Abstract
Background
Intra-abdominal abscesses (IAA) are complications of Crohn’s disease, which often result in hospitalization, surgery, and increased cost. Initial management may include medical therapy, percutaneous drainage (PD), or surgery, although the optimal management of IAA in children is unclear.
Methods
Retrospective review of all pediatric patients with Crohn’s disease who developed an IAA from January 1, 2000 to April 30, 2012. Three groups, based on initial IAA treatment modality (medical, PD, and surgery), were compared.
Results
Thirty cases of IAA were identified (mean age at IAA diagnosis, 15.4 ± 2.6 yr, 67% female, median Crohn’s disease duration, 2.6 mo). Computed tomography was the most common initial (93%) and follow-up (47%) imaging. The average time to follow-up imaging was 8.5 days. For initial management, 18 received medical therapy, 10 PD, and 2 had surgery. The medical therapy group received more computed tomography scans for follow-up imaging than the PD group (12 [67%] versus 2 [20%], P = 0.046). There were no significant differences in abscess characteristics or management of posttreatment course between these 2 groups. Surgical resection occurred in 3 patients (17%) in the medical group and 2 (20%) in the PD group during index hospitalization. No significant differences were identified among treatment groups for readmissions, complications, or abscess recurrence. By 1 year, 12 of the 18 medically managed patients (67%) had surgery, and 6 of the 10 patients (60%) treated with initial PD ultimately had surgery.
Conclusions
The majority of patients with IAA require definitive surgical treatment, and there were no clear predictors of those who did not.
Keywords: inflammatory bowel disease, Crohn’s disease, pediatrics, intra-abdominal abscess
Crohn’s disease (CD) is a chronic, relapsing inflammatory disorder that primarily affects the digestive tract of approximately 1.4 million people in North America, with 25% being diagnosed in childhood. Inflammation within the intestinal tract may lead to intra-abdominal fistulae, phlegmon, and intra-abdominal abscesses (IAA). IAA occurs in 7% to 28% of patients with CD and often results in hospitalization, surgery, and increased cost of care.1–6
The management of IAA is complex and poses multiple diagnostic and therapeutic dilemmas, specifically regarding imaging modalities, drainage, antibiotic use, medications, surgical intervention, and feeding/nutritional approaches. Recently, the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) published a clinical report on the evaluation and treatment of pediatric patients with IAA.7 This report is an important effort to help standardize IAA management; however, there is a paucity of pediatric data reported in the literature and considerable variation in the clinical approach of pediatric gastroenterologists.8 Extrapolation from adult studies is often not appropriate because there are many differences between adult and pediatric CD, including that children tend to present with more severe and more extensive disease compared with adults at time of CD diagnosis.9–11
The main objective of this study was to critically review the management and outcomes of IAA, according to initial treatment group in a cohort of pediatric patients with CD at Nationwide Children’s Hospital over the last 12 years. We describe the treatment and outcomes by initial treatment group including potential predictors of surgery.
MATERIALS AND METHODS
Study Design and Variables
This study was a retrospective medical record review of all pediatric patients with CD who developed an IAA and were treated at Nationwide Children’s Hospital from January 1, 2000 to April 30, 2012. A list of potential subjects was made by identifying patients with both ICD-9 code for CD (555.XX) and one or more ICD-9 codes for appendicitis, peritonitis, and/or abscess (540.X, 567.XX, or 569.5). Appendicitis and peritonitis codes were included in the initial population file to identify any cases of IAA that could have been erroneously coded. Inpatient and outpatient records for all potential subjects were then reviewed. Patients were included in the study cohort if they had evidence for both diagnosis of CD and IAA not due to appendicitis. Data were abstracted by H.B. using detailed case report forms developed and tested by the authors. Case report forms were audited by J.L.D.
Data collected included basic demographics (age, gender, race/ethnicity), characteristics of CD diagnosis (disease location and behavior), previous abdominal surgery, laboratory tests (white blood cell count, hemoglobin, erythrocyte sedimentation rate, c-reactive protein, and albumin), and imaging studies (x-ray, ultrasound, computed tomography (CT), magnetic resonance imaging) at IAA diagnosis and follow-up, the use of interventional radiology–placed drainage devices, antibiotic timing and duration of therapy (days), medical management of underlying CD, and surgical intervention (method and timing of intervention). Patients were followed for 12 months after IAA diagnosis.
Patients were divided into 3 groups (medical management only [no drainage], percutaneous drainage [PD] of the abscess by interventional radiology, or immediate surgical resection) based on the treatment modality used during the first 72 hours of hospitalization. Initial imaging was defined as all imaging studies completed within 72 hours of IAA diagnosis. Follow-up imaging was defined as occurring greater than 72 hours after initial IAA diagnosis. We did include postoperative imaging in the study.
Statistical Analyses
Baseline and demographic characteristics were summarized using standard descriptive statistics. We compared clinical factors and outcomes between treatment groups using chi-square test or Fisher’s exact test, where appropriate, for categorical data, and continuous variables were analyzed using the nonparametric Kruskal–Wallis test (Wilcoxon 2-sample test was used when comparing the 2 treatment groups). To test the effects of select demographic variables and clinical factors as predictors of surgery, patients were defined as having surgery if they had a surgical procedure (local resection) during the initial IAA hospitalization or during any re-admission within the 1-year follow-up period. Univariate logistic regression was used to test the effects of each predictor on the binary outcome (surgery: yes/no). All tests were conducted using SAS 9.3 (SAS Institute Inc. Cary, NC). A P < 0.05 was considered statistically significant. This study was approved by the Nationwide Children’s Hospital Institutional Review Board.
RESULTS
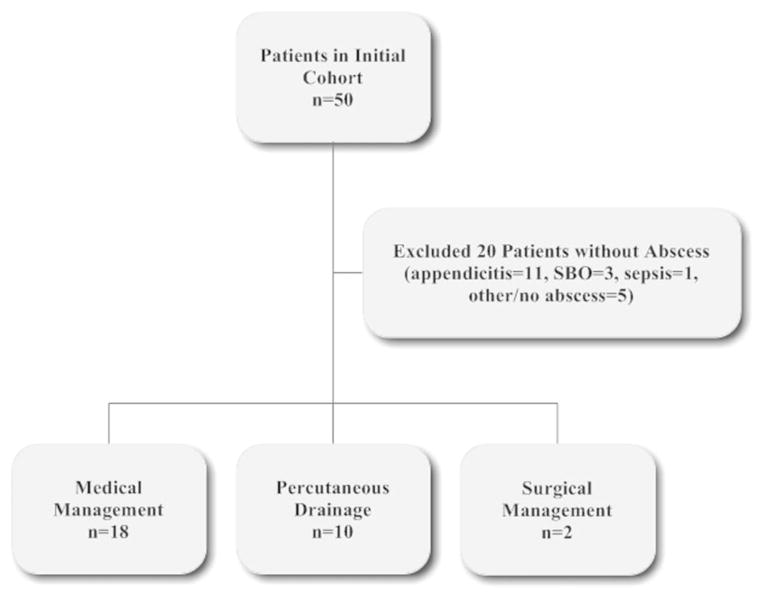
Fifty patients were identified by ICD-9 coding in the preliminary cohort, with 20 subsequent exclusions (Fig. 1). The 30 remaining patients were divided into 3 groups based on initial IAA treatment modality. Because there were only 2 patients in the initial surgical management group, we excluded this group and compared medical management only to PD for subsequent analyses. Most patients were female (67%), with a mean age of 13.5 ± 2.8 years at CD diagnosis and a mean age of 15.4 ± 2.6 years at IAA diagnosis. The majority of IAA occurred shortly after the initial CD diagnosis and initiation of induction therapy. The median disease duration at the time of IAA diagnosis was 2.6 months (18 patients had an IAA diagnosed <90 d of initial CD diagnosis). Nine patients were not yet receiving any therapy because they presented with an IAA at the time of their CD diagnosis, many patients were receiving immunosuppressant medications at the time of admission (Table 1). None of the patients received 5-aminosalicylates as monotherapy. Summaries of the demographics at the time of CD diagnosis and time of IAA diagnosis are illustrated in Table 1.
FIGURE 1.
Study flowchart. SBO, small bowel obstruction.
TABLE 1.
Demographics at CD Diagnosis and IAA Diagnosis
| Variable | |
|---|---|
| Demographics at CD Diagnosis | n (%) |
| Total no. patients | 30 |
| Gender | |
| Female | 20 (67) |
| Age (yr ± SD) | 13.5 ± 2.8 |
| Race/ethnicitya | |
| White | 20 (80) |
| Black | 4 (16) |
| Hispanic or Latino | 0 (0) |
| Other | 1 (4) |
| Disease locationb | |
| L1: distal ½ ileum ± limited cecal | 9 (30) |
| L2: colonic | 0 (0) |
| L3: ileocolonic | 21 (70) |
| L4a: upper disease proximal to the Ligament of Treitz | 5 (17) |
| L4b: upper disease distal to the Ligament of Treitz | 6 (20) |
| L4a and L4b | 2 (7) |
| Disease Behaviorb | |
| B1: nonstricturing, nonpenetrating | 3 (10) |
| B2: stricturing | 8 (27) |
| B3: penetrating | 16 (53) |
| B2B3: stricturing and penetrating | 2 (7) |
| Perianal disease | 8 (27) |
| History of any abdominal surgery | 5 (17) |
| Demographics at IAA Diagnosis | Mean ± SD |
|---|---|
| Age (yr) | 15.4 ± 2.6 |
| Disease duration (mo) (median) | 17.2 ± 27.7 (2.6) |
| Laboratory studies | |
| Mean WBC (K/cu mm) | 16.5 ± 6.7 |
| Mean Hemoglobin (g/dL) | 11.7 ± 1.6 |
| Mean ESR (mm/h) | 37.9 ± 18.1 |
| Mean CRP (mg/dL) | 12.0 ± 8.3 |
| Mean Albumin (g/dL) | 3.5 ± 0.4 |
| Medications, n (%) | |
| 5-ASAc | 8 (27) |
| Corticosteroidsd | 14 (47) |
| 6-MP/AZA | 12 (40) |
| Methotrexate | 1 (3) |
| Anti-TNFα | 3 (10) |
Five patients with missing race/ethnicity.
Paris classification.
No patients were on 5-ASA as monotherapy.
One patient received budesonide.
5-ASA, mesalamine; 6-MP, 6-mercaptopurine; AZA, azathioprine, anti-TNFα, using infliximab or adalimumab; CRP, c-reactive protein; ESR, erythrocyte sedimentation rate; WBC, white blood cell count.
Initial Management
Within the first 72 hours of hospitalization, initial management included either medical therapy only (n = 18), PD performed by interventional radiology (n = 10), or immediate surgical intervention (n = 2).
Summary results for the 2 surgically managed patients included a mean abscess size of 6.1 cm, 1 had a fistula, the other experienced multiple abscesses, median time to resection was 1.5 days, mean antibiotic duration was 7.5 days, 1 had a central line for 21 days, and the median length of stay was 8.5 days. One patient was readmitted because of IAA recurrence. At a 1-year follow-up, neither of the patients underwent further surgical intervention, 1 patient was in remission, the other had mild disease activity, 1 was on 6-mercaptopurine, and the other was receiving anti-TNFα therapy.
For patients in the medical and PD groups, there was a trend toward smaller abscesses being managed medically, but this did not reach statistical significance. In addition to PD occurring in all patients of the PD group, there were 3 patients in the medical group who underwent subsequent PD. Indwelling drainage catheters were placed in all of the patients in the PD group and in 2 of the 3 patients in the medical therapy group who underwent PD during their index hospitalization (P < 0.0001). The median duration of drain placement for the PD and medical groups was 6 and 22 days, respectively (P = 0.83). There was no difference between groups for subsequent surgical resection (P = 0.60) (Table 2).
Table 2.
Course and Management of IAA During Index Hospitalization According to Initial Intervention
| Variable | Medical, n (%) | PD, n (%) | P |
|---|---|---|---|
| Number in initial treatment group | 18 | 10 | |
| Mean abscess size (max diameter [cm] ± SD) | 4.0 ± 1.6 | 7.4 ± 4.9 | 0.06 |
| Multiple abscesses | 4 (22) | 4 (44) | 0.37 |
| Loculated abscess | 3 (17) | 4 (44) | 0.18 |
| Presence of documented fistula | 6 (33) | 4 (40) | 1.00 |
| Mean IV antibiotics (d ± SD, [median]) | 15.9 ± 28.0 (9) | 14.9 ± 12.7 (8.5) | 0.58 |
| Central line–placed | 11 (61) | 9 (90) | 0.19 |
| Median duration (d) | 34 | 26 | 0.65 |
| TPN | 0 | 2 (20) | 0.12 |
| PD | 3 (17) | 10 (100) | <0.0001 |
| Drain placed | 2 (11) | 10 (100) | <0.0001 |
| Median duration (d) | 22 | 6 | 0.83 |
| Subsequent surgical resection | 3 (17) | 2 (20) | 0.60 |
| Median time to resection (d) | 10 | 17 | 1.00 |
| Median LOS (d) | 7 | 10 | 0.41 |
Duration of antibiotics is for inpatient and outpatient; time to surgical resection is from the date of IAA diagnosis to the date of surgery.
LOS, length of stay; TPN, total parenteral nutrition.
Bolded P-values represent significant findings.
Imaging
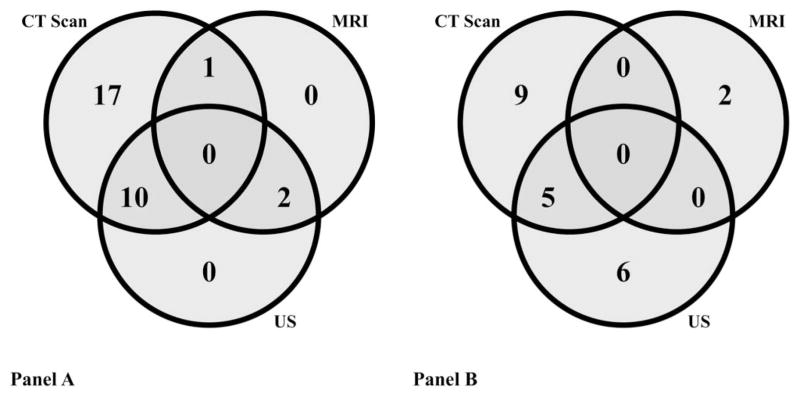
When suspecting an IAA, the most commonly performed initial study for the entire study cohort (n = 30) was a CT scan (93% of patients), which was also the most common follow-up imaging study (CT scan, 47%) (Fig. 2). The medical therapy group received more CT scans for follow-up imaging compared with the PD group (12 [67%] versus 2 [20%], P = 0.046). In this cohort, magnetic resonance imagings were not performed until 2010. Most patients received more than 1 initial (median = 2 per patient, range: 1–3) and follow-up (median = 1 per patient, range: 0–4) imaging study. The average number of days from initial imaging study to the first follow-up imaging study was 8.3 days (median = 6 d, range: 2–31).
FIGURE 2.
The number of patients (n = 30) who had each imaging modality (CT scan, MRI, and/or US) at diagnosis (A) and at follow-up (B) of IAA. “At follow-up” = imaging study done >72 hours after diagnosis; “at diagnosis” = imaging study done <72 hours after diagnosis; MRI, magnetic resonance imaging; US, ultrasound.
Posttreatment Course
Events occurring within 1 year after the initial hospitalization discharge date were considered part of the patient’s posttreatment course. Of the 28 patients, 25 had at least 12 months of follow-up, 2 had 10 months of follow-up, and 1 was lost to follow-up shortly after surgery. There were no differences between treatment groups after index hospitalization regarding readmissions, complications, fistula presence, abscess recurrence, need for further PD, central line placement, CD treatment, or disease severity (physician global assessment) at 1-year follow-up (Table 3).
Table 3.
Complications and Management Within 1-year After IAA Index Hospitalization According to Initial Intervention
| Variable | Medical, n (%) | PD, n (%) | P |
|---|---|---|---|
| Readmission | 9 (50) | 6 (60) | 0.56 |
| No. patients with complications after index hospitalization | 8 (44) | 7 (70) | 0.32 |
| Total no. complications (n) | 18 | 10 | 0.32 |
| Presence of documented fistula | 7 (39) | 3 (10) | 0.33 |
| Abscess recurrence | 6 (33) | 4 (40) | 0.69 |
| Median time to recurrence (d) | 37 | 22.5 | 0.40 |
| PD | 3 (17)a | 2 (50) | 1.00 |
| Central line placements | 7 (39) | 5 (50) | 1.00 |
| Surgical Resection for IAA | 9 (50) | 4b (40) | 0.14 |
| Median time to resection (d) | 47 | 30.5 | 0.14 |
| PGA at 1-yr follow-up visit | 0.33 | ||
| Remission | 12 (67) | 6 (60) | |
| Mild | 5 (28) | 1 (10) | |
| Moderate | 1 (6) | 2 (20) | |
| Severe | 0 | 0 | |
| Medications at 1-yr follow-up visitc | |||
| 5-ASA | 4 (22) | 2 (20) | 1.00 |
| Sulfasalazine | 1 (6) | 1 (10) | 1.00 |
| Corticosteroids | 0 | 0 | n/a |
| 6-MP/AZA | 10 (56) | 3 (30) | 0.25 |
| Methotrexate | 3 (17) | 1 (10) | 1.00 |
| Anti-TNFα | 6 (33) | 5 (28) | 0.44 |
One patient had 2 drainage procedures.
One patient had 2 surgeries.
One patient lost to follow-up in the PD group, so missing PGA at 1 yr.
5-ASA, mesalamine; 6-MP, 6-mercaptopurine; AZA, azathioprine; anti-TNFα, using infliximab or adalimumab; PGA, physician global assessment.
At 1-year follow-up, 12 of the 18 medically managed patients (67%) and 6 of the 10 patients (60%) initially treated with PD ultimately underwent surgery (total: 18 of the 28 [64%]). Total complications were tallied and are depicted in Figure 3.
FIGURE 3.
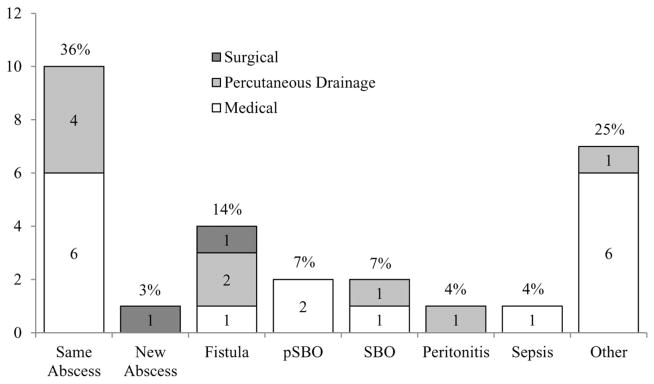
Complications after IAA diagnosis by initial treatment modality. Percentages represent the total percent of each complication for the entire study cohort. Other, terminal ileum stricture, drain site leakage, vomiting, disease recurrence at anastomotic site, pancreatitis, rectal wall fluid collection, or thrombus; pSBO, partial small bowel obstruction; SBO, small bowel obstruction.
Univariate Analysis
The factors considered as potential predictors of surgery included abscess size, abscess number, loculated abscess, fistula presence, gender, age, race, previous abdominal surgery, CD behavior (Paris classification), and laboratory studies (as noted previously). None of these factors were significant in univariate analyses (data not shown). Because none of these potential predictors played a significant role in predicting surgery, we did not fit a multivariate logistic regression model.
DISCUSSION
There is a paucity of research and lack of evidence-based practice guidelines for the optimal management of children with IAA, which contributes to the observed variability in the clinical approach to these patients.8 The recently published clinical report by NASPGHAN is the first attempt to provide guidance in this complex disease process; however, it is limited by the modest pediatric data.7
In our study, the largest single-center review of children and adolescents with CD and IAA to date, there were no predictors of initial treatment modality, although the medical treatment of smaller abscesses approached significance. Further, there was no difference in outcomes whether patients underwent medical treatment or PD for IAA. Given the technical challenge in draining some smaller abscesses, attempting to treat them without surgery or PD is clinically reasonable and is consistent with the recent NASPGHAN clinical report. However, regardless of initial treatment, little data are available regarding the ultimate outcome of pediatric patients with IAA treated medically.
One-year outcomes in our patients were similar in both the medical and PD treatment groups. Specifically, there were no differences in 1-year complications or outcomes among the initial treatment groups, with 67% of patients in the medical group and 60% of patients in the PD group eventually undergoing surgery. However, it should be noted that 1-year outcomes may be significantly influenced by factors other than initial abscess management, including the ongoing medical treatment of their CD. In addition, there was no difference in hospital length of stay between groups, which is consistent with other studies.6,12 We did not identify any risk factors (e.g., abscess size, loculation, number, fistula presence, previous abdominal surgery, demographics), that could predict which patients would ultimately fail medical or PD therapy, thus subsequently require surgery. Both groups also had a high rate of readmission and abscess recurrence.
Although patients initially managed by medical therapy alone had similar length of stay and rates of subsequent surgery compared with the PD group, they did seem to require additional imaging. Specifically, these patients underwent substantially more follow-up CT studies, with the associated increased radiation exposure, perhaps indicating increased clinical concerns in these patients.
PD has also been proposed as a way of avoiding surgery. Although the majority of our patients with PD did require surgical resection, 40% did not. However, we were unable to identify any factors predictive of which patients would eventually require surgical resection. The issue of which patients will eventually require surgery is even less clear in the era of biologics. Specifically, in patients where there has been adequate drainage of the abscess as well as clinical and radiologic resolution, it is uncertain as to which patients would benefit from early surgical resection and which would derive long-term benefit from anti-TNF therapy without resection. Unfortunately, this study is not powered to address this issue. Interestingly, a recent article reported a higher likelihood of surgery for perforating disease in children who never received immunomodulators or infliximab suggesting that these medications may alter disease behavior.13 Given the study spans a 12-year period, the practice patterns of prescribing anti-TNF therapy have likely changed. Ultimately, medical management through a risk stratification approach for children at the time of CD diagnosis, particularly those with more severe disease, may alter disease behavior and reduce complications such as IAA and the need for subsequent surgical intervention.
Although our study is the largest pediatric IAA review to date in the literature, it is limited by the small sample size and single-center design, which limits its generalizability. The small sample size may also have prevented us from recognizing factors that are, indeed, predictive of need for subsequent surgery. The main strength of this study was the comprehensive review of diagnosis and management of IAA in a pediatric cohort with CD.
In conclusion, there were no predictors of successful medical or PD therapy. Surgical intervention was the definitive treatment for the majority of patients with CD who developed an IAA. This study suggests a need for additional primary research to determine the safest, most efficacious, and cost-effective management of IAA. A larger multicenter compilation of cases may help inform the treatment guidelines and outline the appropriate management of IAA in pediatric patients with CD.
Acknowledgments
Supported by Award Number Grant UL1TR000090 from the National Center for Advancing Translational Sciences.
J. L. Dotson was supported by the NASPGHAN Foundation/Crohn’s and Colitis Foundation of America Young Investigator Development Award. W. V. Crandall serves as a consultant and speaker for Abbott and has received research support from AbbVie.
Footnotes
The remaining authors have no conflicts of interest to disclose.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
References
- 1.Keighley MR, Eastwood D, Ambrose NS, et al. Incidence and microbiology of abdominal and pelvic abscess in Crohn’s disease. Gastroenterology. 1982;83:1271–1275. [PubMed] [Google Scholar]
- 2.Greenstein AJ, Sachar DB, Greenstein RJ, et al. Intraabdominal abscess in Crohn’s (ileo) colitis. Am J Surg. 1982;143:727–730. doi: 10.1016/0002-9610(82)90046-0. [DOI] [PubMed] [Google Scholar]
- 3.Ribeiro MB, Greenstein AJ, Yamazaki Y, et al. Intra-abdominal abscess in regional enteritis. Ann Surg. 1991;213:32–36. doi: 10.1097/00000658-199101000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poritz LS, Koltun WA. Percutaneous drainage and ileocolectomy for spontaneous intraabdominal abscess in Crohn’s disease. J Gastrointest Surg. 2007;11:204–208. doi: 10.1007/s11605-006-0030-x. [DOI] [PubMed] [Google Scholar]
- 5.Steinberg DM, Cooke WT, Alexander-Williams J. Abscess and fistulae in Crohn’s disease. Gut. 1973;14:865–869. doi: 10.1136/gut.14.11.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ayuk P, Williams N, Scott NA, et al. Management of intra-abdominal abscesses in Crohn’s disease. Ann R Coll Surg Engl. 1996;78:5–10. [PMC free article] [PubMed] [Google Scholar]
- 7.Pfefferkorn MD, Marshalleck FE, Saeed SA, et al. NASPGHAN clinical report on the evaluation and treatment of pediatric patients with internal penetrating Crohn disease: intraabdominal abscess with and without fistula. J Pediatr Gastroenterol Nutr. 2013;57:394–400. doi: 10.1097/MPG.0b013e31829ef850. [DOI] [PubMed] [Google Scholar]
- 8.Dotson JL, Nwomeh B, Andridge R, et al. Variation in management of intra-abdominal abscesses in children with Crohn’s disease. Inflamm Bowel Dis. 2013;19:818–825. doi: 10.1097/MIB.0b013e3182802a1f. [DOI] [PubMed] [Google Scholar]
- 9.Kelsen J, Baldassano RN. Inflammatory bowel disease: the difference between children and adults. Inflamm Bowel Dis. 2008;14(suppl 2):S9–S11. doi: 10.1002/ibd.20560. [DOI] [PubMed] [Google Scholar]
- 10.Van Limbergen J, Russell RK, Drummond HE, et al. Definition of phenotypic characteristics of childhood-onset inflammatory bowel disease. Gastroenterology. 2008;135:1114–1122. doi: 10.1053/j.gastro.2008.06.081. [DOI] [PubMed] [Google Scholar]
- 11.Vernier-Massouille G, Balde M, Salleron J, et al. Natural history of pediatric Crohn’s disease: a population-based cohort study. Gastroenterology. 2008;135:1106–1113. doi: 10.1053/j.gastro.2008.06.079. [DOI] [PubMed] [Google Scholar]
- 12.Gutierrez A, Lee H, Sands BE. Outcome of surgical versus percutaneous drainage of abdominal and pelvic abscesses in Crohn’s disease. Am J Gastroenterol. 2006;101:2283–2289. doi: 10.1111/j.1572-0241.2006.00757.x. [DOI] [PubMed] [Google Scholar]
- 13.Zimmerman LA, Shamberger RC, Valim C, et al. The effect of immunomodulators and biologics on indication for surgical bowel resection in children with Crohn’s disease. Inflamm Bowel Dis. 2014;20:1015–1020. doi: 10.1097/MIB.0000000000000042. [DOI] [PubMed] [Google Scholar]