Abstract
Background
Contrast-enhanced intra-operative ultrasound (CE-IOUS) for colorectal liver metastases (CLMs) has become a part of clinical practice. Whether it should be selectively or routinely applied remains unclear. The aim of this study was to define criteria for the use of CE-IOUS.
Methods
One-hundred and twenty-seven patients underwent a hepatectomy for CLMs using IOUS and CE-IOUS. All patients underwent computed tomography (CT) and/or magnetic resonance imaging (MRI) within 2 weeks prior to surgery. The reference was histology, and imaging at 6 months after surgery. Univariate and multivariate analyses were performed. Statistical significance was set at P = 0.05.
Results
Using IOUS an additional 172 lesions in 51 patients were found. CE-IOUS found 14 additional lesions in 6 patients. Seventy-eight CLMs in 38 patients appeared within 6 months after surgery. The sensitivity, specificity, positive- and negative-predictive value were 63%, 98%, 100% and 27% for pre-operative imaging, 87%, 100%, 100% and 52% for IOUS, and 89%, 100%, 100% and 56% for IOUS+CE-IOUS, respectively. CE-IOUS allowed better tumour margin definition in 23 patients (18%), thus assisting resection. Analyses indicated that the presence of multiple (P = 0.014), and isoechoic CLMs (P = 0.049) were independently correlated with new findings at CE-IOUS.
Conclusions
Compared with IOUS, CE-IOUS improved detection and resection guidance. These additions are significant and demand its use in cases with multiple and isoechoic CLMs.
Introduction
Contrast-enhanced intra-operative ultrasound (CE-IOUS) was proposed in 2004 for both colorectal liver metastasis (CLM) and hepatocellular carcinoma (HCC) detection1 to improve the results of IOUS. However, the preliminary results remained inconclusive for CLMs.2–4 Fioole et al. 4 concluded that CE-IOUS did not appear necessary in CLM surgery because it did not significantly improve the accuracy of pre-operative CT and IOUS. However, we demonstrated the accuracy of IOUS and CE-IOUS for CLMs in a more significant single-centre series using a well-established pre-operative work-up, histology and adequate minimal follow-up as reference standards.5 For sure, although CE-IOUS originally detected new findings in 44–77% of cases,2,3 the rate has decreased to <20%.4,5 Meanwhile, pre-operative imaging has improved, and new contrast agents have been introduced.6 Therefore, the place of CE-IOUS in CLM staging needs to be redefined, and criteria for its selective adoption rather than its indiscriminate use must be determined.
Patients and methods
Definitions
Histology of resected specimens and post-operative follow-up were used as reference standards. Any new CLMs detected within the first 6 months of follow-up were considered as early post-operative recurrence and as lesions not detected by pre- and intra-operative imaging.
On IOUS, a ‘bright’ liver displayed liver–kidney contrast, and the blurring of both the intrahepatic vessels and diaphragm was related to diffusely increased liver echogenicity.7
The presence of at least three lesions was considered multiple CLMs.
Pre-operative work-up
The pre-operative imaging work-up consisted of an abdominal US, abdominal magnetic resonance imaging (MRI) and/or computed tomography (CT) and chest spiral CT. CT/MRI was always performed within 2 weeks of surgery.
A three-phase CT examination was performed with a Philips Brilliance 64 (Philips, Eindhoven, The Netherlands). CT enhancement was obtained using an iodinated contrast agent (Iomeron 300; Bracco SpA, Milan, Italy).
MRI was performed initially with two different 1.5T magnets (Philips Achieva, Eindhoven, The Netherlands or Siemens Symphony, Erlangen, Germany) with phase array coils using dynamic acquisition sequences and liver-specific MR contrast agents (MultiHance; Bracco SpA and more recently, Primovist: Bayer, Leverkusen, Germany). In October 2010, diffusion-MRI became available and was included in all MRI examinations. More recently, 18-fluordeoxyglucose-positron emission tomography (FDG-PET) was also adopted for pre-operative imaging.
IOUS and CE-IOUS
IOUS and CE-IOUS were performed using an Aloka Alpha 10 (Aloka Ltd, Tokyo, Japan) equipped with a standard 3–6 MHz convex probe and an Esaote MyLab Twice (Esaote Ltd, Genoa, Italy) equipped with a micro T-shaped 3–11 MHz trapezoid scanning transducer. In all patients, an anaesthesiologist injected 2.4 ml of sulfur-hexafluoride microbubbles (SonoVue®; Bracco SpA) into a peripheral vein. Such use of the contrast agent is regularly approved in Italy, and specific informed consent was obtained from all patients.
Inclusion criteria
Patients who underwent surgery for CLM were prospectively enrolled for systematic exploration with IOUS and CE-IOUS.
Patients who underwent an explorative laparotomy alone after IOUS and CE-IOUS were excluded from the analysis because there was no histological tumour confirmation and most were lost to surgical follow-up.
A minimum 6-month post-operative follow-up was required to start the analyses.
Patient follow-up
The patients were followed-up in our institution every 3 months by an expert hepatobiliary team who checked liver function and serum carcinoembryonic antigen (CEA) and performed a physical examination, US (2×/year), CT/MRI (2×/year), colonoscopy (1×/year) and PET (1×/year). At the 6-month follow-up, each patient underwent CT/MRI unless the 3-month US discovered a new lesion, in which case CT/MRI was performed earlier.
End-points
The primary end-point was the identification of factors that could maximize the benefits of CE-IOUS, thus allowing its selective use during CLM surgery.
The secondary end-points were the overall sensitivity, specificity, positive- and negative-predictive values (PPV and NPV, respectively) of IOUS and IOUS plus CE-IOUS compared with pre-operative imaging.
Statistical analysis
Sensitivity, specificity, PPV and NPV were calculated for pre-operative diagnostic imaging, IOUS, and CE-IOUS based on histological results and evidence of new liver lesions within the first 6 months post-operatively.
For univariate analyses, continuous and categorical variables were compared with a two-tailed Student’s t-test and the chi-square test, respectively.
Multivariate analysis was performed using binary logistic regression.
Analyses were performed to identify factors influencing IOUS and CE-IOUS detection and rate of missed lesions diagnosed at follow-up: (i) pre-operative MRI compared with CT; (ii) pre-operative diagnostic work-up with/without PET; (iii) multinodular pattern (≥3) at pre-operative imaging; (iv) pre-operative chemotherapy; (v) ‘bright’ (steatosis) liver at IOUS; (vi) isoechoic CLM at IOUS; (vii) hyperechoic CLM at IOUS; and (viii) hypoechoic CLM at IOUS. The P-value was set at 0.05.
Patients
Between October 2007 and March 2011, 136 consecutive patients underwent a laparotomy after a diagnosis of CLM. Nine patients (10%) were excluded from the analysis because they had undergone an explorative laparotomy alone owing to peritoneal carcinomatosis (6 patients) or lymph node metastasis (3 patients). In total, 127 consecutive patients [77 males and 50 females; mean age 61 years (median 65; range 29–85)] underwent a liver resection using IOUS and CE-IOUS. The imaging diagnostic work-up for all enrolled patients included abdominal US followed by contrast-enhanced CT in 52 (41%) patients and MRI in 75 (59%); 11 (9%) of the latter 75 patients also underwent diffusion-weighted imaging. Twelve patients also received percutaneous contrast-enhanced US (CEUS), and 10 underwent fine-needle biopsy. Ninety-two (72%) patients also underwent 18-FDG-PET. The background liver showed diffuse steatosis in 53 (42%) patients, 4 patients (3%) were cirrhotic, 4 (3%) had chronic hepatitis and the remaining 66 (52%) patients had a normal liver; all patients with liver steatosis had undergone previous systemic chemotherapy.
The total number of tumours at pre-operative imaging was 447 (median 2; mean 3.5; range 1–30), with a mean diameter of 3.8 (median 3.3; range 1–14) cm for the largest tumour. Sixty-two patients (49%) had multiple CLMs at pre-operative imaging. Table 1 reports the characteristics of the patients’ subject of the study.
Table 1.
Characteristics of patients in the study
| Variable | Data |
|---|---|
| Age (years) | 61 (65; 29–85) |
| Gender | |
| Men | 77 (60) |
| Women | 50 (40) |
| Imaging diagnostic work-up | |
| US | 127 (100) |
| CT | 52 (41) |
| MRI | 75 (59) |
| 18-FDG-PET | 92 (72) |
| Liver tumours | |
| At pre-operative imaging | 447 (2; 3.5; 1–30) |
| At IOUS | 617 (4.9; 3; 1–48) |
| At IOUS & CE-IOUS | 631 (5; 3; 1–48) |
| New tumours | |
| At IOUS | 172 (3,4; 2; 1–28) |
| At CE-IOUS | 14 (2.3; 2.5; 1–4) |
| New tumours | |
| At IOUS | 51 (40) |
| At CE-IOUS | 6 (5) |
| Multiple tumours (≥3) | |
| At pre-operative imaging | 62 (49) |
| At IOUS | 76 (60) |
| At IOUS & CE-IOUS | 77 (61) |
| Largest tumour dimension (cm) | |
| At pre-operative imaging | 3.8 (3.3; 1–14) |
| New at IOUS | NA |
| New at CE-IOUS | 0.3–1.1 |
| Tumour topography | |
| Left hemi-liver | 15 (12) |
| Right hemi-liver | 45 (35) |
| Bilobar | 67 (53) |
| Macrovascular invasion | |
| Yes | 22 (17) |
| No | 105 (83) |
| Lymph node metastasis | |
| Yes | 7 (6) |
| No | 120 (94) |
| Tumour echogenicity | |
| Isoechoic | 59 (45) |
| Hyperechoic | 21 (17) |
| Hypoechoic | 36 (28) |
| ‘Bright’ liver | |
| Yes | 23 (18) |
| No | 104 (82) |
| Background liver | |
| Steatosis | 53 (42) |
| Cirrhosis | 4 (3) |
| Chronic hepatitis | 4 (3) |
| Normal | 66 (52) |
| Chemotherapy | |
| Pre-operative | 95 (75) |
| Post-operative | 57 (4%) |
| Both | 42 (33) |
Data are expressed as mean (median; range) or as a number (percentage).
FDG-PET; fluordeoxyglucose-positron emission tomography; US, ultrasound; CT, computed tomography; MRI, magnetic resonance imaging; IOUS, intra-operative ultrasound; CE-IOUS, contrast-enhanced IOUS; NA, not applicable.
Results
Detection
At IOUS, 617 lesions were detected (mean 4.9; median 3; range 1–48), and 172 were new lesions in 51 patients (40%). IOUS detected 79 new lesions in 23/52 patients who underwent pre-operative CT and 93 new lesions in 28 of the 75 patients who underwent MRI (P = 0.6); of the latter, 11 (12%) were also examined with diffusion-weighted analyses. IOUS excluded the pre-operative diagnosis of CLM for two nodules in two patients. IOUS identified multiple CLMs in 76 patients (60%).
CE-IOUS discovered 14 additional CLMs not observed on IOUS (mean 2.3, median 2.5, range 1–4) in 6 patients (5%) (Fig. 1), among whom 1 had no additional sites on IOUS. The two lesions pre-operatively diagnosed as CLMs but not confirmed by IOUS were not visualized by CE-IOUS. The diameter of CE-IOUS-only detected lesions was 0.3–1.1 cm.
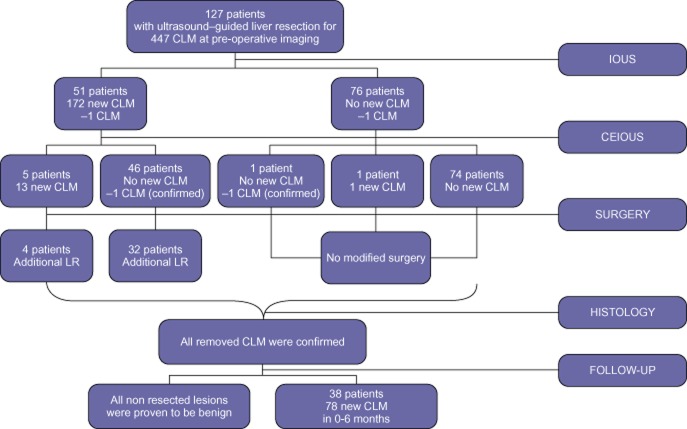
Figure 1.
Flow chart showing the additional findings of intra-operative ultrasound (IOUS) and contrast-enhanced (CE)-IOUS and their output in terms of modified surgery
Overall, IOUS and CE-IOUS identified 631 CLMs (mean 5, median 3, range 1–48), and 186 were new lesions not detected by pre-operative imaging in 52 patients (41%). IOUS and CE-IOUS detected 83 new lesions in 23 of the 52 patients who underwent pre-operative CT and 103 new lesions in 29 of the 75 patients who underwent MRI (P = 0.9) (Fig. 1).
IOUS and CE-IOUS identified 77 patients (61%) with multiple CLMs. A significantly greater number of new lesions were detected by IOUS and CE-IOUS in patients with multiple pre-operative CLMs than in the remaining patient population (4 versus 2.2; P = 0.01)
Among the 23 patients with a ‘bright’ liver, 19 new lesions were detected by IOUS in 6 patients and by CE-IOUS in 1 patient.
Univariate and multivariate analyses indicated that pre-operative chemotherapy, and a pre-operative diagnosis of multiple CLMs significantly influenced CLM detection by IOUS (univariate: P = 0.026 and P = 0.01, respectively; multivariate: P = 0.044 and P = 0.016, respectively) whereas multiple CLMs and isoechoic lesions significantly influenced CLM detection by CE-IOUS (univariate: P = 0.023 and P = 0.041, respectively; multivariate: P = 0.014 and P = 0.049, respectively) (Table 2).
Table 2.
Uni- and multivariate analysis of factors influencing colorectal liver metastases (CLM) detection at intra-operative ultrasound (IOUS) and contrast-enhanced (CE)-IOUS
| No Nr (%) | Yes Nr (%) | χ2 (P-value) | Logistic regression (P-value) | OR | 95% CI | |
|---|---|---|---|---|---|---|
| New CLM at IOUS | ||||||
| MRI versus CT | ||||||
| MRI | 47 (63) | 28 (37) | .436 | NS | ||
| CT | 29 (56) | 23 (44) | ||||
| PET | ||||||
| No | 26 (74) | 9 (26) | .061 | NS | ||
| Yes | 50 (54) | 42 (46) | ||||
| Multiple CLM (≥3) (pre-operative imaging) | ||||||
| No | 47 (72) | 18 (28) | .010 | .016 | 2.5 | 1.18–5.28 |
| Yes | 29 (47) | 33 (53) | ||||
| Pre-operative CHTa | ||||||
| No | 23 (77) | 7 (23) | .026 | .044 | 2.66 | 1.02–6.94 |
| Yes | 51 (54) | 44 (46) | ||||
| Bright liver | ||||||
| No | 59 (57) | 45 (43) | .128 | NS | ||
| Yes | 17 (74) | 6 (26) | ||||
| Isoechoic CLMb | ||||||
| No | 37 (65) | 20 (35) | .421 | NS | ||
| Yes | 34 (58) | 25 (42) | ||||
| Hyperechoic CLMb | ||||||
| No | 58 (61) | 37 (39) | .942 | NS | ||
| Yes | 13 (62) | 8 (38) | ||||
| Hypoechoic CLMb | ||||||
| No | 47 (59) | 33 (41) | .418 | NS | ||
| Yes | 24 (67) | 12 (33) | ||||
| New CLM at CE-IOUS | ||||||
| MRI versus CT | ||||||
| MRI | 71 (95) | 4 (5) | .698 | NS | ||
| CT | 50 (96) | 2 (4) | ||||
| PET | ||||||
| No | 33 (94) | 2 (6) | .746 | NS | ||
| Yes | 88 (96) | 4 (4) | ||||
| Multiple CLM (≥3) (IOUS) | ||||||
| No | 51 (100) | 0 (0) | .023 | .014 | 1.92 | 1.13–6.43 |
| Yes | 70 (92) | 6 (8) | ||||
| Pre-operative CHTa | ||||||
| No | 30 (100) | 0 (0) | .200 | NS | ||
| Yes | 90 (95) | 5 (5) | ||||
| Bright liver | ||||||
| No | 99 (95) | 5 (5) | .925 | NS | ||
| Yes | 22 (96) | 1 (4) | ||||
| Isoechoic CLMb | ||||||
| No | 56 (98) | 1 (2) | .041 | .049 | 2.84 | 1.72–8.96 |
| Yes | 54 (91) | 5 (9) | ||||
| Hyperechoic CLMb | ||||||
| No | 89 (94) | 6 (6) | .237 | NS | ||
| Yes | 21 (100) | 0 (0) | ||||
| Hypoechoic CLMb | ||||||
| No | 75 (94) | 5 (6) | .435 | NS | ||
| Yes | 35 (97) | 1 (3) |
MRI, magnetic resonance imaging; CT, computed tomography; PET; positron emission tomography; CHT, chemotherapy; OR, odds-ration; CI, confidence interval.
Data are not available in two patients.
Data are not available in 11 patients.
Resection guidance
CE-IOUS allowed better visualization of the primary tumour margins in 23 of 127 patients (18%), resulting in easier resection guidance (Fig. 2).
Figure 2.
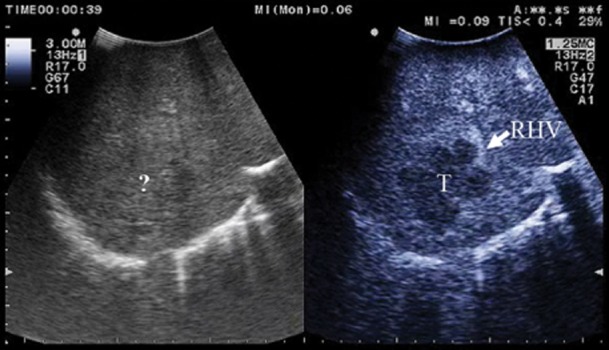
(a) the margins of the lesion invading the right hepatic vein (RHV) are unclear (?) at intra-operative ultrasound (IOUS); (b) at contrast-enhanced (CE)-IOUS the lesion (T) becomes clearly visible by its margins and its relation with the right hepatic vein (RHV)
Surgical outcome
All 186 new liver lesions detected by IOUS and CE-IOUS in 52 patients were resected with 445 pre-operatively diagnosed CLMs and intra-operatively confirmed by IOUS and CE-IOUS. The lesion distribution was bilobar in 67 patients, limited to the right hemi-liver in 45 and limited to the left hemi-liver in 15. Five patients (4%) underwent a major resection (≥3 segments). In 36 (70%) of the 52 patients with new lesions detected by IOUS and CE-IOUS, the surgical approach was modified to an additional limited resection; 3 of the 6 patients with new CLMs detected by CE-IOUS were included. Histology confirmed all removed nodules as metastases and detected no additional CLMs.
After a mean follow-up of 19 months (median 14 months; range 6–48 months), 72 patients (57%) had recurrent disease; in 67 (53%), the liver was involved. Both the mean disease-free survival and mean hepatic recurrence-free survival were 12 (median 7; range 1–48) months; 16 patients (13%) died during follow-up. Thirty-eight patients (30%) developed 78 new liver lesions (mean 2; median 1; range 1–10) in another liver segment within 6 months post-operatively; these nodules were all <2 cm in diameter at detection and were considered metastases missed by pre- (CT/MRI) and intra-operative (IOUS and CE-IOUS) imaging. Of these, 8 patients (6%) with 21 new CLMs at follow-up had additional adverse factors at the time of surgery, including lymph node metastases in 3 (2%) patients, major vascular invasion in 4 (3%) and both in 1 (0.8%).
Seventeen patients (13%) had new CLMs (all <2 cm in diameter) within 12 months after surgery.
Of the 2 patients with pre-operatively diagnosed CLMs not confirmed by IOUS and CE-IOUS and therefore not removed, 1 is currently alive without recurrence at 27 months after surgery, and 1 had 2 lesions in other liver segments 18 months post-operative and underwent a re-resection. Neither of the lesions detected post-operatively were at the site excluded by IOUS and CE-IOUS.
At the univariate and multivariate analyses of factors influencing early post-operative recurrence, multiple CLMs at pre-operative imaging and at IOUS/CEIOUS as well as pre- and post-operative chemotherapy were found to be significant only at the univariate (P = 0.034, P = 0.019, P = 0.016, respectively).
Table 3 shows the sensitivity, specificity, PPV and NPV of pre-operative imaging, IOUS and IOUS+CE-IOUS in detecting CLMs. Pre-operative imaging had significantly lower sensitivity and NPV than IOUS and IOUS+CE-IOUS (P < 0.05). The PPV was not significant in any comparison. The sensitivity, specificity, PPV and NPV were not significantly different between IOUS and IOUS+CE-IOUS.
Table 3.
Sensitivity, specificity, positive- (PPV) and negative-predictive values (NPV) of pre-operative imaging, intra-operative ultrasound (IOUS) and contrast enhanced (CE)-IOUS in patients with colorectal liver metastases
| Preoperative Imaging (CT+MRI) | IOUS | IOUS+CEIOUS | |
|---|---|---|---|
| Sensitivity | 63 | 87 | 89 |
| Specificity | 98 | 100 | 100 |
| PPV | 100 | 100 | 100 |
| NPV | 27 | 52 | 56 |
Adverse reactions and costs
No clinically evident adverse reactions were reported. The additional cost of CE-IOUS to the surgical procedure, considering the use of one sample of contrast agent per patient, was 61.36 euro/patient.
Discussion
Complete surgical clearance of multiple CLMs is justified even in the presence of vascular infiltration because significant benefits in long-term survival have been reported.8 Therefore, tumour staging is crucial, and CE-IOUS was considered to improve IOUS sensitivity in detecting new CLMs in 21% of patients.2 These results were partially confirmed by multi- and single-centre experiences.3,4 However, the conclusions were not consistent. Leen et al.3 considered CE-IOUS a useful addition to IOUS. Fioole et al.4 came to the opposite conclusion. These studies were based on preliminary experiences with small numbers of patients with CE-IOUS and used different criteria for patient enrolment and data analysis. Furthermore, the reference standards differed among the series, and the comparability of results was reduced, decreasing the impact of comparative analyses.
In 2008, we reported the results of 47 patients operated on for CLM using a well-established pre-operative diagnostic flow chart, using histological findings and 6-month post-operative follow-up as the reference standards. CE-IOUS identified 10 new lesions in 4 patients (9% of those examined, 5% of the CLMs removed and 19% of the new CLMs identified by intra-operative imaging).5 This series provided findings similar to those of Fioole et al.4 but quite different from those reported initially (Table 4).2,3 The heterogeneity of the results and the progressive decrease in the rate of new findings made it necessary to identify criteria for the more selective use of CE-IOUS. Therefore, we performed this study, excluding patients who were part of the previously reported analysis.2,5 Nevertheless, the 127 consecutive patients enrolled represent the largest reported series of CE-IOUS for CLMs.
Table 4.
Patients operated for colorectal liver metastases who presented new lesions at intra-operative ultrasound (IOUS) and constrast enhanced(CE)-IOUS as reported in the literature
| Pts | Preop. CLM | Confirmed CLM | Pts with NL IOUS | NL at IOUS | Pts with NL CEIOUS | NL at CEIOUS | Pts with NL IOUS+CEIOUS | Total CLM | Pts with NL F-UP | |
|---|---|---|---|---|---|---|---|---|---|---|
| Torzilli et al., (7) | 24 | 45 | 44 | 5 | 10 | 5 | 8 | 8 | 62 | 0 |
| Leen et al., (8) | 57 | NA | 79 | NA | 5 | 13 | 17 | NA | 103 | NA |
| Fioole et al., (9) | 39 | 160 | 85 | 10 | 20 | 2 | 4 | NA | 137 | NA |
| Torzilli et al., (10) | 47 | 129 | 127 | 20 | 43 | 4 | 10 | 21 | 194 | 10 |
| Present series | 127 | 447 | 445 | 51 | 172 | 6 | 14 | 52 | 709 | 38 |
Pts, patients; CLM, colorectal liver metastases, NL, new lesions; NA, not available.
In addition to the larger size of this series, other important points also differentiate it from most previous reports.
All patients underwent pre-operative imaging within 2 weeks prior to surgery. Although MRI is currently the best tool for lesion detection, CT remains useful and was when this study was planned.9–12 Furthermore, the CT and MRI results did not differ significantly (Table 2).
All lesions detected intra-operatively in this series were removed, and histological confirmation was obtained, whereas a significant proportion of patients received intra-operative thermal ablation of the new sites in previous reports.3,4
Both histology and 6-month post-operative follow-up were used as reference standards.
The results of this study were compared with those of previous studies (Table 4). In the first two reports, new CLMs were identified by CE-IOUS at rates of 44–77%.2,3 The rate decreased to 17–19% in more recent reports4,5 and to 8% in the present report. The more recent use of contrast agents [e.g. perfluorobutane microbubbles (Sonazoid®; GE Healthcare, Oslo, Norway)] for Kupffer-phase imaging6 has not resulted in significant improvements. This may substantiate the progression of pre-operative imaging. However, the number of new CLMs detected by IOUS in this study (24%) was equivalent to previous reports (16–22%).2,4,5 Because a decrease was only observed in the impact of CE-IOUS, it is likely that it has achieved a steady state of detection capability. Consequently, the definition of criteria for selective CE-IOUS use is both justified and mandatory.
We found that patients with multiple CLMs gained a significant benefit regarding staging completeness with both IOUS and CE-IOUS (Table 2). However, multiple CLMs are generally associated with a higher rate of lesions missed by pre-operative imaging.8 If this rate loses relevance once the post-operative follow-up is analysed, it is probable that the careful and routine use of IOUS and CE-IOUS has limited their impact; indeed, although significant in univariate analyses, it lacked significance in a multivariate analysis. This result is important, as the tendency of modern liver surgery is to approach multiple presentations conservatively,13 which explains the high rate of operations modified owing to new findings by IOUS and CE-IOUS (Fig. 1). Effort must be made to precisely clarify liver involvement; the use of CE-IOUS in patients at higher risk of missed CLMs meets this need.
As for multinodularity, isoechoic CLMs were independently associated with CLM detection by CE-IOUS. This ultrasonographic feature of CLMs appears relevant because it may impact patient prognosis after surgery.14 The relevance of CE-IOUS to this issue may also have limited its significance regarding the risk of missed lesions.
In contrast to previous reports2,3,5 and the fact that steatosis may increase the IOUS detectability of CLMs, enhancing their visibility,14 a ‘bright’ liver was not associated with a significantly higher rate of detected CLMs (Table 2). Even if not significant, CE-IOUS allowed the detection of new lesions in five patients with and one without a ‘bright’ liver (Table 2), suggesting its reduced relevance in the event that a ‘bright’ liver is observed by IOUS. However, pre-operative chemotherapy, which should be associated with a higher rate of steatosis and, consequently, indirectly related to the presence of a ‘bright’ liver, was significantly linked to higher detectability by IOUS (Table 2) and, although not significant, at CE-IOUS (Table 2). It remains difficult to discriminate between the potentially opposing roles played by the chemotherapeutic agents, which include (i) shrinking the CLMs and reducing their visibility15 and (ii) changing the echogenic pattern of both CLMs and the surrounding parenchyma, thereby increasing lesion detectability.14 CE-IOUS has shown potential in patients treated with pre-operative chemotherapy.
As in previous reports,2,5 enhancing the contrast between the tumour and the surrounding parenchyma ensured better definition of the tumour margins and the relationship with adjacent vascular structures (Fig. 2), which aided resection guidance.
In conclusion, this study is the first and largest to report that CE-IOUS provides significant findings in patients harbouring multiple and/or isoechoic CLMs on IOUS. The selective use of CE-IOUS for CLMs should maximize the benefit of this diagnostic tool and reduce the cost of its indiscriminate use.
Conflicts of interest
None declared.
References
- Torzilli G, Del Fabbro D, Olivari N, Calliada F, Montorsi M, Makuuchi M. Contrast-enhanced intraoperative ultrasonography during liver surgery. Br J Surg. 2004;91:1165–1167. doi: 10.1002/bjs.4628. [DOI] [PubMed] [Google Scholar]
- Torzilli G, Del Fabbro D, Palmisano A, Donadon M, Bianchi P, Roncalli M, et al. Contrast-enhanced intraoperative ultrasonography during hepatectomies for colorectal cancer liver metastases. J Gastrointest Surg. 2005;9:1148–1153. doi: 10.1016/j.gassur.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Leen E, Ceccotti P, Moug SJ, Glen P, MacQuarrie J, Angerson WJ, et al. Potential value of contrast-enhanced intraoperative ultrasonography during partial hepatectomy for metastases: an essential investigation before resection? Ann Surg. 2006;243:236–240. doi: 10.1097/01.sla.0000197708.77063.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fioole B, de Haas RJ, Wicherts DA, Elias SG, Scheffers JM, van Hillegersberg R, et al. Additional value of contrast enhanced intraoperative ultrasound for colorectal liver metastases. Eur J Radiol. 2008;67:169–176. doi: 10.1016/j.ejrad.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Torzilli G, Botea F, Procopio F, Donadon M, Balzarini L, Lutman F, et al. Use of contrast-enhanced intraoperative ultrasonography during liver surgery for colorectal cancer liver metastases – its impact on operative outcome. Analysis of a prospective cohort study. Eur J Cancer. 2008;6:16–23. [Google Scholar]
- Nanashima A, Tobinaga S, Abo T, Kunizaki M, Takeshita H, Hidaka S, et al. Usefulness of sonazoid-ultrasonography during hepatectomy in patients with liver tumors: a preliminary study. J Surg Oncol. 2011;103:152–157. doi: 10.1002/jso.21782. [DOI] [PubMed] [Google Scholar]
- Chen CH, Lin ST, Yang CC, Yeh YH, Kuo CL, Nien CK. The accuracy of sonography in predicting steatosis and fibrosis in chronic hepatitis C. Dig Dis Sci. 2008;53:1699–1706. doi: 10.1007/s10620-007-0048-2. [DOI] [PubMed] [Google Scholar]
- Minagawa M, Makuuchi M, Torzilli G, Takayama T, Kawasaki S, Kosuge T, et al. Extension of the frontiers of surgical indications in the treatment of liver metastases from colorectal cancer: long-term results of our experience. Ann Surg. 2000;231:487–499. doi: 10.1097/00000658-200004000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong KO, Leen E. Radiological staging of colorectal liver metastases. Surg Oncol. 2007;16:7–14. doi: 10.1016/j.suronc.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Kim YK, Lee MW, Lee WJ, Kim SH, Rhim H, Lim JH, et al. Diagnostic accuracy and sensitivity of diffusion-weighted and of gadoxetic acid-enhanced 3-T MR imaging alone or in combination in the detection of small liver metastasis (≤1.5 cm in diameter) Invest Radiol. 2012;47:159–166. doi: 10.1097/RLI.0b013e31823a1495. [DOI] [PubMed] [Google Scholar]
- Muhi A, Ichikawa T, Motosugi U, Sou H, Nakajima H, Sano K, et al. Diagnosis of colorectal hepatic metastases: comparison of contrast-enhanced CT, contrast-enhanced US, superparamagnetic iron oxide-enhanced MRI, and gadoxetic acid-enhanced MRI. J Magn Reson Imaging. 2011;34:326–335. doi: 10.1002/jmri.22613. [DOI] [PubMed] [Google Scholar]
- Kulemann V, Schima W, Tamandl D, Kaczirek K, Gruenberger T, Wrba F, et al. Preoperative detection of colorectal liver metastases in fatty liver: MDCT or MRI? Eur J Radiol. 2011;79:e1–e6. doi: 10.1016/j.ejrad.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Torzilli G, Procopio F, Botea F, Marconi M, Del Fabbro D, Donadon M, et al. One-stage ultrasonographically guided hepatectomy for multiple bilobar colorectal metastases: a feasible and effective alternative to the 2-stage approach. Surgery. 2009;46:60–71. doi: 10.1016/j.surg.2009.02.017. [DOI] [PubMed] [Google Scholar]
- Van Vledder MG, Torbenson MS, Pawlik TM, Boctor EM, Hamper UM, Olino K, et al. The effect of steatosis on echogenicity of colorectal liver metastases on intraoperative ultrasonography. Arch Surg. 2010;145:661–667. doi: 10.1001/archsurg.2010.124. [DOI] [PubMed] [Google Scholar]
- Robinson PJ. The effects of cancer chemotherapy on liver imaging. Eur Radiol. 2009;19:1752–1762. doi: 10.1007/s00330-009-1333-6. [DOI] [PubMed] [Google Scholar]