Abstract
Stem cells show the capability to proliferate in an undifferentiated state with long-term self-renewal, which gives the cells advantages for use as bioactive material (BM) for embryo culture in vitro. The objective of this experiment was to investigate the effect of two BMs—human adipose tissue–derived mesenchymal stem cell BM (hAT-MSC-BM) and human embryonic stem cell–derived BM (hESC-BM)—on porcine embryo development compared to commonly used bovine serum albumin (BSA) or serum treatment groups. In vitro–fertilized (IVF) embryos were cultured in PZM-5 with 4 mg/mL BSA until day 4 and equally divided into four groups. Starting from day 4 (until day 6), each group was treated with the following protein additives: 4 mg/mL BSA (control), 10% fetal bovine serum (FBS), 10% hAT-MSC-BM, or 10% hESC-BM. Our results show FBS- and two other BM-treated groups showed significant increases in blastocyst formation rate, hatching rate, and total cell number compared with the control group (p<0.05). The hAT-MSC-BM and hESC-BM treatment groups presented better-quality embryo development, especially from the middle expanding stage to hatching. In particular, the hAT-MSC-BM–treated group showed the highest developmental potential of all groups and formed the most expanding-stage blastocysts. The relative expression of reprogramming-related transcription factor (POU5F1, SOX2, DPPA5, and CDH1), antioxidant (PRDX5), and apoptosis (BCL2L1 and BIRC5) genes also increased in two types of BMs compared to the control. In addition, we investigated the protein synthesis of the tight junction– and gap junction–related genes, connexin 43 and zonula occludens-1 (ZO-1); these increased more than in the control. These results demonstrate that stem cell–derived BMs accelerate porcine preimplantation embryo development and that the BMs would be helpful in the development of preimplantation embryos.
Introduction
It is important to find a culture condition that is similar to in vivo conditions to produce excellent-quality blastocysts. In the preimplatation embryo, it has been demonstrated that good gap junctional communication is essential for the maintenance of compaction and subsequent development (Becker and Davies, 1995; Bevilacqua and Abrahamsohn, 1989; Buehr et al., 1987; Lee et al., 1987). There are many studies that improve in vitro culture (IVC) conditions by adding extra protein supplements or carbohydrates (Yoshino et al., 2002; zur Nieden et al., 2005). Antioxidant reagents are added to reduce antioxidant effects on embryos (Takahashi, 2012). Also, co-culturing with a feeder layer produces other growth factors and cytokines (Lapree-Delage et al., 1999). However, the most popular method is to add additional protein supplements to basic culturing media to supply extra nutrients to the embryos. The most frequently used serums are bovine serum albumin (BSA), fetal bovine serum (FBS), and serum replacement (SR). Despite active research, an in vivo–like culturing system has not yet been established.
Stem cells are characterized by their ability to differentiate into many lineage-specific cell types and have been considered for many years to be the major source of cells for tissue engineering applications. Stem cells also secrete various growth factors that repair and replace defective surrounding cells. Some of these growth factors benefit skin regeneration, re-epithelialization, wound healing, and wrinkle prevention. Previously in our studies, we examined the effect of bioactive material (BM) supplements— human embryonic stem cell–derived BM (hESC-BM) and human adipose tissue–derived mesenchymal stem cell BM (hAT-MSC-BM)—for embryonic development in vitro (Kim et al., 2011; Park et al., 2013). In one BM-related study, adding hESC-BM to bovine IVC systems caused an increase in total cell number in bovine blastocysts (Kim et al., 2011). It particularly increased the inner cell mass (ICM) compared to the basic bovine culturing system. Other researchers have reported that they collected stem cell culture medium to use as a protein supplement for embryo culture (Ma et al., 2012; Meng et al., 2001; Shahdadfar et al., 2012).
Stem cells have the capability to proliferate undifferentially and with long-term self-renewal (Marinaro et al., 2011), which gives the cells advantages for use as BM for embryo culture in vitro. In another BM-related study, we investigated the effect of human hAT-MSCs on parthenogenetic porcine embryo development. hAT-MSCs are more abundant and easier to harvest than other types of stem cells. Also, AT-MSCs secrete several growth factors, such as interleukin-10 (IL-10) and fibroblast growth factor 2 (FGF2) (Park et al., 2013). Secreted factors from AT-MSCs are used in cosmetic skin care products and in the protein drug industries (Brohem et al., 2013). Thus, BM derived from hAT-MSCs may provide a more suitable IVC system for the growth of preimplantation embryonic cells.
The objective of this study was to investigate the effects of 10% hAT-MSC-BM or 10% hESC-BM treatment on the developmental capacity of porcine embryos as well as to understand apoptosis-, development-, pluripotency-, antioxidant-, and adhesion structure-related genes better.
Materials and Methods
Chemicals and reagents
All chemicals and reagents were purchased from Sigma Chemical (St. Louis, MO, USA) unless otherwise stated.
Preparation of hAT-MSC-BM
hAT was donated from 23 healthy women with informed consent, as approved by the Institutional Review Board of Medical Liposuction. To generate human hAT-MSCs, hAT was dissociated into small clumps by mechanical dissection and then washed twice with hMSC culture medium [80% Dulbecco's Modified Eagle Medium (DMEM) Nutrient Mixture F-12 (1:1, Gibco), 10% FBS (Gibco), 0.1 mM β-mercaptoethanol, 1% nonessential amino acids, and 4 ng/mL human basic fibroblast growth factor (hbFGF, KOMA Biotech, Inc.)]. Small hAT clumps (100–500 cells/clump) were seeded onto Matrigel- (1:20, Becton, Dickinson and Company, Bedford, MA, USA) coated plates at ∼45–50 clumps per square and cultured in hMSC culture medium. On reaching confluence (7–10 days), the hAT-MSCs were detached from the dishes with trypsin treatment for 7 min at 37°C. Viable cells were counted (Trypan Blue solution, GIBCO) under a light microscope, and 1×106 cells were seeded onto Matrigel-coated plates (passage 1 hAT-MSCs). Culture passage 3 to passage 5 hAT-MSCs were used for generation of hAT-MSC-BM. To generate hAT-MSC-BM, hAT-MSCs were seeded at 50,000 cells/cm2 in hAT-MSC culture medium. One day later, the medium was replaced with fresh hMSC culture medium. Culture supernatant was harvested from expanded, confluent (>90%) hAT-MSCs and filtered using a 0.22-μm filter.
Preparation of hESC-BM
JNU-hES-01 cells (15–20 passages), a hESC line established at Jeju National University using a method modified from Kim et al. (2011), were maintained in an undifferentiated state by culturing the cells on a 10 μg/mL mitomycin C–treated STO cell feeder layer [American Type Culture Collection (ATCC), USA) in hESC culture medium [80% DMEM, Nutrient Mixture F-12 (1:1, Gibco), 20% KnockOut Serum Replacement (Gibco), 0.1 mM β-mercaptoethanol, 1% nonessential amino acids, and 4 ng/mL hbFGF (KOMA Biotech, Inc.)].
To generate hESC-BM, large, expanded hESC colonies (6–7 days after subculture) were recovered by treatment with 0.5 mg/mL collagenase IV for 5 min, dissociated into small clumps by mechanical dissection, and then washed twice with hESC medium. Small hESC clumps (100–500 cells/clump) were seeded (∼45–50 clumps per square) onto Matrigel- (1:20, Becton, Dickinson and Company, Bedford, MA, USA) coated plates and cultured in conditioned medium (CM) prepared from STO cells containing 8 ng/mL hbFGF. Culture supernatant, for use in the hESC-BM, was harvested from expanded, confluent (>90%) hESCs grown in a feeder-free environment. STO-CM was prepared as follows: 10 μg/mL mitomycin C–treated STO cells were seeded at 50,000 cells/cm2 in medium containing 90% DMEM and 10% FBS (Hyclone, Logan, UT, USA). One day later, the medium was replaced with hESC culture medium, and STO-CM was collected daily for 5 days and filtered using a 0.22-μm filter.
Oocyte collection and in vitro maturation
Porcine ovaries were collected from gilts at a local slaughterhouse and transported to the laboratory in saline supplemented with 75 mg/mL penicillin G and 50 mg/mL streptomycin sulfate at 32–35°C within 2 h. Cumulus–oocyte complexes (COCs) were aspirated from follicles 3–7 mm in diameter with an 18-gauge needle attached to a 10-mL disposable syringe. COCs with a minimum of two layers of cumulus cells were selected, washed three times with HEPES buffer, and washed three times with tissue culture medium-199 (TCM-199) supplemented with 0.1% BSA. Fifty COCs were matured in a four-well dish with 0.5 mL of the TCM-199 supplemented with 10% porcine follicular fluid, 10 ng/mL epidermal growth factor (EGF), 0.5 μg/mL lutenizing hormone (LH), and 0.5 μg/mL follicle-stimulating hormone (FSH) for 44 h in 5% CO2 at 38.8°C.
Production of IVF embryos and IVC
At 42 h of maturation culture, oocytes were semi-denuded of cumulus cells using 0.1% hyaluronidase by mechanical pipetting and transferred back into the maturation culture four-well dish for another 2 h for a recovery period. At 44 h, the semi-denuded oocytes were washed three times with porcine in vitro fertilization (P-IVF) medium, which consisted of 4 mg/mL BSA, 2.5 mM of caffeine, and modified Tris-buffered medium (1 mM NaCl, 3 mM KCl, 7.5 mM CaCl2 2H2O, 20 mM Tris, 11 mM glucose, 5 mM sodium pyruvate, and no antibiotics). Every 25 semi-denuded matured oocytes were randomly placed in 50 μL of P-IVF medium fertilization drops. Fresh semen was washed two times with 6 mL of Dulbecco's phosphate-buffered saline (D-PBS) supplemented with 0.1% BSA. The sperm pellet was resuspended in modified Tris-buffered medium (mTBM) to give a concentration of 5×105 cells/mL. A final sperm concentration of 2.5×104 cells per 50 μL of sperm suspension was added to each fertilization drop for insemination (a total of 100 μL per drop) and co-incubated with gametes for 5 h.
For IVC, after 5 h of incubation, the embryos were washed three times with porcine in vitro culture (P-IVC) medium (4 mg/mL fatty acid–free BSA with PZM-5 medium) and transferred into 50-μL P-IVC medium drops until day 2. The embryos were cultured in 5% CO2 at 38.8°C. On day 2, all embryos were examined visually and sorted into two groups. Group 1 consisted of two- to four-cell cleaved embryos and group 2 consisted of six- to eight-cell cleaved embryos. The total number of embryos in each group was equally divided into four subgroups and then cultured in 20 μL of P-IVC medium until day 4. On day 4, each group was treated for several days with a protein additive as follows; Control (4 mg/mL BSA), 10% FBS, 10% hAT-MSC-BM, or 10% hESC-BM.
Assessment of embryo quality
The total cell number in the blastocysts at day 7 was determined after nuclear staining with 20 mg/mL Hoechst 33342 for 15 min. Stained blastocysts were immediately transferred into glycerol to remove excess stain, mounted onto a glass microscope slide with a drop of glycerol, and examined under ultraviolet light using an epifluorescence microscope (Olympus).
Extraction of mRNA
For real-time PCR analysis, mRNA was prepared from the blastocysts using magnetic beads (Dynabeads mRNA Purification Kit, Dynal, Oslo, Norway) according to the manufacturer's instructions. Briefly, in each treatment group, IVF day 6 blastocysts were resuspended in 100 μL of lysis/binding buffer [100 mM Tris-HCl (pH 7.5), 500 mM LiCl, 10 mM EDTA (pH 8.0), 1% lithium dodecyl sulfate (LiDS), and 5 mM dithiothreitol (DTT)] and vortexed at room temperature for 5 min to completely lyse the embryos. A 50-μL aliquot of an oligo(dT)25 magnetic bead suspension was added, and the samples were incubated at room temperature for 5 min. The hybridized mRNA and oligo(dT) beads were washed twice using wash buffer A [10 mM Tris-HCl (pH 7.5), 0.15 M LiCl, 1 mM EDTA, and 1% LiDS] and then washed once with wash buffer B [10 mM Tris-HCl [pH 7.5], 0.15 M LiCl, and 1 mM EDTA]. Samples of mRNA were eluted from beads in 15 μL of double-distilled diethylpyrocarbonate (DEPC)-treated water.
Real-time RT-PCR with SYBR Green
Extraction of mRNA was performed as described above, and standard cDNA was synthesized using an oligo(dT) primer and SuperScript Reverse Transcriptase (Invitrogen, Carlsbad, CA, USA). Real-time RT-PCR was performed using the primer sets shown in Table 1 in a Bio-Rad Chromo4 Real-Time RT-PCR machine. In all experiments, histone H2a mRNA served as an internal standard. The threshold cycle (Ct) value represents the cycle number at which the sample fluorescence rose statistically above the background noise. To monitor the reactions, we followed the protocol described in the DyNAmo SYBR Green qPCR Kit, which contains a modified Tbr DNA polymerase, SYBR Green, optimized PCR buffer, 5 mM MgCl2, and a deoxyribonucleotide triphosphates (dNTP) mix that includes 2′-deoxyuridine, 5′-triphosphate (dUTP; Finnzyme Oy, Espoo, Finland).
Table 1.
Details of the Primer Used for Real-Time RT PCR
| Genes | Genbank accession no. | Primer sequence | Annealing temp(°C) | Product size (bp) |
|---|---|---|---|---|
| BCL2L1 | NM_214285 | F, GAAACCCCTAGTGCCATCAA | 60 | 196 |
| R, GGGACGTCAGGTCACTGAAT | ||||
| BIRC5 | NM_214141 | F, CCTGGCAGCTCTACCTCAAG | 60 | 233 |
| R, GAAAGCACAACCGGATGAAT | ||||
| CASP3 | NM_214131 | F, GAGGCAGACTTCTTGTATGC | 55 | 236 |
| R, CATGGACACAATACATGGAA | ||||
| CDH1 | EU805482 | F, CTGTATGTGGCAGTGACTAAC | 55 | 174 |
| R, AGTGTAGGATGTGATCTCCAG | ||||
| DPPA5 | FJ436413.1 | F, ATGACATCCTGTCTTGGGTAG | 55 | 200 |
| R, GTAAGGACCGTAAACCATGAC | ||||
| GAPDH | AF017079 | F, GGGCATGAACCATGAGAAGT | 60 | 230 |
| R, AAGCAGGGATGATGTTCTGG | ||||
| GJA1 | AY382593 | F, GAACAAGAAAGAGGAGGAACTC | 55 | 175 |
| R, GACAGACTTGAAGAGGATACTG | ||||
| GJD1 | NM_001097519 | F, CCCTCATAAGATAGACTGCTTC | 55 | 170 |
| R, CTTCCAGTTCCCTCCTTTTAC | ||||
| NANOG | DQ447201 | F, TTCCTTCCTCCATGGATCTG | 60 | 214 |
| R, ATCTGCTGGAGGCTGAGGTA | ||||
| POU5F1 | NM_001113060 | F, AGTGAGAGGCAACCTGGAGA | 60 | 166 |
| R, TCGTTGCGAATAGTCACTGC | ||||
| PRDX5 | AF110735.2 | F, GGCATGTCTGAGTGTTAATGAC | 55 | 152 |
| R, CAAAGAGAGACACCAAGGAATC | ||||
| SOD1 | GU944822.1 | F, GCCACTGTGTACATCGAAGAT | 55 | 173 |
| R, GTGATCCCAATTACACCACAG | ||||
| SOX2 | EU503117 | F, GCCCTGCAGTACAACTCCAT | 60 | 216 |
| R, GCTGATCATGTCCCGTAGGT | ||||
| ZO-1 | XM_003480423 | F, GATACCAGTAAGTCGTCCTGA | 55 | 167 |
| R, GAGACAGACTCTTATCCCTACTG |
F, forward; R, reverse.
For the PCR protocol, the cycling conditions were 94°C for 3 min, followed by amplification and quantification cycles that were repeated 40 times at 94°C for 30 sec, 54°C or 60°C for 30 sec, and 72°C for 30 sec. The reactions were subject to a single fluorescence measurement, a melting curve program of 65–95°C with a heating rate of 0.2°C /sec, and continuous fluorescence measurement. Samples were then cooled to 12°C. SYBR Green fluorescence was measured after the extension step. PCR products were then analyzed by generating a melting curve. Because the melting curve of a product is sequence specific, it can be used to distinguish nonspecific from specific PCR products. To do this, it is necessary to determine the crossing points (CP) for each transcript, which were defined as the points at which fluorescence rises appreciably above the background noise. Gene expression was quantified by the 2−ΔΔCt method (Livak and Schmittgen, 2001).
Western blot analysis
The protocol was basically the same as the one described previously (Lee et al., 2012). In brief, blastocysts (50 blastocysts per sample) were solubilized in 20 mL of 1×sodium dodecyl sulfate (SDS) sample buffer [62.5 mM Tris–HCl (pH 6.8) at 25°C, 2% (wt/vol) SDS, 10% (vol/vol) glycerol, 50 mM DTT, and 0.01% (wt/vol) Bromophenol Blue or Phenol Red] and heated for 5 min at 95°C. For western blotting, proteins were resolved on a 5–12% Tris-SDS-polyacrylamide gel electrophoresis (PAGE) gel for 1.5 h at 80–100 V. Samples were then transferred onto a nitrocellulose membrane (Amersham, Hybond-ECL, Buckinghamshire, UK) at 300 mA for 2 h in transfer buffer [25 mM Tris, 200 mM glycine, 20% methanol (pH 8.5)]. After blocking with 5% (wt/vol) nonfat milk in phosphate-buffered saline (PBS) for 1 h, the membrane was incubated for at least 2 h with an anti-zonula occludens-1 (ZO-1) tight junction protein antibody (Abcam, Cambridge, UK) or connexin 43 antibody (Cell Signaling Technology, Danvers, MA, USA) diluted 1:500 in blocking solution [1×TBS (Tris-buffered saline), 0.1% (vol/vol) Tween®-20, 5% (wt/vol) nonfat milk], washed three times in TBST [20 mM Tris–HCl (pH 7.5), 250 mM NaCl, 0.1% (vol/vol) Tween®-20], and incubated for 1 h with anti-rabbit immunoglobulin G–horseradish peroxidase (IgG-HRP; Cell Signaling Technology) diluted 1:2000 in blocking solution. After three washes with TBST, antibody binding was detected with chemiluminescence luminol reagent (Invitrogen).
Statistical analysis
The general linear model (GLM) procedure within the SAS software package was used to analyze data from all experiments. Significant differences were determined by Tukey multiple range tests. A paired Student t-test was used to compare relative gene expression. p values of <0.05 were considered significant.
Results
BMs enhance the porcine IVF embryo development
As indicated in Table 2, 1462 embryos were used in this experiment. On day 2, 64.3% of embryos were cleaved, and they were equally divided into four groups as follows: Control (4 mg/mL BSA), 10% FBS, 10% hAT-MSC-BM, or 10% hESC-BM. On day 4, the protein supplements were added to each group and cultured until day 6. On day 6, the 10% hAT-MSC-BM group's blastocyst development rate was significantly higher (38.7±4.2%, p<0.05) than the control and 10% FBS group (18.7±2.2% and 27.7±3.2%, respectively). There was no difference compared to 10% hESC-BM group (33.6±4.3%). The 10% hESC-BM group blastocyst development rate was significantly higher than the control, but there was no significant difference with 10% FBS and 10% hAT-MSC-BM (Table 2).
Table 2.
Effect of Different Protein Supplements on Developmental Capacity of Porcine IVF Embryos (r=4)
| Treatment | No. of IVF oocytes | No. (%) of cleaved embryos on day 2 | No. of assigned embryos on day 4 | No. (%)* of blastocysts on day 6 | No. of total cells on day 7 (mean±SEM)* |
|---|---|---|---|---|---|
| Control (4 mg/mL BSA) | 1462 | 940 (64.3) | 235 | 44 (18.7±2.2)a | 42.76±3.86a |
| 10% FBS | 235 | 65 (27.7±3.2)b | 59.79±4.84b | ||
| 10% hAT-MSC-BM | 235 | 91 (38.7±4.2)c | 77.95±6.03c | ||
| 10% hESC-BM | 235 | 79 (33.6±4.3)bc | 69.90±5.56bc |
p<0.05 for a–c superscripts.
IVF, in vitro fertilized; SEM, standard error of the mean; BSA, bovine serum albumin; FBS, fetal bovine serum; hAT-MSC-BM, human adipose tissue–derived mesenchymal stem cell bioactive material; hESC-BM, human embryonic stem cell bioactive material.
To examine the effect of hESC-BM on the total number of cells, day 7 blastocysts in the control and treatment groups were stained differentially (Table 2). Day 7 embryos cultured in the presence of 10% hAT-MSC-BM had the highest total cell number (control, 42.76±3.86; 10% FBS, 59.79±4.84; 10% hAT-MSC-BM, 77.95±6.30 and 10% hESC-BM, 69.90±5.56, Table 2, p<0.05).
The BMs increase speed of the blastocyst expansion and hatching following IVF
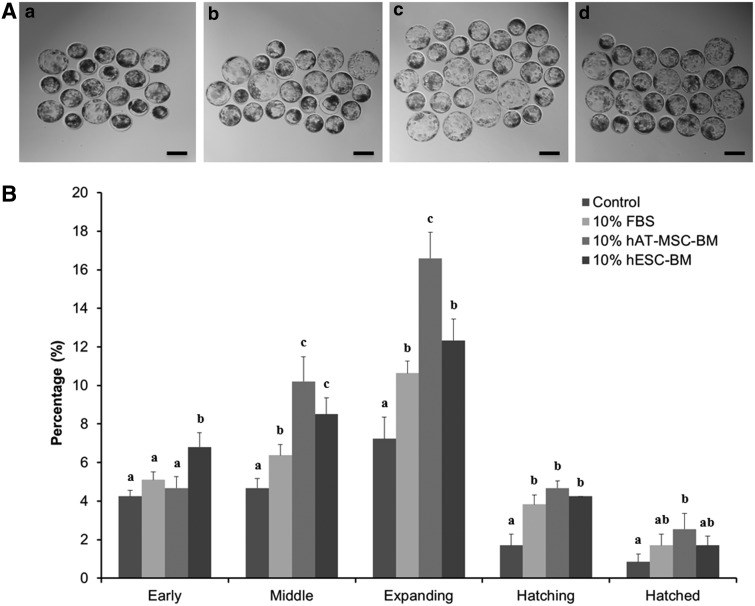
The developmental morphology of blastocysts in the 10% hAT-MSC-BM group was best, and both the 10% FBS and 10% hESC-BM groups were better compared to the control group (Fig. 1A).
FIG. 1.
Blastocyst development morphology (A) in different treatment groups—control (a), 10% FBS (b), 10% hAT-MSC-BM (c), and 10% hESC-BM (d)—and developmental proportion (B) of the five classified blastocysts following early, middle, expanding, hatching, and hatched blastocysts in porcine IVF embryos. Significant differences from control oocytes are indicated (a–cp<0.05). Values are presented as means±standard error of the mean (SEM) of independent experiments. Bar, 200 μm.
To confirm the acceleration of IVF embryo development by differently treated BMs, we investigated the blastocyst development proportion, which was classified as early, middle, expanding, hatching, or hatched blastocysts (Fig. 1B). Overall, the blastocyst development proportion of the treated groups (10% FBS, 10% hAT-MSC-BM, and 10% hESC-BM) was approximatively higher than in the control group. Among the treated groups, 10% hAT-MSC-BM produced the highest development proportion and 10% hESC-BM was higher than 10% FBS (Fig. 1B).
We specifically analyzed each of the five classified blastocysts (early, middle, expanding, hatching, and hatched blastocysts) (Fig. 1B). The percentage in the early blastocyst stage in the 10% hESC-BM group was significantly higher than the other groups. The percentage of the control, 10% FBS, and 10% hAT-MSC-BM groups was the same. The percentage of the 10% hAT-MSC-BM and 10% hESC-BM groups in the middle blastocyst stage was significantly highest, followed by the 10% FBS and control groups. The percentage of the 10% hAT-MSC-BM and 10% hESC-BM groups was significantly the same. The percentage of the 10% hAT-MSC-BM group in the expanding blastocyst stage was significantly highest, followed by 10% hESC-BM, 10% FBS, and the control groups. The percentage of 10% FBS and 10% hESC-BM groups had the same significance. The percentage of the control was significantly lowest. The percentage of 10% FBS, 10% hAT-MSC-BM, and 10% hESC-BM groups in the hatching blastocyst stage was significantly the same and was significantly higher than that of the control. The percentage of the 10% hAT-MSC-BM group in the hatched blastocyst stage was significantly the highest. The percentage of the 10% FBS group was significantly higher than that of the control.
The BMs affect the relative expression of developmentally important genes
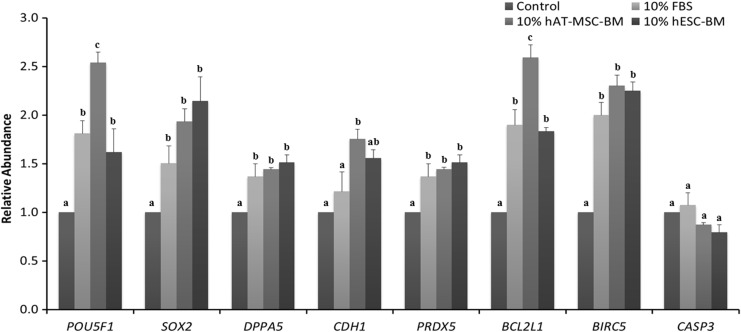
The relative abundance of the expression of reprogramming-related transcription factor genes, POU5F1, SOX2, DPPA5, and CDH1, is shown in Figure 2. POU5F1 mRNA expression was significantly highest in the 10% hAT-MSC-BM group. Both 10% FBS and 10% hESC-BM, the second highest groups, were higher than the control group. There was no significant difference between the 10% FBS and 10% hESC-BM groups. SOX2 and DPPA5 mRNA expression was significantly increased in the 10% FBS, 10% hAT-MSC-BM, and 10% hESC-BM groups compared to the control group. There was no significant difference among the 10% FBS, 10% hAT-MSC-BM, and 10% hESC-BM groups. CDH1 expression was highest in the 10% hAT-MSC-BM group and the 10% hESC-BM group was higher than the 10% FBS group. However, the 10% FBS group was not significantly different than the control group.
FIG. 2.
Relative abundance of reprogramming-related transcription factor, antioxidant, and apoptosis mRNA expression in blastocyst developed from different treatment groups. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal standard. Significant differences from control oocytes are indicated (a–cp<0.05). Values are presented as means±standard error of the mean (SEM) of independent experiments.
The relative abundance of the antioxidant-related gene PRDX5 is shown in Figure 2. PRDX5 mRNA expression significantly increased in the 10% FBS, 10% hAT-MSC-BM, and 10% hESC-BM groups compared to the control group. There was no significant difference between the 10% FBS, 10% hAT-MSC-BM, and 10% hESC-BM groups.
The expression of apoptosis-related genes, BCL2L1, BIRC5, and CASP3, is shown in Figure 2. The 10% hAT-MSC-BM group expressed BCL2L1 significantly higher than the others. Both 10% FBS and 10% hESC-BM, the second highest groups, were higher than the control group. There was no significant difference between the 10% FBS and 10% hESC-BM groups. Expression of another anti-apoptosis gene (BIRC5) was significantly increased in the 10% FBS, 10% hAT-MSC-BM, and 10% hESC-BM groups compared to the control group. There was no significant difference among the 10% FBS, 10% hAT-MSC-BM, and 10% hESC-BM groups. There was no significant difference in CASP3 mRNA expression between the treated groups and the control group.
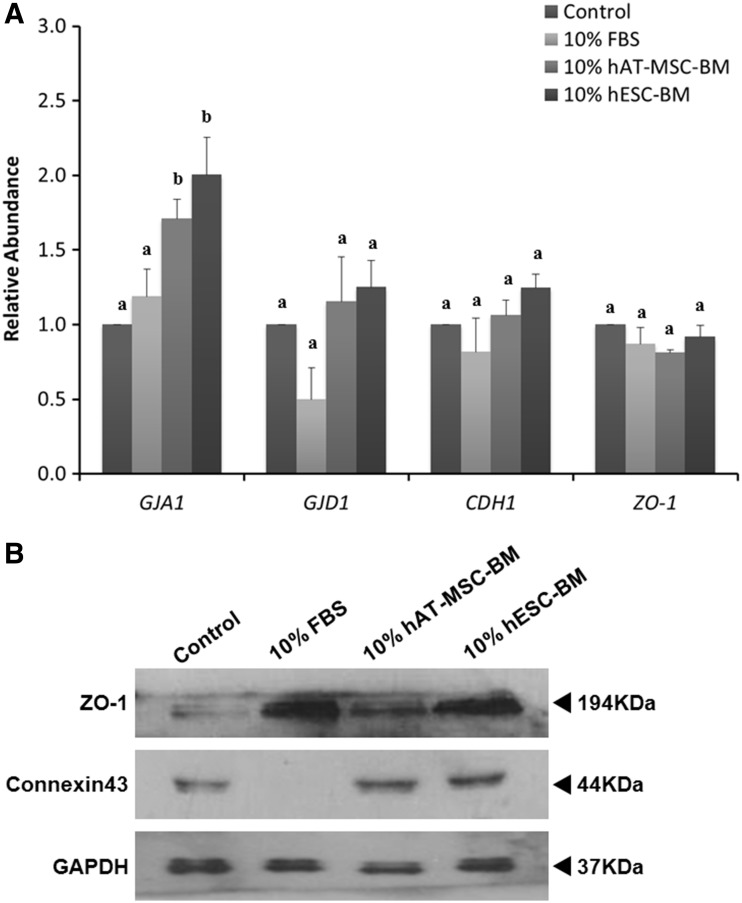
BMs boosted connexin 43 and ZO-1 protein synthesis in porcine IVF embryos
The relative expression of tight junction– and gap junction–related genes varied significantly in the different treatments (Fig. 3A). The expression of gap junction protein, alpha 1, and connexin 43 (GJA1) was significantly higher in the 10% hAT-MSC-BM and 10% hESC-BM groups than in the control group. There was no significant difference between the 10% hAT-MSC-BM and 10% hESC-BM groups. The other treated group, 10% FBS, was not significantly different from the control group. There was no significant difference among these three higher groups. The expression of gap junction protein, gamma 1, connexin 45 (GJC1), cadherin 1, type 1, E-cadherin (CDH1), and ZO-1 genes, was not significantly different in many of the groups.
FIG. 3.
Relative abundance of gap junction and tight junction mRNA expression (A) and protein synthesis (B) in blastocysts developed from different treatment groups. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal standard. Significant differences from control oocytes are indicated (a–cp<0.05). Values are presented as means±standard error of the mean (SEM) of independent experiments.
We investigated connexin 43 and ZO-1 activity (Fig. 3B). The connexin 43 band was detected and expressed similarly in the 10% hESC-BM, 10% hAT-MSC-BM, and control groups. However, the expression was not detected in the 10% FBS group. The amount of connexin 43 in both the 10% hAT-MSC-BM and 10% hESC-BM groups increased more than that in the control group. The ZO-1–specific band was detected in all groups. Expression of ZO-1 in both the 10% FBS and 10% hESC-BM groups was highest, and although not as high, in the 10% hAT-MSC-BM group compared to them, it was higher than in the control group.
Discussion
This study investigated the most effective serum substituent for embryo development. To improve porcine embryo developmental potential, our team collected two types of BMs—hAT-MSC-BM and hESC-BM—by culturing human stem cells. In our previous study, treatment with hESC-BM or hAT-MSC-BM had a positive influence on the development of bovine or porcine preimplantation embryos (Kim et al., 2011; Park et al., 2013). When performing IVC of porcine IVF embryos in hESC-BM or hAT-MSC-BM, we confirmed noticeably better growth than with other serum and protein supplements. In the present study, inclusion of hAT-MSC-BM and hESC-BM affected the rate of blastocyst formation, hatching rate, and total number of cells. In particular, we investigated the expression of reprogramming-related transcription factor, apoptosis, and antioxidant genes, and protein synthesis of tight junction– and gap junction–related genes (connexin 43 and ZO-1).
In this study, two other BMs were used to investigate the effect on development of porcine embryos. IVF embryos were equally divided into four groups and cultured in PZM-5 with 4 mg/mL BSA for 4 days. Each group was treated with protein additives for 2 days as follows; 4 mg/mL BSA (control), 10% FBS, 10% hAT-MSC-BM, or 10% hESC-BM. The addition of 10% hAT-MSC-BM or 10% hESC-BM to culture medium significantly affected the rate of embryo development and increased cell numbers compared to 10% FBS or control groups (Table 2, p<0.05). The hAT-MSC-BM–treated group had the highest blastocyst formation and total cell number. Visual inspection showed that the developmental morphology of blastocysts was best in the 10% hAT-MSC-BM group, whereas both the 10% FBS and 10% hESC-BM groups were better than the control (Fig. 1A).
To confirm this, BMs were used to investigate blastocyst development. Blastocyst development of the treated groups (10% FBS, 10% hAT-MSC-BM, and 10% hESC-BM) was higher than the control group at early, middle, expanding, hatching, and hatched blastocysts stages. Among the treated groups, the 10% hAT-MSC-BM group was the highest and the 10% hESC-BM group higher than the 10% FBS group (Fig. 1B). Notably, the expanding stage and hatched blastocyst stage percentages of the 10% hAT-MSC-BM group were significantly highest, followed by the 10% hESC-BM, 10% FBS, and the control groups. The present study shows that treatment with two BMs significantly influences the acceleration of porcine IVF embryo development.
Although serum supplement results in a high rate of blastocyst development and high cell numbers, serum lot variation in embryo development can cause alterations in the embryo ultrastructure, impaired compaction, abnormal blastulation, large offspring syndrome, aberrant mRNA expression profiles, and greater incidence of stillbirth and death after birth (Abe et al., 1999; Behboodi et al., 1995; Holm et al., 2002; Wrenzycki et al., 2004). The serum may have effects that range from stimulatory to inhibitory. Many experiments have been conducted to define the specific serum components responsible for these effects. It was found that adiponectin, insulin-like growth factor 1, LH, FSH, and progesterone may positively affect embryonic developmental competence (Chappaz et al., 2008; Kim et al., 2006; Wu et al., 2007).
Nevertheless, although serum is an important protein supplement for in vitro porcine embryo development, the search continues for a better supplement to support IVC and to provide conditions more similar to the in vivo environment. In our study, treatment with 10% hAT-MSC-BM or 10% hESC-BM resulted in significantly higher development rates when compared to 0.4% FAF-BSA (control) and 10% FBS treatments (Table 2 and Fig. 1, p< 0.05). Taken together, these results indicate that the two BMs might even be more supportive than serum for preimplantation porcine embryo development in vitro.
Gene expression is one of the major contributors to embryo development, and its perturbation during culture may limit production of high-quality blastocysts (Kikuchi et al., 2002; Pomar et al., 2005). We examined the relative expression of eight developmentally important genes (POU5F1, SOX2, DPPA5, CDH1, BCL2L1, BIRC5, CASP3, and PRDX5) in the four treatment groups. Transcription factor POU5F1 and other reprogramming-related transcription factor genes, such as SOX2, DPPA5, and CDH1, have essential roles in early development and are required for the propagation of undifferentiated ESCs in culture. POU5F1 mRNA and protein have been identified in the blastomeres of preimplantation embryos, in the ICM of blastocysts, in epiblasts and primordial germ cells, and in most germ cells (Bernstein et al., 2001; Rosner et al., 1990; Scholer et al., 1990). SOX2 and NANOG interact with POU5F1 to regulate the transcription hierarchy that specifies ESC identity (Chambers et al., 2003; Mitsui et al., 2003; Nichols et al., 1998). DPPA5 and CDH1 have been identified as markers for evaluating nuclear reprogramming in cloned pig and mouse embryos (Boiani et al., 2002; Lee et al., 2006; Wolf et al., 2011).
In the present study, significantly higher levels of POU5F1 and SOX2 transcripts were detected in blastocysts that developed in the presence of 10% hAT-MSC-BM and 10% hESC-BM compared to the control (Fig. 2, p<0.05). Although a significant difference of POU5F1 and SOX2 transcripts was detected in FBS groups compared to the 10% hAT-MSC-BM or 10% hESC-BM group, the addition of 10% hAT-MSC-BM or 10% hESC-BM also increased the cell numbers of the ICM and trophectoderm (TE) (Table 2, p<0.05). These results indicate that the 10% hAT-MSC-BM or 10% hESC-BM treatments involved upregulation of selected transcription factors, including POU5F1 and SOX2, in the blastocyst stage.
Interestingly, we observed antioxidant-related gene expression of IVF embryo development by differently treated BMs. Peroxiredoxins (PRDXs), a new family of antioxidants, function in connection to detoxify reactive oxygen species (ROS) and thus provide cytoprotection from internal/external environmental stress (Verdoucq et al., 1999; Wood et al., 2003). PRDX5 is a novel and unusual PRDX with mitochondrial and peroxisomal targeting signals (Verdoucq et al,. 1999; Zhou et al., 2000) and is characterized as a thioredoxin peroxidase. Our data show that PRDX5 mRNA expression in the 10% FBS, 10% hAT-MSC-BM, and 10% hESC-BM groups significantly increased compared to the control group (Fig. 2, p<0.05). A recent study showed that pericytes treated with a high glucose concentration underwent apoptosis, and a supply of PRDX5 or PRDX6 could attenuate the process, suggesting that PRDX5 or PRDX6 protects cells by attenuating apoptotic pathways (Kubo et al., 2009).
Several studies have shown that PRDXs are downregulators of apoptotic pathways (Kubo et al., 2009). In addition, our results indicated that the two BMs affected the expression patterns of anti-apoptosis genes. There was no significant difference in the expression of pro-apoptosis genes, such as CASP3. However, 10% hAT-MSC-BM or 10% hESC-BM treatment significantly increased the expression of anti-apoptosis genes, such as BCL2L1 and BIRC5 (Fig. 2, p<0.05). Collectively, these observations suggest that the two BMs may contribute to embryo development through suppression of apoptosis at the molecular level in preimplantation porcine IVF embryos.
We investigated the effect of BMs treatment on the gap junction and tight junction. Intercellular communication via gap junctions is required to coordinate embryonic development. The fundamental unit of gap junction channels is connexin, a hexamer of protein subunits called connexins (Houghton et al., 2002). Connexin 43 is the main connexin isoform expressed in humans (Bloor et al., 2004) and bovine blastocysts (Rizos et al., 2003). In the present study, the relative expression of GJA1 mRNA and connexin 43 protein increased in the 10% hAT-MSC-BM and 10% hESC-BM groups (Fig. 3, p<0.05). GJC1 mRNA expression did not differ among the groups (Fig. 3, p<0.05). The tight junction–related gene, E-cad, mediated cell-to cell-adhesion and is associated with compaction (Fleming et al., 1984; Pratt et al., 1982). E-cad transcripts and protein were found in the ICM and TE of expanded bovine blastocysts (Barcroft et al., 1998; Shehu et al., 1996). ZO-1 mRNA was found in both the TE and ICM cells of the bovine blastocyst (Wrenzycki et al., 2003), and ZO-1 protein was detected as a continuous ring at the apical points of TE cell contact in bovine and murine embryos (Barcroft et al., 1998; Sheth et al., 1997).
In the present study, although CDH1 and ZO-1 mRNAs expression did not differ among the groups, ZO-1 protein synthesis in both the 10% hESC-BM and 10% FBS groups was highest, and that in the 10% hAT-MSC-BM group was higher than the control group (Fig. 3, p<0.05). Collectively, this suggests that there may be effects between the gap junction and tight junction, demonstrating the involvement of BMs supplement in porcine IVF embryo development.
In conclusion, our results indicate that treatment with hAT-MSC-BM or hESC-BM significantly accelerates the development of porcine IVF embryos. In the present study, we found the serum substituents needed to promote porcine embryo development when added to two BMs; however, further studies are needed regarding the use of these supplements. Finally, our results should be further applied in assisted reproductive technology to produce qualified embryos with good viability and to enhance the implantation rate and pregnancy rate after embryo transfer.
Acknowledgments
This study was supported by the Cooperative Research Program for Agriculture Science & Technology Development, RDA, Republic of Korea (grant no. PJ009103) and Next-Generation BioGreen 21 Program (grant sponsor), RDA, Republic of Korea (grant no. PJ009075).
Author Disclosure Statement
The authors declare that no conflicting financial interests exist.
References
- Abe H., Yamashita S., Itoh T., Satoh T., and Hoshi H. (1999). Ultrastructure of bovine embryos developed from in vitro-matured and -fertilized oocytes: Comparative morphological evaluation of embryos cultured either in serum-free medium or in serum-supplemented medium. Mol. Reprod. Dev. 53, 325–335 [DOI] [PubMed] [Google Scholar]
- Barcroft L.C., Hay-Schmidt A., Caveney A., Gilfoyle E., Overstrom E.W., Hyttel P., and Watson A.J. (1998). Trophectoderm differentiation in the bovine embryo: Characterization of a polarized epithelium. J. Reprod. Fertil. 114, 327–339 [DOI] [PubMed] [Google Scholar]
- Becker D.L., and Davies C.S. (1995). Role of gap junctions in the development of the preimplantation mouse embryo. Microsc. Res. Techn. 31, 364–374 [DOI] [PubMed] [Google Scholar]
- Behboodi E., Anderson G.B., BonDurant R.H., Cargill S.L., Kreuscher B.R., Medrano J.F., and Murray J.D. (1995). Birth of large calves that developed from in vitro-derived bovine embryos. Theriogenology 44, 227–232 [DOI] [PubMed] [Google Scholar]
- Bernstein E., Caudy A.A., Hammond S.M., and Hannon G.J. (2001). Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 409, 363–366 [DOI] [PubMed] [Google Scholar]
- Bevilacqua E.M., and Abrahamsohn P.A. (1989). Trophoblast invasion during implantation of the mouse embryo. Arch. Biol. Med. Exp. (Santiago) 22, 107–118 [PubMed] [Google Scholar]
- Bloor D.J., Wilson Y., Kibschull M., Traub O., Leese H.J., Winterhager E., and Kimber S.J. (2004). Expression of connexins in human preimplantation embryos in vitro. Reprod. Biol. Endocrinol. 2, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boiani M., Eckardt S., Scholer H.R., and McLaughlin K.J. (2002). Oct4 distribution and level in mouse clones: Consequences for pluripotency. Genes Dev. 16, 1209–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brohem C.A., de Carvalho C.M., Radoski C.L., Santi F.C., Baptista M.C., Swinka B.B., de A.U.C., de Araujo L.R., Graf R.M., Feferman I.H., and Lorencini M. (2013). Comparison between fibroblasts and mesenchymal stem cells derived from dermal and adipose tissue. Int. J. Cosmet. Sci. 35, 448–457 [DOI] [PubMed] [Google Scholar]
- Buehr M., Lee S., McLaren A., and Warner A. (1987). Reduced gap junctional communication is associated with the lethal condition characteristic of DDK mouse eggs fertilized by foreign sperm. Development 101, 449–459 [DOI] [PubMed] [Google Scholar]
- Chambers I., Colby D., Robertson M., Nichols J., Lee S., Tweedie S., and Smith A. (2003). Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell 113, 643–655 [DOI] [PubMed] [Google Scholar]
- Chappaz E., Albornoz M.S., Campos D., Che L., Palin M.F., Murphy B.D., and Bordignon V. (2008). Adiponectin enhances in vitro development of swine embryos. Domest. Anim. Endocrinol. 35, 198–207 [DOI] [PubMed] [Google Scholar]
- Fleming T.P., Warren P.D., Chisholm J.C., and Johnson M.H. (1984). Trophectodermal processes regulate the expression of totipotency within the inner cell mass of the mouse expanding blastocyst. J. Embryol. Exp. Morphol. 84, 63–90 [PubMed] [Google Scholar]
- Holm P., Booth P.J., and Callesen H. (2002). Kinetics of early in vitro development of bovine in vivo- and in vitro-derived zygotes produced and/or cultured in chemically defined or serum-containing media. Reproduction 123, 553–565 [PubMed] [Google Scholar]
- Houghton F.D., Barr K.J., Walter G., Gabriel H.D., Grummer R., Traub O., Leese H.J., Winterhager E., and Kidder G.M. (2002). Functional significance of gap junctional coupling in preimplantation development. Biol. Reprod. 66, 1403–1412 [DOI] [PubMed] [Google Scholar]
- Kikuchi K., Onishi A., Kashiwazaki N., Iwamoto M., Noguchi J., Kaneko H., Akita T., and Nagai T. (2002). Successful piglet production after transfer of blastocysts produced by a modified in vitro system. Biol. Reprod. 66, 1033–1041 [DOI] [PubMed] [Google Scholar]
- Kim E.Y., Lee J.B., Park H.Y., Jeong C.J., Riu K.Z., and Park S.P. (2011). The use of embryonic stem cell derived bioactive material as a new protein supplement for the in vitro culture of bovine embryos. J. Reprod. Dev. 57, 346–354 [DOI] [PubMed] [Google Scholar]
- Kim S., Lee S.H., Kim J.H., Jeong Y.W., Hashem M.A., Koo O.J., Park S.M., Lee E.G., Hossein M.S., Kang S.K., Lee B.C., and Kwang W.S. (2006). Anti-apoptotic effect of insulin-like growth factor (IGF)-I and its receptor in porcine preimplantation embryos derived from in vitro fertilization and somatic cell nuclear transfer. Mol. Reprod. Dev. 73, 1523–1530 [DOI] [PubMed] [Google Scholar]
- Kubo E., Singh D.P., Fatma N., and Akagi Y. (2009). TAT-mediated peroxiredoxin 5 and 6 protein transduction protects against high-glucose-induced cytotoxicity in retinal pericytes. Life Sci. 84, 857–64 [DOI] [PubMed] [Google Scholar]
- Lapree-Delage G., Volante M., Frydman R., and Chaouat G. (1999). Interleukin-6 levels in co-culture of human in vitro fertilization embryos with Vero cells are not predictive of future successful development. Am. J. Reprod. Immunol. 41, 164–167 [DOI] [PubMed] [Google Scholar]
- Lee E., Lee S.H., Kim S., Jeong Y.W., Kim J.H., Koo O.J., Park S.M., Hashem M.A., Hossein M.S., Son H.Y., Lee C.K., Hwang W.S., Kang S.K., and Lee B.C. (2006). Analysis of nuclear reprogramming in cloned miniature pig embryos by expression of Oct-4 and Oct-4 related genes. Biochem. Biophys. Res. Commun. 348, 1419–1428 [DOI] [PubMed] [Google Scholar]
- Lee S., Gilula N.B., and Warner A.E. (1987). Gap junctional communication and compaction during preimplantation stages of mouse development. Cell 51, 851–860 [DOI] [PubMed] [Google Scholar]
- Lee S.E., Sun S.C., Choi H.Y., Uhm S.J., and Kim N.H. (2012). mTOR is required for asymmetric division through small GTPases in mouse oocytes. Mol. Reprod. Dev. 79, 356–366 [DOI] [PubMed] [Google Scholar]
- Livak K.J., and Schmittgen T.D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- Ma H.Y., Yao L., Yu Y.Q., Li L., Ma L., Wei W.J., Lu X.M., Du L.L., and Jin Y.N. (2012). An effective and safe supplement for stem cells expansion ex vivo: Cord blood serum. Cell Transplant. 21, 857–869 [DOI] [PubMed] [Google Scholar]
- Marinaro C., Butti E., Bergamaschi A., Papale A., Furlan R., Comi G., Martino G., and Muzio L. (2011). In vivo fate analysis reveals the multipotent and self-renewal features of embryonic AspM expressing cells. PloS One 6, e19419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng G.L., Teng L., Zou J.Z., Xue Y.F., and Shang K.G. (2001). [The medium conditioned incubation with rat heart cells maintains the properties of ES cells]. Yi chuan xue bao=Acta genetica Sinica 28, 911–920 [PubMed] [Google Scholar]
- Mitsui K., Tokuzawa Y., Itoh H., Segawa K., Murakami M., Takahashi K., Maruyama M., Maeda M., and Yamanaka S. (2003). The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell 113, 631–642 [DOI] [PubMed] [Google Scholar]
- Nichols J., Zevnik B., Anastassiadis K., Niwa H., Klewe-Nebenius D., Chambers I., Scholer H., and Smith A. (1998). Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell 95, 379–391 [DOI] [PubMed] [Google Scholar]
- Park H.Y., Kim E.Y., Lee S.E., Choi H.Y., Moon J.J., Park M.J., Son Y.J., Lee J.B., Jeong C.J., Lee D.S., Tiu K.J., and Park S.P. (2013). Effect of human adipose tissue-derived mesenchymal-stem-cell bioactive materials on porcine embryo development. Mol. Reprod. Dev. 80, 1035–1047 [DOI] [PubMed] [Google Scholar]
- Pomar F.J., Teerds K.J., Kidson A., Colenbrander B., Tharasanit T., Aguilar B., and Roelen B.A. (2005). Differences in the incidence of apoptosis between in vivo and in vitro produced blastocysts of farm animal species: A comparative study. Theriogenology 63, 2254–2268 [DOI] [PubMed] [Google Scholar]
- Pratt H.P., Ziomek C.A., Reeve W.J., and Johnson M.H. (1982). Compaction of the mouse embryo: An analysis of its components. J. Embryol. Exp. Morphol. 70, 113–132 [PubMed] [Google Scholar]
- Rizos D., Gutierrez-Adan A., Perez-Garnelo S., De La Fuente J., Boland M.P., and Lonergan P. (2003). Bovine embryo culture in the presence or absence of serum: Implications for blastocyst development, cryotolerance, and messenger RNA expression. Biol. Reprod. 68, 236–243 [DOI] [PubMed] [Google Scholar]
- Rosner M.H., Vigano M.A., Ozato K., Timmons P.M., Poirier F., Rigby P.W., and Staudt L.M. (1990). A POU-domain transcription factor in early stem cells and germ cells of the mammalian embryo. Nature 345, 686–692 [DOI] [PubMed] [Google Scholar]
- Scholer H.R., Dressler G.R., Balling R., Rohdewohld H., and Gruss P. (1990). Oct-4: A germline-specific transcription factor mapping to the mouse t-complex. EMBO J. 9, 2185–2195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahdadfar A., Haug K., Pathak M., Drolsum L., Olstad O.K., Johnsen E.O., Petrovski G., Moe M.C., and Nicolaissen B. (2012). Ex vivo expanded autologous limbal epithelial cells on amniotic membrane using a culture medium with human serum as single supplement. Exp. Eye Res. 97, 1–9 [DOI] [PubMed] [Google Scholar]
- Shehu D., Marsicano G., Flechon J.E., and Galli C. (1996). Developmentally regulated markers of in vitro-produced preimplantation bovine embryos. Zygote 4, 109–121 [DOI] [PubMed] [Google Scholar]
- Sheth B., Fesenko I., Collins J.E., Moran B., Wild A.E., Anderson J.M., and Fleming T.P. (1997). Tight junction assembly during mouse blastocyst formation is regulated by late expression of ZO-1 alpha+ isoform. Development 124, 2027–2037 [DOI] [PubMed] [Google Scholar]
- Takahashi M. (2012). Oxidative stress and redox regulation on in vitro development of mammalian embryos. J. Reprod. Dev. 58, 1–9 [DOI] [PubMed] [Google Scholar]
- Verdoucq L., Vignols F., Jacquot J.P., Chartier Y., and Meyer Y. (1999). In vivo characterization of a thioredoxin h target protein defines a new peroxiredoxin family. J. Biol. Chem. 274, 19714–19722 [DOI] [PubMed] [Google Scholar]
- Wolf X.A., Serup P., and Hyttel P. (2011). Three-dimensional localisation of NANOG, OCT4, and E-CADHERIN in porcine pre- and peri-implantation embryos. Dev. Dynamics 240, 204–210 [DOI] [PubMed] [Google Scholar]
- Wood Z.A., Schroder E., Robin Harris J., and Poole L.B. (2003). Structure, mechanism and regulation of peroxiredoxins. Trends Biochem. Sci. 28, 32–40 [DOI] [PubMed] [Google Scholar]
- Wrenzycki C., Herrmann D., and Niemann H. (2003). Timing of blastocyst expansion affects spatial messenger RNA expression patterns of genes in bovine blastocysts produced in vitro. Biol. Reprod. 68, 2073–2080 [DOI] [PubMed] [Google Scholar]
- Wrenzycki C., Herrmann D., Lucas-Hahn A., Lemme E., Korsawe K., and Niemann H. (2004). Gene expression patterns in in vitro-produced and somatic nuclear transfer-derived preimplantation bovine embryos: Relationship to the large offspring syndrome? Anim. Reprod. Sci. 82–83, 593–603 [DOI] [PubMed] [Google Scholar]
- Wu J., Xu B., and Wang W. (2007). Effects of luteinizing hormone and follicle stimulating hormone on the developmental competence of porcine preantral follicle oocytes grown in vitro. J. Assist. Reprod. Genet. 24, 419–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino A., Suzuki K., Urano T., Aoki K., Takada Y., Kazui T., and Takada A. (2002). Enhanced secretion of tissue plasminogen activator by simultaneous use of retinoic acid and ascorbic acid from tissue cultured gastroepiploic artery. Life Sci. 70, 1461–1470 [DOI] [PubMed] [Google Scholar]
- Zhou Y., Kok K.H., Chun A.C., Wong C.M., Wu H.W., Lin M.C., Fung P.C., Kung H., and Jin D.Y. (2000). Mouse peroxiredoxin V is a thioredoxin peroxidase that inhibits p53-induced apoptosis. Biochem. Biophys. Res. Communications 268, 921–927 [DOI] [PubMed] [Google Scholar]
- Zur Nieden N.I., Kempka G., Rancourt D.E., and Ahr H.J. (2005). Induction of chondro-, osteo- and adipogenesis in embryonic stem cells by bone morphogenetic protein-2: Effect of cofactors on differentiating lineages. BMC Dev. Biol. 5, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]