Abstract
Background
Chronic liver injury triggers a progenitor-cell repair-response, and liver fibrosis occurs when repair becomes de-regulated. Previously, we reported that reactivation of the Hedgehog (Hh) pathway promotes fibrogenic liver-repair. Osteopontin (OPN) is a Hh-target, and a cytokine that is highly upregulated in fibrotic tissues, and regulates stem-cell fate. Thus, we hypothesized that OPN may modulate liver progenitor-cell response, and thereby, modulate fibrotic outcomes. We further evaluated the impact of OPN-neutralization on murine liver fibrosis.
Methods
Liver progenitors (603B and BMOL) were treated with OPN-neutralizing aptamers in the presence or absence of TGF–β, to determine if (and how) OPN modulates liver progenitor function. Effects of OPN-neutralization (using OPN-aptamers or OPN-neutralizing antibodies) on liver progenitor-cell response and fibrogenesis were assessed in three models of liver fibrosis (carbon tetrachloride, methionine-choline deficient diet, 3, 5,-diethoxycarbonyl-1,4-dihydrocollidine diet) by qRTPCR, Sirius-Red staining, hydroxyproline assay, and semi-quantitative double-immunohistochemistry. Finally, OPN expression and liver progenitor response were corroborated in liver tissues obtained from patients with chronic liver disease.
Results
OPN is over-expressed by liver progenitors in humans and mice. In cultured progenitors, OPN enhances viability and wound-healing by modulating TGF-β signaling. In vivo, OPN-neutralization attenuates the liver progenitor-cell response, reverses epithelial-mesenchymal-transition in Sox9+ cells, and abrogates liver fibrogenesis.
Conclusions
OPN upregulation during liver injury is a conserved repair-response, and influences liver progenitor-cell function. OPN-neutralization abrogates the liver progenitor-cell response and fibrogenesis in mouse models of liver fibrosis.
Keywords: fibrosis, hepatic, cytokine, treatment
Introduction
The occurrence of fibrosis (scar tissue accumulation) in chronic liver disease (CLD) presents a vast unmet clinical challenge. At present, there is no proven anti-fibrotic treatment that halts or reverses the progression of liver fibrosis (1, 2). Individuals with liver fibrosis are therefore, at risk of developing cirrhosis, and complications such as liver cancer and liver failure, for which the only potential treatment is a liver transplant (3, 4). Furthermore, the prevalence of CLD is predicted to increase in coming decades due to the global epidemic of major risk factors for non-alcoholic fatty liver disease (NAFLD), including type 2 diabetes mellitus and obesity (5). As such, fibrosis and cirrhosis complicating CLD is a major health and economic burden, and critically requires the identification and development of novel anti-fibrotic strategies.
Liver fibrosis is an excessive wound-healing or repair-response to CLD (6, 7), which includes (non)-alcoholic fatty liver disease (NAFLD or ALD), viral hepatitis (B and C), primary biliary cirrhosis (PBC), and primary sclerosing cholangitis. During CLD restoration of liver mass and function in response to hepatocyte-loss involves activation of progenitor cells within the liver (i.e. progenitor-associated repair response or ductular reaction) (8 – 10), which proliferate and differentiate into new hepatocytes and cholangiocytes (11, 12). This pool of progenitors is heterogeneous, compromising the resident liver progenitor cell (LPC) or oval cell residing in the Canals of Hering (12, 13), bone marrow-derived progenitors (14, 15), as well as hepatic stellate cells (HSC) (16). HSC are liver fibroblasts which normally transition into collagen-producing myofibroblasts when activated (17, 18), but have recently been recognized as a multi-potent progenitor that interacts with the liver progenitor pool (16); thus, providing an explanation for the fibrogenic outcomes which accompany progenitor-cell expansion during persistent liver injury (i.e. fibrogenic-repair). These cell culture and mouse observations are supported by longitudinal human studies, which show that the ductular reaction is predictive of subsequent fibrosis (9, 19). Hence, targeting the progenitor-associated repair response may be of value in inhibiting fibrosis in CLD.
Progenitor-cell activation is driven by a milieu of growth factors, and cytokines which accompany chronic liver disease (20, 21). Recently, we reported that injury-related re-activation of the hedgehog (Hh) pathway (a morphogen important for embryonic development) induces liver progenitors to proliferate, undergo epithelial-mesenchymal transition (EMT) (i.e. upregulating mesenchymal, while repressing epithelial genes), and secrete factors that activate neighboring progenitors and matrix-producing cells (22–25). Osteopontin (OPN) is one such molecule secreted by liver progenitors (26).
OPN is a Hh-target, and a matricellular protein that is highly upregulated in fibrotic skin, lungs, kidneys, and joints (27–30). Mice genetically-deficient in OPN develop less fibrosis after certain injuries, suggesting that OPN may be a direct effector of the fibrotic process. Not surprisingly, HSC express high levels of OPN to auto-regulate their fibrogenic phenotype (26). However, the highest expression of OPN is seen in cells located in the liver periportal regions, and recent studies show that OPN is a key regulator of bone-marrow progenitor/stem-cell fate (31). These led us to hypothesize that OPN modulates the progenitor-associated fibrogenic repair response during liver injury.
Despite prevailing data giving credence to OPN being an attractive anti-fibrotic target, and humanized antibodies to OPN being developed for inflammatory-joint diseases (30), no study has yet evaluated the impact of OPN neutralization in the treatment of fibrosis complicating CLD. Therefore, to evaluate these hypotheses, we studied the direct effects of OPN in cultures of liver progenitors, examined the effects of OPN-neutralization (by OPN-specific aptamers or OPN-neutralizing antibodies) in three murine models of liver fibrosis, and corroborated findings with analysis of liver tissues from patients with CLD.
Materials and Methods
Mice: Adult C57BL/6 wild-type (WT)
Models of Hepatic Fibrosis
Carbon Tetrachloride (CCL4)
Mice (n = 5/group) received twice-weekly intra-peritoneal injections of CCl4 (0.5 mg/kg, Sigma-Aldrich) for 6 weeks to induce liver fibrosis (32), or vehicle (mineral oil)
Methionine-Choline Deficient (MCD) diet
Mice (n = 5/group) were fed the MCD diet for 5 weeks to induce nonalcoholic steatohepatitis (NASH)-fibrosis, or control chow (24).
Model of Biliary Fibrosis
3, 5,-Diethoxycarbonyl-1,4-dihydrocollidine (DDC) diet
Mice (n =5/group) were fed the DDC-diet for 3 weeks to induce biliary-type fibrosis (33).
Osteopontin neutralization
OPN-specific aptamers
Three additional studies were performed (4th study: CCL4; 5th study: MCD; 6th study: DDC) (n =10/study; 5/group). OPN-specific aptamers (which specifically neutralize circulating-extracellular OPN) or sham-aptamers (negative control with mutated active binding site) (34) were administered to mice by tail-vein injections (total of 4 injections per mouse), during the final week of dietary or chemical challenge. A 200ug dose of sham or OPN-aptamers (in 100ul of PBS) was used as this was the dose previously shown to exhibit efficacy in vivo (34, 35). All mice were sacrificed 24 hours after the final dose of aptamers.
OPN-neutralizing antibodies
MCD-fed mice (n=5/group) were injected either control (IgG) or anti-OPN (R&D) in the final week, as described above (4 injections; 50ug/injection), using an amount of anti-OPN previously shown to be effective in reducing insulin-resistance in obese mice (36), and sacrificed 24 hours after the final injection.
Mice were housed in 12-hour-light/dark cycle with food and water ad libitum. Liver samples were obtained for RNA analyses and immunohistochemistry. Animal care and procedures were as per the NIH “Guide for the Care and Use of Laboratory Animals”, and approved by relevant institutions: Duke University Institutional Animal Care and Use Committees, Vrije Universiteit Brussel, Belgium (LA 123 02 12), University of Calgary Animal Care Committee, and the United Kingdom Home Office approval in accordance with the Animals (Scientific Procedures) Act of 1986 (University of Birmingham, PPL 40/3201).
Human study
FFPE liver sections were from de-identified controls and explanted liver tissues from individuals undergoing liver transplantation for NASH-cirrhosis, alcoholic liver disease (ALD)-cirrhosis, or primary biliary cirrhosis (PBC). Normal tissues were obtained from excess split-liver grafts. Freshly explanted and snap-frozen NASH, ALD, and PBC liver tissues (n =5/group) were used for total liver RNA analyses.
All studies using material from Duke University Hospital were conducted in accordance with NIH and Institutional guidelines for human subject research. Samples acquired from the Hepatobiliary Unit in Birmingham were studied in accordance with local ethical approval 04/Q2708/41 and REC 2003/242 from the South-Birmingham Research Ethics Committee, UK, and those obtained from University Hospital Essen, Germany under the local ethics commission (09-4252).
Cell culture / treatments, and Functional studies
Liver Immunohistochemistry & Molecular Techniques
See Suppl. Materials and Methods and Supplemental Tables 1–3.
Statistics
All data were expressed as mean ± SEM. Statistical analysis was performed using Student’s t test or one-way ANOVA as indicated. All analysis was conducted using Graph-Pad Prism 4 software (GraphPad Software Inc.). Significant differences were considered at P ≤ 0.05
Results
Osteopontin (OPN) is upregulated in human CLD and is expressed by Sox9+ liver progenitors
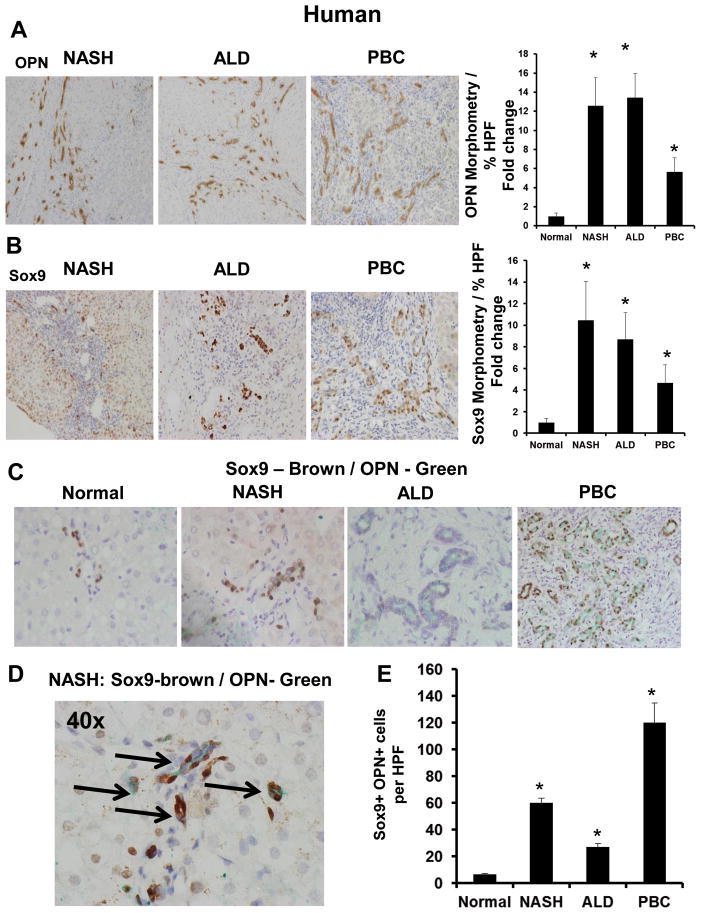
Coded liver sections from patients with NASH-cirrhosis (n=5), ALD-cirrhosis (n=5), and PBC (n=5) were stained to demonstrate OPN, Sox9, and K19. Compared with healthy livers, cirrhotic livers exhibit up to 15-fold more OPN protein (Fig 1A, Suppl Fig 1A) and 10-fold more OPN mRNA (Suppl Fig 1F). The highest level of OPN is expressed by cells located in the peri-portal regions. As expected, cirrhotic livers express more TGF-β mRNA than healthy livers (Suppl Fig 1F)
Figure 1. Osteopontin (OPN) is upregulated in human CLD and is expressed by Sox9+ liver progenitors.
Liver sections from patients with NASH-cirrhosis (n = 5), ALD-cirrhosis (n = 5), PBC (n = 5), and excess normal donor livers (n = 3), were stained for OPN, and Sox9. OPN-staining was analyzed by morphometry; the number of Sox9+ cells (stained nuclei) was counted in 20 randomly-chosen high-power fields (HPF)/section. Representative photomicrographs are shown. (A) OPN staining and OPN morphometry. (B) Sox9 staining and Sox9 morphometry. (C) Sox9 (Brown) and OPN (Green)–double-immunostaining. (D) High magnification of Sox9 / OPN–double-immunostaining in NASH-cirrhosis (black arrows) (x400). Sox9 / OPN quantification by cell counting; number of double-positive cells per HPF. Results are expressed as fold change relative to normal liver and graphed as mean ± SEM. *p<0.05 vs. normal liver
Because Sox9+ cells are bi-potent liver progenitors that arise from peri-portal Canals of Hering (37), we examined this LPC (oval cell) marker. As expected, cirrhotic livers contain up to 10-times more Sox9+ cells (Fig 1B, Suppl Fig 1B) and upregulate Sox9 mRNA by up to 5-fold (Suppl Fig 1F). Sox9+ cells also express K19 (another ductular progenitor marker) (Suppl Fig 1D). Compared with healthy control livers, cirrhotic livers accumulate up to 20-fold more Sox9/K19 double-positive liver progenitors (Suppl Fig 1D, E). Interestingly, the highest amount of OPN is expressed by liver progenitors (Fig 1C–E). Double immuno-labelling confirm that OPN+ cells co-localize with Sox9+ cells (Fig 1C–D), and cirrhotic livers are enriched with OPN/Sox9 double-positive progenitors by over 15-fold (Fig 1E).
OPN regulates viability/proliferation of liver progenitors
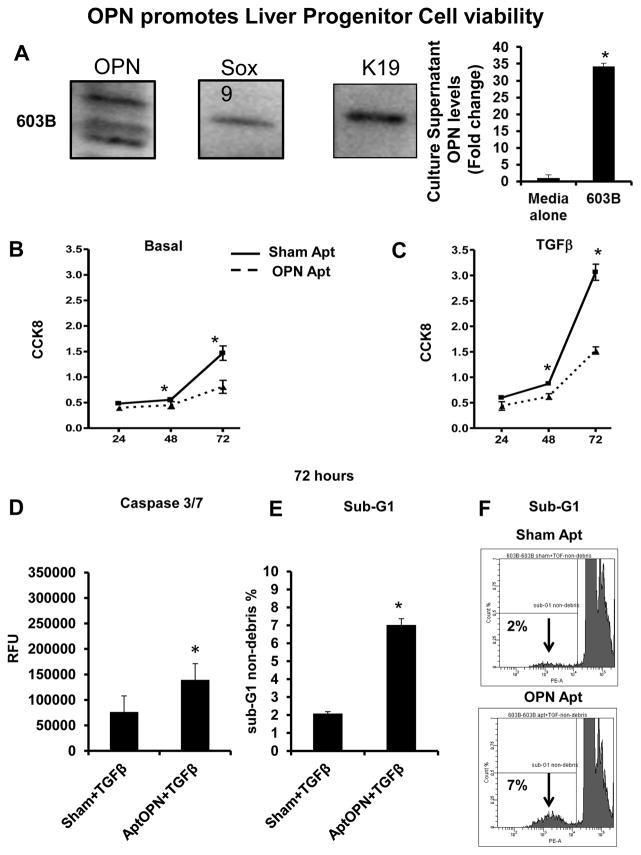
As the highest amounts of OPN were expressed by Sox9+ LPC in vivo (Fig 1), we evaluated the importance of OPN in LPC viability/proliferation. We utilized 603B cells (ductular progenitors) (24, 38), which co-express Sox9, K19 (markers of LPC), and OPN (Fig 2A). 603B supernatants contain high levels of OPN (Fig 2A). Treating 603B with OPN-aptamers or OPN-neutralizing antibodies (R&D) lowered OPN levels in culture supernatants (Suppl Fig 2A, B). OPN-aptamers reduced cell viability by 24 h, but this was most pronounced at 72 h (up to 2-fold reduction) when quantified by both CCK8 and cell count methods (Fig 2B and Suppl Fig 2C). Because liver fibrosis and cirrhosis is associated with upregulated TGF-β (Suppl Fig 1F) (and TGF-β modulates progenitor-cell differentiation (39)), we repeated OPN-neutralizing experiments under conditions of TGF-β stimulation. OPN-neutralization under pro-fibrogenic stimulation led to greater suppression of cell proliferation (3-fold; Fig 2C and Suppl Fig 2D), and markedly increased 603B apoptosis, as measured by the caspase 3/7 assay (Fig 2D, Suppl 2E); sub-G1 analysis by FACS (detecting DNA fragmentation) confirmed comparable increases in 603B apoptosis (3-fold; Fig 2E–F and Suppl Fig 2F).
Figure 2. OPN promotes 603B-LPC viability.
603B-LPC were analyzed for OPN, Sox9 and K19 by western blot, and 603B-conditioned medium (CM) assayed using OPN ELISA. In separate experiments, LPC were treated with sham or OPN-aptamers, in the presence or absence of TGF-β. Viability/proliferation was assessed using the CCK8 assay, and apoptotic activity evaluated by caspase-3/7 activity and by sub-G1 analysis (A) OPN, Sox9, and K19 protein expression; OPN levels in 603B-CM. (B) Cell numbers under basal (non-TGF-β) conditions; 24–72 h. (C) Cell numbers under TGF-β conditions; 24–72 h (solid line: sham-aptamer-treated; dashed line: OPN-aptamer-treated). Mean ± SEM (O.D) are graphed. (D) Caspase-3/7 under TGF-β conditions; 72 h. Mean ± SEM (RFU) are graphed. (E) Sub-G1 analysis under TGF-β conditions; 72 h. % of cells in sub-G1 are graphed (F) Representative sub-G1 histograms from sham or OPN-aptamer treated 603B at 72 h. All experiments were performed in triplicate. *p<0.05 vs. respective baseline
OPN enhances progenitor-associated wound-healing
OPN effects on LPC
Cumulative data suggest that liver progenitors are capable of re-programming into myofibroblasts (22, 24, 40). Therefore, we evaluated if changes in OPN levels would lead to similar alterations in progenitor-phenotype. 603B-LPC were treated with recombinant OPN or OPN-aptamers. OPN-neutralization led to a decrease in progenitor-associated fibrogenic genes, Snail and Collagen 1αI (Fig 3A), while upregulating epithelial genes, E-Cadherin and Id2 (Fig 3B). The addition of exogenous OPN, on the other hand, showed minimal effects on pro-EMT genes (data not shown), because 603B already express high levels of OPN (Fig 2A) and pro-EMT genes (26, 41).
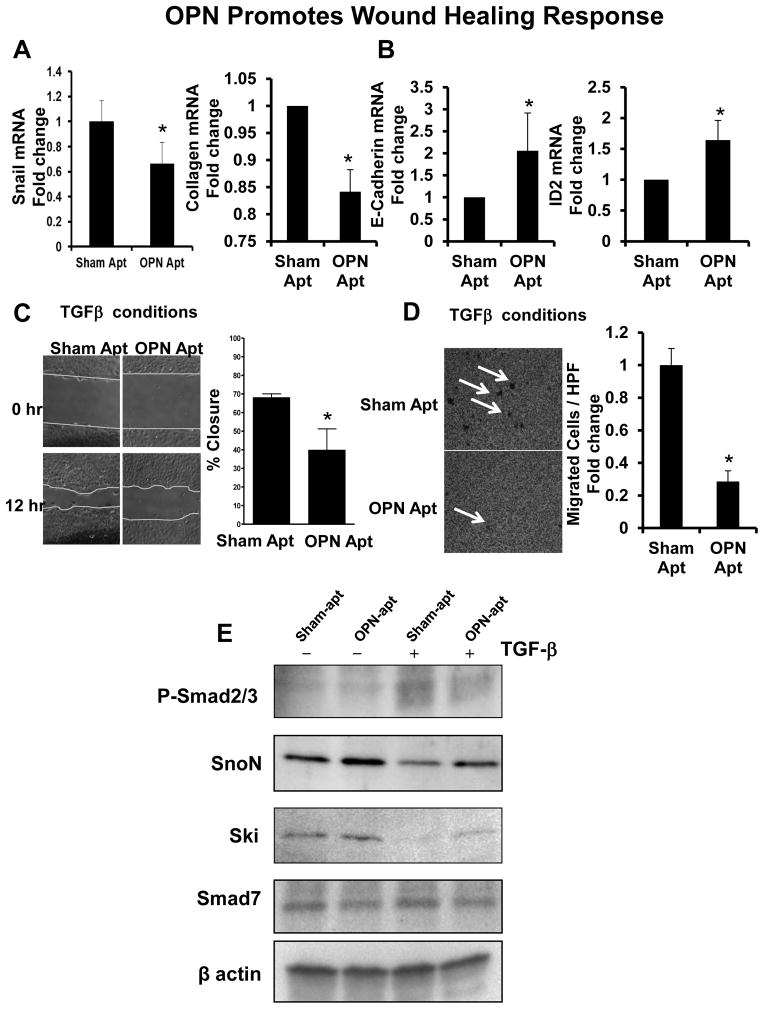
Figure 3. OPN enhances 603B progenitor-associated wound healing responses.
603B-LPC were treated with sham or OPN-aptamers, in the presence or absence of TGF-β for 48 h. Cells were analyzed for EMT markers. (A) Snail and Collagen 1αI mRNA. (B) E-cadherin and Id2 mRNA. Mean ± SEM were graphed. Wound-healing and transmigration studies were performed using conditions described (C–D). (C) Wound-healing under TGF-β conditions at time 0 (time of scratch) and 12 h later; migration was quantified by measuring the distance dividing the two sides of the monolayer. Mean (% wound closure) ± SEM were graphed. (D) Transmigration under TGF-β conditions was evaluated 24 h after seeding of cells, by counting crystal violet-stained cells on the underside membrane in 15 random HPF. Mean cell numbers ± SEM were graphed. *p<0.05 vs sham-aptamer. To determine if OPN modulated TGF-β signaling, 603B were treated as above, and protein analyzed. (E) Representative western blot: phospho-Smad 2/3, SnoN, Ski, Smad 7, and beta-actin. Quantitative data for these studies are shown in Supplemental Fig 5.
Increased cell-motility/migration are key phenotypic changes that accompany EMT (22, 42). Therefore, we compared 603B migration in OPN-neutralizing or control conditions. Migration was assessed by semi-quantitating the dimensions of a wound dividing the confluent monolayer 12 h after the scratch (Fig 3C, Suppl Fig 3A–B). Treatment with TGF-β led to enhanced wound shrinkage compared with vehicle (data not shown). OPN-neutralization under basal conditions led to ~30% less wound healing compared with controls (p=0.057) (Suppl Fig 3A–B), but this effect was significantly enhanced (>50% less wound healing) after TGF-β (Fig 3C). Comparable results were observed in the modified cell-invasion assay (Fig 3D and Suppl Fig 3C–D). OPN-neutralization under basal conditions led to 20% fewer 603B cells invading across the insert membrane (Suppl Fig 3C–D), but this was enhanced under TGF-β stimulation, where OPN-neutralization led to a repression of nearly 70% (Fig 3D).
To exclude the possibility that observed responses were 603B-specific, we repeated experiments using the bi-potential mouse oval liver (BMOL) line (43). We confirmed that BMOL cells resembled 603B-LPC, expressed OPN, Sox9, and K19 proteins (Suppl Fig 4A), and proliferated in response to OPN (Suppl Fig 4B). BMOL exhibited comparable wound-healing responses: OPN-neutralization inhibited wound closure and transmigration (Suppl Fig 4C), repressed Collagen 1αI (Suppl Fig 4D), while inducing E-Cadherin and Id2 mRNA (Suppl Fig 4E–F).
OPN is a complex molecule which consists of intracellular and extracellular/soluble OPN isoforms, which exhibit overlapping, but also different and opposing functions (44). OPN-aptamers neutralize only soluble OPN isoforms. We evaluated if additional loss of intracellular OPN by RNAi might reproduce OPN-aptamer effects on LPC phenotype. Compared with control (603B-shScr) (603B infected with lentiviral particles containing non-targeting scrambled shRNA), OPN knockdown (~50%) (603B-shOPN) was associated with a 2–3 fold reduction in cell proliferation under basal and TGF-β conditions (Suppl Fig 5A–D), with minimal changes in LPC apoptosis. shOPN abrogated 603B-LPC transmigration by 50% (Suppl Fig 5E), but did not affect wound healing response even under TGF-β conditions (data not shown), thus indicating functional differences between extracellular and intracellular OPN.
OPN modulates TGF-β signaling
Because the fibrotic liver is enriched with high levels of TGF-β (Suppl Fig 1), and the greatest effects of OPN-neutralization were observed under TGF-β conditions (Fig 2–3), we evaluated if OPN effects on LPC were mediated by modulating TGF-β signaling. Treating 603-LPC with TGF-β led to the accumulation of phospho-Smad-2/3 proteins (Fig 3E, Suppl Fig 6A), and to the preservation of Ski and SnoN (Fig 3E, Suppl Fig 6B–C), transcriptional co-repressors which inhibit transcriptional activity of TGF-β-dependent Smad-2/3-complexes under basal conditions (45). Both Smad phosphorylation and Ski and SnoN levels were unaffected by the addition of the sham-aptamers. In contrast, treatment with OPN-specific aptamers led to reduced levels of phospho-Smad-2/3 proteins (Fig 3E, Suppl Fig 6A), while protecting Ski and SnoN from TGF-β-induced degradation (Fig 3E, Suppl Fig 6B-C). No changes were observed with Smad7 protein (Fig 3E, Suppl Fig 6D), another negative feedback mechanism that regulates the TGF-β signal (46).
To better define how OPN modulates TGF-β signaling, we targeted (putative extracellular OPN-receptors on progenitor cells) (47), treating 603B-LPC with CD44-neutralizing antibody, and αvβ3 antagonist XJ735. CD44 and αvβ3 blockade resulted in a 30% reduction in phospho-Smad-2/3 expression (Suppl Fig 6E), resembling the effects of OPN neutralization with OPN-aptamers. However, general depletion of OPN (intracellular) using shOPN (i.e. 603B-shOPN) did not repress phospho-Smad-2/3 expression (data not shown), implying that widespread OPN knockdown could only recapitulate some effects of OPN-neutralization with OPN-aptamers, and reinforcing the concept that extracellular OPN is different from intracellular OPN.
OPN effects on Hepatic Stellate Cell
Liver progenitors comprise not only of LPC (oval cell), but HSC, a fibroblast and recently recognized multi-potent progenitor (16). When activated, HSC undergo transition to become myofibroblasts (25, 40). LPC and HSC are in close proximity, suggesting that both progenitor-cell populations are capable of crosstalk. We therefore evaluated whether reduced levels of OPN in the microenvironment alters the ‘LPC- secretome’ that influences HSC phenotype. 603Bs were treated with OPN-aptamers or sham-aptamers, in the presence of TGF-β for 48 hours; 603B-conditioned media (CM) were then harvested and added to primary HSC for 24 hours. As expected, the addition of 603B-CM to HSC resulted in activation of HSC by upregulating αSMA and collagen 1α1 mRNA by 2 fold (Suppl Fig 7A, B). HSC activation was enhanced when treated with TGF-β–stimulated 603B-CM. The addition of OPN-aptamers to 603B resulted in a significantly altered secretome: CM from OPN-aptamer-treated 603B induced a significantly attenuated fibrogenic-response in HSC (to almost quiescent) compared with CM obtained from sham-aptamer-treated 603B, with 4-fold and 5-fold less αSMA and collagen 1α1 mRNA under basal and TGF-β–stimulated conditions, respectively (Suppl Fig 7A, B). Experiments repeated using the human liver myofibroblast line (Lx2) revealed comparable findings: CM from OPN-aptamer-treated 603B had diminished activating capacity on Lx2 cells (lower αSMA mRNA by 40% and lower collagen 1αI mRNA by 50%) (Suppl Fig 7C, D).
To address concerns that effects on HSC could be related to residual OPN-aptamers in the 603B-CM, we used CM obtained from 603B-shOPN (OPN knockdown) and 603B-shScr (control) cells, treated with or without TGF-β (Suppl Fig 7A, B). Under basal conditions, CM derived from 603B-shOPN activated primary HSC (αSMA and collagen 1α1 mRNA) approximately 2-fold less than CM-603B-shScr (Suppl Fig 7A, B). These differences in CM-effects were reduced under TGF-β conditions (~1.5 fold). In a further experiment, 603B-shOPN and 603B-shScr were treated with either sham-aptamers or OPN-aptamers for 48 hours, and respective CMs collected to treat HSC. The addition of OPN-aptamers virtually abolished HSC activation (αSMA and collagen 1α1 mRNA levels were lower than untreated HSC). In aggregate, these data implicate the importance of OPN in modulating the LPC response.
Finally, to confirm that OPN neutralization has a direct impact on HSC, we treated primary HSC directly with sham-aptamers or OPN-aptamers. OPN neutralization with OPN-aptamers led to 50% decrease in αSMA and collagen 1α1 mRNA under basal conditions (Suppl Fig 7E, F), but up to 4-fold downregulation under TGF-β stimulation.
In sum, OPN is a critical factor that modulates the LPC niche, by modulating progenitor-cell and HSC responses, via direct and indirect mechanisms.
OPN neutralization ameliorates the liver progenitor-cell response and fibrogenesis in Mice
a) Accumulation of OPN+ (Sox9+) LPC in liver fibrosis (validation)
CCl4 and MCD models
i) Fibrosis
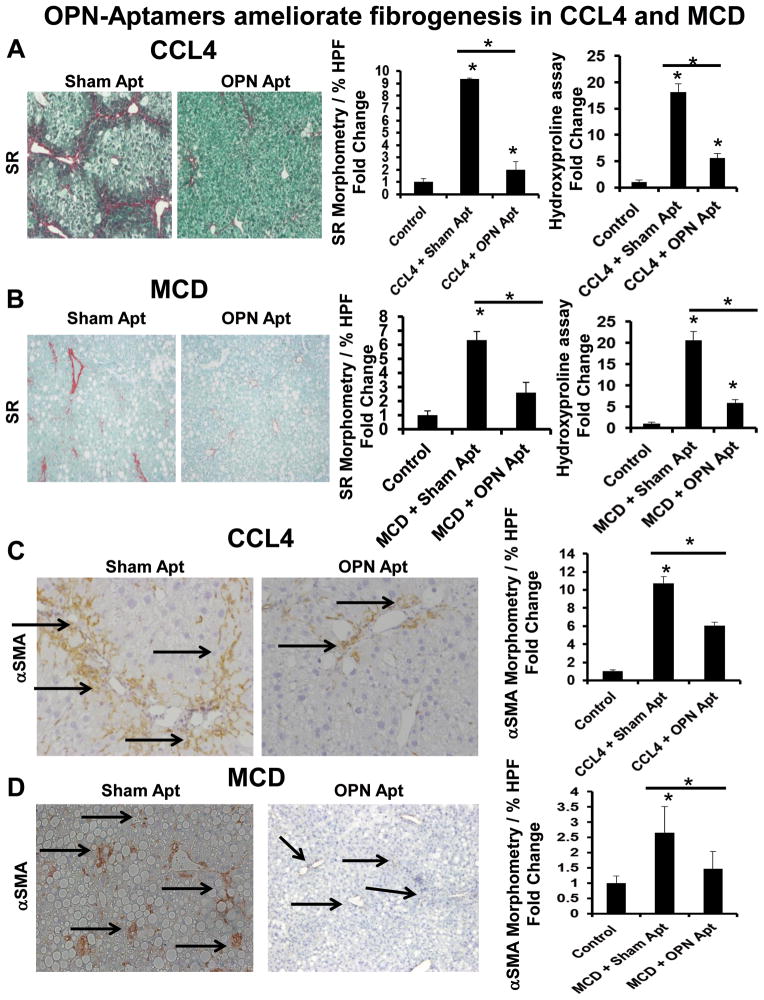
CCl4 and MCD-treated mice developed significant liver fibrosis (Fig 4, Suppl Fig 8, 9). This was demonstrated by increased Sirius Red (SR)-staining (8-fold) (Fig 4A, B) and hepatic hydroxyproline quantification. Collagen deposition was accompanied by the accumulation of αSMA+ cells (10-fold) (Fig 4C, D), and induction of key fibrogenic genes, αSMA, Collagen 1αI, and TGF-β1 (Suppl Fig 9).
Figure 4. OPN-aptamers ameliorate fibrogenesis in CCL4 and MCD diet-treated mice.
CCL4: In one group, mice (n =5/group) received twice-weekly injections of olive oil (control) or CCl4 for 6 weeks. In another group, mice receiving CCL4 were administered sham or OPN-aptamers (n =5/group) during the final week of CCL4. MCD: one group of mice (n=5/group) was fed control-chow or the methionine-choline deficient (MCD) diet for 5 weeks. Another group fed the MCD diet was treated with sham or OPN-aptamers (n =5/group) in the final week of dietary-challenge. Mice were sacrificed 24 h after final dose of aptamers, and livers analyzed. Representative staining are shown. (A–B) Sirius-red staining with morphometry, and liver hydroxyproline measurements in CCL4-treated (A), and MCD-fed (B) mice. (C–D) αSMA immunoreactivity (black arrows) and morphometry (20x fields for analysis) in CCL4-treated (C), and MCD-fed (D) mice. Results were expressed as fold change relative to control mice and graphed as mean ± SEM. *p<0.05 vs. control mice
ii) Liver progenitors
Pertinently, liver fibrosis was associated with a 6–10 fold increase in OPN+ cells (Fig 5A, 6A, Suppl Fig 8C), and an exuberant progenitor-response: 5-fold more Sox9+ LPC (Fig 5B, 6B, Suppl Fig 8D), 4-fold more Sox9 mRNA (Suppl Fig 10A, C), and ~2-fold more K19 mRNA (Suppl Fig 10B, D). Specifically, there was a 5 to 10-fold enrichment of OPN+ cells which co-expressed Sox9, the LPC marker (Fig 5C, 6C).
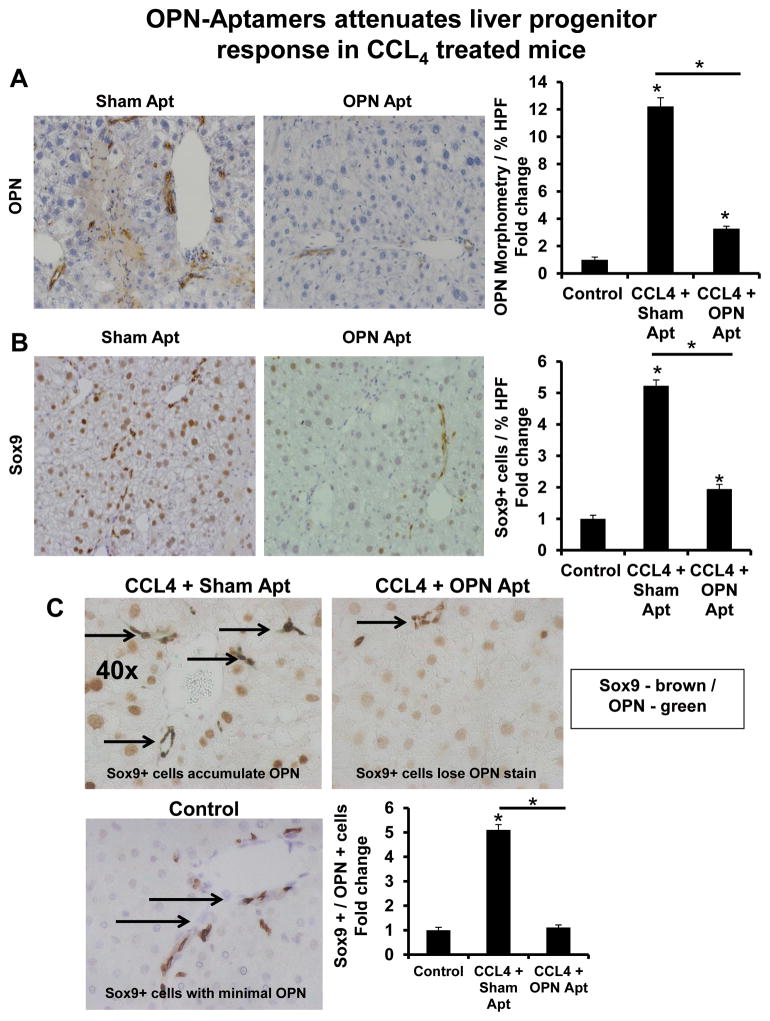
Figure 5. OPN-aptamers attenuate LPC response in CCL4-treated mice.
Mice were treated as described in Figure legend 4. Livers were harvested for IHC. Representative staining displayed. (A) OPN-staining and OPN-quantification (B) Sox9 staining and Sox9-quantification; % of positive cells per HPF. (C) Double immuno-staining for Sox9 (Brown) and OPN (Green). (Bottom left panel) Sox9 / OPN double staining in control mice. (Top left panel) Sox9 / OPN double staining (accumulation of Sox9 / OPN double staining) in CCL4-treated mice receiving sham-aptamers. (Top right panel) Sox9 / OPN double staining (loss of OPN staining) in CCL-treated mice receiving OPN-aptamers. (Bottom right) Sox9 / OPN quantification by cell counting; number of double-positive cells per HPF. Results are expressed as fold change relative to control mice and graphed as mean ± SEM. *p<0.05 vs. control mice. Black arrows indicate Sox9 positive cells which co-express (or should co-express) OPN.
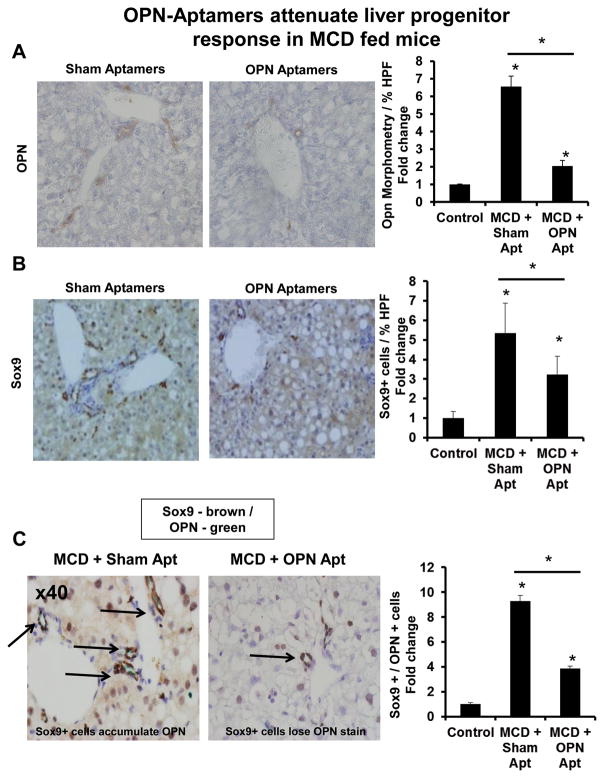
Figure 6. OPN-aptamers attenuate LPC response in MCD diet-fed mice.
Mice were fed control chow or the MCD diet for 5 weeks, in the presence of sham or OPN-aptamers, as described in Figure legend 4. Livers were harvested for IHC. Representative staining are shown. (A) OPN staining and OPN quantification by morphometric analysis. (B) Sox9 staining and Sox9 quantification by cell counting; % of positive cells per HPF. (C) Sox9 (Brown) and OPN (Green) – double immunostaining (magnification x400). (Left panel) Accumulation of Sox9 / OPN double positive cells in MCD-fed mice receiving sham-aptamers; (Right panel) Fewer Sox9 / OPN double positive cells in MCD-fed mice receiving OPN-aptamers. Graph shows Sox9 / OPN quantification by cell counting; number of double-positive cells per HPF. Results are expressed as fold change relative to control mice and graphed as mean ± SEM. *p<0.05 vs. control mice. Black arrows indicate Sox9 positive cells which co-express (or should co-express) OPN.
iii) Liver progenitor re-programming
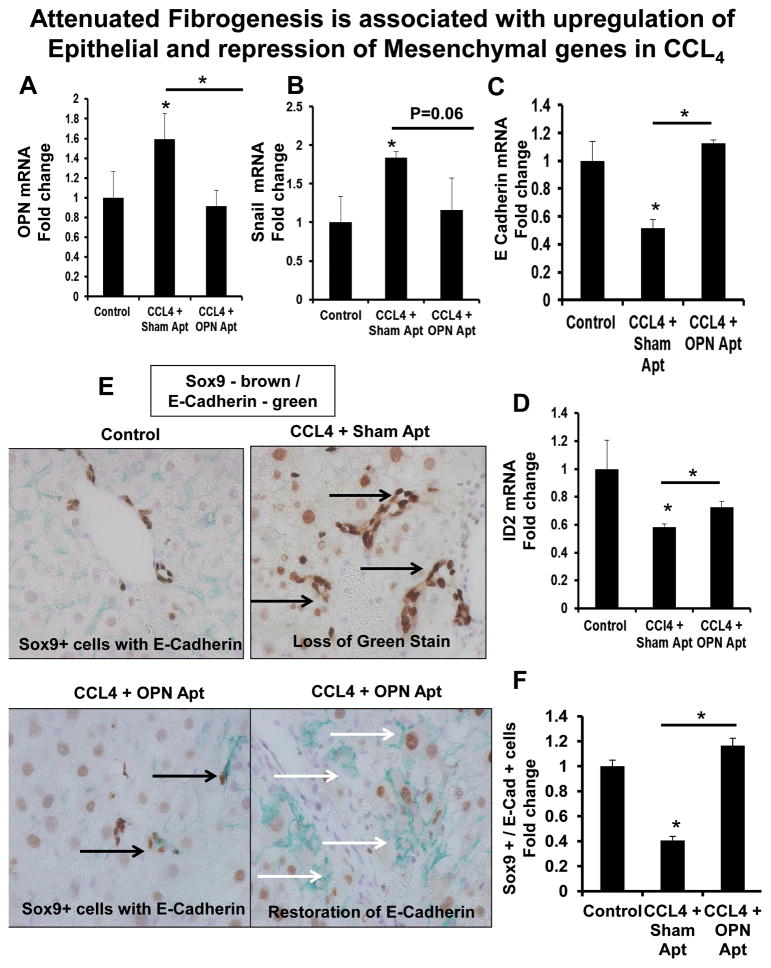
Liver fibrosis was accompanied by the upregulation of mesenchymal markers, OPN and Snail (Fig 7A–B, Suppl Fig 11A–B), and a downregulation of epithelial markers, E-cadherin and Id2 (Fig 7C–D, Suppl Fig 11C–D). Double immuno-labelling identified Sox9+ LPC which co-expressed E-Cadherin (Fig 7E–F, Suppl Fig 11E–F) under basal conditions; expression of E-Cadherin was significantly repressed during fibrogenesis.
Figure 7. OPN-aptamers upregulate epithelial and repress mesenchymal genes in CCL4-treated mice.
Mice were treated as described in Figure legend 4. (A) OPN mRNA. (B) Snail mRNA. (C) E-Cadherin mRNA. (D) Id2 mRNA. Results were expressed as fold change relative to control-treated mice and graphed as mean ± SEM. Livers were stained for Sox9 (Brown) and E-Cadherin (Green) (E-F). (E) (Top left) Sox9/E-Cadherin double-staining in control mice; (Top right) Loss of E-Cadherin staining in CCL4 mice with sham-aptamers; (Bottom left) Re-expression of E-Cadherin in Sox9+ cells in OPN-aptamer-treated mice; (Bottom right) Re-expression of membranous-E-Cadherin in OPN-aptamer-treated mice. (F) Sox9/E-Cadherin quantification by cell counting; number of double-positive cells/HPF. Results are expressed as fold change relative to control mice and graphed as mean ± SEM. *p<0.05 vs. control mice. Black arrows indicate Sox9 positive cells which co-express (or should co-express) E-Cadherin. White arrows indicate membranous-expression of E-Cadherin.
DDC model
Biliary-type fibrosis was induced by the DDC-supplemented diet (33). Increased SR-staining and liver hydroxyproline content (Suppl Fig 12A) was similarly associated with a greater than 10-fold enrichment in the number of αSMA+ cells (Suppl Fig 12B) and an upregulation in αSMA, Collagen 1αI, and TGF-β1 mRNA (Suppl Fig 12C–E). There was an accumulation of OPN+ and Sox9+ cells (over 10-fold) (Suppl Fig 13A, B), and OPN/Sox9 double-positive cells (up to 6-fold) (Suppl Fig 13C). These changes were accompanied by upregulation in OPN (~14-fold) (Suppl Fig 13D), and Sox9 mRNA (~40% increase) (Suppl Fig 13E).
b) OPN neutralization attenuates the liver progenitor response and ameliorates fibrogenesis (interventional)
CCl4 and MCD models
i) Fibrosis
Aptamers were administered during the final week of liver injury. No mice died. OPN-neutralization ameliorated liver fibrosis in both CCl4-treated, and MCD-fed mice. OPN-aptamers significantly repressed hepatic hydroxyproline content and the amount of SR-stained fibrils (Fig 4A, B) by 4–5 fold, reduced αSMA+ cells by 50–80% (Fig 4C, D), and downregulated αSMA (80–90%), Collagen 1αI (50–100%), and TGF-β1 (50–90%) mRNA (Suppl Fig 9).
ii) Liver progenitors
Inhibited fibrogenesis was accompanied by fewer liver progenitors (Fig 5, 6, Suppl Fig 10). OPN+ cells and Sox9+ cells were ~4-fold and ~2–3-fold fewer, respectively, after OPN-neutralization (Fig 5A, B, 6A, B). There was parallel repression of Sox9 and K19 mRNA levels (Suppl Fig 10).
iii) Liver progenitor re-programming
OPN-neutralization also downregulated mesenchymal markers, OPN and Snail (Fig 7A, B, Suppl Fig 11A, B) while upregulating epithelial markers, E-cadherin and Id2 (Fig 7C–D, Suppl Fig 11C, D). Immunostaining further revealed the restored-expression of membranous-E-Cadherin, and greater number of Sox9/E-Cadherin double-positive cells in OPN-aptamer-treated mice, to near normal levels (Fig 7E–F, Suppl Fig 11E, F). This was mirrored by a reduction in Sox9/OPN double-positive cells to basal levels (Fig 5C, 6C), thus confirming reversal of the fibrogenic phenotype.
DDC model
Comparable outcomes were noted in DDC-fed mice: OPN-aptamer treatment led to ~4 fold less hepatic hydroxyproline, ~2-fold less SR-stained fibrils (Suppl Fig 12A), ~3-fold fewer αSMA+ cells (Suppl Fig 12B), and repression of αSMA (3-fold), Collagen 1αI (~25%), and TGF-β (~30%) mRNA (Suppl Fig 12C–E). This was accompanied by an attenuated LPC response: fewer OPN+ (~4-fold) (Suppl Fig 13A), Sox9+ (~3-fold) (Suppl Fig 13B), and Sox9/OPN double-positive cells (~2-fold) (Suppl Fig 13C). There was a comparable downregulation in OPN (3-fold), and Sox9 (2.5-fold) mRNA (Suppl Fig 13D, E). By contrast, Sox9/E-Cadherin double-positive cells increased nearly 2-fold (Suppl Fig 14A, B) during OPN-neutralization.
Because OPN can potentially bind to matrix metalloproteinases (MMP) (48), we evaluated expression of extracellular matrix (ECM) regulation genes. OPN neutralization led to the down-regulation of tissue inhibitor of metalloprotease-1 (TIMP1) and lysyl oxidase (LOX, mediates collagen cross-linking) in all 3 models (by ~3 fold; p <0.05), MMP2 in CCL4 (by ~2 fold; p<0.05), MMP9 in all 3 models (by ~3 to 5 fold; p<0.05), and MMP13 in the MCD-treated mice (by ~2 fold; p<0.05). Furthermore, OPN-neutralization increased MMP2: TIMP1 ratio in the MCD and DDC models (by ~2 fold), and increased MMP13: TIMP1 ratio in the CCL4 and DDC models (by ~2 fold).
OPN is an immune-cell chemoattractant (44), and could modulate the degree of hepatic injury. Thus, we evaluated if OPN-neutralization could lead to differences in serum alanine aminotransferase (ALT). Treatment with OPN-aptamers led to greater reductions in ALT levels in MCD (385±42 to 102±22 IU/L; p<0.05) and DDC (846±75 to 133±15 IU/L; p<0.05)-fed mice, compared with CCL4-treated mice (438±44 to 321±71). These aggregate data demonstrate that OPN-aptamers ameliorate liver fibrogenesis via multiple pathways: i) directly – by modulating the liver progenitor cell and HSC responses, ii) indirectly - by reducing hepatic injury and/or regulating matrix degradation.
The outcomes of OPN-aptamer-studies were verified using OPN-neutralizing antibodies. MCD-fed mice that received OPN-antibodies accumulated 4–5 fold less SR-stained fibrils (Suppl Fig 15A), 60% fewer αSMA+ cells (Suppl Fig 15B), and downregulated αSMA, Collagen 1αI, TGF-β1 mRNA by up to 60% (Suppl Fig 15C–E). This was associated with fewer OPN+ and Sox9+ cells (~80%) (Suppl Fig 16A, B), and reduced OPN and Sox9 mRNA (~50%) (Suppl Fig 16C, D).
In summary, targeting OPN using two neutralizing approaches, and in three liver disease models, led to an attenuated liver progenitor-cell response, and an amelioration of murine liver fibrosis.
Discussion
This is the first study to show that OPN-neutralization is effective in treating murine liver fibrosis. Treatment with OPN-aptamers or OPN-neutralizing antibodies attenuated liver progenitor-cell response, and repressed fibrogenesis to levels comparable with control-fed mice, suggesting that one week of ‘anti-OPN’ treatment is safe, and effective in reversing fibrogenic outcomes. Mechanistically, we show that OPN is an important viability factor for liver progenitors, and directly regulates progenitor-cell phenotype by up-regulating mesenchymal genes while repressing epithelial ones. Importantly, OPN-neutralization significantly inhibited progenitor-cell migration in wound healing and transmigration assays, key features of EMT (42).
EMT describes the process by which epithelial-progenitors acquire a more mesenchymal phenotype that facilitates their migration into the stroma (49). This process is critical for development and is characteristic of invasive-cancers. Fate-mapping studies in three distinct mouse-strains and in two models of chronic liver injury provide compelling evidence that EMT occurs during liver regeneration and repair (16, 40, 50). Comparable features of EMT are detected in human diseased livers (22, 24). In this study, OPN-neutralization led to fewer Sox9+ LPC, and significantly less hepatic K19 and Sox9 mRNA (i.e. attenuated liver progenitor response) than sham-treated mice. Importantly, Sox9+ LPC lost E-Cadherin expression with fibrosis progression, but regained epithelial-type expression during OPN-neutralization. Sox9+ LPC also lost expression of the mesenchymal marker, OPN, during fibrosis regression. The collective in vitro and in vivo data support the hypothesis that OPN regulates progenitor cell-wound healing responses.
The liver progenitor niche comprises the LPC (oval cell), bone marrow-derived progenitors, and liver fibroblasts (HSC) (51). Previously, we showed that co-cultures of LPC with HSC led to enhanced progenitor-cell proliferation and EMT (22), while LPC-derived Hh ligands and OPN activate HSC into myofibroblasts (26). Here, we showed that OPN-neutralization resulted in a ‘less-fibrogenic’ LPC-secretome in vitro and a ‘less-fibrogenic’ progenitor cell microenvironment in vivo, highlighting the importance of OPN within the progenitor niche. This finding is particularly relevant given our recent study identifying HSC as a resident multi-potent progenitor (16, 40), and provides an explanation for their shared phenotype (i.e. LPC and HSC express similar progenitor markers, and undergo EMT) (24, 25). Thus, LPC and HSC interact to replenish the progenitor pool (16), and identify OPN as a key modulator of the liver progenitor-associated response during injury.
OPN binds to multiple receptors (44). As such, it is likely that OPN-neutralization has other effects, apart from modulating LPC and stellate cell responses. Preliminary studies show that OPN can promote immune cell entry into the liver, and perpetuate hepatic injury. Others have reported that OPN can directly regulate immune-cell functions (52). Our findings that OPN-neutralization resulted in the downregulation of key ECM regulators, TIMP1, LOX, and MMPs, while increasing the ratios of MMP: TIMP1 is unsurprising, as OPN and its family members are known to bind to MMPs (48). Nevertheless, these observations explain in part, how OPN-neutralization could lead to such impressive reversibility in fibrosis. Further studies however, will be needed to evaluate how OPN modulates MMP activities and whether OPN regulates specific immune-subsets recruitment and function (fibrosis-promoting vs. fibrosis-regressing) in vivo (53).
TGF-β is a pro-fibrogenic cytokine, and promotes progenitor-cell EMT (39). Given that OPN also regulates the liver progenitor-response and EMT, it is not surprising that TGF-β and OPN may interact. Previously, we showed that TGF-β mRNA expression is Hh-regulated (24). In this study, we confirmed that OPN-neutralization in mice led to reduced TGF-β1 mRNA. Intriguingly, OPN-neutralization also decreased levels of phospho-Smad-2/3-complexes in LPC. This was mirrored by increased Ski and SnoN, two potent transcriptional co-repressors of the TGF-β pathway (45). Under basal conditions, Ski and SnoN inhibit gene transcription; TGF-β stimulation leads to Ski and SnoN degradation, thereby, allowing phospho-Smad-2/3 to bind to target genes. The results imply that OPN may be a novel regulator of SnoN and Ski levels during fibrogenic liver-repair, and OPN-neutralization decreases levels of phospho-Smad-2/3 which, in turn, triggers proteosomal-degradation of Ski and SnoN (54). This is consistent with observations that Ski and SnoN over-expression are associated with amelioration of renal fibrosis, and resistance to renal-tubular EMT (55). Our preliminary studies further suggest that OPN-mediated effects may occur via OPN-CD44 and OPN-αvβ3 interactions (putative OPN-receptors), and/or via MZF1 regulation of TGF-β mRNA (a zinc finger transcription factor) (Mi Z, personal communications). The aggregate findings show that OPN effects are mediated in part, by modulating TGF-β signaling, complementing and extending earlier evidence which positioned both OPN and TGF-β down-stream of Hh during liver fibrogenesis (24, 26).
To date, studies have utilized OPN-knockout (genetically deficient) mice (26, 33, 56). Despite their usefulness in providing proof-of-concept, translating findings from these animals to humans are limited, as genetic modifications may result in contradictory outcomes when subjected to chronic injury. These disparities could be explained by the presence of distinct OPN isoforms (intracellular and extracellular/soluble) which exhibit overlapping, but also differing functions (44). Soluble OPN behaves as a cytokine while intracellular OPN is an important viability and cytoskeletal protein that regulates intracellular protein functions. In support, our cell culture data show that the reduction of intracellular OPN expression (in 603B-shOPN) leads to similar but non-identical outcomes. In vivo, OPN-knockout mice developed more fibrosis after chronic CCL4 (56), but less fibrosis in dietary-induced NASH (26). Similar divergent outcomes have been reported in OPN-knockout mice with lung or rheumatological disease (57, 58). OPN-aptamers or OPN-neutralizing antibodies used in this study is potentially safer because it negates the excess circulating OPN without directly abolishing the expression of intracellular OPN necessary for key cellular function (47). Furthermore, in clinical practice, individuals are more likely to need treatment for advanced-fibrosis, as opposed to prophylactic anti-fibrotics; therefore, the administration of OPN-neutralizing therapies once fibrosis has developed is more likely to be clinically relevant (1).
Although no mice died in this study, future studies need to specifically evaluate the potential risks of OPN-neutralization. OPN over-expression, however, occurs during tissue inflammation and fibrosis (skin, lung, kidneys, bone marrow), and in individuals with metabolic risk factors such as obesity, endothelial dysfunction, and diabetes (44, 59). Thus, neutralizing and lowering excess extracellular OPN under such circumstances could be beneficial. This concept and therapeutic safety is supported by a recent phase 1/2 study of OPN-neutralization in patients with advanced rheumatoid arthritis (30).
In summary, our analyses in cell-culture, mice and humans show that OPN upregulation during liver injury is a conserved repair-response, and influences liver progenitor-cell function by modulating TGF-β signaling. OPN-neutralization using two neutralizing-modalities, and in three different models of mice abrogated the liver progenitor-cell response and liver fibrosis. Future studies will be necessary to evaluate the importance of alternative mechanisms by which OPN modulates fibrogenesis, and to evaluate if extended periods of treatment could lead to even better anti-fibrotic outcomes. As humanized-antibodies to OPN and OPN-specific aptamers are currently being developed, future studies will also be needed in humans to evaluate the safety and efficacy of anti-OPN treatment.
Supplementary Material
Summary Box.
What is already known about the subject?
Progressive liver fibrosis is associated with a liver progenitor-cell response (also known as the ductular reaction in human)
Liver progenitor cells participate in the wound healing (fibrogenic) response through direct epithelial-mesenchymal transition changes and / or indirect paracrine crosstalk with hepatic stellate cells
Osteopontin is a cytokine and matrix molecule that is highly expressed in inflamed and fibrotic tissues throughout the body, and directly activate hepatic stellate cells
There is currently no licensed anti-fibrotic therapy for use in patients with advanced liver fibrosis or cirrhosis
What are the new findings?
Osteopontin is an important regulator of the liver progenitor-cell response
Neutralizing Osteopontin leads to the reversal of the liver progenitor-cell response (i.e. reduced progenitor cell numbers and impaired wound healing / transmigration)
Neutralizing Osteopontin modulates TGF-β signaling by downregulating TGF-β mRNA and protecting levels of Ski and SnoN (two transcriptional co-repressors of TGF-β signaling).
Neutralizing Osteopontin using 2 different approaches (aptamer or neutralizing antibody) results in a dramatically attenuated liver progenitor-cell response and liver fibrosis in 3 different mouse models of liver fibrosis
Osteopontin neutralization is safe in mice with liver fibrosis
How might it impact on clinical practice in the foreseeable future?
Osteopontin-specific aptamers are available and other neutralizing antibodies / small molecule compounds are being developed
Osteopontin neutralization may be a useful strategy for the treatment of individuals with advanced liver fibrosis.
Acknowledgments
Dr. G. J. Gores (Mayo Clinic, Rochester, MN) and Yoshiyuki Ueno (Tohoku University, Sendai, Japan) for providing the murine immature ductular cell line (603B), Dr Scott L Friedman (Mount Sinai School of Medicine, NY) for providing the human HSC line, LX-2, and Dr George Yeoh (University of Western Australia) for providing the BMOL line. We are grateful to Dr Mari Shinohara (Duke University Medical Center, NC) and Dr T Uede (Hokkaido University, Japan) for helpful discussions.
Funding:
This study was funded predominantly by CORE-UK (WKS), BRET (WKS), EASL (WKS), The Foundation for Liver Research London (WKS), and the University of Birmingham (WKS). Additional funding was provided by the National Institute of Health 5K08DK080980 (SSC), R01 DK077794 (AMD), Belgian Federal Science Policy Office (Interuniversity Attraction Poles program - P6/20 and P7/83-HEPRO) (LD, LAvG), the Brussels Capital Region (INNOVIRIS Impulse programme-Life Sciences 2007 and 2011; BruStem) (LD, LAvG), the Institute for the Promotion of Innovation through Science and Technology in Flanders (SBO-IWT-090066 HEPSTEM) (LD, LAvG), the Natural Sciences and Engineering Research Council of Canada Postgraduate Doctoral Scholarship (DR, BE), the Alberta Innovates Technology Futures Graduate Scholarship (DR, BE), DFG (German Research Association) CA267/8-1 (AC), and the Wilhelm Lapitz Foundation (AC).
Abbreviations
- NAFLD
nonalcoholic fatty liver disease
- Hh
Hedgehog
- EMT
epithelial-to-mesenchymal transition
- OPN
Osteopontin
- TGF-β
transforming growth factor β
- K19
keratin 19
- αSMA
alpha smooth muscle actin
- Id
inhibitor of differentiation
- MCD diet
methionine choline deficient diet
- DDC
3, 5,-diethoxycarbonyl-1,4-dihydrocollidine diet
- CCL4
chronic carbon tetrachloride
- FFPE
formalin-fixed, paraffin embedded
- shScr
short-hairpin RNA against non-target (scrambled) sequence
- shOPN
short-hairpin RNA against OPN sequence
Footnotes
The Corresponding author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence on a worldwide basis to the BMJ publishing group Ltd and its Licensees to permit this article to be published in Gut Editions and any other BMJPGL products to exploit all subsidiary rights, as set out in our licence.
Disclosures / Competing Interests
On behalf of authors, I declare no Competing Interests
Contributions:
JC, MSS, LD, DR, BE, LC, MOB, SS, OH, AR, SC, SSC performed experiments and contributed intellectually to the study; SP, ZM, PCK, RW, AC, DHA, LvanG, AMD, SSC provided samples, and contributed to the design and funding of the studies. JC, MBO, RW, and AMD co-wrote the manuscript. WKS is the lead investigator, who designed and supervised the overall project and sub- studies, performed experiments, wrote the manuscript, and is the senior author and guarantor of the manuscript.
References
- 1.Friedman SL, Sheppard D, Duffield JS, et al. Therapy for fibrotic diseases: nearing the starting line. Sci Transl Med. 2013;5:167sr161. doi: 10.1126/scitranslmed.3004700. [DOI] [PubMed] [Google Scholar]
- 2.Schuppan D, Kim YO. Evolving therapies for liver fibrosis. J Clin Invest. 2013;123:1887–1901. doi: 10.1172/JCI66028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838–851. doi: 10.1016/S0140-6736(08)60383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rahimi RS, Rockey DC. End-stage liver disease complications. Curr Opin Gastroenterol. 2013;29:257–263. doi: 10.1097/MOG.0b013e32835f43b0. [DOI] [PubMed] [Google Scholar]
- 5.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013 Sep 17; doi: 10.1038/nrgastro.2013.171. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 6.Diehl AM, Chute J. Underlying potential: cellular and molecular determinants of adult liver repair. J Clin Invest. 2013;123:1858–1860. doi: 10.1172/JCI69966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghosh AK, Quaggin SE, Vaughan DE. Molecular basis of organ fibrosis: potential therapeutic approaches. Exp Biol Med (Maywood) 2013;238:461–481. doi: 10.1177/1535370213489441. [DOI] [PubMed] [Google Scholar]
- 8.Roskams T, Desmet V. Ductular reaction and its diagnostic significance. Semin Diagn Pathol. 1998;15:259–269. [PubMed] [Google Scholar]
- 9.Richardson MM, Jonsson JR, Powell EE, et al. Progressive fibrosis in nonalcoholic steatohepatitis: association with altered regeneration and a ductular reaction. Gastroenterology. 2007;133:80–90. doi: 10.1053/j.gastro.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Yang S, Koteish A, Lin H, et al. Oval cells compensate for damage and replicative senescence of mature hepatocytes in mice with fatty liver disease. Hepatology. 2004;39:403–411. doi: 10.1002/hep.20082. [DOI] [PubMed] [Google Scholar]
- 11.Espanol-Suner R, Carpentier R, Van Hul N, et al. Liver progenitor cells yield functional hepatocytes in response to chronic liver injury in mice. Gastroenterology. 2012;143:1564–1575. e1567. doi: 10.1053/j.gastro.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 12.Dolle L, Best J, Mei J, et al. The quest for liver progenitor cells: a practical point of view. J Hepatol. 2010;52:117–129. doi: 10.1016/j.jhep.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 13.Santoni-Rugiu E, Jelnes P, Thorgeirsson SS, et al. Progenitor cells in liver regeneration: molecular responses controlling their activation and expansion. APMIS. 2005;113:876–902. doi: 10.1111/j.1600-0463.2005.apm_386.x. [DOI] [PubMed] [Google Scholar]
- 14.Petersen BE, Bowen WC, Patrene KD, et al. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168–1170. doi: 10.1126/science.284.5417.1168. [DOI] [PubMed] [Google Scholar]
- 15.Oh SH, Witek RP, Bae SH, et al. Bone marrow-derived hepatic oval cells differentiate into hepatocytes in 2-acetylaminofluorene/partial hepatectomy-induced liver regeneration. Gastroenterology. 2007;132:1077–1087. doi: 10.1053/j.gastro.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Swiderska-Syn M, Syn WK, Xie G, et al. Myofibroblastic cells function as progenitors to regenerate murine livers after partial hepatectomy. Gut. 2013 doi: 10.1136/gutjnl-2013-305962. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arthur MJ, Stanley A, Iredale JP, et al. Secretion of 72 kDa type IV collagenase/gelatinase by cultured human lipocytes. Analysis of gene expression, protein synthesis and proteinase activity. Biochem J. 1992;287 (Pt 3):701–707. doi: 10.1042/bj2870701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iredale JP, Goddard S, Murphy G, et al. Tissue inhibitor of metalloproteinase-I and interstitial collagenase expression in autoimmune chronic active hepatitis and activated human hepatic lipocytes. Clin Sci (Lond) 1995;89:75–81. doi: 10.1042/cs0890075. [DOI] [PubMed] [Google Scholar]
- 19.Wood MJ, Gadd VL, Powell LW, et al. The ductular reaction in hereditary haemochromatosis: The link between hepatocyte senescence and fibrosis progression. Hepatology. 2013 Aug 26; doi: 10.1002/hep.26706. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 20.Holt AP, Salmon M, Buckley CD, et al. Immune interactions in hepatic fibrosis. Clin Liver Dis. 2008;12:861–882. doi: 10.1016/j.cld.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carpino G, Renzi A, Onori P, et al. Role of hepatic progenitor cells in nonalcoholic Fatty liver disease development: cellular cross-talks and molecular networks. Int J Mol Sci. 2013;14:20112–20130. doi: 10.3390/ijms141020112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Omenetti A, Porrello A, Jung Y, et al. Hedgehog signaling regulates epithelial-mesenchymal transition during biliary fibrosis in rodents and humans. J Clin Invest. 2008;118:3331–3342. doi: 10.1172/JCI35875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Omenetti A, Syn WK, Jung Y, et al. Repair-related activation of hedgehog signaling promotes cholangiocyte chemokine production. Hepatology. 2009;50:518–527. doi: 10.1002/hep.23019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Syn WK, Jung Y, Omenetti A, et al. Hedgehog-mediated epithelial-to-mesenchymal transition and fibrogenic repair in nonalcoholic fatty liver disease. Gastroenterology. 2009;137:1478–1488. e1478. doi: 10.1053/j.gastro.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi SS, Omenetti A, Witek RP, et al. Hedgehog pathway activation and epithelial-to-mesenchymal transitions during myofibroblastic transformation of rat hepatic cells in culture and cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2009;297:G1093–1106. doi: 10.1152/ajpgi.00292.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Syn WK, Choi SS, Liaskou E, et al. Osteopontin is induced by hedgehog pathway activation and promotes fibrosis progression in nonalcoholic steatohepatitis. Hepatology. 2011;53:106–115. doi: 10.1002/hep.23998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takahashi F, Takahashi K, Okazaki T, et al. Role of osteopontin in the pathogenesis of bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2001;24:264–271. doi: 10.1165/ajrcmb.24.3.4293. [DOI] [PubMed] [Google Scholar]
- 28.Liaw L, Birk DE, Ballas CB, et al. Altered wound healing in mice lacking a functional osteopontin gene (spp1) J Clin Invest. 1998;101:1468–1478. doi: 10.1172/JCI1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagao T, Okura T, Irita J, et al. Osteopontin plays a critical role in interstitial fibrosis but not glomerular sclerosis in diabetic nephropathy. Nephron Extra. 2012;2:87–103. doi: 10.1159/000337330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boumans MJ, Houbiers JG, Verschueren P, et al. Safety, tolerability, pharmacokinetics, pharmacodynamics and efficacy of the monoclonal antibody ASK8007 blocking osteopontin in patients with rheumatoid arthritis: a randomised, placebo controlled, proof-of-concept study. Ann Rheum Dis. 2012;71:180–185. doi: 10.1136/annrheumdis-2011-200298. [DOI] [PubMed] [Google Scholar]
- 31.Nilsson SK, Johnston HM, Whitty GA, et al. Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood. 2005;106:1232–1239. doi: 10.1182/blood-2004-11-4422. [DOI] [PubMed] [Google Scholar]
- 32.Choi SS, Sicklick JK, Ma Q, et al. Sustained activation of Rac1 in hepatic stellate cells promotes liver injury and fibrosis in mice. Hepatology. 2006;44:1267–1277. doi: 10.1002/hep.21375. [DOI] [PubMed] [Google Scholar]
- 33.Fickert P, Stoger U, Fuchsbichler A, et al. A new xenobiotic-induced mouse model of sclerosing cholangitis and biliary fibrosis. Am J Pathol. 2007;171:525–536. doi: 10.2353/ajpath.2007.061133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mi Z, Guo H, Russell MB, et al. RNA aptamer blockade of osteopontin inhibits growth and metastasis of MDA-MB231 breast cancer cells. Mol Ther. 2009;17:153–161. doi: 10.1038/mt.2008.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Talbot LJ, Mi Z, Bhattacharya SD, et al. Pharmacokinetic characterization of an RNA aptamer against osteopontin and demonstration of in vivo efficacy in reversing growth of human breast cancer cells. Surgery. 2011;150:224–230. doi: 10.1016/j.surg.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kiefer FW, Zeyda M, Gollinger K, et al. Neutralization of osteopontin inhibits obesity-induced inflammation and insulin resistance. Diabetes. 2010;59:935–946. doi: 10.2337/db09-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Furuyama K, Kawaguchi Y, Akiyama H, et al. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet. 2011;43:34–41. doi: 10.1038/ng.722. [DOI] [PubMed] [Google Scholar]
- 38.Ishimura N, Bronk SF, Gores GJ. Inducible nitric oxide synthase up-regulates Notch-1 in mouse cholangiocytes: implications for carcinogenesis. Gastroenterology. 2005;128:1354–1368. doi: 10.1053/j.gastro.2005.01.055. [DOI] [PubMed] [Google Scholar]
- 39.Thenappan A, Li Y, Kitisin K, et al. Role of transforming growth factor beta signaling and expansion of progenitor cells in regenerating liver. Hepatology. 2010;51:1373–1382. doi: 10.1002/hep.23449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Michelotti GA, Xie G, Swiderska M, et al. Smoothened is a master regulator of adult liver repair. J Clin Invest. 2013;123(6):2380–94. doi: 10.1172/JCI66904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pritchett J, Harvey E, Athwal V, et al. Osteopontin is a novel downstream target of SOX9 with diagnostic implications for progression of liver fibrosis in humans. Hepatology. 2012;56:1108–1116. doi: 10.1002/hep.25758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee JM, Dedhar S, Kalluri R, et al. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol. 2006;172:973–981. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tirnitz-Parker JE, Tonkin JN, Knight B, et al. Isolation, culture and immortalisation of hepatic oval cells from adult mice fed a choline-deficient, ethionine-supplemented diet. Int J Biochem Cell Biol. 2007;39:2226–2239. doi: 10.1016/j.biocel.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 44.Uede T. Osteopontin, intrinsic tissue regulator of intractable inflammatory diseases. Pathol Int. 2011;61(5):265–80. doi: 10.1111/j.1440-1827.2011.02649.x. [DOI] [PubMed] [Google Scholar]
- 45.Deheuninck J, Luo K. Ski and SnoN, potent negative regulators of TGF-beta signaling. Cell Res. 2009;19:47–57. doi: 10.1038/cr.2008.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hayashi H, Abdollah S, Qiu Y, et al. The MAD-related protein Smad7 associates with the TGFbeta receptor and functions as an antagonist of TGFbeta signaling. Cell. 1997;89:1165–1173. doi: 10.1016/s0092-8674(00)80303-7. [DOI] [PubMed] [Google Scholar]
- 47.Chiu CC, Sheu JC, Chen CH, et al. Global gene expression profiling reveals a key role of CD44 in hepatic oval-cell reaction after 2-AAF/CCl4 injury in rodents. Histochem Cell Biol. 2009;132(5):479–89. doi: 10.1007/s00418-009-0634-9. [DOI] [PubMed] [Google Scholar]
- 48.Fedarko NS, Jain A, Karadag A, et al. Three small integrin binding ligand N-linked glycoproteins (SIBLINGs) bind and activate specific matrix metalloproteinases. FASEB J. 2004;18(6):734–6. doi: 10.1096/fj.03-0966fje. [DOI] [PubMed] [Google Scholar]
- 49.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 50.Yang L, Jung Y, Omenetti A, et al. Fate-mapping evidence that hepatic stellate cells are epithelial progenitors in adult mouse livers. Stem Cells. 2008;26:2104–2113. doi: 10.1634/stemcells.2008-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roskams T, Katoonizadeh A, Komuta M. Hepatic progenitor cells: an update. Clin Liver Dis. 2010;14:705–718. doi: 10.1016/j.cld.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 52.Wang KX, Denhardt DT. Osteopontin: role in immune regulation and stress responses. Cytokine Growth Factor Rev. 2008;19(5–6):333–45. doi: 10.1016/j.cytogfr.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 53.Pellicoro A, Ramachandran P, Iredale JP, et al. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat Rev Immunol. 2014;14(3):181–94. doi: 10.1038/nri3623. [DOI] [PubMed] [Google Scholar]
- 54.Stroschein SL, Wang W, Zhou S, et al. Negative feedback regulation of TGF-beta signaling by the SnoN oncoprotein. Science. 1999;286:771–774. doi: 10.1126/science.286.5440.771. [DOI] [PubMed] [Google Scholar]
- 55.Fukasawa H, Yamamoto T, Togawa A, et al. Ubiquitin-dependent degradation of SnoN and Ski is increased in renal fibrosis induced by obstructive injury. Kidney Int. 2006;69:1733–1740. doi: 10.1038/sj.ki.5000261. [DOI] [PubMed] [Google Scholar]
- 56.Lorena D, Darby IA, Gadeau AP, et al. Osteopontin expression in normal and fibrotic liver. altered liver healing in osteopontin-deficient mice. J Hepatol. 2006;44:383–390. doi: 10.1016/j.jhep.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 57.Xanthou G, Alissafi T, Semitekolou M, et al. Osteopontin has a crucial role in allergic airway disease through regulation of dendritic cell subsets. Nat Med. 2007;13(5):570–8. doi: 10.1038/nm1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weber GF, Cantor H. Differential roles of osteopontin/Eta-1 in early and late lpr disease. Clin Exp Immunol. 2001;126(3):578–83. doi: 10.1046/j.1365-2249.2001.01702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Musso G, Paschetta E, Gambino R, et al. Interactions among bone, liver, and adipose tissue predisposing to diabesity and fatty liver. Trends Mol Med. 2013 Sep;19(9):522–35. doi: 10.1016/j.molmed.2013.05.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.