Abstract
Oligotrich ciliates are common marine microplankters, but their biodiversity and evolutionary relationships have not been well-documented. Morphological descriptions and small subunit rRNA gene sequences of two new species representing two new strombidiid genera, Sinistrostrombidium cupiformum gen. nov., sp. nov. and Antestrombidium agathae gen. nov., sp. nov. are presented, and their taxonomy and molecular phylogeny are analyzed. Sinistrostrombidium gen. nov. is characterized by a sinistrally spiraled girdle kinety and a longitudinal ventral kinety. Antestrombidium gen. nov. is distinguished by tripartite somatic kineties (circular and ventral kineties plus dextrally spiraled girdle kinety). Sinistrostrombidium and Antestrombidium branched separately from one another in phylogenetic trees, clustering with different clades of strombidiids. The new genera added to the diversities of ciliary patterns and small subunit rRNA gene sequences in strombidiids leads to presentation of a new hypothesis about evolution of the 12 known strombidiid genera, based on ciliary pattern and partly supported by molecular evidence. In addition, our new morphological and molecular analyses support establishment of a new order Lynnellida ord. nov., characterized by an open adoral zone of membranelles without differentiation of anterior and ventral membranelles, for Lynnella, but we remain unable to assign the genus to a subclass with confidence.
Introduction
Oligotrich ciliates are common marine microplankters that usually contribute to the energy flow of the microbial loop [1–3]. More than 100 species of oligotrichs in 15 genera have been reported in marine and brackish water, and recent investigations demonstrated that the group is much more divergent than previously realized [4–11].
The family Strombidiidae is the major group of oligotrichs, characterized by a three-part oral ciliature (endoral membrane, anterior and ventral membranelles) and the stomatogenesis occurring within a transient tube [12, 13]. The somatic ciliature of strombidiids typically consists of only girdle (GK) and ventral (VK) kineties; however, an impressive diversity of ciliary patterns has originated from these two kineties and is regarded as important generic characters. Since Claparède & Lachmann [14] erected the well-known type-genus Strombidium, ten other strombidiid genera have been described [8, 12, 15–20]. The rich diversity of ciliary patterns in the family Strombidiidae raises questions about whether some patterns developed convergently and how they evolved. Evolutionary relationships of the main ciliary patterns of oligotrichs have been outlined in previous studies, based on purely morphological data [12, 19, 21]. However, hypotheses suggested by these studies were broadly descriptive of evolutionary lineages within the subclass Oligotrichia rather than elucidating specific patterns within the family Strombidiidae.
In the present paper, we describe two new species of oligotrichs that were isolated from coastal waters of southern China. All available evidence indicates that they represent two new genera in the family Strombidiidae, and their phylogenetic positions are analyzed using both morphological characters and SSrDNA trees. The new genera add to the diversity of ciliary patterns known in strombidiids and provide an opportunity for a better analysis of evolutionary relationships within the Strombidiidae. The phylogenetic position of the enigmatic genus Lynnella is also reconsidered based on new evidence.
Materials and Methods
Sample Collection, Observation and Terminology
Samples were collected from an interitdal zone and a mangrove wetland in 250 ml, wide-mouth bottles. The sampling locations are pulbic areas, thus no specific permissions were required to collect the materials necessary for the present study. No known endangered or protected species were involved in the present study.
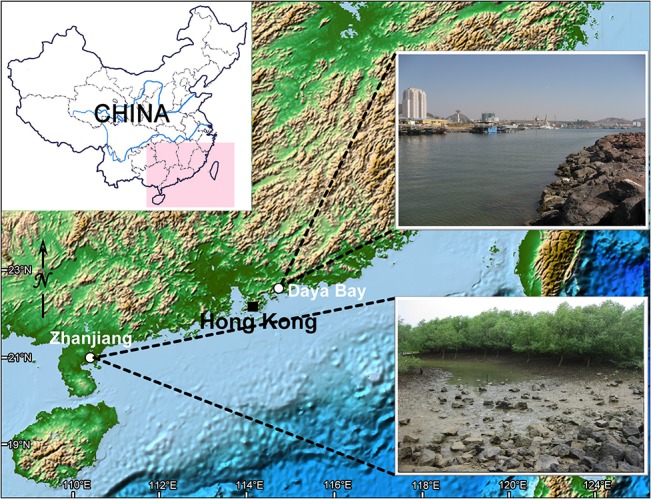
Sinistrostrombidium cupiformum gen. nov., sp. nov. was isolated from the littoral zone of Daya Bay (22°71′N; 114°54′E), Guangdong Province, China, on 27 May 2009 (Fig 1). The water temperature was 27.8°C, salinity 31.0 psu, and pH 8.6. Antestrombidium agathae gen. nov., sp. nov. was discovered in a mangrove wetland near Zhanjiang (21°36′N; 110°43′E), Guangdong Province, China, on 26 March 2010 (Fig 1). The water temperature was 19.7°C, salinity 23.9 psu, and pH 7.8.
Fig 1. Location and habitats of sampling sites.
The samples were transferred to Petri dishes (9 cm across; water depth 1 cm). The behaviour of the species was observed at about 20°C and then specimens were immediately isolated under a stereo microscope (Guiguang XTL-200, China) for further study in the laboratory. No cultures were established. Isolated living specimens were examined using bright field and differential interference contrast microscopy (Nikon Eclipse-80i, Tokyo, Japan). Staining with protargol according to the method of Wilbert [22] was used to reveal the infraciliature and nuclear apparatus. Counts and measurements on protargol-impregnated cells were performed at 1,000× magnification; in vivo measurements were made on ten cells for each species at 40–1,000× magnification. All measurements were done with a filar micrometer. Drawings of live specimens were based on direct observation and photomicrographs of ten cells for each species. Drawings of protargol-stained cells were made with a camera lucida at 1,000× magnification. Terminology follows Agatha [23], in which orientation of the somatic dikinetids is determined by whether the anterior or posterior kinetosome is ciliated, and classification follows Lynn [13].
Extraction, Amplification and Sequencing of DNA
For each species, five cells were collected and rinsed according to the protocol of Xu et al. [24]. Genomic DNA was extracted with REDExtract-N-Amp Tissue PCR Kit (Sigma, St. Louis). The PCR was performed according to Liu et al. [25] with the universal eukaryotic primers EukA (5'-AACCTGGTTGATCCTGCCAGT-3') and EukB (5'-TGATCCTTCTGCAGGTTCACCTAC-3') [26]. The PCR product was purified with a TIAN gel Midi Purification Kit (Tiangen Bio. Co., Shanghai, China) and inserted into a pUCm-T vector (Sangon Bio. Shanghai, China). DNA from plasmids was harvested using a Mini-prep Spin Column Kit (Sangon) and sequenced by the Invitrogen sequencing facility (Shanghai, China).
Phylogenetic Analysis
The SSrRNA gene sequences of all ciliates used in this study are available from the GenBank database. The SSrRNA gene sequences were aligned with Clustal X 1.83 [27] and the variable regions that could not be aligned unambiguously were removed manually from the initial alignment using Bioedit 7.0 [28], leaving a final alignment of 1,556 characters that was used to construct phylogenetic trees. The GTR + I + G evolutionary model was selected by both MrModeltest v.2 [29] and Modeltest v.3.7 [30] under the Akaike Information Criterion and used for Bayesian inference (BI) and maximum likelihood (ML) analyses, respectively. Bayesian trees were constructed with MrBayes 3.1.2 [31]. Four simultaneous chains were run for 1,500,000 generations, with sampling every 100 generations, and the first 3,750 (25%) trees discarded as burn-in. All remaining trees were used to calculate posterior probabilities using a majority rule consensus. The ML tree was constructed with PhyML v2.4.4 [32], including bootstrapping (1,000 replicates). A Maximum parsimony (MP) tree incorporating 1,000 bootstrap replicates was constructed with PAUP* 4.0b 10 [33] using a heuristic search with all characters coded as unordered. Phylogenetic trees were visualized with TreeView v1.6.6 [34] and MEGA 4.0 [35].
PAUP* 4.0b 10 was used to generate constrained ML trees under the GTR + I + G model to test the hypothesis that Lynnella clusters with subclass Oligotrichia and Choreotrichia, respectively. The best constraint trees (i.e. with lowest-lnL values) were compared with unconstrained ML trees using the approximately unbiased (AU) and Shimodaira-Hasegawa (SH) tests [36] implemented in CONSEL v0.1i [37]. Similarities and pairwise distances between SSrRNA gene sequences of Sinistrostrombidium cupiformum gen. nov., sp. nov., Antestrombidium agathae gen. nov., sp. nov. and typical species of other strombidiid genera were analysed using Bioedit 7.0.
Nomenclatural Acts
The electronic edition of this article conforms to the requirements of the amended International Code of Zoological Nomenclature, and hence the new names contained herein are available under that Code from the electronic edition of this article. This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the ICZN. The ZooBank LSIDs (Life Science Identifiers) can be resolved and the associated information viewed through any standard web browser by appending the LSID to the prefix "http://zoobank.org/". The LSID for this publication is: urn:lsid:zoobank.org:pub: 0240D079-D523-4AE5-8639-D127609D18EA. The electronic edition of this work was published in a journal with an ISSN, and has been archived and is available from the following digital repositories: PubMed Central, CLOCKSS.
Results
Morphological Descriptions
Class Spirotrichea Bütschli, 1889
Subclass Oligotrichia Bütschli, 1887
Order Strombidiida Petz & Foissner, 1992
Family Strombidiidae Fauré-Fremiet, 1970
Genus Sinistrostrombidium gen. nov. urn:lsid:zoobank.org:act: 3C0AC3A7-5A5F-438A-96A9-00139B065581
Diagnosis: Members of Strombidiidae with ventral kinety and sinistrally spiraled girdle kinety; oral primordium develops below left end of girdle kinety.
Etymology: Composite of the Latin sinistro- (left) and the generic name Strombidium, referring to the sinistrally spiraled girdle kinety and the general similarity to the genus Strombidium with respect to other characters. Neuter gender.
Type species: Sinistrostrombidium cupiformum gen. nov., sp. nov. urn:lsid:zoobank.org:act: 8F5C8BD0-B8A3-4405-B6E8-8AE6DFE5AC3A
Sinistrostrombidium cupiformum gen. nov., sp. nov. (Figs 2 and 3; Table 1)
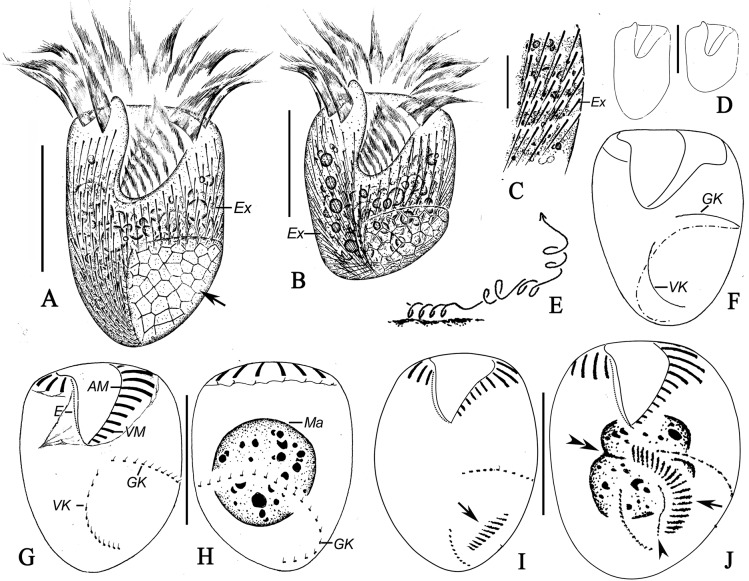
Fig 2. Sinistrostrombidium cupiformum gen. nov., sp. nov. from life (A-E) and after staining with protargol (F-J).
A, B. Ventral view of different specimens; arrow marks the cortical platelets; C. Detailed view showing the distribution of extrusomes; D. Variations in shape of the body; E. Swimming trace; F. Pattern of somatic ciliature; G, H. Ventral (G) and dorsal (H) views of the same specimens showing the ciliary pattern and macronuclear nodules; I, J. Ventral views of cells in the early (I) and middle (J) stages of division, arrows indicate the oral primordium, arrowhead marks the new endoral membrane, and double-arrowheads note the replication band of the macronucleus. Legend: AM-anterior membranelles; E-endoral membrane; Ex-extrusomes; GK-girdle kinety; Ma-macronucleus; VK-ventral kinety; VM-ventral membranelles. Scale bars: A, B, D, G, H. 20 μm; C. 5μm.
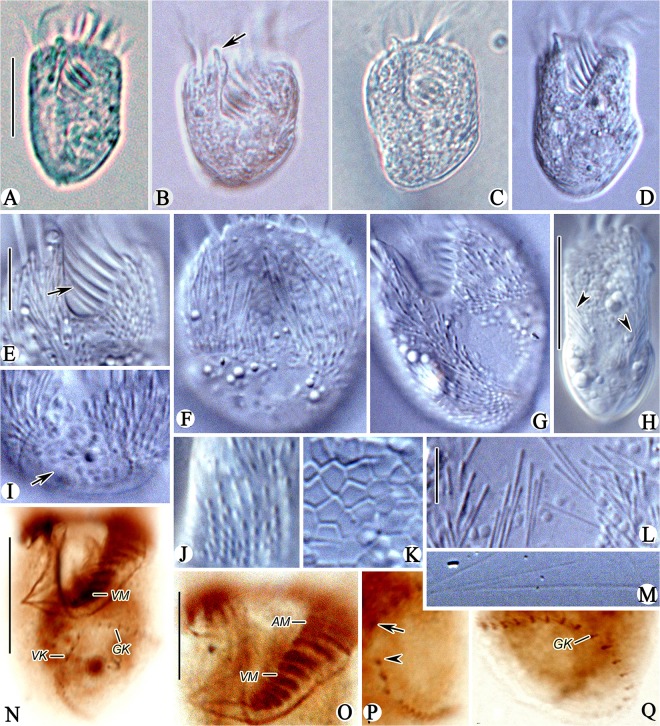
Fig 3. Photomicrographs of Sinistrostrombidium cupiformum gen. nov., sp. nov. from life (A-M) and after staining with protargol (N-Q).
A. Ventral view of a typical individual; B-D. Variation in shape of the body, note the apical protrusion (arrow); E. Ventral view of anterior part of cell showing the VM (arrow); F-H. Dorsal (F), ventral (G) and lateral (H) views showing the distribution of extrusomes (arrowheads); I. Ventral view of posterior part of cell showing the hemitheca (arrow); J. Outer ends of extrusomes; K. Detail of hemitheca with polygonal cortical platelets; L. Undischarged extrusomes; M. Discharged extrusomes; N. Ventral view showing the somatic kineties and AZM; O. Ventral view of anterior part of cell showing the AM and VM; P. Ventral view, arrowhead marks the anterior end of VK, arrow indicates the anterior end of GK; Q. Dorsal view showing the GK. Legend: AM-anterior membranelles; GK-girdle kinety; VK-ventral kinety; VM-ventral membranelles. Scale bars: A-D, H, N. 20 μm; E-G, I, O-Q. 10 μm; J-M. 5μm.
Table 1. Morphometric data of Sinistrostrombidium cupiformum gen. nov., sp. nov. (first row) and Antestrombidium agathae gen. nov., sp. nov. (second row).
| Characters | Min | Max | Mean | SD | n |
|---|---|---|---|---|---|
| Length of cell (μm) | 27 | 34 | 30.3 | 2.20 | 20 |
| 41 | 51 | 45.1 | 3.36 | 11 | |
| Width of cell (μm) | 20 | 28 | 24.4 | 1.98 | 20 |
| 21 | 29 | 24.1 | 2.74 | 11 | |
| Distance from anterior end to cytostome (μm) | 12 | 14 | 13.3 | 0.80 | 20 |
| 15 | 21 | 17.3 | 2.06 | 10 | |
| Length of ventral kinety (μm) | 8 | 12 | 10.5 | 1.51 | 13 |
| 6 | 11 | 9.14 | 1.77 | 7 | |
| Number of anterior membranelles | 11 | 13 | 11.9 | 0.49 | 20 |
| 15 | 21 | 18.1 | 1.87 | 11 | |
| Number of ventral membranelles | 7 | 8 | 7.6 | 0.51 | 20 |
| 7 | 9 | 7.8 | 0.72 | 12 | |
| Number of dikinetids in girdle kinety | 24 | 31 | 27.2 | 1.96 | 13 |
| 37 | 52 | 43.3 | 3.86 | 10 | |
| Number of dikinetids in circular kinety | — | — | — | — | — |
| 25 | 34 | 28.5 | 2.58 | 11 | |
| Number of dikinetids in ventral kinety | 9 | 14 | 11.8 | 1.54 | 13 |
| 10 | 16 | 11.9 | 1.76 | 9 | |
| Diameter of macronucleus (μm) | 14 | 17 | 15.6 | 0.94 | 17 |
| — | — | — | — | — | |
| Length of macronucleus (μm) | — | — | — | — | — |
| 13 | 17 | 15.1 | 1.45 | 11 | |
| Width of macronucleus (μm) | — | — | — | — | — |
| 8 | 12 | 9.7 | 1.27 | 11 |
All data based on specimens stained with protargol; Max-maximum value observed; Mean-arithmetic mean; Min-minimum value observed; n-sample size; SD-standard deviation.—: data unavailable.
Diagnosis: Size approximately 40 × 30 μm for living cells and 30 × 25 μm after staining with protargol; body cupiform to broadly cylindroid. Hemitheca covering left side of posterior part of cell, and extrusomes scattered all over cell surface except area beneath hemitheca. Girdle kinety consisting of approximately 27 dikinetids, extending from center of ventral side to dorsal side, curving toward left and terminating at posterior end of cell. Ventral kinety consisting of approximately 12 dikinetids, curving slightly toward left side of cell and terminating at posterior end. Adoral zone of membranelles composed of approximately 12 anterior and 8 ventral membranelles. Single spheroidal macronucleus.
Etymology: The Latin word “cupiformum” refers to the shape of the body, which is generally cylindroid or barrel-shaped, with a rounded posterior end.
Type locality: Littoral zone of Daya Bay (22°71′N; 114°54′E), Guangdong, China.
Deposition of Type-specimens: One slide containing the protargol-stained holotype specimen was deposited at the Natural History Museum, London, with registry number NHMUK 2011.11.23.1. Two paratype slides (protargol preparations) were deposited in the collection of the Laboratory of Protozoology, Ocean University of China, with registry numbers LWW2009052704-01 and LWW2009052704-02
Gene sequence: A sequence of the SSrRNA gene of S. cupiformum has been deposited in the GenBank database with accession number JX310366.
Description: Living cells measured 35–45 × 20–35 μm and protargol-stained cells 27–34 × 20–28 μm. Shape of body slightly variable, generally cupiform to broadly cylindrical (Figs 2A, 2B and 2D; 3A–3D). Anterior end transversely truncated, with 3 μm-long apical protrusion at the right end of the peristome in living cells that is undetectable after fixation (Figs 2A and 2B; 3B–3D arrow). Posterior end bluntly rounded and slanted slightly to the right (Figs 2A, 2B; 3A and 3C). Subequatorial area usually widest. Cell dorsoventrally flattened, with thickness/width ratio of approximately 4/5 (Fig 3H).
Pellicle thin, left side of posterior half of cell covered by hemitheca composed of polygonal platelets approximately 2 μm in diameter (Fig 2A and 2B, arrows; 3F-I, arrow, K). Hemitheca covers left 2/3 of posterior half of dorsal side and left 1/2 of posterior half of ventral side. Hemitheca located posterior to GK, where cell surface is distinctly distended. Cytoplasm is colourless and usually filled with numerous food vacuoles measuring 2–3 μm in diameter (Fig 2B). Extrusomes distributed over all of cell surface except area beneath hemitheca in irregular, longitudinal rows of 5–15 individuals (Figs 2A and 2B; 3F, 3G and 3H arrowheads). Some extrusomes densely packed together near edge of hemitheca, but rest sparsely distributed in anterior portion of cell (Figs 2C; 3G, 3I and 3J). Extrusomes near edge of hemitheca transversely oriented and those in anterior part of cell oriented at a slightly oblique angle (Fig 3H, arrowheads). Undischarged extrusomes needle-like, measuring approximately 5 × 0.4 μm, with rounded anterior and sharply pointed posterior end (Fig 3L). Discharged extrusomes thread-like and approximately 35 μm long (Fig 3M). Macronucleus spheroidal and centrally located, measuring approximately 15 μm in diameter after staining with protargol (Fig 2H). Micronucleus, contractile vacuole and cytopyge not observed. In Petri dish with in situ water at room temperature, cells always swimming in spirals (about 50 μm across) by rotating around their longitudinal axis with sudden changes in direction when disturbed (Fig 2E).
Somatic cilia arranged as girdle (GK) and ventral kineties (VK) (Figs 2G and 2H; 3Q). Girdle kinety composed of 24–31 dikinetids and following sinistrally directed, helical path around posterior half of cell; GK extending from center of ventral side below the oral cavity, across left ventral and dorsal sides, slanting toward posterior end, and curving toward left to terminate at posterior end of cell (Figs 2F–2H; 3Q). Left kinetosome of each dikinetid in GK bears short, cylindroid cilium measuring approximately 2 μm long. Ventral kinety extends from posterior 1/3–1/4 of cell, curves slightly toward left side, and terminates at posterior end of cell (Figs 2F and 2G; 3N and 3P). Ventral kinety composed of 9–14 densely arranged dikinetids, 2–3 of which are usually widely spaced at anterior end (Fig 2G). Each dikinetid of VK possesses one short cilium, approximately 2 μm long, on anterior kinetosome.
Oral apparatus occupies entire anterior end of cell. Buccal field wide and deep, extending obliquely for 2/5 of length of cell (Figs 2A and 2B; 3D and 3E). Adoral zone of membranelles (AZM) composed of 11–13 anterior (AM) and 7–8 ventral membranelles (VM) (Figs 2G; 3O). Anterior membranelles extend from right side of peristome below apical protrusion, around dorsal rim of peristome, to join with VM on left side of peristome (Figs 2G; 3N and 3O). Cilia of AM approximately 18–22 μm long in living cells, with tips splayed obliquely outward in swimming cells (Fig 2A and 2B). Cilia of VM approximately 3–5 μm in length. Bases of AM approximately 5 μm long and distinctly longer than those of VM, which gradually shorten from upper to lower (approximately 2–3 μm) (Figs 2G; 3O). All membranelles comprise three rows of basal bodies except for two posteriormost membranelles, which probably comprise only two rows. No thigmotactic membranelle observed. Endoral membrane approximately 7 μm long, located on inner wall of buccal cavity, and apparently composed of single row of kinetosomes (Fig 2G). System of argentophilic fibres developed and associated with adoral membranellar zone. Pharyngeal fibres up to 5 μm long extend obliquely rightward (Figs 2G and 2H; 3N).
Two divisional stages observed. One cell in early stages of division formed oral primordium left of VK and below anterior end of GK (Fig 2I, arrow). Another cell in mid- to-late stages of division developed oral primordium further toward posterior half of cell at point in time when posterior portion of newly built AZM bends to right and anterior part passes through gap between the GK and VK (Fig 2J, arrow). In this cell, endoral membrane could be seen originating to right of new AZM (Fig 2J, arrowhead), and replication band was recognizable in center of macronuclear nodule (Fig 2J, double-arrowheads)
Genus Antestrombidium gen. nov. urn:lsid:zoobank.org:act: 5356F427-ACA8-4632-A26E-203C786B4599
Diagnosis: Members of Strombidiidae with three somatic kineties—i.e., dextrally spiraled girdle kinety, circle kinety and ventral kinety.
Etymology: Composite of the Latin Ante- (before) and the generic name Strombidium. Neuter gender.
Type species: Antestrombidium agathae gen. nov., sp. nov. urn:lsid:zoobank.org:act: 1B630D57-DD65-488B-8C39-FD0A44F2C6FE
Antestrombidium agathae gen. nov., sp. nov. (Figs 4 and 5; Table 1)
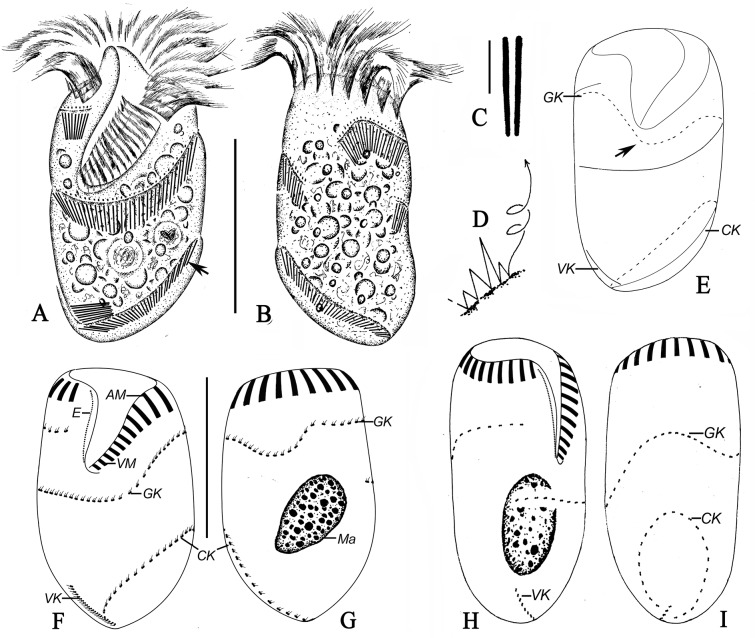
Fig 4. Antestrombidium agathae gen. nov., sp. nov. from living observations (A-D) and after staining with protargol (E-I).
A, B. Ventral and dorsal views of a typical specimen, arrows mark the extrusomes; C. Undischarged extrusomes; D. Swimming trace; E. Pattern of somatic ciliature; F, G. Ventral (F) and dorsal (G) views of the same specimen showing the ciliary pattern and macronucleus; H, I. Right (H) and left (G) lateral views showing the ciliary pattern. Legend: AM-anterior membranelles; CK-circular kinety; E-endoral membrane; GK-girdle kinety; Ma-macronucleus; VK-ventral kinety; VM-ventral membranelles. Scale bars: A, B, E-I. 30 μm; C. 3 μm.
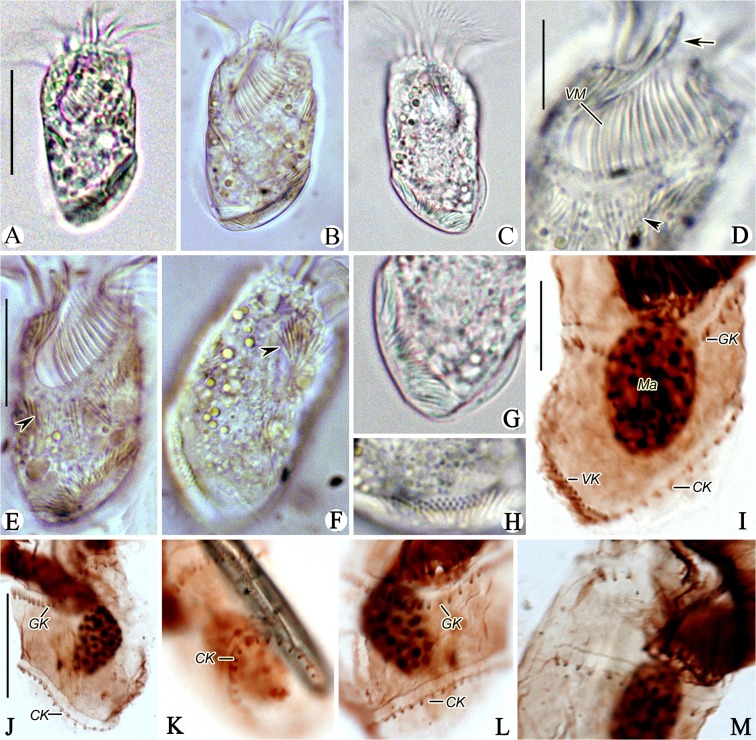
Fig 5. Photomicrographs of Antestrombidium agathae gen. nov., sp. nov. from life (A-H) and after staining with protargol (I-M).
A, B. Ventral views of two individuals; C. Dorsal view; D. Ventral view of anterior part of cell, arrow marks the apical protrusion and arrowhead indicates the extrusomes; E, F. Ventral (E) and Dorsal (F) views showing the distribution of extrusomes (arrowheads); G, H. Posterior portion of cell showing the distribution of extrusomes; I, J. Ventral and dorsal views of posterior portion of cell, showing the somatic kineties; K, L. Left and ventral views showing the GK and CK; M. Right lateral view showing the GK. Legend: CK-circular kinety; GK-girdle kinety; Ma-macronucleus; VK-ventral kinety; VM-ventral membranelles. Scale bars: A-C. 30 μm; D, G-I, L, M. 10 μm; E, F, J, K. 20 μm.
Diagnosis: Cell size measure approximately 55 × 25 μm in vivo and 45 × 25 μm after protargol-stained; body elliptical, with obvious apical protrusion. Extrusomes arranged in closely spaced series alongside somatic kineties. Girdle kinety making one helical circuit around cell following dextral path, composed of approximately 43 dikinetids; circle kinety surrounding left posterior part of cell, composed of approximately 29 dikinetids; ventral kinety extending obliquely across right posterior part of ventral surface of cell, composed of approximately 12 dikinetids. Adoral zone of membranelles consisting of approximately 18 anterior and 8 ventral membranelles. Single ovoid macronucleus.
Dedication: We dedicate this new species to Dr. Sabine Agatha, University of Salzburg, Austria, in recognition of her significant contributions to knowledge of oligotrich and choreotrich ciliates.
Type locality: Brackish water from a mangrove wetland near Zhanjiang (21°36′N; 110°43′E), Guangdong, China.
Deposition of Type-specimens: One slide containing the protargol-stained holotype specimen and paratype specimens was deposited in the Laboratory of Protozoology, Ocean University of China, with registry number LWW2010032606.
Gene sequence: A sequence of the SSrRNA gene of A. agathae has been deposited in the GenBank database with accession number JX310365.
Description: Living cells measured 50–60 × 20–30 μm and protargol-stained specimen 41–51 × 21–29 μm. Body elliptical, with anterior end truncated and right end of peristome projecting to form anterior protrusion approximately 4 μm high and undetectable after fixation. Posterior end of cell tapered slightly and tilted toward right (Figs 4A and 4B; 5A–5C).
Cells usually dark at low magnifications owing to presence of numerous food granules in the cytoplasm that are 2–3 μm across (Fig 4A and 4B). Cytoplasm colourless and apparently lacking cortical platelets. Extrusomes closely spaced to form bands along GK, VK and circle kinety (CK). Their attachment sites arranged in one row above GK, VK and CK, where cell surface is elevated to form conspicuous ridges (Fig 4A and 4B, arrow; 5D-H, arrowheads). Undischarged extrusomes rod-shaped, measuring approximately 6 × 0.6 μm (Fig 4C). Macronucleus ovoid, located in posterior half of cell, and measuring approximately 10 × 15 μm after staining with protargol (Figs 4G and 4H; 5I). Micronucleus, contractile vacuole and cytopyge not observed. In Petri dish with in situ water at room temperature, cell normally moves by crawling over debris but swims away by rotation about longitudinal axis in spirals (about 70 μm across) when disturbed (Fig 4D).
Somatic ciliature composed of the following three parts: GK, CK, and VK (Fig 4E). Girdle kinety, consisting of 37–52 dikinetids, curves toward posterior on dorsal side and makes one helical circuit around cell in dextral direction. GK extends from right end of AZM, crosses dorsal surface of cell, continues around left side, and crosses ventral surface to point almost directly posterior to its anterior end (Figs 4E–4I; 5I–5M). Circular kinety consisting of 25–34 dikinetids, occupying posterior 1/3 of left side of cell and forming circle with small gap in posterior end of cell through which one end of VK passes (Figs 4E and 4I; 5J–5L). Both kinetosomes of each dikinetid in GK and CK bear fusiform cilium, with left (anterior) one usually longer (approximately 3 μm long) than right (posterior) one (approximately 1 μm long) (Fig 5M). Ventral kinety located on posterior portion of right side of cell, extending from gap in CK along right side to posterior 1/5 of cell (Figs 4E, 4F and 4H; 5I). Ventral kinety composed of 10–16 dikinetids, with left (anterior) kinetosome of each dikinetid bearing short cilium approximately 2 μm long.
Buccal field conspicuously broad and deep, obliquely spanning 2/5 of length of cell (Figs 4A; 5A and 5C). Adoral zone of membranelles consists of 15–21 AM and 7–9 VM, which are continuous (Fig 4F). Cilia of AM approximately 15–20 μm long in living cells, splayed outward horizontally when cell is swimming (Figs 4A; 5B). Cilia of VM approximately 3–5 μm long. Bases of AM slightly longer (approximately 6 μm) than those of VM (approximately 4 μm) (Fig 4F). All membranelles comprise three kinety rows, except for two posteriormost membranelles which probably comprise only two rows. Endoral membrane on inner wall of buccal cavity extends to center of apical protrusion, approximately 10 μm long, apparently composed of single row of kinetosomes (Fig 4F). Pharyngeal fibres and thigmotactic membranelle not observed.
Molecular Phylogenetics
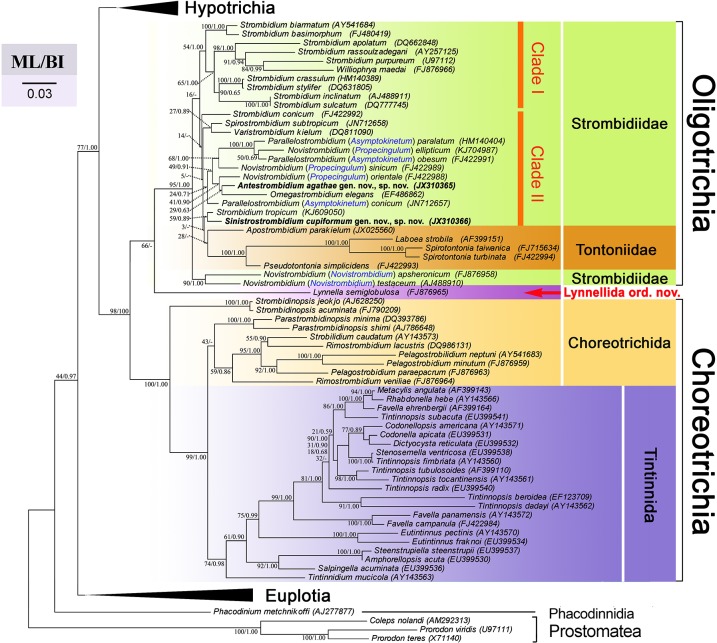
The SSrRNA gene sequences of Sinistrostrombidium cupiformum and Antestrombidium agathae were deposited into GenBank with accession numbers JX310366 and JX310365, respectively. In all our phylogenetic trees (Fig 6), monophyly of the subclasses Oligotrichia and Choreotrichia was supported strongly at their basal nodes, respectively (95% ML, 1.00 BI; 100% ML, 1.00 BI). Lynella semiglobosa branches basally off the Oligotrichia with low support (66% ML).
Fig 6. Maximum Likelihood tree inferred from SSrRNA gene sequences.
Numbers at the nodes are support values presented in the following order: Maximum Likelihood (ML) bootstrap values and Bayesian inference (BI) posterior probabilities. Nodes absent from BI tree are indicated by a hyphen. The scale bar indicates the number of substitutions per 10 nucleotides.
In the oligotrich clade, Novistrombidium apsheronicum and N. testaceum are basal to all other members of the clade. Most species of Strombidium form a poorly resolved assemblage (clade I, Fig 6) comprising three relatively well-supported subclades, with a clade consisting of S. basimorphum and S. biarmatum occupying a basal position. Williophrya maedai nests within one of these subclades (Fig 6). All other strombidiid species, together with tontoniids, form a second, poorly supported assemblage (clade II, Fig 6), consisting of four subclades. Strombidium conicum does not cluster with its congeneric species but branches at the base of the clade II, representing the first subclade. Spirostrombidium subtropicum and Varistrombidium kielum branch separately, forming the second subclade. Sinistrostrombidium cupiformum gen. nov., sp. nov. clusters with Strombidium tropicum on an isolated basal branch that is an unresolved sister to the poorly supported subclade containing tontoniids and Apostrombidium parakielum. The fourth subclade is composed of three groups. Antestrombidium agathae gen. nov., sp. nov. associates with Omegastrombidium elegans, which then clusters with Novistrombidium orientale (Fig 6). This group is sister to a poorly supported group containing Parallelostrombidium paralatum, P. ellipticum, P. obesum and N. sinicum. Parallelostrombidium conicum represents the third group in this subclade (Fig 6).
Discussion
Comparison of Sinistrostrombidium and Antestrombidium to Other Strombidiid Genera
The arrangement and composition of somatic kineties are regarded as important diagnostic characters of strombidiid genera [12, 23]. Ten genera are presently in the family Strombidiidae (Table 2) [8, 11, 12, 17–20].
Table 2. Morphological comparison of somatic kineties among the known genera in the family Strombidiidae.
| Genus | Number of somatic kinety fragments | Pattern of girdle or other kineties | ventral kinety | Source of data |
|---|---|---|---|---|
| Strombidium | 1 or 2 | Transverse | present | [18] |
| Novistrombidium | 2 | Dextrally spiraled | present | [17] |
| Spirostrombidium | 2 or 3 | Dextrally spiraled, with posterior portion of GK recurved and inversely parallel to VK | present | [12] |
| Parallelostrombidium | 2 | Dextrally spiraled, with posterior portion of GK parallel to VK | present | [12] |
| Omegastrombidium | 2 or 3 | Ω-shaped | present | [12] |
| Apostrombidium | 2 or 3 | Curved to posterior end of cell on both ventral and dorsal sides | absent | [11] |
| Varistrombidium | 5 | Obliquely and parallel across ventral side, with longest two extending to posterior end of dorsal side | absent | [20] |
| Opisthostrombidium | 2 | Oral primordium anterior to GK and extrusome stripe | present | [19] |
| Foissneridium | 2 | Oral primordium between GK and extrusome stripe | present | [19] |
| Williophrya | 2 | Horizontally oriented and bipartite | absent | [8] |
| Sinistrostrombidium gen. nov. | 2 | Sinistrally spiraled | present | Present work |
| Antestrombidium gen. nov. | 3 | Dextrally spiraled GK and circular CK | present | Present work |
CK-circle kinety; GK-girdle kinety; VK-ventral kinety;
Sinistrostrombidium gen. nov. can be distinguished easily from Novistrombidium, Parallelostrombidium, and Spirostrombidium by its sinistrally directed, helical GK, compared to the dextrally helical GKs of the latter genera [12, 17]. The GKs of Foissneridium, Omegastrombidium, Opisthostrombidium, and Strombidium are transversely circular or Ω-shaped rather than helical, and thus separate them from Sinistrostrombidium [12, 19]. Williophrya, Apostrombidium, and Varistrombidium have a GK that is split into two or more parts; thus, all three differ in a fundamental way from Sinistrostrombidium with a complete GK [8, 18, 20].
The pattern of the GK in Sinistrostrombidium represents a unique morphological novelty among members of the family Strombidiidae and, therefore, justifies establishment of the new genus. Furthermore, the validity of Sinistrostrombidium is supported by the fact that it branches separately from other strombidiid genera in our phylogenetic analyses, and its SSrRNA sequence shows relatively low similarity (93.0–97.8%) to and high pairwise distance (0.020–0.066) from those of other strombidiid species (Table 3).
Table 3. Similarities (first row) and pairwise distances (second row) between SSrRNA gene sequences of Sinistrostrombidium cupiformum gen. nov., sp. nov., Antestrombidium agathae gen. nov., sp. nov. and typical species of other strombidiid genera.
| Species | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| S. cupiformum | 97.0% | 93.0% | 95.8% | 97.6% | 97.8% | 95.6% | 97.5% |
| 0.026 | 0.066 | 0.038 | 0.021 | 0.020 | 0.039 | 0.021 | |
| A. agathae | 97.0% | 93.4% | 95.3% | 97.6% | 97.7% | 96.4% | 96.8% |
| 0.026 | 0.062 | 0.044 | 0.022 | 0.022 | 0.031 | 0.029 |
1, Strombidium conicum; 2, Williophrya maedai; 3, Novistrombidium testaceum; 4, Spirostrombidium subtropicum; 5, Parallelostrombidium conicum; 6, Omegastrombidium elegans; 7, Varistrombidium kielum.
The somatic kineties are composed of a GK and a VK or a GK alone in all strombidiid genera except Varistrombidium and Antestrombidium gen. nov. In the latter genus, the somatic kineties are distinctive by being differentiated into three parts: GK, VK and CK, a pattern that also differs markedly from the five somatic kineties of Varistrombidium [20]. Thus, A. agathae represents another morphological novelty within the family Strombidiidae, justifying establishment of a new genus for it. Likewise, its SSrRNA sequence shows relatively low similarity (93.4–97.6%) to and high pairwise distance (0.022–0.062) from those of other strombidiids (Table 3), which supports the validity of the new genus.
Evolutionary Hypothesis of Strombidiids Based on Molecular and Morphological Evidence
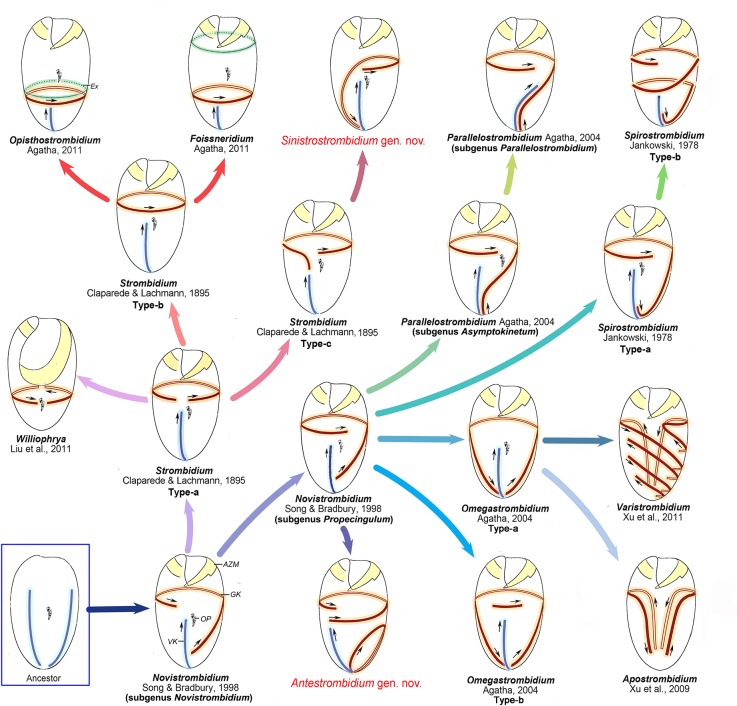
Up to now, 16 different ciliary patterns can be identified in 12 genera belonging to the family Strombidiidae (Fig 7, previous analyses suggested that Laboea should represent a member of family Tontoniidae rather than Strombidiidae and thus its ciliary pattern was not included here [21, 38, 39]) and SSrRNA gene sequences of 28 species of oligotrichs are available for analysis, which prompts us to offer an update of the evolutionary hypothesis of oligotrichs and relate it to morphotypes of strombidiids.
Fig 7. Hypothetical evolution of Strombidiidae based on an analysis of somatic ciliature.
Small arrows mark the orientation of the kineties. Legend: AZM-adoral zone of membranelles; Ex-extrusomes; GK-girdle kinety; OP-oral primordium; VK-ventral kinety.
The ontogenetic process can provide important evidence to understand the evolution of ciliary patterns in oligotrichs [12]. However, the ontogenetic data of most strombidiids are not available at present. On the other hand, the differences of somatic kineties in the location, length, number and spiral extent, which reflect the different triggers of ciliary pattern development during the ontogenesis such as split, migration and extension, could be important clues to deduce their evolutionary stages. Therefore, based on the comparison of ciliary patterns in strombidiids, their possible developing causes during evolution are presumed, and their evolutionary stages as well as evolutionary relevance are proposed.
In previous papers [12, 19, 21], the common ancestor of oligotrichs was hypothesized to have several longitudinal kineties which then were reduced to two kineties. These two kineties were assumed to be situated close to one another and shift to generate the pattern of Parallelostrombidium which was considered to be plesiomorphic relative to the pattern of kineties in other oligotrichs. However, this assumption does not agree with present and previous phylogenetic analyses based on SSrRNA gene sequences, in which species of Parallelostrombidium always branch late, while the subgenus Novistrombidium branches basally to other strombidiids with strong support [8, 25]. We agree with the general hypothesis that the somatic kineties in ancestral oligotrich were reduced to two kineties (the origin of GK and VK), but in our opinion, they should be separated rather than close to each other. Because in the ontogeny of strombidiids, the oral primordium is commonly situated between GK and VK, and the destinies of GK and VK are prominently different during the ontogenesis, i.e. GK spiraled with a dextral torsion of the oral primordium while VK remained longitudinally oriented, all this could have happened only when these two kineties were located separately in the ancestor. In addition, two separated kineties are also characteristic in Lynnella semiglobulosa [7] which branches basally to oligotrichs in phylogenetic trees (Fig 6), supporting that separated kineties are a plesiomorphy. Following our hypothesis, one of the two kineties which is near the proximal dextrally spiraled with a dextral torsion of the proximal end of the membranellar zone to form the GK, and the other located far away from the proximal remained longitudinally oriented on the ventral side to form the VK, as in the subgenus Novistrombidium (Fig 7). Based on the above analyses and molecular evidence (Fig 6), we suggest that the subgenus Novistrombidium pattern rather than the Parallelostrombidium pattern probably represents the ancestral state in the evolution of strombidiids. This hypothesis is supported by the ontogenetic pattern of known genera, in which the similar pattern of subgenus Novistrombidium is commonly recapitulated [12, 40]. By contrast, the closely parallel kineties in Parallelostrombidium are thus considered as a genus-specific feature that appears to have evolved convergently.
With further rightward torsion of the proximal in the ancestor, the anterior portion of the GK of the subgenus Novistrombidium pattern would extend to left and terminate at the left side of VK, giving rise to the pattern of the subgenus Propecingulum (Fig 7). This relative position of the anterior ends of GK and VK also appears in Parallelostrombidium and Spirostrombidium, and thus the Propecingulum pattern can be identified as probably ancestral to the patterns of the former genera. These were supported by the ontogenetic similarity in these three genera that the oral primordium is located between the stripes of extrusome attachment sites extending along anterior and posterior parts of girdle kinety respectively. The posterior GK of the Propecingulum pattern curved anteriad to lie parallel to the VK, giving rise to the type-a Spirostrombidium pattern (e.g. S. cinctum) as proposed in previous hypotheses, which then produced the type-b Spirostrombidium pattern (e.g. S. schizostomum) by extending the anterior end of GK to form two whorls (Fig 7). By contrast, the posterior GK of the Propecingulum pattern curved downward to lie parallel to the VK, giving rise to the pattern of the subgenus Asymptokinetum, which then produced the subgenus Parallelostrombidium pattern by orienting the anterior portion of VK obliquely to parallel with GK (Fig 7). In our phylogeny analyses, the close relationship of Asymptokinetum and Propecingulum is well confirmed, although Spirostrombidium is separated from Propecingulum (Fig 6).
We propose that the Antestrombidium pattern probably originated in parallel with Omegastrombidium pattern from the Propecingulum pattern (Fig 7). The strong evidence comes from the following. First, the circle kinety of Antestrombidium appears to be homologous to the Ω-shaped GK in Omegastrombidium. Second, the short extra kinety fragment usually located at the ventral center or around left shoulder area of cell in some individuals of Omegastrombidium (type-b pattern)[41] is generally similar to the GK of Antestrombidium. Furthermore, the orientation of the extra kinety matches that of the GK of Antestrombidium [41].
We hypothesize that the GK of Antestrombidium pattern and the extra kinety fragment of the Omegastrombidium type-b pattern, both of which have a dextrally spiraled orientation, were derived from the extended anterior portion of the GK of Propecingulum. This could have been accomplished by extension of the anterior portion of GK in Propecingulum followed by separation into two parts, the posterior part forming the Ω-shaped kinety by posterior migration of its right end and the anterior part developing into the GK of Antestrombidium and the extra kinety fragment of Omegastrombidium type-b. The Omegastrombidium type-a pattern that lacks an extra kinety fragment would have originated in an ancestor with the Propecingulum pattern in which the GK migrated directly without extending first. This hypothesis is well supported by our phylogenetic analyses, in which species of Omegastrombidium and Antestrombidium cluster together and species of Propecingulum branch basally (Fig 6). As in previous hypotheses [19], the Omegastrombidium type-a pattern gives rise successively to the Apostrombidium and Varistrombidium patterns (Fig 7). All genera derived from the Propecingulum pattern except for Apostrombidium cluster together in our phylogenetic analyses (Fig 6), which confirms their close evolutionary relationship; however, the trees do not reveal the relationship among them as yet.
Strombidium-like patterns constitute the other group in the evolutionary hypothesis of strombidiid oligotrichs (Fig 7). Three variations of the Strombidium pattern can be identified, characterized by the following different conformations of GK: 1) the type-a Strombidium pattern has a C-shaped GK with a small ventral gap, as in S. capitatum; 2) the type-b Strombidium pattern has a somatic ciliature with a closed GK that is typical for Strombidium, as in S. sulcatum; and 3) the Type-c Strombidium pattern has a ventral open GK, with its right end curved slightly toward the posterior, as in S. apolatum. We agree with Agatha’s [19] hypothesis that the type-a Strombidium pattern was derived first from Novistrombidium by migration of the left portion of GK toward the anterior. Three further evolutionary lineages (Fig 7) were suggested by our analyses. First, the C-shaped GK of the type-a Strombidium pattern closed, forming the circular GK of the type-b Strombidium pattern. Foissneridium and Opisthostrombidium then developed from the type-b Strombidium pattern as suggested by Agatha [19] when the horizontal GK migrated toward the posterior below the oral primordium, with the stripe of extrusome attachment sites above or below the oral primordium. Second, the Williophrya pattern originated when the GK of the type-a Strombidium pattern split dorsally into two fragments, together with loss of the VK. This hypothesis is corroborated by our molecular analyses, in which Williophrya nested within Strombidium (Fig 6). Third, the right end of GK in an ancestor with the type-a Strombidium pattern curved toward the posterior, producing the type-c Strombidium pattern. The Sinistrostrombidium pattern was assumed to originate from the Strombidium pattern by elongation and sinistral spiraling of the right portion of GK. This could be confirmed by the transition stage type-c Strombidium patterns. In our phylogenetic trees, the association of Sinistrostrombidium with Strombidium subtropicum which has a type-c pattern [42], strongly supports this hypothesis (Fig 6).
In general, our evolutionary hypothesis of ciliary patterns in strombidiids is supported by the molecular phylogeny. The two hypothetical evolutionary pathways are in basic agreement with the topology of the two main strombidiid clades; however, some evolutionary relationships within these clades (e.g., Apostrombidium and Omegastrombidium patterns are closely related in the hypothesis) are not supported by the molecular tree, and the nesting of tontoniids within strombidiid clade II (Fig 6) does not match the morphological analysis. Previous evolutionary hypotheses also were tested by molecular phylogenetic analyses [19, 21], which yielded the same poor match between morphological and molecular evidence. At present, topologies of trees constructed from available sequences of oligotrichs tend to vary, depending on number of samples included or the analytic methods used. This, as well as the low support values for basal nodes in all molecular studies performed so far, suggests that there are still many gaps in the molecular evidence for SSrRNA trees to provide an appropriately rigorous test of conclusions inferred from morphological inference.
Beside the morphological and molecular evidence, some ontogenetic data supply important support for the evolutionary hypothesis in our study. However, the detailed ontogeny of most species are not available at present. With the increase of more comprehensive data combining morphology, molecular, ontogeny and ultrastructure, we can better understand about the evolution of the girdle kinty patterns in strombidiids.
Remarks on the Phylogenetic Position of Lynnella
The family Lynnellidae was established for the morphospecies Lynnella semiglobulosa [7], but its suprafamilial classification has remained uncertain. Lynnella has an open AZM like that of oligotrichs but lacks the differentiation of AM and VM, which is characteristic of them [7]. Furthermore, its ellipsoidal macronuclear nodules and longitudinally arranged somatic kineties are characteristic features of choreotrichs.
In previous studies, the position of Lynnella has not been stable in phylogenetic trees. It clustered with oligotrichs in some analyses [8, 24] and with choreotrichs in others [7, 9, 21, 39], albeit with relatively higher support in ML and BI trees (95/0.91 and 79/1.00) for the former relationship vs. 65/0.61, 79/1.0 and 71/0.99 for the latter. In the present study, Lynnella clusters with oligotrichs in a basal position in the ML tree with low support, but in BI trees, the relationship between Lynnella, oligotrichs, and choreotrichs is completely unresolved. In addition, our analyses showed that neither hypothesis of Lynnella falling into oligotrich clade nor choreotrich clade was rejected by AU (P = 0.169 and 0.086, respectively) or SH tests (P = 0.742 and 0.438, respectively). In summary, therefore, it remains impossible to assign Lynnella to a subclass. Analysis of molecular characters from the SSrRNA alignment reveals four unique nucleotide identities between Lynnella and oligotrichs, and two between Lynnella and choreotrichs (Fig 8). Moreover, 12 nucleotides in the sequence of Lynnella differ from both oligotrichs and choreotrichs (Fig 8). This evidence suggests that Lynnella may represent an evolutionary lineage separate from both the subclasses Oligotrichia and Choreotrichia, but more data will be needed to confirm this.
Fig 8. Unique nucleotide signatures in the SSrRNA gene sequences of oligotrichs, Lynnella, and choreotrichs.
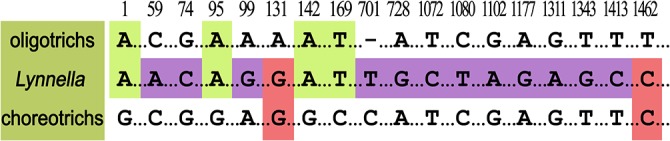
Positions of nucleotides in the alignment are given at the top. Gaps are indicated by dashes.
In regard to its ordinal classification, Lynnella can be excluded from all three known orders in oligotrichs s. lat. by the following differences: 1) Oligotrichida has an adoral zone of membranelles with differentiation of anterior and ventral membranelles, but ventral membranelles are absent in Lynnella; 2) in Choreotrichida, the adoral zone of membranelles form a closed circle, but they are open on the ventral side in Lynnella; and 3) Tintinnida has a lorica covering the cell body, but there is no lorica in Lynnella) [23]. Furthermore, Lynnella does not cluster reliably with any of the known orders of oligotrichs s. lat. in any phylogenetic analysis based on SSrRNA gene sequences [7–9, 21, 25]. Therefore, there is more than enough evidence to justify establishment of a new order, Lynnellida ord. nov. for Lynnella. In a previous paper [11], we classified L. semiglobulosa into the order Lynnellida but did not provide a formal description of the new order. This omission is corrected here.
Class Spirotrichea
Order Lynnellida ord. nov.
Diagnosis. Adoral zone of membranelles open without differentiation of anterior and ventral membranelles.
Type family. Lynnellidae Liu et al., 2011.
Remarks. In choreotrichs, a minute ventral gap like that in Lynnella also appears in the AZM of Parastrombidium and Parastrombidinopsis, and in trees based on morphological characters alone [9], Lynnella is an unresolved sister to both genera in a moderately supported clade. These three genera thus were considered to have a close phylogenetic relationship, and the ventrally opened AZM was interpreted as synapomorphic retrogression to the plesiomorphic (open) state [9, 21]. However, there are obvious differences between Lynnella, Parastrombidium and Parastrombidinopsis in the AZM. In Lynnella, the proximal end of the AZM is located in the oral cavity below the distal end, and its endoral membrane is positioned longitudinally on the inner wall of the buccal lip, which is more like its appearance in oligotrichs and probably represents a plesiomorphy. By contrast, the AZMs of Parastrombidium and Parastrombidinopsis are only slightly opened, with the proximal end on the same horizontal level as the distal end, and the endoral membrane is absent or extends across the peristomial field, which is characteristic of other choreotrichs and considered to be a secondary apomorphy. These differences suggest that Lynnella is not closely related to Parastrombidium and Parastrombidinopsis, and may represent a divergent, plesiomorphic taxon either within the Oligotrichia or independent of both oligotrichs and choreotrichs. In our phylogenetic analyses, Lynnella is separated from Parastrombidinopsis (Fig 6), which indicates a deep divergence between them.
Data Availability
SSrDNA sequences files are available from the GenBank database (accession number(s) JX310365, JX310366). The remaining relevant data may be found within the paper and its Supporting Information files.
Funding Statement
Financial support was provided by the National Natural Science Foundation of China (projects numbers: 31222050, 31172060, 31201696, 31430077, 31471973), Research Fund for the Outstanding Young Teachers Program of Higher Education in Guangdong (Yq2013052), and the International Research Coordination Network for Biodiversity of Ciliates funded jointly by the U.S. National Science Foundation (oDEB) and the National Natural Science Foundation of China (project number: 31111120437). http://isisn.nsfc.gov.cn/egrantindex/funcindex/prjsearch-list; http://www.gdhed.edu.cn/publicfiles/business/htmlfiles/gdjyt/tzgg/201402/205392.html. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Dale T, Dahl E. Mass occurrence of planktonic oligotrichous ciliates in a bay in southern Norway. J Plankton Res. 1987;9:871–879. [Google Scholar]
- 2. Pierce RW, Turner JT. Ecology of planktonic ciliates in marine food webs. Rev Aquat Sci. 1992;6:139–181. [Google Scholar]
- 3. Fenchel T. The microbial loop– 25 years later. J Exp Mar Biol Ecol. 2008;366:99–103. [Google Scholar]
- 4. Liu W, Xu D, Lin X, Li J, Gong J, Al-Rasheid KA, et al. Novistrombidium sinicum n. sp. and Novistrombidium orientale n. sp. (Protozoa: Ciliophora): Two new oligotrich ciliates from a mangrove wetland, South China. J Eukaryot Microbiol. 2009;56:459–465. 10.1111/j.1550-7408.2009.00425.x [DOI] [PubMed] [Google Scholar]
- 5. McManus GB, Xu D, Costas BA, Katz LA. Genetic identities of cryptic species in the Strombidium stylifer/apolatum/oculatum cluster, including a description of Strombidium rassoulzadegani n. sp. J Eukaryot Microbiol. 2010;57:369–378. 10.1111/j.1550-7408.2010.00485.x [DOI] [PubMed] [Google Scholar]
- 6. Agatha S. Global diversity of aloricate Oligotrichea (Protista, Ciliophora, Spirotricha) in marine and brackish sea water. PloS ONE. 2011;6:e22466 10.1371/journal.pone.0022466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu W, Yi Z, Lin X, Al-Rasheid KA. Morphologic and molecular data suggest that Lynnella semiglobulosa n. g., n. sp. represents a new family within the subclass Choreotrichia (Ciliophora, Spirotrichea). J Eukaryot Microbiol. 2011;58:43–49. 10.1111/j.1550-7408.2010.00519.x [DOI] [PubMed] [Google Scholar]
- 8. Liu W, Yi Z, Warren A, Al-Rasheid KAS, Al-Farraj SA, Lin X, et al. Taxonomy, morphology and molecular systematics of a new oligotrich ciliate, Williophrya maedai gen. nov., sp. nov., with redescriptions of Strombidium basimorphum and Pseudotontonia simplicidens (Protozoa, Ciliophora, Oligotrichia). Syst Biodiver. 2011;9:247–258. [Google Scholar]
- 9. Agatha S, Struder-Kypke MC. Reconciling cladistic and genetic analyses in choreotrichid ciliates (ciliophora, spirotricha, oligotrichea). J Eukaryot Microbiol. 2012;59:325–350. 10.1111/j.1550-7408.2012.00623.x [DOI] [PubMed] [Google Scholar]
- 10. Xu D, Sun P, Shin MK, Kim YO. Species boundaries in tintinnid ciliates: a case study—morphometric variability, molecular characterization, and temporal distribution of Helicostomella species (Ciliophora, Tintinnina). J Eukaryot Microbiol. 2012;59:351–358. 10.1111/j.1550-7408.2012.00625.x [DOI] [PubMed] [Google Scholar]
- 11. Song W, Li J, Liu W, Jiang J, Al-Rasheid KA, Hu X. Taxonomy, morphology and molecular systematics of three oligotrich ciliates, including a description of Apostrombidium parakielum spec. nov. (Ciliophora, Oligotrichia). Inter J Syst Evol Microbiol. 2013;63:1179–1191. [DOI] [PubMed] [Google Scholar]
- 12. Agatha S. Evolution of ciliary patterns in the Oligotrichida (Ciliophora, Spirotricha) and its taxonomic implications. Zoology. 2004;107:153–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lynn D. The Ciliated Protozoa. Characterization, Classification, and Guide to the Literature. New York: Springer; 2008. 605 p. [Google Scholar]
- 14. Claparède E, Lachmann J. Études sur les Infusoires et les Rhizopodes. Mém Inst Nat Genevois. 1859;6:261–482. [Google Scholar]
- 15. Petz W, Song W, Wilbert N. Taxonomy and ecology of the ciliate fauna (Protozoa, Ciliophora) in the endopagial and pelagial of the Weddell Sea, Antarctica. Stapfia. 1995;40:1–223. [Google Scholar]
- 16. Song W, Bradbury PC. Studies on some new and rare reported marine planktonic ciliates (Ciliophora: Oligotrichia) from coastal waters in North China. J Mar Biol Ass UK. 1998;78:767–794. [Google Scholar]
- 17. Agatha S. Morphology and ontogenesis of Novistrombidium apsheronicum nov. comb. and Strombidium arenicola (Protozoa, Ciliophora): a comparative light microscopical and SEM study. Europ J Protistol. 2003;39:245–266. [Google Scholar]
- 18. Song W, Warren A, Hu X. Free-living ciliates in the Bohai and Yellow Seas Beijing: Science Press; 2009. 518 p. [Google Scholar]
- 19. Agatha S. Updated hypothesis on the evolution of oligotrichid ciliates (Ciliophora, Spirotricha, Oligotrichida) based on somatic ciliary patterns and ontogenetic data. Europ J Protistol. 2011;47:51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xu D, Sun P, Clamp J, Ma H, Song W. The establishment of a new oligotrich genus Varistrombidium gen. nov. and the morphology and phylogeny of a marine ciliate, Varistrombidium kielum (Maeda and Carey, 1985) nov. comb. (Protista, Ciliophora). Acta Zootaxon Sin. 2011;36:502–511. [Google Scholar]
- 21. Agatha S, Struder-Kypke M. What morphology and molecules tell us about the evolution of Oligotrichea (Alveolata, Ciliophora). Acta Protozool. 2014;53:77–90. [Google Scholar]
- 22. Wilbert N. Eine verbesserte Technik der Protargolimprägnation für Ciliaten. Mikrokosmos. 1975;64:171–179. [Google Scholar]
- 23. Agatha S. A Cladistic Approach for the classification of oligotrichid ciliates (Ciliophora: Spirotricha). Acta Protozool. 2004;43:201–217. [PMC free article] [PubMed] [Google Scholar]
- 24. Xu D, Sun P, Warren A, Noh JH, Choi DL, Shin MK, et al. Phylogenetic investigations on ten genera of tintinnid ciliates (Ciliophora: Spirotrichea: Tintinnida), based on small subunit ribosomal RNA gene sequences. J Eukaryot Microbiol. 2013;60:192–202. 10.1111/jeu.12023 [DOI] [PubMed] [Google Scholar]
- 25. Liu W, Yi Z, Li J, Warren A, Al-Farraj SA, Lin X. Taxonomy, morphology and phylogeny of three new oligotrich ciliates (Protozoa, Ciliophora, Oligotrichia) from southern China. Inter J Syst Evol Microbiol. 2013;63:4805–4817. [DOI] [PubMed] [Google Scholar]
- 26. Medlin L, Elwood HJ, Stickel S, Sogin ML. The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene. 1988;71:491–499. [DOI] [PubMed] [Google Scholar]
- 27. Jeanmougin F, Thompson J, Gouy M, Higgins D, Gibson T. Multiple sequence alignment with Clustal X. Trends Biochem Sci. 1998;23:403–405. [DOI] [PubMed] [Google Scholar]
- 28. Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- 29. Nylander JA. MrModeltest Ver.2. Evolutionary Biology Centre, Uppsala University, Sweden: 2004. [Google Scholar]
- 30. Posada D, Crandall KA. Modeltest: testing the model of DNA substitution. Bioinformatics. 1998;14:817–818. [DOI] [PubMed] [Google Scholar]
- 31. Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. [DOI] [PubMed] [Google Scholar]
- 32. Guindon S, Gascuel O. A simple, fast and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol. 2003;52:696–704. [DOI] [PubMed] [Google Scholar]
- 33.Swofford DL. PAUP*. Phylogenetic analysis using parsimony (*and other methods). Version 4. Sunderland, MA. 2002.
- 34. Page RDM. TREEVIEW: an application to view phylogenetic trees on personal computers. Comput Appl Biosci. 1996;12:357–358. [DOI] [PubMed] [Google Scholar]
- 35. Tamura K, Dudley J, Nei M, Kumar S. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. [DOI] [PubMed] [Google Scholar]
- 36. Shimodaira H. An approximately unbiased test of phylogenetic tree selection. Syst Biol. 2002;51:492–508. [DOI] [PubMed] [Google Scholar]
- 37. Shimodaira H, Hasegawa M. CONSEL: for assessing the confidence of phylogenetic tree selection. Bioinformatics. 2001;17:1246–1247. [DOI] [PubMed] [Google Scholar]
- 38. Gao S, Gong J, Lynn D, Lin X, Song W. An updated phylogeny of oligotrich and choreotrich ciliates (Protozoa, Ciliophora, Spirotrichea) with representative taxa collected from Chinese coastal waters. Syst Biodiver. 2009;7: 235–242. [Google Scholar]
- 39. Li J, Liu W, Gao S, Warren A, Song W. Multigene-based analyses of the phylogenetic evolution of oligotrich ciliates, with consideration of the internal transcribed spacer 2 secondary structure of three systematically ambiguous genera. Eukaryot Cell. 2013;12: 430–437. 10.1128/EC.00270-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xu D, Song W, Warren A. Morphology and infraciliature of two new species of marine oligotrich ciliates (Ciliophora: Oligotrichida) from China. J Nat Hist. 2006;40:1287–1299. [Google Scholar]
- 41. Song W, Wang M, Warren A. Redescriptions of three marine ciliates, Strombidium elegans Forentin,1901, Strombidium sulcatum Claparede & Lachmann,1859 and Heterostrombidium paracalkinisi Lei, Xu & Song, 1999 (Ciliophora, Oligotrichida). Europ J Protistol. 2000;36:327–342. [Google Scholar]
- 42.Liu W, Yi Z, Lin X, Li J, Al-Farraj AS, Al-Rasheid KA, et al. Morphology and molecular phylogeny of three new oligotrich ciliates (Protozoa, Ciliophora) from the South China Sea. Zool J Linn Soc. 2015;Accepted.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
SSrDNA sequences files are available from the GenBank database (accession number(s) JX310365, JX310366). The remaining relevant data may be found within the paper and its Supporting Information files.