Abstract
Background
Current pre-operative staging methods for gallbladder cancer (GBC) are suboptimal in detecting metastatic disease. Positron emission tomography (PET) may have a role but data are lacking.
Methods
Patients with GBC and PET assessed by a hepatobiliary surgeon in clinic between January 2001 and June 2013 were retrospectively reviewed. Computed tomography (CT)/magnetic resonace imaging (MRI) were correlated with PET scans and analysed for evidence of metastatic or locally unresectable disease. Medical records were reviewed to determine if PET scanning was helpful by preventing non-therapeutic surgery or enabling resection in patients initially deemed unresectable.
Results
There were 100 patients including 63 incidental GBC. Thirty-eight patients did not proceed to surgery, 35 were resected and 27 patients were explored but had unresectable disease. PET was positive for metastatic disease in 39 patients (sensitivity 56%, specificity 94%). Five patients definitively benefitted from PET: in 3 patients PET found disease not seen on CT, and 2 patients with suspicious CT findings had negative PET and successful resections. In a further 12 patients PET confirmed equivocal CT findings. Three patients had additional invasive procedures performed owing to PET avidity in other sites. Utility of PET was higher in patients with suspicious nodal disease on CT [odds ratio (OR) 7.1 versus no nodal disease, P = 0.0004], and in patients without a prior cholecystectomy (OR 3.1 versus post-cholecystectomy, P = 0.04).
Conclusion
Addition of PET to conventional cross-sectional imaging has a modest impact on management pre-operatively particularly in patients without a prior cholecystectomy and to confirm suspicious nodal disease on CT.
Introduction
Gallbladder cancer (GBC) is the fifth most common gastrointestinal cancer in the United States with an estimated incidence of 1.2 per 100 000 persons per year.1 Most patients have advanced or unresectable disease at diagnosis.2,3 Early stage cancer is often incidentally diagnosed after a cholecystectomy for presumed benign disease.4 GBCs have a tendency to metastasize early and widely, spreading via lymphatics, hematogenously and intraperitoneally.5 While the overall prognosis is poor,2 a good outcome after a complete resection is possible for early disease (T1/T2, N0).6,7 The role of surgery for locally advanced disease (T3/T4) and regional nodal disease (N1) is more controversial, but surgery remains the only chance for long-term survival for these patients.3,4,7–10 Distant metastatic disease and nodal disease beyond the hepatoduodenal ligament (N2) are generally considered contraindications to surgery because of poor survival outcomes after resection.
Pre-operative staging is important to identify patients with locally advanced or metastatic disease in whom surgery is ineffective. Unfortunately, it is still common for a surgeon to embark on surgery only to find small liver metastases, peritoneal disease, or distant nodal disease not seen on pre-operative imaging. Current staging tools include ultrasound, computed tomography (CT), magnetic resonance imaging (MRI), 18F-FDG-PET (18-fluorodeoxyglucose positron emission tomography) and laparoscopy. In recent years, PET has been used in staging a number of malignancies such as colorectal metastases, oesophageal cancer, lymphoma and melanoma. It is particularly useful for detecting occult metastatic disease and to further characterize equivocal lesions seen on CT or MRI.
There is relatively sparse data in the literature pertaining to the use of PET in GBC. Most published series of utility of PET in GBC have a small number of patients, often combined with cholangiocarcinoma.11–17 Our group previously published a series of patients who had PET for biliary tract malignancies between March 2001 and October 2003, including 41 gallbladder cancers. Thirty-one of those patients had PET as part of their workup prior to intended surgery. The sensitivity for the primary tumour was 86% and for detecting metastatic disease 87%. For detecting recurrence, the sensitivity and specificity were 89% and 100%, respectively. Overall PET changed management in 7 out of 31 (23%) patients.18
In this study, we sought to determine if the utility of PET in GBC has changed given continual improvement in multimodality cross-sectional imaging. Specifically data were analysed for any additional metastatic disease noted on PET that was not seen on CT or MRI that would change surgical management, or PET findings that help confirm equivocal lesions on conventional imaging.
Patients and methods
The Institutional Review Board at Memorial Sloan Kettering Cancer Center (MSKCC) granted a waiver of consent for this retrospective study. Patients with a clinical or pathological diagnosis of gallbladder cancer and who had underwent PET between January 2001 and June 2013 were identified from the institutional administrative database at MSKCC, supplemented by patients from departmental databases. A total of 146 patients were identified and their electronic medical records were reviewed. Eleven patients were excluded because three had PET for reasons other than GBC, and eight patients did not have CT or MRI to correlate PET findings. Of the remaining 135 patients, 100 had been assessed by a hepatobiliary surgeon and considered for surgery, and 35 had PET for follow-up. The 100 patients who had pre-operative PET comprised our study population. Twenty-seven patients in this study had been included in a previously published analysis.18
The majority of patients were referred from another institution after a cholecystectomy for presumed benign disease but were incidentally found to have GBC on pathological evaluation. Histological slides were obtained for these patients and reviewed by pathologists at MSKCC to confirm the diagnosis of GBC. Eighty-two patients had cross-sectional imaging performed or repeated at MSKCC, and 12 were done at an outside institution. The scans were performed clinically per standard institutional protocol. Ninety-four out of 100 PET scans were performed at MSKCC. The technique of 18F-FDG-PET at our institution has been previously described.18 Briefly, patients were fasted for 6 h then injected with 10–15 mCi of 18F-FDG. Standardized uptake values (SUV) were calculated and a cutoff of greater than two was considered abnormal. Abnormal PET avidity was noted in the primary site (gallbladder or gallbladder resection bed), lymph nodes (regional or distant) and distant sites. Eighty-seven PET scans were performed as part of a PET-CT using a low-dose CT protocol for attenuation correction and anatomical localization.
Demographic, clinical and pathological data, as well as treatment and follow-up details were obtained from electronic medical records. Cancer classification and staging were based on the 7th edition of AJCC Staging Manual and wherever possible pathological T classifications were reported. For patients without pathological confirmation, accurate clinical T classifications were inherently difficult; however, evidence of gross invasion of the liver on imaging was taken as evidence of T3 disease. For scans done at MSKCC, radiology images and reports for cross-sectional imaging (CT or MRI) and PET were reviewed for evidence of unresectable disease. For scans done at referring institutions, data were collected from reports. Resectability was determined on a case-by-case basis but contraindications to resection included distant metastases, discontiguous liver metastases, nodal metastases beyond the hepatoduodenal ligament and unresectable T4 disease that invaded major vascular structures or multiple organs. CT/MRI and PET results were classified as positive, suspicious, or negative. The utility of PET was defined by whether PET provided additional information to conventional imaging that influenced management. PET was considered helpful if it avoided a non-therapeutic operation, or it lead to exploration and successful resection in patients deemed unresectable by CT/MRI. In cases where PET lead to unnecessary procedures, the negative impact of PET was reported separately. Disease resectability was confirmed at surgery, and unexplored metastatic disease was confirmed by progression on follow-up imaging or biopsy.
The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for PET to detect metastatic disease were calculated for metastases to any site, and to the peritoneum, lymph nodes, liver and lung. Whenever possible, pathological confirmation of metastatic disease by resection or biopsy was used. When no pathological material was available, metastatic disease was determined by follow-up imaging. True positives included patients with disease confirmed by surgical exploration or by follow-up imaging showing disease progression. True negatives were confirmed by surgical exploration, or if follow-up imaging showed no evidence of metastatic disease at that site. All PET false positives were confirmed histologically by surgical excision or biopsies. False negatives were confirmed by surgical exploration, or in correlation with CT/MRI done concurrently or within 1 month of PET, showing metastatic disease which subsequently progressed on follow-up imaging.
Statistical analyses were performed using Prism (version 6.0; GraphPad Software, Inc., La Jolla, CA, USA). Fisher’s exact tests were performed on categorical data; P-values of <0.05 were considered significant.
Results
One hundred patients were analysed. The median age was 67 years, with a slight female predominance. Sixty-three patients had incidental gallbladder cancer detected after a cholecystectomy, whereas 37 had no prior cholecystectomy. The majority of patients (64%) had T3 disease.
Thirty-eight patients did not proceed to surgical exploration owing to a pre-operative finding of unresectable disease. Of those who proceeded to surgery, 35/62 (56%) were able to be resected. Among the 27 patients deemed unresectable at surgery, peritoneal disease was found in 13, distant nodal disease in 9, liver metastases in 8 and locally advanced disease in 7. The patient, disease and treatment factors are summarized in Table 1.
Table 1.
Patient demographics, clinical and pathological characteristics
| Total patients | 100 |
| Age years, median (interquartile range) | 67 (60–74) |
| Male gender, n | 44 |
| No prior cholecystectomy, n | 37 |
| Post-cholecystectomy, n | 63 |
| T classificationa, n | |
| 1b | 6 |
| 2 | 19 |
| 3 | 64 |
| 4 | 11 |
| Surgery, n | |
| No surgery | 38 |
| Explored, unresectable | 27 |
| Explored, resectable | 35 |
| Unresectable disease found at surgery, n | |
| Peritoneal metastases | 13 |
| Distant nodal metastases | 9 |
| Liver metastases | 8 |
| Locally advanced disease | 7 |
Pathological T classification if histology available. Clinical classification if no histology available.
Table 2 shows the yield of PET for detecting unresectable disease by T classification. The overall yield was 39/100 patients. The yield was comparable among T2, T3 and T4 disease, but there was an increasing trend from T1 to T4 (0%, 37%, 42% and 45%, respectively). No patient with T1 disease had a positive PET for unresectable disease. The yield for patients without a prior cholecystectomy was 47%, compared with 34% for patients with a prior cholecystectomy (incidental GBC), however this difference did not reach statistical significance (P = 0.29).
Table 2.
Yield of positron emission tomography (PET) (positive or suspicious for unresectable disease) by T classification
| T classification | Gallbladder resected (incidental GBC) | % Yield | Gallbladder not resected | % Yield | Total | % Yield |
|---|---|---|---|---|---|---|
| T1 | 0/5 | 0 | 0/1 | 0 | 0/6 | 0 |
| T2 | 5/17 | 29 | 2/2 | 100 | 7/19 | 37 |
| T3 | 13/37 | 35 | 14/27 | 52 | 27/64 | 42 |
| T4 | 4/5 | 80 | 1/6 | 17 | 5/11 | 45 |
| All | 22/64 | 34 | 17/36 | 47 | 39/100 | 39 |
GBC, gallbladder cancer.
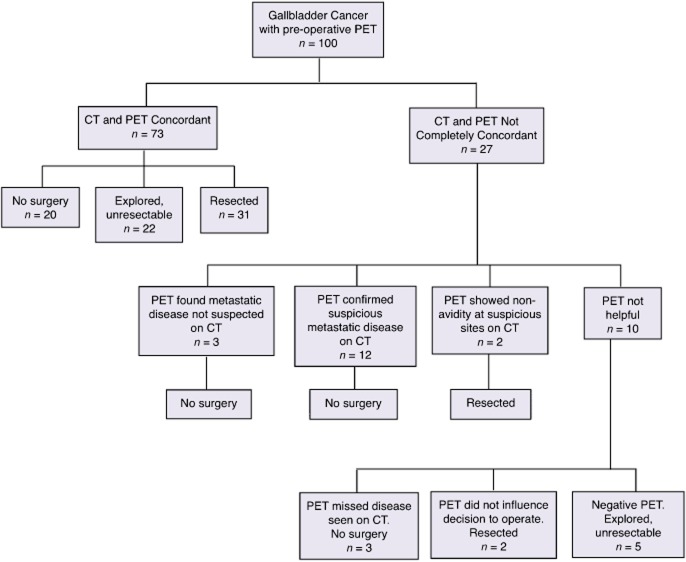
Fig. 1 summarizes the utility of PET. In 73 patients CT and PET were completely concordant and PET did not add any information. In 27 patients, CT and PET results were disconcordant. In 3 patients, PET found metastatic disease not suspected on CT (liver, lung, mediastinal nodes in one patient each). In 12 patients, CT were suspicious for unresectable disease, including distant nodal metastases in 11 and locally advanced T4 disease in one patient. In the absence of PET, these patients may have undergone an exploratory laparotomy and been unresectable. Hence PET contributed to avoiding unnecessary surgery. In two other patients, CT was equivocal for distant metastases (one retroperitoneal nodal and one omental) but PET was negative, leading to surgical exploration and successful resection.
Figure 1.
Flow diagram showing the utility of positron emission tomography (PET). In 73 patients computed tomography (CT) and PET were completely concordant and PET provided no additional information. In 27 patients CT and PET were not completely concordant and in 17 patients PET provided addition information that influenced management
Overall, 5 patients benefitted from the additive information from PET scanning, and in a further 12 patients there was probably a benefit by confirming suspicious findings on CT. Of the 15 patients who avoided surgery based on PET avidity alone or in concordance with CT results, 8 had subsequent radiological progression of disease, 1 had metastatic disease confirmed on biopsy, and 6 patients were lost to follow-up.
When patients with definitively helpful PET and confirmatory PET were analysed together, PET was found to be more useful in patients with nodal disease seen on CT [38% versus 8% if no nodal disease, odds ratio (OR) 7.1, P = 0.0004). A higher proportion of patients without a prior cholecystectomy also had their management changed by PET compared with post-cholecystectomy patients (31% versus 13%, OR 3.1, P = 0.035). The T classification of primary cancer did not influence the utility of PET (24% for T1/T2 versus 17% for T3/T4, OR 1.5, P = 0.56). The influence of these factors on the utility of PET is summarized in Fig. 2. There was a non-significant trend towards increased utility of PET when the CT or MRI was done outside (6/12 versus 11/71, OR 3.2, P = 0.08).
Figure 2.
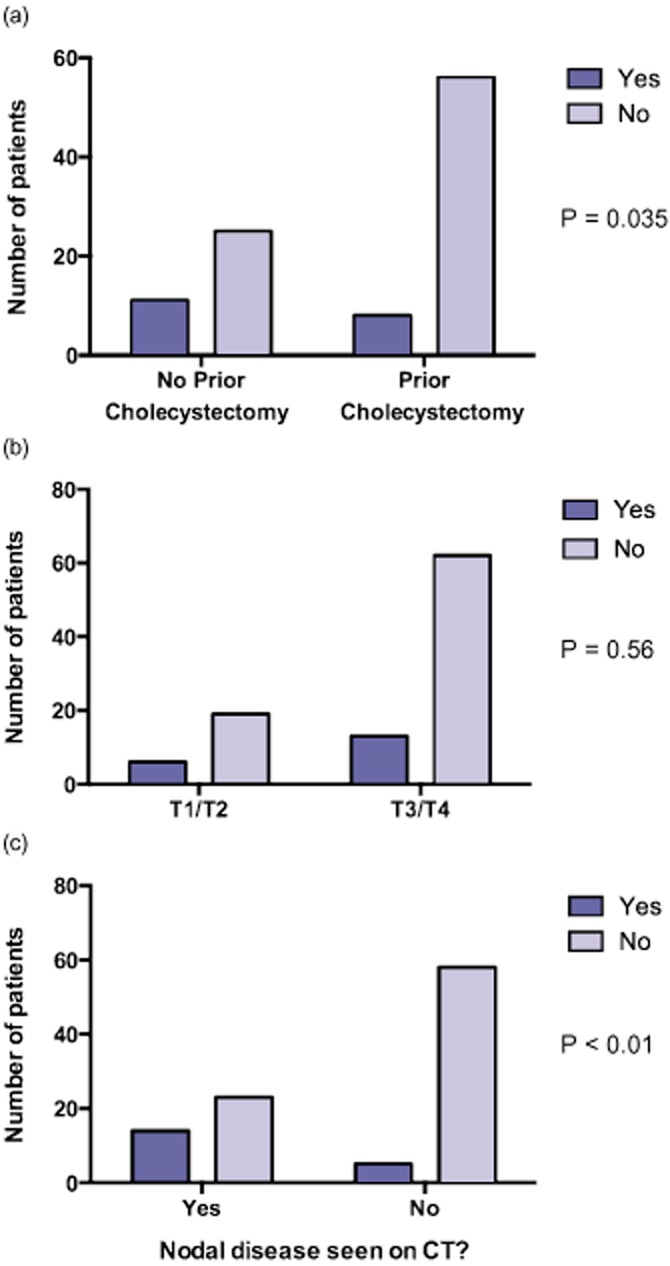
The utility of positron emission tomography (PET) to provide additional information for patients with (a) no prior cholecystectomy compared with prior cholecystectomy, (b) early T classification compared with advanced T classification, and (c) nodal disease seen on computed tomography (CT) compared with no nodal disease
In three patients, PET detected three unrelated findings not seen on CT, including one secondary malignancy and two false-positive lesions. One patient had a PET-avid cancer within a polyp in the sigmoid colon which was resected colonoscopically and confirmed to be malignant. While PET correctly identified this second malignancy, it however missed the liver metastases and T4 disease invading multiple organs and failed to prevent a non-therapeutic laparotomy. The second patient had PET avid mediastinal lymph nodes which led to a mediastinoscopy and biopsy. Results showed benign disease only. PET also missed the liver and peritoneal metastases in this patient. The third patient had PET avid lung nodules which led to a lung resection. Histology showed bronchiolitis obliterans organizing pneumonitis. The gallbladder cancer was successfully resected. Therefore in these three patients PET resulted in invasive procedures that ultimately did not benefit the patient. PET did not help avoid surgery in these cases.
Of the 27 patients who proceeded to the operating room but were not resected, 6 had a laparoscopy which found unresectable disease thus avoiding a non-therapeutic laparotomy, 6 had a laparoscopy but unresectable disease was only found after conversion to a laparotomy, and 15 patients proceeded directly to a laparotomy.
The presence or absence of metastatic disease was able to be determined via surgical exploration +/- biopsies in 64 patients. A further 23 patients who were not explored had adequate follow-up imaging to detect disease progression. Thirteen patients were lost to follow-up and therefore the accuracy of their PET results could not be confirmed. The sensitivity and specificity of PET for metastatic disease (including regional and distant lymph nodes) were 57% and 94%, respectively, with a PPV of 94% and NPV of 58%. When individual sites of metastases were considered, the PPV was highest for peritoneum, followed by the liver, lymph nodes and lung (100%, 93%, 91% and 67%, respectively). The NPV was highest for the lung, followed by the liver, lymph nodes and peritoneum (99%, 93%, 88% and 84%, respectively). Twenty-three patients had false-negative PET, including 13 with peritoneal disease, 6 with retroperitoneal nodal disease and 4 with liver metastases. These results are summarized in Table 3.
Table 3.
Diagnostic performance of positron emission tomography (PET) for metastatic disease (n = 88)
| All metastases (including nodal) | Peritoneal | Nodal (regional and distant) | Liver | Lung | |
|---|---|---|---|---|---|
| Patients with positive PET, n | 33 | 5 | 21 | 15 | 3 |
| Patients with true metastases, n | 53 | 18 | 27 | 19 | 3 |
| Sensitivity, % | 57 | 28 | 70 | 74 | 67 |
| Specificity, % | 94 | 100 | 97 | 99 | 99 |
| Positive Predictive Value, % | 94 | 100 | 91 | 93 | 67 |
| Negative Predictive Value, % | 58 | 84 | 88 | 93 | 99 |
Discussion
Positron emission tomography has been successfully used as an adjunct to standard cross-sectional imaging such as CT and MRI for staging of a number of malignancies. In colorectal liver metastasis, one meta-analysis found that PET changed management in 31.6% of patients.19 Other studies showed a similar influence on management in 38.2% of oesophageal and oesophagogastric cancers,20 30.9% of head and neck cancers21 and 49% of melanoma.22
The sensitivity of PET in distinguishing a benign from a malignant gallbladder mass has been reported as 75–80%, and specificity of 82–88%.13–15 The sensitivity of PET for detecting extrahepatic disease seems to be somewhat poorer, ranging from 56%15 to 100%.16,23 Two studies that compared CT with PET have shown comparable sensitivity for the primary tumour and regional disease, but PET appears to be superior in detecting distant metastases.12,16 However, these studies have the same limitations common to many studies involving PET and gallbladder cancer, namely a small number of patients and combining gallbladder cancer with cholangiocarcinoma in their analyses. Our study here shows a somewhat lower sensitivity at 57% for detecting metastases. This is likely to be influenced by the high false-negative rate for peritoneal disease (16%). Small tumour volume (<1 cm) peritoneal disease may be difficult to diagnose on PET pre-operatively, as shown in some studies of gastric24 and ovarian cancer,25 which are similar to gallbladder cancer in their propensity for transperitoneal spread. In recent years, combined or fused PET/CT has become more common and likely lead to improvement in diagnostic accuracy.26
A study by Petrowsky et al.16 showed that PET changed management in 8 of 48 (17%) patients with biliary tract cancer, including 16 patients with GBC. Butte et al.11 reported their series of 32 patients with incidental gallbladder cancer after a cholecystectomy and found that PET changed management in 25% owing to finding of disseminated disease. Corvera et al. from our group previously reported that PET changed management in 7/31 (23%) of pre-operative GBC patients.18 These seven patients had metastatic disease seen on PET that was not seen on CT. In comparison, in the present study, only two patients had distant metastatic disease that was completely missed by CT. It should be noted that our current study included some patients reported in the earlier study. This difference may be attributed to improving CT and MRI technology and thus increasing accuracy of standard cross-sectional imaging. Furthermore, the definition of metastatic disease differs between the two studies. Corvera et al. included some patients with regional nodal disease in their unresectable metastatic group, whereas many surgeons now consider regional nodal disease resectable, albeit with a poorer prognosis.
We found here that PET was more useful in patients without a prior cholecystectomy compared with patients who already had their primary resected. This may be as a result of referral bias, as patients referred for definitive management after incidental diagnosis of GBC would have passed the test of surgical exploration during their initial cholecystectomy, therefore were less likely to have metastatic disease of sufficient volume to be visible on PET. Another important consideration is that PET in the post-operative setting is difficult to interpret given the inflammatory or hypermetabolic effects associated with wound healing.
We hypothesized that patients with higher T classifications (T3/T4) may benefit from PET more than patients with lower T classifications (T1/T2). Somewhat unexpectedly the results showed no statistical significance. In contrast, the yield of PET (chance of positivity) for unresectable disease increased with T classification. No patient with T1 primary had a positive PET. Patients with T2 and T3 disease had a 35% yield, whereas patients with T4 disease had a 45% yield. Therefore routine use of PET scans in T1 disease without other suspicious lesions is probably not cost effective. However, an increasing yield with more advanced T classifications did not translate to an equivalent increase in benefit to patients. This may be because patients with T3/T4 disease were more likely to have peritoneal disease or small liver metastases, both of which are often missed by PET. Therefore for higher T classifications, even although the rate of true positives for PET increased, so did the false negatives.
Historically, gallbladder cancer has traditionally been treated with nihilism. As a result of a perceived poor prognosis, surgery for T3 and T4 disease had been controversial. A survey-based study by Cubertafond et al. involving 724 surgically treated patients in Europe and Asia in the 1980s showed that patients with carcinoma in situ had good outcomes, ones with T1 and T2 disease did poorly, and ones with T3 and T4 cancers had a median survival of only 3–6 months and no survivors beyond 3 years.8 However modern surgery, where the importance of a liver resection and regional lymphadenectomy is recognized, has resulted in improved survival in selected patients. D’Angelica et al. (2009) reported their series of 104 patients after a resection, where patients with T3 and T4 disease had a median survival of 24 months and 5-year disease-specific survival of 25%.7 Therefore even although a long-term cure may not be possible in these patients, many surgeons will now advocate a radical resection if technically resectable.
The resectability of patients with nodal disease is also controversial. In the study by D’Angelica et al. nodal positivity was associated with a much worse prognosis, with a median survival of 18 months and 5 years disease-specific survival of 17%, compared with 65 months and 51%, respectively, for node-negative patients.7 Several studies have shown that regional nodal involvement, although still a poor prognostic factor, represents a better group than distant nodal disease.13,27,28 In the 7th edition of AJCC Cancer Staging Manual (2009), nodal disease is now separated into N1 (regional nodes including portal and hepatoduodenal) and N2 (distant nodes including periaortic, pericaval, superior mesenteric artery and celiac), with the latter upstaging the patient from stage III in AJCC 6th edition to stage IVA.29 On the contrary, there have been some Japanese studies that showed the number of positive nodes but not the location of the nodes independently predicted survival.30,31 There is probably no benefit in resecting extensive nodal disease, but these patients may benefit from a neoadjuvant approach and pre-operative PET may help identify these patients.
Overall the use of PET was definitively helpful in 5%, confirmatory in 12% and harmful in 3% of our patients. Our data suggests that with modern high-quality cross-sectional imaging, it is uncommon for PET findings to be the sole determinant of resectability. However, we found that PET was more useful in a confirmatory role, particularly in patients with equivocal N2 disease, where PET positivity would be a contraindication to resection. The risk of PET doing harm owing to false positivity by PET must also be considered. Although false-positive PET scans that prevent a patient from having curative surgery are probably rare, false-positives that result in unnecessary additional procedures occurs occasionally (3% in this study).
In spite of the use of CT/MRI and PET, 27 patients still underwent non-therapeutic surgery. Laparoscopy spared six of those patients from a laparotomy. A study by Weber et al. (2002) involving 44 patients with gallbladder cancer who had a staging laparoscopy showed a yield of 48% and an accuracy of 58% in detecting unresectable disease.32 More recently, a study by Agarwal et al. involving patients with 409 primary gallbladder cancer showed a yield of 23.2%, overall accuracy of 55.9% and for detecting surface liver and peritoneal metastases a accuracy of 94.1%.33 These results suggest that there should be a low threshold for laparoscopy in GBC. These studies also showed that the yield of laparoscopy increased with higher T classifications, but not the accuracy, owing to increased false negatives with higher T classifications, a finding analogous with our current results with a different diagnostic modality.
The retrospective nature of this study poses some limitations on our results. Whether the addition of PET changed management was retrospectively and subjectively assessed and the multitude of factors that influenced the clinician’s decision at the time may not be fully realized. As a group our selection criteria for surgery has been well defined and consistent, hence the decision-making process should be fairly reproducible. Nonetheless, for patients where PET appeared to be confirmatory for equivocal metastatic lesions, it is difficult to be certain if PET truly changed management. Another limitation is that the PET scans were not reviewed by a blinded radiologist. As the PET scans were often performed after the CT scans were reported, the interpretation of the PET may be biased. Furthermore, the true accuracy of the imaging studies cannot be confirmed in all patients, as 36/100 patients did not undergo surgical exploration. In 23 patients with adequate imaging follow-up, progression of disease was used as indirect evidence for confirmation of initial imaging findings. Thirteen patients were lost to follow-up hence the accuracy of their imaging is unknown.
Finally, the utility of PET over conventional imaging in any study must be interpreted with the quality of the standard cross-sectional imaging in mind, as apparent usefulness of PET may be a reflection of poor detection rates with CT/MRI. In this study, 18% of CT/MRI and 6% of PET scans were performed at another institution, which may introduce some heterogeneity in the interpretation and reporting of metastatic lesions. In our experience, the quality of CT or MRI scans done outside tertiary oncology referral centres are often suboptimal, and this may lead to an overestimation of the the utility of PET. However, we found no statistically significant difference in the utility of PET between patients whose CT/MRI scans were done at MSKCC or at an outside hospital. The role of PET may diminish as the quality of CT and MRI continue to improve, enabling small liver, lung, or peritoneal metastases to be detected. However, the determination of metastatic involvement of lymph nodes is still problematic on CT and MRI. There are little data specifically examining the size of regional lymph nodes and their correlation with histological nodal positivity in gallbladder cancer. One study found that in patients with biliary cancer, regional nodes with a short-axis diameter >16 mm had a PPV of only 56% for metastatic involvement.34 PET may help overcome this limitation, especially in patients with enlarged N2 nodes in the celiac, retropancreatic, aortocaval and para-aortic regions, where PET avidity will likely render them unresectable. Another potential area where PET may become more useful in the future is in the setting of neoadjuvant chemotherapy, which has not been widely practiced for biliary cancers owing to the lack of evidence for its efficacy. However, with improving systemic agents, the assessment of response may become more important in selecting patients for subsequent resection with curative intent.
Conclusion
PET is highly specific for metastatic gallbladder cancer but not sensitive, particularly for small volume peritoneal disease. The addition of PET to standard staging CT may be helpful in 17% of patients to improve classification of equivocal lesions by CT or MRI and identify distant metastatic disease, but may cause harm in 3% of patients. FDG-PET appears to be complimentary rather than definitive in many patients with GBC, and its role is limited in patients with negative CT/MRI and T1 disease. While the routine use of PET in GBC is probably not cost effective, we believe that PET should be used when there are suspicious findings on CT/MRI, such as large tumours, questionable nodes or peritoneal infiltration.
Funding
None. There are no financial disclosures from any authors.
Conflicts of interest
None declared.
References
- Jayaraman S, Jarnagin WR. Management of gallbladder cancer. Gastroenterol Clin North Am. 2010;39:331–342. doi: 10.1016/j.gtc.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Duffy A, Capanu M, Abou-Alfa GK, Huitzil D, Jarnagin W, Fong Y, et al. Gallbladder cancer (GBC): 10-year experience at Memorial Sloan-Kettering Cancer Centre (MSKCC) J Surg Oncol. 2008;98:485–489. doi: 10.1002/jso.21141. [DOI] [PubMed] [Google Scholar]
- Dixon E, Vollmer CM, Sahajpal A, Cattral M, Grant D, Doig C, et al. An aggressive surgical approach leads to improved survival in patients with gallbladder cancer. Ann Surg. 2005;241:385–394. doi: 10.1097/01.sla.0000154118.07704.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong Y, Jarnagin W, Blumgart LH. Gallbladder cancer: comparison of patients presenting initially for definitive operation with those presenting after prior noncurative intervention. Ann Surg. 2000;232:557–569. doi: 10.1097/00000658-200010000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerma EJ. Towards an oncological resection of gall bladder cancer. Eur J Surg Oncol. 1994;20:537–544. [PubMed] [Google Scholar]
- de Aretxabala X, Roa I, Burgos L, Losada H, Roa JC, Mora J, et al. Gallbladder cancer: an analysis of a series of 139 patients with invasion restricted to the subserosal layer. J Gastrointest Surg. 2006;10:186–192. doi: 10.1016/j.gassur.2005.11.003. [DOI] [PubMed] [Google Scholar]
- D’Angelica M, Dalal KM, DeMatteo RP, Fong Y, Blumgart LH, Jarnagin WR. Analysis of the extent of resection for adenocarcinoma of the gallbladder. Ann Surg Oncol. 2009;16:806–816. doi: 10.1245/s10434-008-0189-3. [DOI] [PubMed] [Google Scholar]
- Cubertafond P, Gainant A, Cucchiaro G. Surgical treatment of 724 carcinomas of the gallbladder. Results of the French Surgical Association Survey. Ann Surg. 1994;219:275–280. doi: 10.1097/00000658-199403000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett DL, Fong Y, Fortner JG, Brennan MF, Blumgart LH. Long-term results after resection for gallbladder cancer. Implications for staging and management. Ann Surg. 1996;224:639–646. doi: 10.1097/00000658-199611000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SY, Poon RT, Lo CM, Ng KK, Fan ST. Management of carcinoma of the gallbladder: a single-institution experience in 16 years. J Surg Oncol. 2008;97:156–164. doi: 10.1002/jso.20885. [DOI] [PubMed] [Google Scholar]
- Butte JM, Redondo F, Waugh E, Meneses M, Pruzzo R, Parada H, et al. The role of PET-CT in patients with incidental gallbladder cancer. HPB (Oxford) 2009;11:585–591. doi: 10.1111/j.1477-2574.2009.00104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SW, Kim HJ, Park JH, Park DI, Cho YK, Sohn CI, et al. Clinical usefulness of 18F-FDG PET-CT for patients with gallbladder cancer and cholangiocarcinoma. J Gastroenterol. 2010;45:560–566. doi: 10.1007/s00535-009-0188-6. [DOI] [PubMed] [Google Scholar]
- Koh T, Taniguchi H, Yamaguchi A, Kunishima S, Yamagishi H. Differential diagnosis of gallbladder cancer using positron emission tomography with fluorine-18-labeled fluoro-deoxyglucose (FDG-PET) J Surg Oncol. 2003;84:74–81. doi: 10.1002/jso.10295. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Fernandez A, Gomez-Rio M, Llamas-Elvira JM, Ortega-Lozano S, Ferron-Orihuela JA, Ramia-Angel JM, et al. Positron-emission tomography with fluorine-18-fluoro-2-deoxy-D-glucose for gallbladder cancer diagnosis. Am J Surg. 2004;188:171–175. doi: 10.1016/j.amjsurg.2003.12.070. [DOI] [PubMed] [Google Scholar]
- Anderson C. Fluorodeoxyglucose PET imaging in the evaluation of gallbladder carcinoma and cholangiocarcinoma. J Gastrointest Surg. 2004;8:90–97. doi: 10.1016/j.gassur.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Petrowsky H, Wildbrett P, Husarik DB, Hany TF, Tam S, Jochum W, et al. Impact of integrated positron emission tomography and computed tomography on staging and management of gallbladder cancer and cholangiocarcinoma. J Hepatol. 2006;45:43–50. doi: 10.1016/j.jhep.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Yamada I, Ajiki T, Ueno K, Sawa H, Otsubo I, Yoshida Y, et al. Feasibility of (18)F-fluorodeoxyglucose positron-emission tomography for preoperative evaluation of biliary tract cancer. Anticancer Res. 2012;32:5105–5110. [PubMed] [Google Scholar]
- Corvera CU, Blumgart LH, Akhurst T, DeMatteo RP, D’Angelica M, Fong Y, et al. 18F-fluorodeoxyglucose positron emission tomography influences management decisions in patients with biliary cancer. J Am Coll Surg. 2008;206:57–65. doi: 10.1016/j.jamcollsurg.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Wiering B, Krabbe PF, Jager GJ, Oyen WJ, Ruers TJ. The impact of fluor-18-deoxyglucose-positron emission tomography in the management of colorectal liver metastases. Cancer. 2005;104:2658–2670. doi: 10.1002/cncr.21569. [DOI] [PubMed] [Google Scholar]
- Blencowe NS, Whistance RN, Strong S, Hotton EJ, Ganesh S, Roach H, et al. Evaluating the role of fluorodeoxyglucose positron emission tomography-computed tomography in multi-disciplinary team recommendations for oesophago-gastric cancer. Br J Cancer. 2013;109:1445–1450. doi: 10.1038/bjc.2013.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming AJ, Jr, Smith SP, Jr, Paul CM, Hall NC, Daly BT, Agrawal A, et al. Impact of [18F]-2-fluorodeoxyglucose-positron emission tomography/computed tomography on previously untreated head and neck cancer patients. Laryngoscope. 2007;117:1173–1179. doi: 10.1097/MLG.0b013e31805d017b. [DOI] [PubMed] [Google Scholar]
- Gulec SA, Faries MB, Lee CC, Kirgan D, Glass C, Morton DL, et al. The role of fluorine-18 deoxyglucose positron emission tomography in the management of patients with metastatic melanoma: impact on surgical decision making. Clin Nucl Med. 2003;28:961–965. doi: 10.1097/01.rlu.0000099805.36471.aa. [DOI] [PubMed] [Google Scholar]
- Ramos-Font C, Gomez-Rio M, Rodriguez-Fernandez A, Jimenez-Heffernan A, Sanchez RS, Llamas-Elvira JM. Ability of FDG-PET/CT in the detection of gallbladder cancer. J Surg Oncol. 2014;109:218–224. doi: 10.1002/jso.23476. [DOI] [PubMed] [Google Scholar]
- Lim JS, Kim MJ, Yun MJ, Oh YT, Kim JH, Hwang HS, et al. Comparison of CT and 18F-FDG pet for detecting peritoneal metastasis on the preoperative evaluation for gastric carcinoma. Korean J Radiol. 2006;7:249–256. doi: 10.3348/kjr.2006.7.4.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannu HK, Cohade C, Bristow RE, Fishman EK, Wahl RL. PET-CT detection of abdominal recurrence of ovarian cancer: radiologic-surgical correlation. Abdom Imaging. 2004;29:398–403. doi: 10.1007/s00261-003-0118-7. [DOI] [PubMed] [Google Scholar]
- Dirisamer A, Schima W, Heinisch M, Weber M, Lehner HP, Haller J, et al. Detection of histologically proven peritoneal carcinomatosis with fused 18F-FDG-PET/MDCT. Eur J Radiol. 2009;69:536–541. doi: 10.1016/j.ejrad.2007.11.032. [DOI] [PubMed] [Google Scholar]
- Oh TG, Chung MJ, Bang S, Park SW, Chung JB, Song SY, et al. Comparison of the sixth and seventh editions of the AJCC TNM classification for gallbladder cancer. J Gastrointest Surg. 2013;17:925–930. doi: 10.1007/s11605-012-2134-9. [DOI] [PubMed] [Google Scholar]
- Meng H, Wang X, Fong Y, Wang ZH, Wang Y, Zhang ZT. Outcomes of radical surgery for gallbladder cancer patients with lymphatic metastases. Jpn J Clin Oncol. 2011;41:992–998. doi: 10.1093/jjco/hyr072. [DOI] [PubMed] [Google Scholar]
- Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A American Joint Committee on Cancer, American Cancer Society. AJCC Cancer Staging Handbook: From the AJCC Cancer Staging Manual. 7th edn. New York: Springer; 2010. [Google Scholar]
- Shirai Y, Sakata J, Wakai T, Ohashi T, Ajioka Y, Hatakeyama K. Assessment of lymph node status in gallbladder cancer: location, number, or ratio of positive nodes. World J Surg Oncol. 2012;10:87. doi: 10.1186/1477-7819-10-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo I, Shimada H, Tanabe M, Fujii Y, Takeda K, Morioka D, et al. Prognostic significance of the number of positive lymph nodes in gallbladder cancer. J Gastrointest Surg. 2006;10:999–1007. doi: 10.1016/j.gassur.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Weber SM, DeMatteo RP, Fong Y, Blumgart LH, Jarnagin WR. Staging laparoscopy in patients with extrahepatic biliary carcinoma. Analysis of 100 patients. Ann Surg. 2002;235:392–399. doi: 10.1097/00000658-200203000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal AK, Kalayarasan R, Javed A, Gupta N, Nag HH. The role of staging laparoscopy in primary gall bladder cancer-an analysis of 409 patients: a prospective study to evaluate the role of staging laparoscopy in the management of gallbladder cancer. Ann Surg. 2013;258:318–323. doi: 10.1097/SLA.0b013e318271497e. [DOI] [PubMed] [Google Scholar]
- Noji T, Kondo S, Hirano S, Tanaka E, Suzuki O, Shichinohe T. Computed tomography evaluation of regional lymph node metastases in patients with biliary cancer. Br J Surg. 2008;95:92–96. doi: 10.1002/bjs.5920. [DOI] [PubMed] [Google Scholar]