Abstract
Human immunodeficiency virus (HIV)-1 infection of the central nervous system occurs in the vast majority of HIV infected patients. HIV-associated dementia (HAD) represents the most severe form of HIV-related neuropsychiatric impairment and is associated with neuropathology involving HIV proteins and activation of proinflammatory cytokine circuits. Interferon-γ (IFN-γ) activates the JAK/STAT1 pathway, a key regulator of inflammatory and apoptotic signaling, and is elevated in brains of HIV-1 infected brains progressing to HAD. Recent reports suggest that green tea derived (-)-epigallocatechin-3-gallate (EGCG) can attenuate neuronal damage mediated by this pathway in conditions such as brain ischemia. In order to investigate the therapeutic potential of EGCG to mitigate the neuronal damage characteristic of HAD, IFN-γ was evaluated for its ability to enhance well-known neurotoxic properties of HIV-1 proteins gp120 and Tat in primary neurons and mice. Indeed, IFN-γ enhanced the neurotoxicity of gp120 and Tat via increased JAK/STAT signaling. Additionally, primary neurons pretreated with a JAK1 inhibitor or those derived from STAT1-deficient mice were largely resistant to the IFN-γ enhanced neurotoxicity of gp120 and Tat. Moreover, EGCG treatment of primary neurons from normal mice reduced IFN-γ enhanced neurotoxicity of gp120 and Tat, while attenuating JAK/STAT1 pathway activation. EGCG was also found to attenuate the neurotoxic properties of HIV-1 proteins in the presence of IFN-γ in vivo. Taken together, these data suggest that EGCG attenuates the neurotoxicity of IFN-γ augmented neuronal damage from HIV-1 gp120 and Tat both in vitro and in vivo. Thus EGCG may represent a novel, and naturally occurring compound for the prevention and treatment this disease.
Keywords: Green tea EGCG, AIDS dementia complex, HIV associated dementia, HIV encephalopathy, neurotoxicity, HIV-1 gp120 and Tat proteins, IFN-γ
1. Introduction
Epidemiologic studies indicate that some 60% of HIV-1 infected patients suffer some form of related neuropsychiatric impairment [22, 24] characterized by cognitive, motor, and/or behavioral symptoms. HIV-associated dementia [HAD; 9, 20, 7], represents the most severe form of AIDS-related neuropsychiatric impairment [23] and the average survival after symptom onset is 6 months [21]. In the early phases of HIV infection, virus invades the central nervous system (CNS) tissues from peripheral cell populations sustained particularly by infected T cells and monocytes/macrophages. Through this process HIV effectively establishes a viral reservoir early after primary infection that is resistant to highly active anti-retroviral therapy [HAART; 20]. Later in the symptomatic phase of HAD, usually coinciding with CD4+ T-cell depletion to levels lower than 200 cells/mm3, the virus is sustained in the CNS primarily by resident microglia and macrophages that have invaded from peripheral tissues. These cells seemingly serve as both viral factories and as mediators for inflammatory events resulting in neuropathology and subsequent neuropsychiatric impairment; the sequelae of HAD linked to “AIDS dementia-associated neuron damage” [1, 14, 23, 31]. Indeed, pathologic CNS immune dysfunction has been widely explored in many past studies of microglia; the primary host cells for HIV-1 in the CNS [8, 13, 32, 33]. In addition, considering that HIV-1 rarely infects neurons [17], many investigations have focused on neurotoxic effects of viral proteins including HIV-1 gp120 and Tat, acting in concert with proinflammatory circuits mediated by reactive immune cells and soluble factors able to induce or precipitate neuron death in HAD [31].
Able to directly induce neuron damage through apoptosis [11], the actions of HIV-1 proteins gp120 and Tat may be enhanced by cytokine-mediated signaling. For example in HAD, cytokines including IFN-γ, TNF-α, and IL-1β augment the neurotoxic properties of HIV-1 gp120 [22]. A similar role has been suggested to be at work in Alzheimer's disease where IFN-γ has been demonstrated to augment neuronal death in response to amyloid-beta [2]. Indeed several studies have implicated the Th1 cytokine IFN-γ, in the pathophysiology of AIDS dementia [3]. IFN-γ binding to its receptor causes Janus associated kinases (JAKs) to phosphorylate tyrosine residues on the intracytoplasmic side of the IFN-γ receptor leading to signal transducer and activator of transcription (STAT) proteins activation and migration to the nucleus; a system known collectively as the JAK/STAT pathway [10]. In normal cells, IFN-γ-mediated JAK/STAT1 activation is a transient, lasting from several minutes to several hours.
It has been suggested that this key regulatory system of proinflammatory and apoptotic signaling is dysfunctional in patients with HIV encephalopathy such that it is in a recurring state of inflammatory cytokine-mediated apoptotic signaling, leading to widespread neuron damage [12, 16, 22, 23]. Pervious studies support a role for JAK/STAT activation in the mediation of neuronal damage in HAD [4] as well as stroke [26]. Given that green tea (-)-epigallocatechin-3-gallate (EGCG) can act to attenuate neuronal JAK/STAT-regulated neuronal damage [30], we wished whether EGCG could modulate HAD-like neuronal damage by inhibition of JAK1/STAT1 activation. Therefore, we first tested the ability of IFN-γ to enhance neuronal damage inflicted by HIV-1 proteins gp120 and Tat in mice. We report that HIV-1 protein-induced neuronal damage was enhanced by IFN-γ in vitro and in vivo; an effect associated with increased JAK/STAT1 signaling. Primary neurons treated with JAK1 inhibitor or STAT1 deficient neurons were accordingly resistant to IFN-γ enhanced neurotoxicity of gp120 and Tat. Further, EGCG treatment was able to ameliorate HAD-like neuronal injury mediated by HIV-1 proteins: gp120 and Tat in the presence of IFN-γ in vitro and in vivo.
2. Materials & Methods
2.1 Reagents
Green tea-derived EGCG (>95% purity by HPLC) was purchased from Sigma (St. Louis, MO) and murine recombinant IFN-γ was obtained from R&D systems, (Minneapolis, MN). Monoclonal mouse anti-neuronal nuclei antibody was obtained from Chemicon (Temecula, CA). Donkey anti-mouse IgG Alexa Fluor 594 was purchased from Molecular Probes (Eugene, OR). Tris-buffered saline was obtained from Bio-Rad (Hercules, CA) and luminol reagent was obtained from Pierce Biotechnology. Anti-phospho-STAT1/anti-phospho-JAK1, anti-total-STAT1/anti-total-JAK1, anti-Bcl-xL, and anti-Bax antibodies were purchased from Upstate (Lake Placid, NY). Anti-actin antibody was obtained from Roche. JAK inhibitor I was purchased from EMD Biosciences, Inc. (San Diego, CA). Recombinant HIV-1 proteins gp120 (HIV-1CN54 gp120) and Tat were obtained from The National Institutes of Health (NIH) AIDS Research & Reference Reagent Program (Rockville, MD).
2.2 Mice
Breeding pairs of C57BL/6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and STAT1 deficient mice were purchased from Taconic (Hudson, NY). Animals were housed and maintained at the College of Medicine Animal Facility of the University of South Florida, and all experiments were in compliance with protocols approved by the University of South Florida Institutional Animal Care and Use Committee.
2.3 In vitro neurotoxicity analysis
Primary cultures of mouse cortical neurons were prepared as described previously [5]. Briefly, neuronal cells were isolated from newborn C57BL/6 mice and seeded in 6-well tissue-culture plates at 2 × 105 cells/well for 48 hours. Cells were then treated with gp120 (250 ng/ml) or Tat (250 ng/ml) in the presence or absence of IFN-γ (100 U/ml; Pierce Endogen) for 12 hours. In addition, to test whether EGCG could inhibit JAK/STAT1 signaling and neuronal damage induced by gp120 or/and Tat in the presence of IFN-γ, EGCG was also employed as the co-treatment. Cell culture supernatants were used for LDH assay while cell lysates were used for Western blot analysis of Bcl-x and Bax proteins.
2.4 In vivo neurotoxicity analysis
Animals were anesthetized using isoflurane (chamber induction at 4 - 5% isoflurane, intubation and maintenance at 1 - 2%). After reflexes were checked to ensure that mice were unconscious, they were positioned on a stereotaxic frame (Stoelting Lab Standard) with ear-bars positioned and jaws fixed to a biting plate. The axis coordinates were taken from a mouse brain atlas, and a 5 mm sterile plastic guide cannula (21 GA; Plastic One, Inc., Roanoke, VA) was implanted into the left lateral ventricle delimited from the stereotaxic coordinates (coordinates relative to bregma: − 0.6 mm anterior/posterior, + 1.2 mm medial/lateral, and − 3.0 mm dorsal/ventral) using the stereotaxic device (Stoelting Lab Standard) and an attached probe (cannula) holder. IFN-γ (200 U/mouse) with HIV-1 protein gp120 (500 ng/mouse) or Tat (500 ng/mouse) or PBS (10 μL) was administered at the rate of 1 μL/min using a Hamilton syringe (modified with a solder stop to prevent over insertion of the needle) through the implanted cannula. Correctness of the injection was confirmed by trypan blue dye administration and histological examination. The wounds were closed with 1-2 staples and mice were all observed until anesthesia had cleared. For testing EGCG effect on inhibiting Tat or/and gp120/IFN-γ neurotoxicity, the EGCG (50 mg/kg) or vehicle was intraperitoneally (i.p.) administered immediately after intracerebroventricular (i.c.v.) injection. Twenty four hours after the i.c.v. injections animals were sacrificed with isolfluorane and brain tissues collected. All dissected brain tissues were rapidly frozen for subsequent NeuN staining, Western blot, and LDH analysis.
2.5 JAK/STAT1 signaling analyses
Normal C57BL/6 primary cultured neuronal cells as well as STAT1 deficient primary neuronal cells were isolated and cultured as described previously [5]. Normal cells were cotreated with either gp120 or Tat (250 ng/mL) with or without IFN-γ (100 U/mL) and/or JAK inhibitor (50 nM). STAT1 deficient cells were treated with HIV-1 gp120 or HIV-1 Tat (250 ng/mL) in the presence or absence of IFN-γ. (100 U/mL) for 12 hours. At the end of the treatment period, neuronal cells were washed in ice-cold PBS three times and lysed in ice-cold lysis buffer. After incubation for 30 min on ice, samples were centrifuged at high speed for 15 min, and supernatants were collected. Total protein content was estimated by using the Bio-Rad protein assay. For phosphorylation of JAK1, membranes were first hybridized with phosphospecific Tyr1022/1023 JAK1 antibody (Cell Signaling Technology, Beverly, MA) and then stripped and finally analyzed by total JAK1. For STAT1 phosphorylation, membranes were probed with a phospho-Ser727 STAT1 antibody (Cell Signaling Technology), stripped with stripping solution, and then re-probed with an antibody that recognizes total STAT1 (Cell Signaling Technology). Alternatively, membranes with identical samples were probed either with phospho-JAK or STAT1 or with an antibody that recognizes total JAK or STAT1. Immunoblotting was performed with a primary antibody, followed by an anti-rabbit HRP-conjugated IgG secondary antibody as a tracer. After being washed in TBS, the membranes were incubated in the luminol reagent and exposed to film.
2.6 LDH Assay
LDH release assay (Promega, Madison, WI) was performed as previously described [28]. Briefly, after treatment at a variety of conditions, cell cultured media were collected for LDH release assay. Total LDH release was represent maximal lysis of target cells with 5% Triton X-100. Data are presented as mean ± SD of LDH release.
2.7 Western blot analysis
Western Blot was performed as described previously [28]. Briefly, for the in vivo studies left hemispheres of mouse brains were lysed in ice-cold lysis buffer and an aliquot corresponding to 50 μg of total protein was electrophoretically separated using 16.5% Tris-tricine gels. Electrophoresed proteins were then transferred to PVDF membranes (Bio-Rad), washed in dH2O, and blocked for 1 hour at ambient temperature in Tris-buffered saline containing 5% (w/v) non-fat dry milk. After blocking, membranes were hybridized for 1 hour at ambient temperature with various primary antibodies. Membranes were then washed three times (five minutes each) in dH2O and incubated for 1 hour at ambient temperature with the appropriate HRP-conjugated secondary antibody (1:1,000). All antibodies were diluted in TBS containing 5% (w/v) non-fat dry milk. Blots were developed using the luminol reagent. Densitometric analysis was done using the Fluor-S MultiImagerTM with Quantity OneTM software (Bio-Rad). Antibodies used for Western blot included: anti-Bcl-xL antibody (1:1,000), anti-Bax antibody (1:1,000), anti-phospho-STAT1 (1:500), anti-total-STAT1 (1:500), anti-phospho-JAK1 (1:500), anti-total-JAK1 (1:500), and anti-actin antibody (1:1,500). Similar procedures were followed for the in vitro studies using cell culture supernatant aliquots corresponding to 50 μg of total protein.
2.8 NeuN immunochemistry analysis
At sacrifice, mice were anesthetized with isofluorane and transcardially perfused with ice-cold physiological saline containing heparin (10 U/mL). Brains were rapidly isolated and separated into left and right hemispheres using a mouse brain slicer (Muromachi Kikai, Tokyo, Japan). The right hemispheres were used for cryostate sectioning and subsequent NeuN immunochemistry analysis according to brain region (frontal, prefrontal, parietal, occipital, and subcortical). NeuN staining was performed under standard immunofluorescence-labeling procedures. Briefly, frozen tissue sections were washed in PBS and blocked in 10% horse serum for 1 hour, then incubated overnight in primary antibody, monoclonal mouse anti-neuronal nuclei antibody (1:100). The following day, sections were washed in PBS 3 times (10 min each), and then incubated for 1 hour in the dark with secondary antibody, donkey anti-mouse IgG Alexa Fluor 594 at 1:100. After another cycle of washing, floating sections were mounted onto slides, dehydrated and coverslipped with Vectashield fluorescence mounting media (Vector Labs., Burlingame, CA). Slides were visualized under dark field using an Olympus BX-51 microscopy.
2.9 Statistical analysis
All data were normally distributed; therefore, in instances of single mean comparisons, Levene's test for equality of variances followed by t-test for independent samples was used to assess significance. In instances of multiple mean comparisons, analysis of variance (ANOVA) was used, followed by post-hoc comparison using Bonferonni's method. Alpha levels were set at 0.05 for all analyses. The statistical package for the social sciences release 10.0.5 (SPSS Inc., Chicago, Illinois) was used for all data analysis.
3. Results
3.1 IFN-γ enhances neuronal injury induced by gp120 and Tat in vitro and in vivo
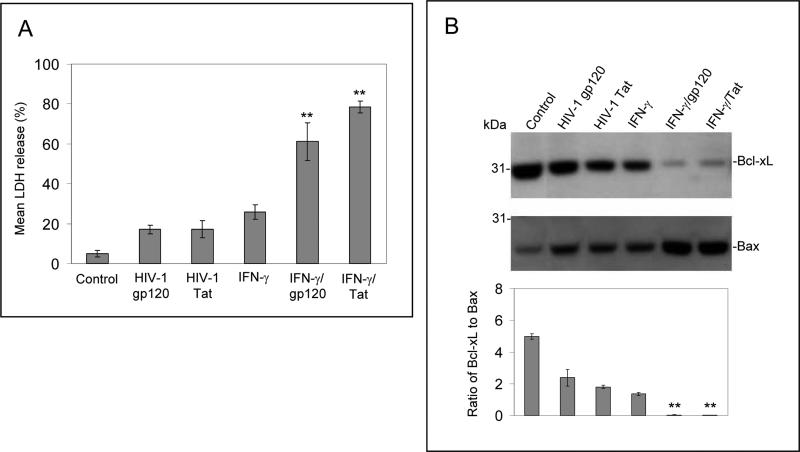
It has been reported that neuronal cells express IFN-γ receptor [2]. In support of these studies, we also found that IFN-γ receptor mRNA and protein are expressed by both neuron-like cells (N2a cells) and primary cultured cells (data not shown). In this study, we first wished to test whether IFN-γ could play a role in modulation of HIV-1 proteins gp120 and Tat -induced neuronal injury. According to a method previously described [28], we isolated primary cultured neuronal cells from newborn mice (1 to 2-day-old, wild-type C57BL/6). These cells were treated with gp120 or Tat (250 ng/ml) in the presence or absence of IFN-γ (100 U/mL) for 12 hours. Cell cultured media were collected for LDH assay and cell lysates were prepared for neuronal injury examination by Western blot analysis [28]. As shown in Figure 1A, B, the presence of IFN-γ significantly increases LDH release and reduces the band density ratio of Bcl-xL to Bax in primary neuronal cells challenged with HIV-1 proteins gp120 or Tat.
Figure 1.
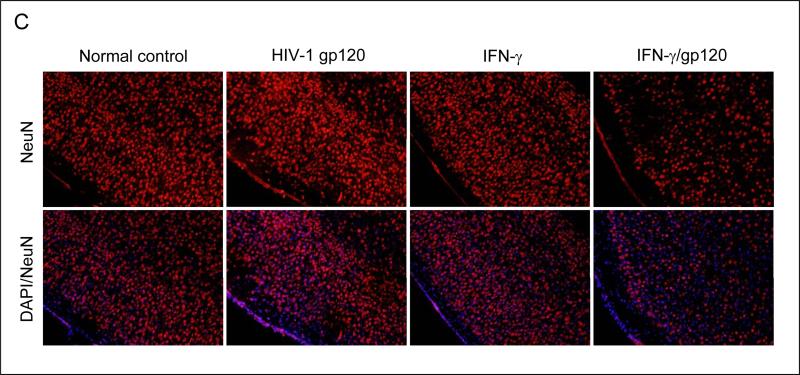
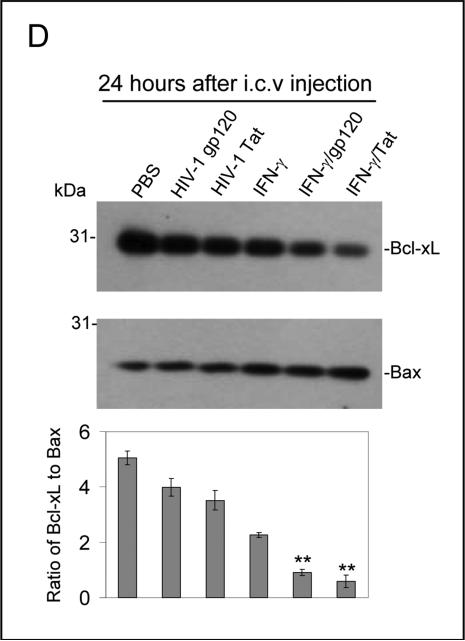
IFN-γ enhances neuronal injury induced by HIV-1 proteins gp120 or Tat in vitro and in vivo. Primary cultured neuronal cells were treated with gp120, Tat, IFN-γ alone or gp120, Tat (250 ng/mL) in combination with IFN-γ (100 U/mL; IFN-γ/gp120/ or IFN-γ/Tat) for 12 hours. Cell cultured media were collected for LDH assay (A) and cell lysates were prepared for neuronal injury examination by Western blot analysis (B). Data are presented as mean ± SD of LDH release and Western blot band density ratio of Bcl-xL to Bax (n = 3). One-way ANOVA followed by post hoc comparison revealed significant differences between gp120 or Tat and HIV-1 gp120 or Tat plus IFN-γ (**P < 0.001) for both LDH release and band density ratio of Bcl-xL to Bax. (C) Mouse brain coronal frozen sections were stained with NeuN and NeuN/DAPI and there are marked neuronal damage in gp120 plus IFN-γ condition compared to controls. There is a similar result we also observed in Tat plus IFN-γ condition (data not shown). (D) Bcl-xL and Bax protein levels in mouse brain homogenates were analyzed by Western blot. Data are presented as mean ± SD of Western blot band density ratio of Bcl-xL to Bax (n = 8; 4 male/4 female). One-way ANOVA followed by post hoc comparison revealed significant differences between gp120 or Tat and gp120 or Tat plus IFN-γ in band density ratio of Bcl-xL to Bax (**P < 0.001).
Furthermore, to test whether IFN-γ could enhance neuronal cell injury induced by gp120 and Tat in vivo, we treated C57BL/6 mice (n = 8; 4 male/4 female) with gp120 or Tat (500 ng/mouse) in the presence of IFN-γ (200 U/mouse) via an i.c.v. route. Twenty four hours after i.c.v. injection, these mice were sacrificed and brain tissues were collected. All dissected brain tissues were rapidly frozen for subsequent biochemical and immunochemistry analyses. Mouse brain sections from cortex regions were stained with NeuN and NeuN/DAPI. As shown in Figure 1C, the results indicate a marked increase in neuronal damage in cortex regions of the brains from mice i.c.v injected with gp120 plus IFN-γ condition compared to controls. There is a similar result also observed in the Tat plus IFN-γ condition (data not shown). In addition, brain homogenates from these mice were prepared for Western blot analysis of Bcl-xL and Bax protein expresses. Consistently, there is a significant reduction in the ratio of Bcl-xL to Bax (Figure 1D) in IFN-γ/gp120 or IFN-γ/Tat. For Figure 1D, one-way ANOVA followed by post hoc comparison revealed significant differences between gp120 or Tat and gp120 or Tat plus IFN-γ in Western blot band density ratio of Bcl-xL to Bax.
3.2 Critical involvement of JAK/STAT1 signaling in neuronal damage induced by gp120 or Tat in the presence of IFN-γ
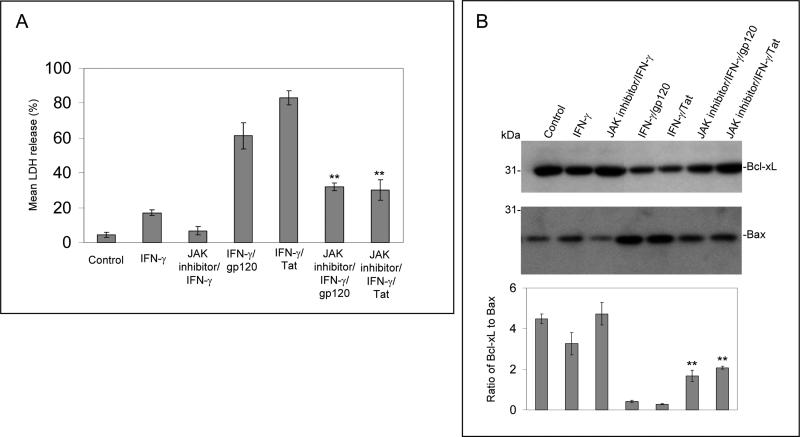
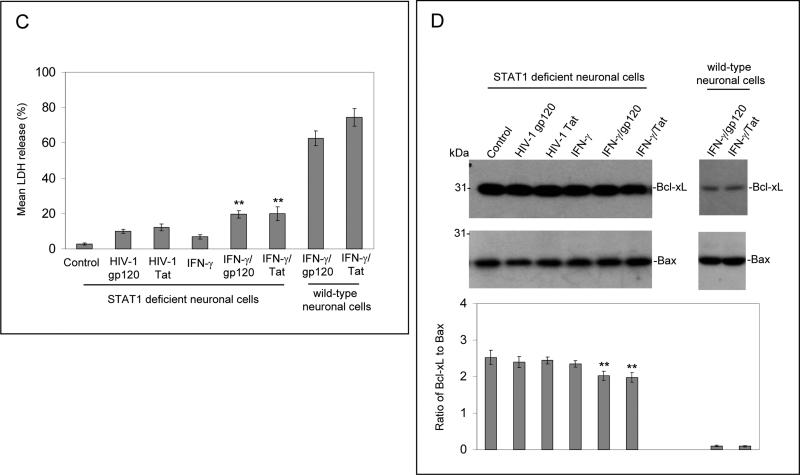
In order to further investigate IFN-γ enhanced neuronal injury induced by gp120 and Tat, involvement of the IFN-γ activated JAK/STAT1 signaling was analyzed. We treated primary cultured neuronal cells with PBS, gp120 (250 ng/mL), Tat (250 ng/mL), IFN-γ (100 U/mL), and/or JAK inhibitor (50 nM) for 12 hours. Interestingly, the neuronal injury was significantly inhibited by the presence of JAK inhibitor (Figure 2A, B). One-way ANOVA followed by post hoc comparison revealed significant differences between IFN-γ/gp120 or IFN-γ/Tat and JAK inhibitor/IFN-γ /gp120 or JAK inhibitor/IFN-γ /Tat for both LDH release and Western blot band density ratio of Bcl-xL to Bax. Furthermore, we isolated and cultured primary neuronal cells from STAT1 deficient mice. We treated these cells with gp120 or Tat (250 ng/mL) in the presence or absence of IFN-γ (100 U/mL) for 12 hours. Cell cultured media and cell lysates from these cells were subjected to LDH assay and Western blot analysis. The results show that neuronal cell injury was largely attenuated in these STAT1 deficient neurons treated with IFN-γ /gp120 or IFN-γ /Tat (Figure 2C, D). Oneway ANOVA followed by post hoc comparison revealed significant differences between STAT1 deficient neuronal cells and wild-type neuronal cells following treatment with IFN-γ /gp120 or IFN-γ /Tat for both LDH release and Western blot band density ratio of Bcl-xL to Bax.
Figure 2.
JAK/STAT1 signaling is critically involved in enhancement of IFN-γ effect on HIV-1 gp120 and Tat -induced neuronal damage. Primary cultured neuronal cells were co-treated with IFN-γ (100 U/mL) and either gp120 or Tat at 250 ng/mL in the presence of JAK inhibitor (50 nM) for 12 hours. Cell cultured media were collected for LDH assay (A) and cell lysates were prepared for neuronal injury examination by Western blot analysis (B). Data are presented as mean ± SD of LDH release and Western blot band density ratio of Bcl-xL to Bax (n = 3). One-way ANOVA followed by post hoc comparison revealed significant differences between IFN-γ/gp120 or IFN-γ/Tat and JAK inhibitor/IFN-γ/gp120 or JAK inhibitor/IFN-γ/Tat (**P < 0.001). Primary neuronal cells derived from STAT1 deficient mice were treated with gp120 or Tat at 250 ng/mL in the presence or absence of IFN-γ (100 U/mL) for 12 hours. Cell cultured media and cell lysates from these cells were subjected to LDH assay (C) and Western blot analysis (D). Data are presented as mean ± SD of LDH release and Western blot band density ratio of Bcl-xL to Bax (n = 5). One-way ANOVA followed by post hoc comparison revealed significant differences between STAT1 deficient neuronal cells and wild-type neuronal cells following treatment with IFN-γ/gp120 or IFN-γ/Tat for both LDH release and band density ratio of Bcl-xL to Bax (**P < 0.001).
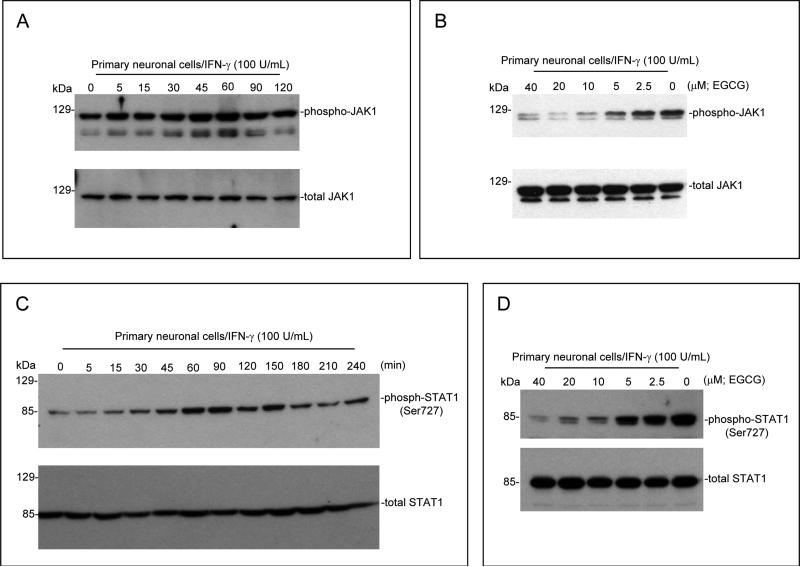
3.3 EGCG inhibits JAK/STAT1 signaling and neuronal damage induced by gp120 or Tat in the presence of IFN-γ in vitro
Primary cultured neuronal cells were treated with IFN-γ (100 U/mL) for different time points as indicated. The results revealed that IFN-γ stimulates phosphorylation of JAK1 (Figure 3A) and STAT1 (Figure 3C) in a time-dependent manner. In order to test whether EGCG could modulate this phosphorylation in neuronal cells, we co-incubated these cells with IFN-γ (100 U/mL) and EGCG at a range of doses as indicated for 60 min. JAK1/STAT1 phosphorylation was analyzed by Western blot. As shown in Fig. 3B, D, the presence of EGCG resulted in dose-dependent inhibition of JAK1/STAT1 phosphorylation.
Figure 3.
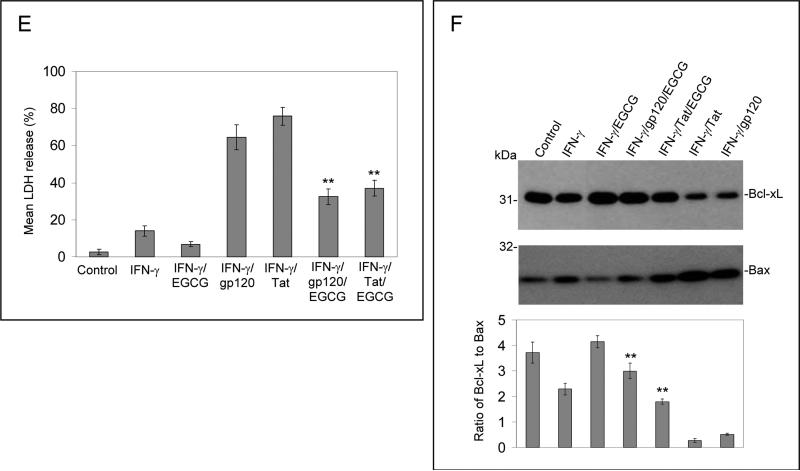
EGCG inhibits IFN-γ-induced JAK/STAT1 phosphorylation and protects neuronal cells against injury induced by IFN-γ/gp120 or IFN-γ/Tat in vitro. JAK1 and STAT1 protein phosphorylations were examined by Western blot. (A, C) Cell lyses were prepared from primary cultured neuronal cells treated with IFN-γ (100 U/mL) for different time points as indicated. (B, D) Cell lyses were prepared from primary cultured neuronal cells co-treated with IFN-γ (100 U/mL) and EGCG at different doses as indicated for 60 min. To examine the putative role of EGCG in opposing neuronal injury induced by IFN-γ/gp120 or IFN-γ/Tat, primary neuronal cells were co-treated with gp120 or Tat at 500 ng/mL in the presence of IFN-γ (100 U/mL) and EGCG (20 μM) for 12 hours. Cell cultured supernatants were collected for LDH assay (E) and cell lysates were prepared for Bcl-xL/Bax Western blot analysis (F). Data are presented as mean ± SD of LDH release and Western blot band density ratio of Bcl-xL to Bax (n = 3). Oneway ANOVA followed by post hoc comparison revealed significant differences between IFN-γ/gp120 or IFN-γ/Tat and EGCG/IFN-γ/gp120 or EGCG/IFN-γ/Tat for both LDH release and band density ratio of Bcl-xL to Bax (**P < 0.001).
It has been reported that EGCG modulates STAT1 activation in vitro [6, 18, 29] and in vivo [25, 30]. To examine the putative role of EGCG in opposing neuronal injury induced by HIV-1 protein gp120 or Tat in the presence of IFN-γ , we co-treated primary neuronal cells with gp120 or Tat (500 ng/mL) in the presence of IFN-γ (100 U/mL) and EGCG (20 μM) for 12 hours. Cell cultured supernatants were collected for LDH assay and cell lysates were prepared for Bcl-xL/Bax Western blot analysis. The results show that EGCG co-treatment markedly attenuates neuronal injury; as evidenced by decreased LDH release (Fig. 3E) and increased ratio of Bcl-xL to Bax (Fig. 3F). One-way ANOVA followed by post hoc comparison revealed significant differences between IFN-γ/gp120 or IFN-γ/Tat and EGCG/IFN-γ/gp120 or EGCG/IFN-γ/Tat for both LDH release and Western blot band density ratio of Bcl-xL to Bax.
3.4 EGCG inhibits neuronal damage mediated by gp120 or Tat in the presence of IFN-γ in vivo
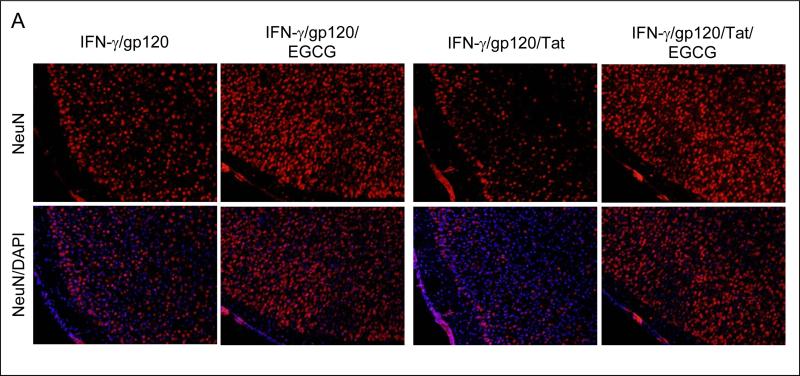
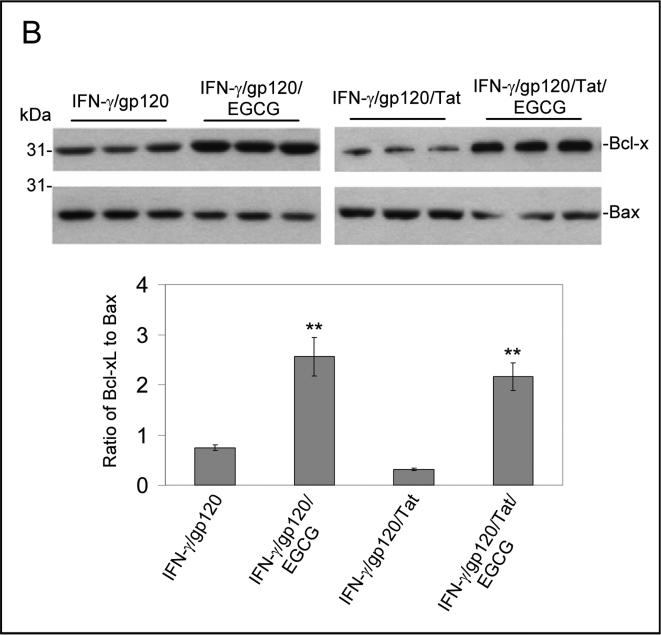
In order to evaluate the ability of EGCG to inhibit neuronal damage induced by HIV-1 proteins in combination with IFN-γ in vivo, we treated C57BL/6 mice (n = 8; 4 male/4 female) with HIV-1 proteins gp120 or Tat (500 ng/mouse) in the presence of IFN-γ (200 U/mouse) via an i.c.v. injection. The EGCG (50 mg/kg) or vehicle was i.p. administered immediately after the i.c.v. injection. Twenty four hours after EGCG treatment, these mice were sacrificed and brain tissues were collected. All dissected brain tissues were rapidly frozen for subsequent biochemical and immunochemistry analyses. Mouse brain sections from cortical regions were stained with NeuN and NeuN/DAPI. As shown in Figure 4A, the results show marked reductions of neuronal damage in cortical regions of the brains from mice i.c.v injected with IFN-γ/gp120 or IFN-γ/gp120/Tat in the presence of EGCG compared to controls. Similar reductions in neuronal injury are also observed in mice treated with IFN-γ/Tat in the presence of EGCG compared with mice treated with IFN-γ/Tat alone (data not shown). In addition, brain homogenates were prepared from these mice for Western blot analysis of Bcl-xL and Bax protein expressions. Consistently, there is a significant increase in the ratio of Bcl-xL to Bax for both IFN-γ/gp120/EGCG and IFN-γ/gp120/Tat/EGCG (Figure 4B) compared with IFN-γ/gp120 and IFN-γ/Tat, respectively. For Fig. 4B, one-way ANOVA followed by post hoc comparison revealed significant differences between IFN-γ/gp120/EGCG or IFN-γ/gp120/Tat/EGCG and IFN-γ/gp120and IFN-γ/gp120/Tat in Western blot band density ratio of Bcl-xL to Bax.
Figure 4.
Mice i.p. injected with EGCG show a significant reduction of neuronal injury after i.c.v. injection of IFN-γ/gp120, IFN-γ/Tat or IFN-γ/gp120/Tat. (A) Mouse brain coronal frozen sections were stained with NeuN (top panels) and NeuN/DAPI (low panels) and analyzed for neuron injury/loss. There is a marked reduction of neuronal damage induced by either IFN-γ/gp120 or IFN-γ/gp120/Tat with EGCG treatment compared to controls. Similar effects of EGCG were also observed in IFN-γ/Tat condition (data not shown). (B) Bcl-xL and Bax protein levels in mouse brain homogenates were analyzed by Western blot. Data are presented as mean ± SD of Western blot band density ratio of Bcl-xL to Bax (n = 8; 4 female/4 male). One-way ANOVA followed by post hoc comparison revealed significant differences in band density ratio of Bcl-xL to Bax observed between gp120/IFN-γ or gp120/Tat/IFN-γ compared with gp120/IFN-γ/EGCG or gp120/Tat/IFN-γ/EGCG, respectively (**P < 0.001).
4. Discussion
Neuronal damage and cognitive impairment found in HAD occurs in the later stages of infection whereas a CNS viral reservoir is initiated early after infection. Development of HAD usually occurs in the later stages of HIV infection, around the time of conversion to full-blown AIDS. The specific components leading to neurological dysfunction in HAD remains unclear. However, current studies aim to differentiate and characterize individual disease mechanisms involved in this complex process comprising chronic inflammatory activation of immune effector cells, and HIV protein-induced dysfunction of neurons resulting in cell death.
In HAD neurons are not killed by direct viral infection but rather viral proteins released from infected CNS mononuclear cells may directly kill neurons or may render them susceptible to cell death signals. Clearly viral proteins can bind to cell surface receptors such as CXCR4 and N-methyl-D-aspartate receptors. Thus HIV-1 proteins gp120 and Tat may trigger neuronal apoptosis and excitotoxicity resulting from altered cellular intracellular calcium concentrations and mitochondrial dysfunction [19]. Inflammation and proinflammatory soluble factors also play important roles in the pathogenesis and progression of HAD. Increasingly, studies point to the central roles played by reactive immune cells including macrophages and microglia in the generation and progression of many disease mechanisms also implicated in the pathology of HAD [1].
In order to effectively investigate components of HAD-like neuronal damage we developed a multifaceted approach involving HIV-1 proteins gp120 and Tat in combination with the proinflammatory cytokine, IFN-γ. Collaboration of proinflammatory cytokines with HIV-1 proteins in the induction of neuron injury appears to be an integral component of the generation of neuron injury in HAD [1, 7, 11, 13, 24, 31]. Here we have demonstrated in vitro that IFN-γ can enhance HAD-like neuronal damage mediated by gp120 and Tat (Figure 1A, B). Moreover, normal mice injected i.c.v. with gp120, Tat, or IFN-γ display neuron loss and pro-apoptotic signaling. Importantly, combinations of gp120 and Tat with IFN-γ resulted in dramatic neuron loss in the cortical regions examined (Figure 1C, D). Indeed we found synergistic proapoptotic effects when IFN-γ was combined with a challenge of HIV-1 gp120 and Tat proteins (Figure 1D). Previous investigations have demonstrated cause and effect relationships between production of HIV-1 proteins gp120 and Tat, and neuronal damage [17, 19, 21]. Consistent with these findings clinical reports detail correlations between HIV-1 proteins, IFN-γ, and neuron cell loss resulting in cognitive decline in HAD patients [14, 19, 23].
Previous studies investigating neurotoxic effects of IFN-γ implicated members of the JAK and STAT families [10, 12, 16]. The JAK1/STAT1 interaction is extensively described in studies investigating apoptosis induced by ischemia/reperfusion in cardiovascular, CNS, and other tissues [5, 15, 25, 27]. In neurons, STAT1 appears to be primed by ischemia/reperfusion and thus rendere=d more sensitive to IFN-γ receptor activation [25, 27]. Occlusion of the middle cerebral artery resulted in rapid co-localization of STAT1 with TUNEL-positive neurons, thereby suggesting a role for STAT1 in cell apoptosis/death [27]. Since HIV infection of the CNS induces marked increases in IFN-γ expression in CNS tissues [23] the involvement of the JAK/STAT pathway in neuronal damage associated with AIDS dementia is likely. Thus we investigated the involvement of JAK1 and STAT1 (Fig. 2A-D) in our observed IFN-γ-enhanced gp120/Tat-induced neuronal damage in primary culture neurons. Clearly activation of JAK1 and STAT1 is markedly evident after treatment with IFN-γ in primary cultured neurons from wild type mice (Figure 3A, D). JAK inhibitor I mitigated neuron damage, inflicted by combinations of IFN-γ and gp120 and Tat proteins, in vitro (Figure 2A B). Both LDH and Bcl-xL/Bax ratios were greatly reduced by addition of JAK inhibitor I, however these indicators of cell death and apoptotic cell changes, respectively, were not returned to baseline levels of the control treatment group when combination of gp120 and Tat were included in the treatment; indicating an alternate pathway/mechanism utilized by these proteins to induce cell damage. Consistent with these above mentioned data, primary neurons from STAT1 deficient mice were highly resistant to IFN-γ-enhanced neuron damage, however in combination with gp120 or Tat greater neuronal damage ensued; albeit dramatically less than the damage observed in wild-type neurons treated with gp120 or Tat in combination with IFN-γ (Figure 2C, D).
Previous studies investigating the properties of green tea-derived polyphenol EGCG indicated this compound was able to attenuate cell death induced by ischemia/reperfusion through downregulation of the JAK/STAT1 pathway [30]. Therefore we investigated whether EGCG could effectively down-regulate IFN-γ mediated JAK/STAT1 signaling; a process that enhanced gp120/Tat-induced neuron damage. Our in vitro studies utilizing primary culture neurons from wild type mice demonstrated marked reductions in both LDH release and in Bcl-xL/Bax ratios after combination treatments including gp120/Tat, IFN-γ, and EGCG (Figure 3E, F). These data suggest that EGCG's ability to reduce JAK/STAT1 signaling in primary culture neurons is protective against IFN-γ-enhanced gp120/Tat-induced HAD-like neuronal damage in vitro.
To evaluate the effects of EGCG on inhibition of neuronal damage induced by HIV-1 proteins gp120 and Tat in the presence of IFN-γ in vivo, we administered control mice i.p. injections of EGCG or PBS (vehicle control) and then delivered i.c.v. injections of HIV-1 proteins, gp120 or Tat, in the presence of IFN-γ. Consistent with our above mentioned results, EGCG was protective against neuron loss induced by i.c.v injected IFN-γ and/or gp120/Tat in cortical regions examined. This was evidenced by increased Bcl-xL/Bax ratios in brain homogenates of mice co-treated with IFN-g/gp120 or IFN-g/Tat (Figure 4B) and reductions in neuron loss in cortical sections by immunohistochemistry (Figure 4A).
Several reports investigating EGCG's ability to block JAK/STAT1 signaling have reported protective effects of the compound against: proinflammatory activation of immune cells, epithelial barrier dysfunction, and neuronal apoptosis after ischemia/reperfusion injury. Thus, JAK/STAT1 interaction may be an important therapeutic target for a variety of CNS disorders [29, 30]. Taken together, our data suggest the JAK/STAT1 pathway may be an important therapeutic target for opposing – the neuronal death seen in HAD brain. Indeed inhibition of the JAK/STAT pathway by green tea derived EGCG or derived compounds may provide an effective therapeutic intervention as an adjunct to HAART for the treatment of AIDS dementia complex and related disorders.
5. Acknowledgements
This work is supported by grants from the NIH/NINDS (JT) and the Johnnie B. Byrd Senior Alzheimer's Center & Research Institute (JT and RDS).
Abbreviations
- EGCG
(-)-epigallocatechin-3-gallate
- HIV-1
human immunodeficiency virus type
- gp120
HIV envelope glycoprotein 120
- Tat
HIV transactivator protein
- JAK
Janus associated kinase
- STAT1
signal transducer and activator of transcription 1
- HAART
highly active antiretroviral therapy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aquaro S, Ronga L, Pollicita M, Antinori A, Ranazzi A, Perno CF. Human immunodeficiency virus infection and acquired immunodeficiency syndrome dementia complex: role of cells of monocyte-macrophage lineage. J Neurovirol. Suppl. 2005;113:58–66. doi: 10.1080/13550280500513416. [DOI] [PubMed] [Google Scholar]
- 2.Bate C, Kempster S, Last V, Williams A. Interferon-gamma increases neuronal death in response to amyloid-beta1-42. J Neuroinflammation. 2006;28:1–7. doi: 10.1186/1742-2094-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benveniste EN. Cytokine circuits in brain. In: Price RW, Perry SW, editors. HIV, AIDS and the brain. Raven Press, Ltd.; New York City: 1994. pp. 71–88. [Google Scholar]
- 4.Bovolenta C, Comrali L, Lorini AL, Ghezzi S, Vicenzi E, Lazzarin A, Poli G. Constitutive Activation of STATs Upon In Vivo Human Immunodeficiency Virus Infection. Blood. 1999;94:4202–4209. [PubMed] [Google Scholar]
- 5.Chin YE, Kitagawa M, Kuida K, Flavell RA, Fu XY. Activation of STAT signaling pathway can cause expression of Caspase 1 and apoptosis. Mol. Cell. Biol. 1997;17:5328–5337. doi: 10.1128/mcb.17.9.5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Prati AC, Ciampa AR, Cavalieri E, Zaffini R, Darra E, Menegazzi M, Suzuki H, Mariotto S. STAT1 as a new molecular target of anti-inflammatory treatment. Curr Med Chem. 2005;1216:1819–28. doi: 10.2174/0929867054546645. [DOI] [PubMed] [Google Scholar]
- 7.Fujimura RK, Bockstahler LE, Goodkin K, Werner T, Brack-Werner R, Shapshak P. Neuropathology and Virology of HIV Associated Dementia. Rev Med Virol. 1996;63:141–150. doi: 10.1002/(SICI)1099-1654(199609)6:3<141::AID-RMV141>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 8.Garden G, Budd S, Tsai E, Hanson L, Kaul M, D'Emilia DM, Friedlander RM, Yuan J, Masliah E, Lipton SA. Caspase Cascades in Human Immunodeficiency Virus-Associated Neurodegeneration. J Neuroscience. 2002;22:4015–4024. doi: 10.1523/JNEUROSCI.22-10-04015.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodkin K, Wilkie FL, Concha M, Hinkin CH, Symes S, Baldewicz TT, Asthana D, Fujimara RK, Lee D, van Zuilen MH, Khamis I, Shapshak P, Eisdorfer C. Aging and neuro-AIDS conditions and the changing spectrum of HIV-1-associated morbidity and mortality. Clin Epidemiol. 2001;54:S35–43. doi: 10.1016/s0895-4356(01)00445-0. Review. [DOI] [PubMed] [Google Scholar]
- 10.Heitmeier MR, Scarim AL, Corbett JA. Prolonged STAT1 activation is associated with interferon-gamma priming for interleukin-1-induced inducible nitric-oxide synthase expression by islets of Langerhans. J Biol Chem. 1999;274:29266–73. doi: 10.1074/jbc.274.41.29266. [DOI] [PubMed] [Google Scholar]
- 11.Kaul M, Garden G, Lipton S. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- 12.Kim TK, Maniatis T. Regulation of interferon-gamma-activated STAT1 by the ubiquitin- proteasome pathway. Science. 1996;273:1717–1719. doi: 10.1126/science.273.5282.1717. [DOI] [PubMed] [Google Scholar]
- 13.Koenig S, Gendelman HE, Orenstein JM. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986;233:1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- 14.Kumar M, Kumar AM, Waldrop D, Antoni MH, Eisdorfer C. HIV-1 infection and its impact on the HPA axis, cytokines, and cognition. Stress. 2003;6:167–72. doi: 10.1080/10253890310001605376. [DOI] [PubMed] [Google Scholar]
- 15.Kumar A, Commane M, Flickinger TW, Horvath CM, Stark GR. Selective TNF-alpha-induced apoptosis in STAT1-null cells due to low constitutive levels of caspases. Science. 1997;278:1578–9. doi: 10.1126/science.278.5343.1630. [DOI] [PubMed] [Google Scholar]
- 16.Lee KY, Anderson E, Madani K, Rosen GD. Loss of STAT1 expression confers resistance to IFN-gamma-induced apoptosis in ME180 cells. FEBS Lett. 1999;459:323–6. doi: 10.1016/s0014-5793(99)01283-1. [DOI] [PubMed] [Google Scholar]
- 17.Li W, Galey D, Mattson MP, Nath A. Molecular and cellular mechanisms of neuronal cell death in HIV dementia. Neurotox Res. 2005;8:119–34. doi: 10.1007/BF03033824. Review. [DOI] [PubMed] [Google Scholar]
- 18.Magro F, Fraga S, Soares de Silva P. Interferon-gamma-induced STAT1-mediated membrane retention of NHE1 and associated proteins ezrin, radixin and moesin in HT-29 cells. Biochem Pharmacol. 2005;70:1312–9. doi: 10.1016/j.bcp.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 19.Mattson MP, Haughey NJ, Nath A. Cell death in HIV dementia. Cell Death Differ. 2005;(Suppl 1):893–904. doi: 10.1038/sj.cdd.4401577. [DOI] [PubMed] [Google Scholar]
- 20.Melton ST, Kirkwood CK, Ghaemi SN. Pharmacotherapy of HIV dementia. Ann Pharmacother. 1997;31:457–473. doi: 10.1177/106002809703100413. [DOI] [PubMed] [Google Scholar]
- 21.Nath A, Conant K, Chen P, Scott C, Major EO. Transient exposure to HIV-1 Tat protein results in cytokine production in macrophages and astrocytes. A hit and run phenomenon. J Biol Chem. 1999:17098–102. doi: 10.1074/jbc.274.24.17098. [DOI] [PubMed] [Google Scholar]
- 22.Ozdener H. Molecular mechanisms of HIV-1 associated neurodegeneration. J Biosci. 2005;30:391–405. doi: 10.1007/BF02703676. [DOI] [PubMed] [Google Scholar]
- 22.Peruzzi F, Bergonzini V, Aprea S, Reiss K, Sawaya BE, Rappaport J, Amini S, Khalili K. Cross talk between growth factors and viral and cellular factors alters neuronal signaling pathways: implication for HIV-associated dementia. Brain Res Brain Res Rev. 2005;50:114–25. doi: 10.1016/j.brainresrev.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 23.Shapshak P, Duncan R, Minagar A, Rodriguez de la Vega P, Stewart RV, Goodwin K. Elevated expression of IFN-gamma in the HIV-1 infected brain. Frontiers in Bioscience. 2004;9:1073–1081. doi: 10.2741/1271. [DOI] [PubMed] [Google Scholar]
- 24.Speth C, Dierich MP, Sopper S. HIV-infection of the central nervous system: The tightrope walk of innate immunity. Mol Immunol. 2005;42:213–28. doi: 10.1016/j.molimm.2004.06.018. Review. [DOI] [PubMed] [Google Scholar]
- 25.Stephanou A. Role of STAT-1 and STAT-3 in ischaemia/reperfusion injury. J Cell Mol Med. 2004;8:519–25. doi: 10.1111/j.1582-4934.2004.tb00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stephanou A, Brar BK, Scarabelli TM, Jonassen AK, Yellon DM, Marber MS, Knight RA, Latchman DS. Ischemia-induced STAT-1 expression and activation play a critical role in cardiomyocyte apoptosis. J Biol Chem. 2000;275:10002–8. doi: 10.1074/jbc.275.14.10002. [DOI] [PubMed] [Google Scholar]
- 27.Takagi Y, Harada J, A Chiarugi MA. Moskowitz, STAT1 is activated in neurons after ischemia and contributes to ischemic brain injury. J Cereb Blood Flow Metab. 2002;22:1311–8. doi: 10.1097/01.WCB.0000034148.72481.F4. [DOI] [PubMed] [Google Scholar]
- 28.Tan J, Town T, Crawford F, Mori T, DelleDonne A, Cresenctini R, Obregon D, Flavell RA, Mullan MJ. Role of CD40 ligand in amyloidosis in transgenic Alzheimer's mice. Nature Neuroscience. 2002;5:1288–1293. doi: 10.1038/nn968. [DOI] [PubMed] [Google Scholar]
- 29.Tedeschi E, Suzuki H, Menegazzi M. Antiinflammatory action of EGCG, the main component of green tea, through STAT-1 inhibition. Ann N Y Acad Sci. 2002;973:435–7. doi: 10.1111/j.1749-6632.2002.tb04678.x. [DOI] [PubMed] [Google Scholar]
- 30.Townsend PA, Scarabelli TM, Pasini E E, Gitti G, Menegazzi M, Suzuki H, Knight RA, Latchman DS, Stephanou A. Epigallocatechin-3-gallate inhibits STAT-1 activation and protects cardiac myocytes from ischemia/reperfusion-induced apoptosis. FASEB J. 2004;18:1621–3. doi: 10.1096/fj.04-1716fje. [DOI] [PubMed] [Google Scholar]
- 31.Xiong H, Zeng YC, Lewis T, Zheng J, Persidsky Y, Gendelman HE. HIV-1 infected mononuclear phagocyte secretory products affect neuronal physiology leading to cellular demise: relevance for HIV-1-associated dementia. J Neurovirol. 2000;6:S14–23. Review. [PubMed] [Google Scholar]
- 32.Yoshioka M, Shapshak P, Sun NC, Nelson SJ, Svenningsson A, Tate LG, Pardo V, Resnick L. Simultaneous detection of ferritin and HIV-1 in reactive microglia. Acta Neuropathol. 1992;84:297–306. doi: 10.1007/BF00227823. [DOI] [PubMed] [Google Scholar]
- 33.Wiley CA, Schrier RD, Nelson JA, Lampert PW, Oldstone MB. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndromepatients. Proc Natl Acad Sci. 1986;83:7089–93. doi: 10.1073/pnas.83.18.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]