocarbamate group resides at the chain end and can be subsequently hydrolyzed to -SH by a base [Eq. (1)].[28] When a dithiocarbamate is used as the polymerization initiator, after hydrolysis, the resulting polymer will possess -SH at both ends of the chain [Eq. (2)]; an example of which is SH-functionalized poly(methacrylic acid) prepared by Garnier and co-workers.[29] In their studies, the polymer was tethered to a gold-coated AFM tip at one end; the density of which was controlled by the addition of mercapto-1-dodecanol during the adsorption of the polymer. Upon exposure to water, the polymer swelled and thus exposed the second -SH group that was then coupled to the gold substrate (Figure 2b). This configuration, with the polymer tethered in between the AFM tip and the substrate, allowed the authors to stretch and rapture the polymer chain and measured the single polymer entropic elasticity and the single bond force profile.[29]
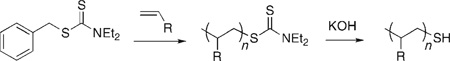 |
(1) |
 |
(2) |
Figure 2.
Immobilization of a) monothiol- and b) dithiol-functionalized polymer on gold.
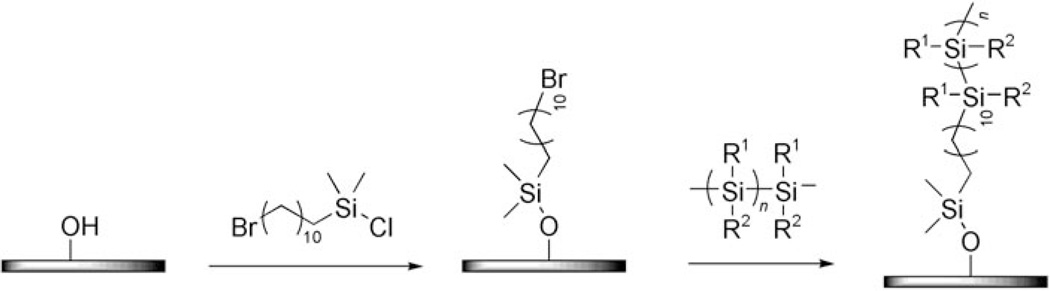
Other grafting methods employ more elaborated chemical derivatization procedures; an example of which is the coupling of polysilanes to silicon oxide surface studied by Furukawa and co-workers.[30] In the process shown in Scheme 1, silanol-activated silicon oxide was first treated with ω-bromodimethylchlorosilane to introduce bromo groups on the substrate. A polysilane anion, obtained by either anionic polymerization or an alkyllithium-initiated scission reaction of a higher molecular weight polysilane, is then attached to the substrate via a nucleophilic substitution reaction. To obtain single polymers, the density of the surface bromo groups was reduced with an alkyldimethylchlorosilane during the silanization reaction. The researchers synthesized a number of polysilanes varying the length and rigidity of the side chains R1, R2. Short alkyl groups yielded flexible polysilanes that collapsed into the pancake conformation showing in the AFM images as “dots”. With bulky branched alkyl groups or phenyl groups, however, “rope”-like structures were observed reflecting the increased rigidity of the resulting polysilanes.
Scheme 1.
Covalent immobilization of polysilanes on silicon oxide.
To obtain single polymers by chemisorption, the polymer solution is often diluted with a small organic molecule. The small molecules serve as spacers separating polymer chains and producing isolated polymer molecules for single molecule studies. Both examples shown in Figure 2b and Scheme 1 adopted such a strategy. Other deposition methods include using AFM tips to deliver single polymer molecules to the substrate. In the work of Vancso et al.,[31] polyamidoamine dendrimers were first adsorbed on an AFM tip. The molecules were then transferred to a substrate that had been functionalized with N-hydroxysuccinimidyl (NHS) ester groups. Amide formation between amino groups on the dendrimer and the surface NHS groups drove the deposition of the dendrimer molecules onto the substrate.
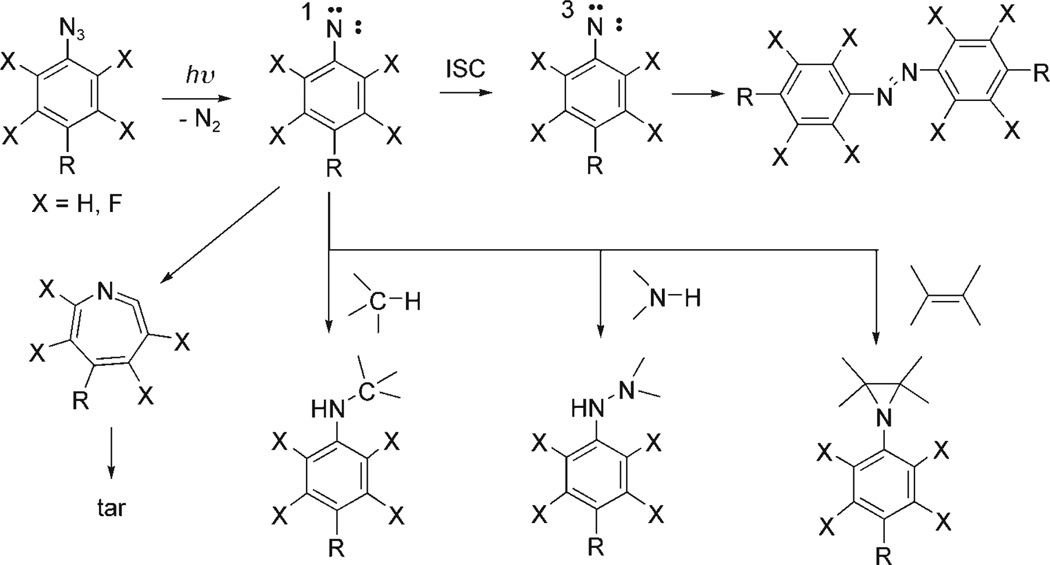
We have developed a photochemical method to generate covalently tethered single polymers. The immobilization chemistry is based on fluorinated phenylazides that act as coupling agents to attach polymer molecules to the substrate surface. The photochemistry of phenylazide is complex. When irradiated, phenylazide decomposes to give the singlet phenylnitrene. This highly reactive intermediate quickly rearranges to the corresponding seven-membered ketenimine which can react with amines to give azepinamines, or produces mainly polymer tars in the absence of a nucleophile (Scheme 2).[32, 33] The singlet phenylnitrene can also relax via intersystem crossing (ISC) to the corresponding triplet phenylnitrene, a process preferred at low temperatures or catalyzed by alcohols. The lower energy triplet state undergoes H-abstraction reactions forming primarily aniline-type products, or biomolecular reactions yielding the corresponding azo compound (Scheme 2). The singlet phenylnitrene is the key intermediate in the photochemistry of phenylazides, dictating whether useful adducts can be formed by the insertion/addition reactions rather than the ring expansion reaction. The F substituents, either perfluorinated or ortho to the azido group, raise the energy barrier of the ring-expansion reaction and greatly increases the lifetime of the corresponding fluorinated singlet phenylnitrenes.[34, 35] The pathway for the adduct formation is therefore favored and insertion reaction yields are greatly enhanced.[36–38] Fluorinated phenylazides have gained increasing popularity in photoaffinity labeling, a technique in which a natural ligand modified with a photosensitive moiety is used to probe the binding site structure of the biological receptor.[39, 40] We began applying perfluorophenylazides (PFPAs) in surface modifications and have since demonstrated that these compounds are highly efficient for the covalent immobilization of a variety of polymers.[41–44]
Scheme 2.
Simplified description of phenylazide photochemistry.
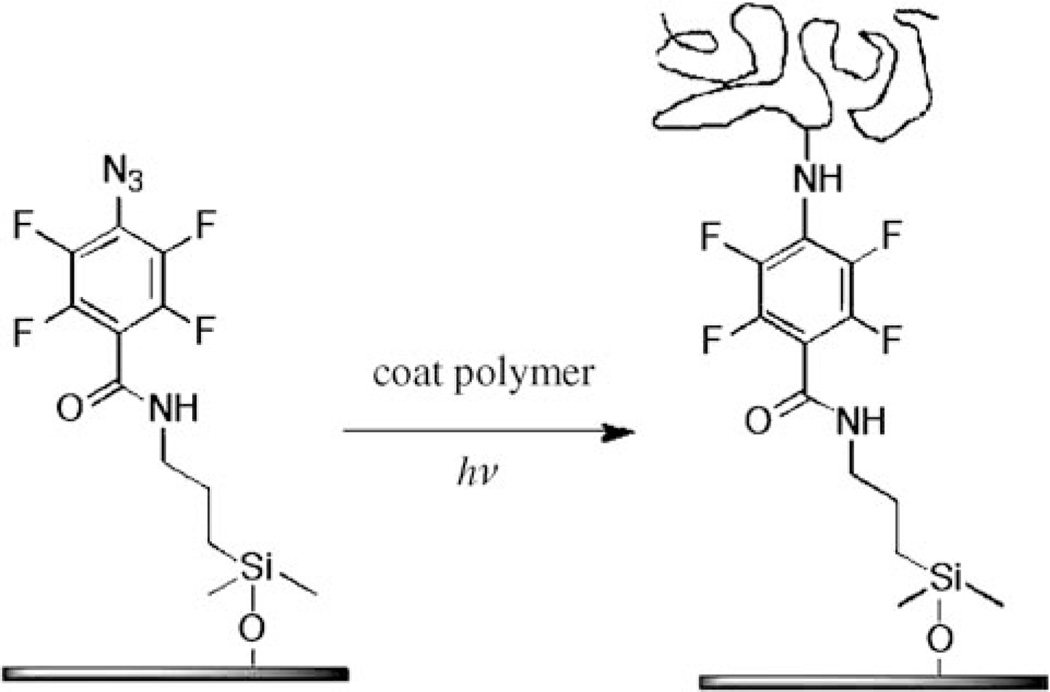
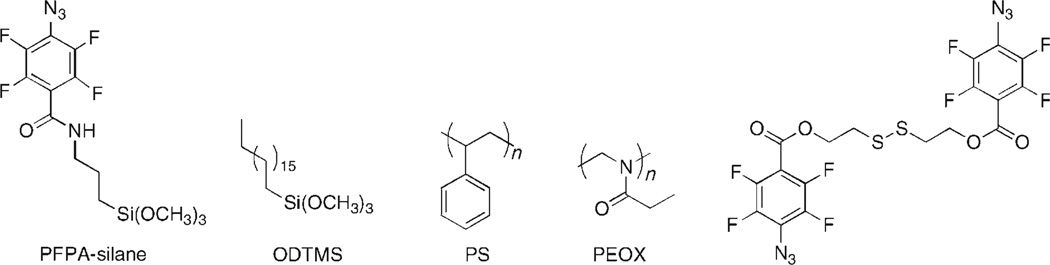
The photochemical immobilization process is illustrated in Figure 3 where a silane-functionalized perfluorophenylazide (PFPA-silane, Figure 4) is used to covalently attach polymers onto silicon oxide surfaces. PFPA-silane is first chemisorbed on silicon oxide, introducing the perfluorophenylazido groups to the substrate. The polymer is then coated and illuminated with UV light. Irradiation generates singlet perfluorophenylnitrenes which undergo C-H or N-H insertion reactions with the polymer to form covalent adducts. Because the reactions occur at the interface of the substrate surface and the neighboring molecules, only a monolayer of polymer is immobilized after the un-attached polymer is removed with a solvent.
Figure 3.
Immobilization of polymer on PFPA-silane-functionalized silicon oxide surface.
Figure 4.
Perfluorophenylazides have shown high efficiencies in the covalent adduct formation with a wide range of polymers. We have demonstrated that the surface immobilization process is highly reproducible and defect-tolerant. Consistent results were obtained from surfaces treated with PFPA-silane at concentrations of a few µm, or when more than 100 times of a non-photoactive silane were added.[45] This is because in principle only one attachment point is necessary to tether the polymer to the substrate. We therefore hypothesized that as the density of the surface azido groups decreases, the immobilized polymer would evolve from a uniform film to eventually isolated single molecules. Indeed, by reducing the density of the azido groups on the substrate, covalently immobilized polymer single molecules were obtained.[46]
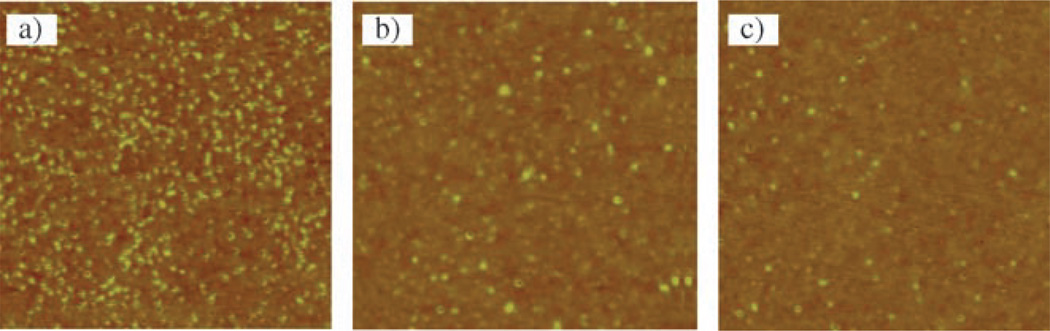
The immobilized polymer molecules appear as “dots” in the AFM images, corresponding to the pancake-like collapsed conformation that is commonly observed with isolated flexible polymers grafted on a surface. Results shown in Figure 5 were obtained by treating silicon wafers with a mixture of PFPA-silane and octadecyltrimethoxysilane (ODTMS). The addition of ODTMS, a non-photoactive silane, decreases the concentration of the surface azido groups. When the concentration was sufficiently low, isolated single molecules resulted. The higher the molar ratio of ODTMS to PFPA-silane, the lower the azide density, the fewer polymer molecules were immobilized (Figure 5). The density of the surface azido groups can also be controlled by the concentration of the PFPA-silane solution. Using this approach, covalently immobilized polystyrene molecules of various molecular weights were obtained by treating silicon wafers with PFPA-silane at very low concentrations.[46] We found that the higher the molecular weight of the polymer, the lower the concentration of PFPA-silane needed to achieve single molecule immobilization (see caption in Figure 6). This is consistent with the immobilization chemistry. As the molecular weight and thus size of the polymer increases, less surface azido groups are needed to attach the polymer. The concentration of PFPA-silane was therefore reduced in order to observe isolated single molecules.
Figure 5.
Polystyrene single molecules immobilized on silicon wafer treated with a solution of ODTMS/PFPA-silane at the molar ratio of a) 500:1, b) 2000:1, and c) 5000:1. Monodisperse polystyrene was used; Mw 223 200. The scan area was 1 µm × 1 µm for all images.
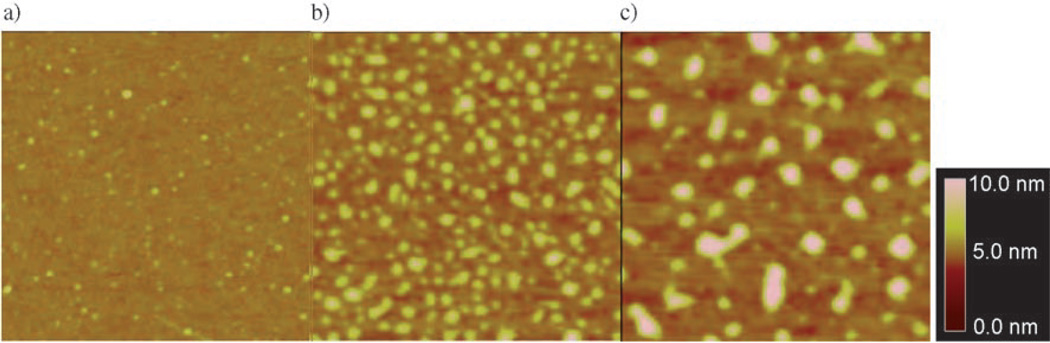
Figure 6.
Polystyrene molecules immobilized on silicon wafers. Mw of the monodisperse sample was a) 223 200, b) 570 000, and c) 1877 000. The wafers were treated with PFPA-silane in toluene at the concentration of a) 5 × 10−5 mg mL−1, b) 1 × 10−5 mg mL−1, and c) 5 × 10−6 mg mL−1. The scan area is 1 µm × 1 µm for all images. Adopted from reference [46].
Interestingly, we observed a preferential immobilization of larger sized molecules as indicated by the higher molecular volumes obtained from AFM measurements than the theoretical values calculated from the average molecular weight of the polymer. This effect was more pronounced with polydisperse samples where the molecular weight of the polymer is more heterogeneous than its monodisperse counterpart. A likely explanation is that on surfaces possessing limited amount of azido groups, larger molecules, that is, polymer of higher molecule weight, would have higher statistical probabilities than smaller molecules to be attached.
Because the immobilization chemistry is based on the insertion/addition reactions of the reactive singlet perfluorophenylnitrenes, no functional groups are required on the polymers to be immobilized so long as they possess C–H, N–H, or C=C bonds. The technique is therefore inherently versatile and is applicable to polymers of various structures, architecture, and physical properties. Especially beneficial are polymers that are difficult to immobilize by the conventional graft-to or graft-from approach due to the lack of functional groups or inability to surface polymerize in situ. Once the PFPA-functionalized substrate is prepared, it could be used as a universal surface to covalently immobilize polymers by a simple and fast irradiation procedure. Using this approach, we have already successfully immobilized single molecules of poly(2-ethyloxazoline) (PEOX)[46] and poly(4-vinylpyridine).[47]
In comparison with other covalent immobilization methods, this approach uses a photochemically initiated process to facilitate the attachment of single polymers. Light offers a highly chemoselective means to activate the photosensitive moieties without affecting other molecular and structural entities. The photosensitive groups can be specifically addressed at mild conditions in the solid state in high yields. By simply tuning the intensity of the light, the immobilization yield and density can be conveniently controlled. Furthermore, the reactions can be locally initiated by focusing the light in the areas of interest thus providing spatial control and resolution.[48, 49] Finally, the photochemistry of phenylazides is among the fastest of common photocrosslinkers; the photoimmobilization is often accomplished in a few minutes using a conventional photochemical setup.
This technique can be considered as a graft-to approach although it differs from the conventional protocol that the polymer is attached to the substrate by insertion reactions which would unlikely occur at the chain ends because of the low statistical probability. A more likely scenario is the attachment via the train segments of the polymer chain. Since the nitrene insertion reaction is non-selective, the point of attachment as well as the number of attachment points is difficult to predict or determine experimentally. The obtained structure is therefore less defined as compared to the end-grafted polymer where the chain extends from the surface from one end of the molecule.
Besides the ability to attach a wide range of polymers, the technique can be further applied to different substrates by derivatizing PFPA with the functional group appropriate for the specific substrate. The PFPA structure can be readily modified to introduce various functional groups as well as further structural variations such as spacers for conformation and topography control. We have synthesized the disulfide derivative of PFPA (Figure 4) that has been applied to modify gold surfaces,[50] and are in the process of preparing new functionalized PFPA derivatives suitable for additional types of substrates. Substrates functionalized with perfluorophenylazido groups can be considered as universal functional surfaces facilitating the attachment of large variety of polymers.
Conclusion
The preparation of immobilized single polymers follow the general approaches to the immobilization of polymers, namely physisorption and chemisorption. Physisorbed polymers are generated by a simple casting procedure and are highly mobile. Covalently tethered single polymers are often prepared by the graft-to approach, resulting in structures that are robust and suitable for experiments under fluidic conditions. The photochemical grafting technique developed in our laboratory aims at creating universal functionalized surfaces that can be used to attach a wide range of polymers regardless of their structures, architecture, and properties. In addition, this new approach adds an external light-induced control to the process that is fast, tuneable, and efficient.
The need to understand molecular level structures of polymers and correlate them with micro- and macroscopic properties demands the availability of a diverse number of materials in chemical composition, molecular structure, architecture, functional groups, and conformation. Beyond the fundamental studies of single polymer properties is developing single polymer-based advanced nanomaterials and nanodevices. Especially difficult in these applications are the precise control in the orientation and conformation of the single polymers, and the ability to generate ordered arrays with tuneable properties. These challenges will continue to drive the development of new and improved surface attachment chemistries.
Acknowledgement
This work has been supported by an NIH AREA award 1R15 GM066279-01A2.
References
- 1.Mather PT. Nat. Mater. 2007;6:93. doi: 10.1038/nmat1834. [DOI] [PubMed] [Google Scholar]
- 2.Zhou F, Huck WTS. Phys. Chem. Chem. Phys. 2006;8:3815. doi: 10.1039/b606415a. [DOI] [PubMed] [Google Scholar]
- 3.Matyjaszewski K, Spanswick J. Mater. Today. 2005;8:26. [Google Scholar]
- 4.Moad G, Rizzardo E, Thang SH. Aust. J. Chem. 2005;58:379. [Google Scholar]
- 5.Cunningham M, Lin M, Smith J-A, Ma J, McAuley K, Keoshkerian B, Georges M. Prog. Colloid Polym. Sci. 2004;124:88. [Google Scholar]
- 6.Otsu T. J. Polym. Sci. Part A: Polym. Chem. 2000;38:2121. [Google Scholar]
- 7.Samori P, Surin M, Palermo V, Lazzaroni R, Leclere P. Phys. Chem. Chem. Phys. 2006;8:3927. doi: 10.1039/b607502a. [DOI] [PubMed] [Google Scholar]
- 8.Samori P. Scanning probe microscopies: beyond imaging—manipulation of molecules and nanostructures. Weinheim: Wiley-VCH; 2006. [Google Scholar]
- 9.Ito S, Aoki H. Adv. Polym. Sci. 2005;182:131. [Google Scholar]
- 10.shang J, Geva E. J. Phys. Chem. B. 2005;109:16340. doi: 10.1021/jp052275c. [DOI] [PubMed] [Google Scholar]
- 11.Minko S, Roiter Y. Curr. Opin. Colloid Interface Sci. 2005;10:9. [Google Scholar]
- 12.Grandbois M, Beyer M, Rief M, Clausen-Schaumann H, Gaub HE. Science. 1999;283:1727. doi: 10.1126/science.283.5408.1727. [DOI] [PubMed] [Google Scholar]
- 13.Al-Maawali S, Bemis JE, Akhremitchev BB, Leecharoen R, Janesko BG, Walker GC. J. Phys. Chem. B. 2001;105:3965. [Google Scholar]
- 14.Kühner F, Erdmann M, Gaub HE. Phys. Rev. Lett. 2006;97:218301. doi: 10.1103/PhysRevLett.97.218301. [DOI] [PubMed] [Google Scholar]
- 15.Kühner F, Erdmann M, Sonnerberg L, Serr A, Morfill J, Gaub HE. Langmuir. 2006;22:11 180. doi: 10.1021/la061704a. [DOI] [PubMed] [Google Scholar]
- 16.Antonietti M, Landfester K. ChemPhysChem. 2001;2:207. doi: 10.1002/1439-7641(20010417)2:4<207::AID-CPHC207>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 17.Baker JRJ, Quintana A, Piehler L, Banazak-Holl M, Tomalia D, Raczka E. Biomed. Microdevices. 2001;3:61. [Google Scholar]
- 18.Yagi M, Kaneko M. Adv. Polym. Sci. 2006;199:143. [Google Scholar]
- 19.Li C, Gunari N, Fischer K, Janshoff A, Schmidt M. Angew. Chem. 2004;116:1121. doi: 10.1002/anie.200352845. Angew. Chem. Int. Ed.2004, 43, 1101. [DOI] [PubMed] [Google Scholar]
- 20.Gallyamov MO, Tartsch B, Mela P, Borner H, Matyjaszewski K, Sheiko S, Khokhlov A, Moller M. Phys. Chem. Chem. Phys. 2007;9:346. doi: 10.1039/b612654e. [DOI] [PubMed] [Google Scholar]
- 21.Zhuang W, Ecker C, Metselaar GA, Rowan AE, Nolte RJM, Samori P, Rabe JP. Macromolecules. 2005;38:473. [Google Scholar]
- 22.Kumaki J, Kawauchi T, Yashima E. Macromolecules. 2006;39:1209. [Google Scholar]
- 23.Pfau A, Schrepp W, Horn D. Langmuir. 1999;15:3219. [Google Scholar]
- 24.Zhu M, Schneider M, Papastavrou G, Akari S, Mohwald H. Langmuir. 2001;17:6471. [Google Scholar]
- 25.Minko S, Kiriy A, Gorodyska G, Stamm M. J. Am. Chem. Soc. 2002;124:3218. doi: 10.1021/ja017767r. [DOI] [PubMed] [Google Scholar]
- 26.Hugel T, Grosholz M, Clausen-Schaumann H, Pfau A, Gaub HE, Seitz M. Macromolecules. 2001;34:1039. [Google Scholar]
- 27.Otsu T, Matsunaga T, Kuriyama A, Yoshioka M. Eur. Polym. J. 1989;25:643. [Google Scholar]
- 28.Ortiz C, Hadziioannou G. Macromolecules. 1999;32:780. [Google Scholar]
- 29.Garnier L, Gauthier-Manuel B, van der Begte EW, Snijders J, Hadziioannou G. J. Chem. Phys. 2000;113:2497. [Google Scholar]
- 30.Furukawa K. Acc. Chem. Res. 2003;36:102. doi: 10.1021/ar020072q. [DOI] [PubMed] [Google Scholar]
- 31.Salazar RB, Shovsky A, Schonherr H, Vancso GJ. Small. 2006;2:1274. doi: 10.1002/smll.200600235. [DOI] [PubMed] [Google Scholar]
- 32.Scriven EFU, editor. Azides and Nitrenes. Academic Press; New York: 1984. [Google Scholar]
- 33.Gritsan NP, Zhu Z, Hadad CM, Platz MS. J. Am. Chem. Soc. 1999;121:1202. doi: 10.1021/ja9944305. [DOI] [PubMed] [Google Scholar]
- 34.Platz MS. Acc. Chem. Res. 1995;28:487. [Google Scholar]
- 35.Britsan NP, Platz MS. Adv. Phys. Org. Chem. 2001;36:255. [Google Scholar]
- 36.Soundararajan N, Platz MS. J. Org. Chem. 1990;55:2034. [Google Scholar]
- 37.Keana JFW, Cai XS. J. Org. Chem. 1990;55:3640. [Google Scholar]
- 38.Poe R, Schnapp K, Young MJT, Grayzar J, Platz MS. J. Am. Chem. Soc. 1992;114:5054. [Google Scholar]
- 39.Brunner J. Annu. Rev. Biochem. 1993;62:483. doi: 10.1146/annurev.bi.62.070193.002411. [DOI] [PubMed] [Google Scholar]
- 40.Pandurangi RS, Karra SR, Kuntz RR, Volkert WA. Photochem. Photobiol. 1997;65:208. doi: 10.1111/j.1751-1097.1996.tb02427.x. [DOI] [PubMed] [Google Scholar]
- 41.Bartlett M, Yan M. Adv. Mater. 2001;13:1449. [Google Scholar]
- 42.Yan M, Ren J. Chem. Mater. 2004;16:1627. [Google Scholar]
- 43.Graupner RK, Yan M. Langmuir. 2004;20:8675. doi: 10.1021/la0494728. [DOI] [PubMed] [Google Scholar]
- 44.Yan M, Ren J. J. Mater. Chem. 2005;15:523. [Google Scholar]
- 45.Liu L, Engelhard MH, Yan M. J. Am. Chem. Soc. 2006;128:14067. doi: 10.1021/ja062802l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu L, Yan M. Angew. Chem. 2006;118:6353. doi: 10.1002/anie.200602097. Angew. Chem. Int. Ed.2006, 45, 6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu L, Yan M. unpublished results. [Google Scholar]
- 48.Yan M, Bartlett M. Nano Lett. 2002;2:275. [Google Scholar]
- 49.Pei Z, Yu H, Theurer M, Walden A, Nilsson P, Yan M, Ramstrom O. ChemBioChem. 2007;8:166. doi: 10.1002/cbic.200600447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pei Y, Yu H, Pei Z, Theurer M, Yan M, Ramstrom O. 2007 unpublished results. [Google Scholar]