Abstract
Background
This single-centre study evaluated the outcome of a pancreatoduodenectomy for Grade 5 injuries of the pancreas and duodenum.
Methods
Prospectively recorded data of patients who underwent a pancreatoduodenectomy for trauma at a Level I Trauma Centre during a 22-year period were analysed.
Results
Nineteen (17 men and 2 women, median age 28 years, range 14–53 years) out of 426 patients with pancreatic injuries underwent a pancreatoduodenectomy (gunshot n = 12, blunt trauma n = 6 and stab wound n = 1). Nine patients had associated inferior vena cava (IVC) or portal vein (PV) injuries. Five patients had initial damage control procedures and underwent a definitive operation at a median of 15 h (range 11–92) later. Twelve had a pylorus-preserving pancreatoduodenectomy (PPPD) and 7 a standard Whipple. Three patients with APACHE II scores of 15, 18, 18 died post-operatively of multi-organ failure. All 16 survivors had Dindo-Clavien grade I (n = 1), grade II (n = 7), grade IIIa (n = 2), grade IVa (n = 6) post-operative complications. Factors complicating surgery were shock on admission, number of associated injuries, coagulopathy, hypothermia, gross bowel oedema and traumatic pancreatitis.
Conclusions
A pancreatoduodenectomy is a life-saving procedure in a small cohort of stable patients with non-reconstructable pancreatic head injuries. Damage control before a pancreatoduodenectomy will salvage a proportion of the most severely injured patients who have multiple injuries.
Introduction
Severe injuries of the pancreatic head, duodenum and bile duct in haemodynamically unstable patients with associated injuries are complex to manage and tax the skill and ingenuity of even the most experienced trauma and pancreatic surgeons.1,2 Previous reports indicate that outcome is determined by the complexity and site of the pancreatic injury, the number, extent, and magnitude of the associated injuries, the amount of blood loss and duration of shock, the rapidity and efficacy of resuscitation, and the speed and quality of surgical intervention.2–7 Overall morbidity rates for maximal pancreatoduodenal injuries are substantial and mortality is directly proportional to the number of injuries sustained and is highest in the elderly and those who are haemodynamically unstable.8 Early mortality is due either to uncontrolled venous bleeding or major adjacent organ injuries.2–4,9 Late mortality is generally a consequence of infection or multiple organ failure.2,3,9
Urgent intervention and resection of the pancreatic head and reconstruction in severely injured patients with complex pancreatic injuries aggravated by hypothermia, coagulopathy and acidosis has in the past resulted in prohibitive mortality rates.4,10 Often life-threatening-associated collateral injuries, especially those involving adjacent large splanchnic veins including the inferior vena cava, portal and superior mesenteric veins take precedence in management.4,10 In addition, there are technical difficulties resecting and reconstructing complex pancreatic injuries which require special surgical skills and expertise.2,4,11 The answers to several issues regarding the role of a pancreatoduodenectomy for major pancreatic injuries are unresolved. These questions include: what is the mortality for emergency Whipple’s resection using modern pancreatic and biliary operative techniques? Is a pylorus-preserving pancreatoduodenectomy (PPPD) technically feasible and appropriate in acute trauma? Is there a beneficial role for a pancreatogastrostomy in selected patients in reconstruction after an emergency Whipple’s resection? Although several substantial reviews12–14 and original data from Cape Town3,9,15 have detailed aspects of the management of pancreatic injuries, no publications have specifically assessed the results of an emergency pancreatoduodenectomy for complex injuries of the pancreas and duodenum when performed by or under the supervision of experienced HPB surgeons. The present study critically evaluated the outcome after a pancreatoduodenectomy for non-reconstructable pancreatic injuries in a cohort of consecutive patients treated at a level I trauma centre using established HPB techniques of resection and reconstruction adapted for the trauma situation.
Patients and methods
Study population
The study design was a single-centre retrospective cohort analysis of prospective data on consecutive patients who had a pancreaticoduodenectomy for trauma between January 1990 and December 2011. The study used a registered fit for purpose departmental database which documents the details of all patients with pancreatic injuries treated at the Level 1 Trauma Centre and the Hepatopancreatobiliary and Surgical Gastroenterology units at Groote Schuur Hospital, Cape Town. The study was approved by the University of Cape Town Ethics and Research Committee and the protocol conforms to the ethical guidelines of the ‘World Medical Association Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Subjects’ adopted by the 18th WMA General Assembly, Helsinki, Finland, June 1964, and revised in Tokyo 2004.
Data collection
During the 22-year study period, 426 patients were treated for pancreatic injuries of whom 19 (4.5%) underwent a pancreaticoduodenectomy for complex non-reconstructable injuries involving the proximal pancreas and duodenum (Fig. 1). Data relating to each patient were entered prospectively on a standardized electronic password protected Microsoft Access data spread sheet and analysed using Microsoft Access and Microsoft Excel. Data fields comprised demographic information including age and gender, mechanism of injury, time from injury to Trauma Centre admission, vital signs on admission including systolic blood pressure in mmHg, heart rate and details of the clinical examination including details of associated extra-abdominal injuries. The trauma scores recorded included the Glasgow Coma Scale (GCS), revised trauma score (RTS), abbreviated injury score (AIS), injury severity score (ISS), APACHE II and P-POSSUM scores. Pre-operative blood gas analysis and arterial blood pH, base deficit, temperature and coagulation profile including the International Normalized Ratio (INR) were recorded. Operative findings and associated intra-abdominal injuries, anatomic location and grade of the pancreatic injury, surgical procedure performed, duration of the operation, post-operative course and duration of hospital stay were recorded. Intra-operative crystalloid and colloid volumes were recorded and the number of packed red cells, fresh frozen plasma and platelet packs given were documented and the accuracy reconciled with blood bank records.
Figure 1.
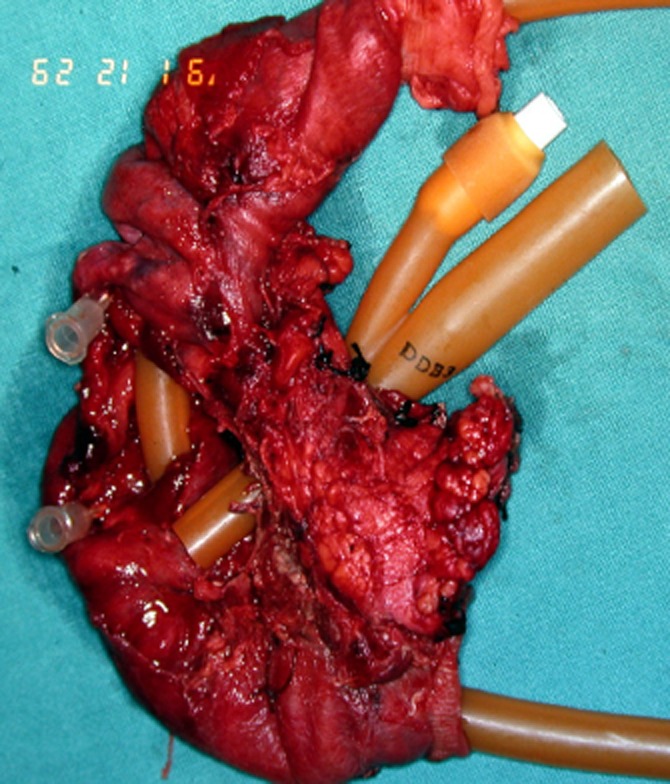
Whipple specimen after resection of a grade 5 pancreatoduodenal injury with disruption of the ampulla and devitalization of the duodenum after blunt trauma in an 18-year-old schoolboy
All patients who had a pancreaticoduodenectomy had grade 5 pancreatic injuries according to the Organ Injury Scaling (OIS) of the American Association for the Surgery of Trauma (AAST).16 Post-operative complications were classified according to the Clavien–Dindo grading system.17 For the purpose of data analysis, post-operative morbidity was subdivided into three categories: (i) pancreas-specific complications which included a pancreatic fistula and pseudocyst; (ii) non-pancreatic abdominal complications including intra-abdominal abscesses, enterocutaneous fistulae and wound infections; and (iii) systemic complications including acute respiratory distress syndrome, pneumonia, renal and multiple organ failure.
Definitions
Shock was defined as a systolic blood pressure less than 90 mmHg measured pre- or intra-operatively. Pancreatic fistulae were graded according to the International Study Group of Pancreatic Fistula classification scheme.18 Infectious complications were defined as a clinical or culture positive nosocomial infections in accordance with the Society of Critical Care Medicine guidelines.19 Post-operative complications recorded as Clavien–Dindo grade III or greater were regarded as severe.17 Mortality was defined as any cause of death occurring in hospital after a pancreatic injury. An initial pH measuring less than 7.3 was defined as acidosis; a temperature less than 35.5°C was defined as hypothermia; coagulopathy was defined as an INR greater than 1.5. The Denver Multiple Organ Failure Scoring System was used to define organ dysfunction and multiple organ failure.20
Operative management of pancreatic injury
Initial resuscitation was according to Advanced Trauma Life Support (ATLS®) guidelines. All patients in this study underwent an urgent laparotomy because of persisting shock with evidence of major intra-abdominal bleeding or an acute abdomen and signs of peritonitis. Operative management of the pancreatic injury was according to a specific operative strategy based on the haemodynamic stability of the patient, the magnitude and extent of associated injuries and the location and severity of the pancreatic injury. Details have been published previously.3,9,15 In brief, the principles applied were urgent control of intra-abdominal bleeding, closure of visceral perforations to prevent contamination of the peritoneal cavity and rapid volume replacement to correct acidosis, coagulopathy and hypothermia. Patients who remained unstable or those in extremis with major associated organ and visceral vascular injuries had an initial damage control operation which comprised a truncated laparotomy followed by continued resuscitation and correction of haemodynamic, metabolic and physiological defects in the intensive care unit and definitive surgery at a later, second or third operation.
A PPPD was done in all patients except in those in whom the injury had irretrievably damaged the pylorus, in which case a classic Whipple resection was done. In patients in whom the jejunum was grossly oedematous, usually after prolonged portal vein clamping and large volume intra-operative crystalloid and blood transfusion, the pancreatic stump was anastomosed to the stomach. The bile duct was joined to the jejunum in the standard fashion for bile duct reconstruction. In situations where the bile duct measured less than 3 mm and gross oedema jeopardized the bile duct to jejunum anastomosis, the gall bladder was preserved and used as the conduit for the biliary enteric anastomosis. In high-risk stented biliary anastomoses, the duodenojejunostomy was created as the first anastomosis using the Imanaga technique to allow post-operative ERCP and biliary stent retrieval.21 All pancreatic anastomoses were stented internally with 8 cm long 5Fr silastic pediatric feeding tubes cut to size.
All biliary and pancreatic anastomoses were drained using closed silastic suction drains. Drainage volumes and amylase levels were measured daily post-operatively. Drains were left in situ while drain amylase levels were elevated or volume measured over 30 ml per day. All patients had intra-operative placement of double or triple lumen internal jugular central lines for venous access and total parenteral nutrition. Nasojejunal low residue enteral feeding was initiated as soon as the patient was haemodynamically stable, inotropes had been discontinued and intestinal continuity re-established. No dietary restrictions were imposed if a pancreatic fistula occurred and oral food intake was continued while the fistula drained. Suspicion of infected intra-abdominal collections post-operatively was investigated by contrast-enhanced computed tomography (CT) scan and treated by ultrasound guided 7-Fr percutaneous catheter drainage.
Results
During the 22-year period from January 1990 to December 2011, 426 patients [389 (91.3%) men, median age 26 years (range 13–69)] had confirmed pancreatic injuries. One hundred and eighteen (27.7%) were caused by blunt trauma (62 motor vehicle accidents, 41 assaults and 15 other), 229 (53.8%) were gunshot wounds and 79 (18.5%) were stab wounds. Of these, 19 (4.5%) had AAST grade V injuries involving the head of the pancreas and duodenum which were not reconstructable and required a pancreatoduodenectomy (Table 1). Thirteen of the 19 had penetrating injuries (12 low-velocity gunshot wounds and 1 stab wound) and 6 had sustained blunt abdominal injuries owing to motor vehicle accidents. Nine patients were in cardiovascular shock on admission to hospital in spite of volume resuscitation by paramedical staff while in transit. On admission to the Trauma Centre, the patients’ median recorded systolic blood pressure was 100 mmHg, (range 0–155) and median pulse rate was 94 per minute (range 80–128). The pre-operative trauma scores are shown in Table 1. The median delay from injury to Trauma Centre admission was 1 h (range 0.5–17). The median delay from admission to initial operation was 2 h (range 1–7).
Table 1.
Demographic and operative data
| Demographic data | |
| Total number of patients | 19 (17 men, 2 women) |
| Median Age | 28 years (range 14–53) |
| Pre-operative data | |
| Revised Trauma Score (RTS) median | 7.84 (range 5.43–7.84) |
| Injury Severity Score (ISS) median | 25 (range 25–75) |
| New Injury Severity Score (NISS) median | 75 (range 49–75) |
| Abdominal trauma Index (ATI) median | 56 (range 33–86) |
| APACHE II Score median | 2 (range 0–18) |
| Intra-operative data | |
| Median duration of surgery | 6h10min (range 4h20–10h45) |
| Median blood loss | 1500 ml (range 800–9000 ml) |
| Median intra-operative blood replacement | 1200 ml (range 0–8325 ml) |
| Median intra-operative crystalloid volume | 6000 ml (range 2000–14000 ml) |
| Median intra-operative colloid volume | 1500 ml (range 500–3000 ml) |
| Median fresh frozen plasma volume | 1040 ml (range 520–2080 ml) |
| Post-operative data | |
| Median duration ICU stay | 5 days (range 1–20 days) |
| Median duration hospital stay | 29 days, (range 14–94 days) |
Associated injuries
Two men who had sustained blunt abdominal trauma had isolated injuries confined to the head of the pancreas and duodenum. In addition to the grade 5 injuries of the pancreas and duodenum, 17 of the 19 patients had a total of 30 associated non-vascular intra-abdominal injuries (median 2, range 1–4) which involved bile ducts and gall bladder (n = 10), liver (n = 9), right kidney and ureter (n = 5), stomach (n = 3) and colon (n = 3). Concurrent extra-abdominal trauma in three patients involved the lung (n = 1), spine (n = 1) and left femur (n = 1). Nine of the 19 patients had 1 or more associated vascular injuries involving the inferior vena cava (IVC) (n = 8), portal vein (PV) (n = 2), superior mesenteric vein (SMV) (n = 2), renal vein (n = 1) and lumbar veins (n = 1).
Surgery
All 19 patients had maximal injuries with destruction of the head of the pancreas involving the main pancreatic duct, the intrapancreatic portion of the distal common bile duct or had disruption or avulsion of the ampulla from the medial wall of the duodenum and duodenal devitalisation. Nine patients had, in addition, as indicated above, exsanguinating retroperitoneal or retropancreatic bleeding owing to associated major splanchnic venous injuries involving the IVC and/or PV, SMV and in one patient the superior mesenteric artery.
Initial damage control operation
Five patients in whom complex pancreatic injuries were aggravated by severe associated injuries and major blood loss, acidosis, coagulopathy, hypothermia and persisting hypotension in spite of vigorous resuscitation, had an initial damage control operation (median duration 102 min, range 92–165), followed by a subsequent pancreatoduodenectomy and reconstruction when stable. These five patients had a median Apache II score of 11 (range 0–18) and received a median of 10 (range 8–12) units of blood intra-operatively. Four of the 5 were shocked on admission to hospital and four had associated vascular injuries. The pancreaticoduodenectomy was completed at a median of 15 h (range 11–96) after the initial damage control laparotomy. Three had relook laparotomies.
Pancreatic resection
Twelve patients had a PPPD and seven a standard Whipple’s resection. In two patients the reconstruction arrangement used the Imanaga technique to allow a post-operative endoscopic retrograde cholangiopancreatography (ERCP) to retrieve or replace the biliary stent because of an associated major liver injury with a segmental intrahepatic ductal injury. In three patients the bile duct measured 3 mm or less in diameter and because the jejunum was grossly oedematous, the bile duct was ligated and the gallbladder was used as the conduit for biliary drainage into the jejunum. In eight patients the back wall of the stomach was used to drain the pancreatic stump with a single layer pancreatogastrostomy and in 11 patients an end-to-side pancreatojejunostomy was used. The relevant intra-operative data are shown in Table 1.
Post-operative course
Morbidity
The Clavien–Dindo complication grades were as follows: 1 patient had a grade I complication, 7 patients had grade II complications, 2 had grade IIIa, 6 had grade IVa, and 3 had grade V complications and died. The surviving 16 patients had a total of 31 complications which included 10 systemic complications (pneumonia n = 5, multi-organ failure n = 2, renal failure n = 1, central line sepsis n = 1, jaundice n = 1), 18 intra-abdominal complications (intra-abdominal and subphrenic abscess n = 6, anastomotic leak n = 2, enterocutaneous fistula n = 2, bowel obstruction n = 1, bile leak n = 1, delayed gastric outlet emptying n = 3 and wound sepsis n = 3). Three patients developed a pancreatic fistula after the pancreatoduodenectomy. All were treated conservatively and resolved spontaneously after a median of 22 (interquartile range 12–38) days. Six patients had infected fluid collections identified on CT scan which were treated with percutaneous ultrasound-guided 8-Fr catheter drainage. Four resolved and two required surgical drainage for persistent multi-locular collections in spite of percutaneous drainage.
Late complications
One patient was admitted to hospital on three occasions over a period of 18 months with acute pancreatitis after an alcohol binge. Each event resolved on conservative treatment. One patient had symptomatic malabsorption which resolved on pancreatic replacement therapy. Three patients required a further operation after discharge from hospital. One patient returned 6 months after the pancreatoduodenectomy for closure of a defunctioning colostomy and two patients in whom the gallbladder had been retained and used for biliary drainage returned 3 and 6 years after the pancreatoduodenectomy with cholangitis as a result hepatic duct stones. Both had a cholecystectomy and a formal hepaticojejunostomy.
Mortality
Three of the 19 patients died, 2 of whom had damage control operations. All 3 were shocked on admission; 2 were in extremis on arrival at the Trauma Centre with no recordable blood pressure and both underwent an initial damage control operation before later definitive surgery. All 3 patients who died had associated major splanchnic venous injuries involving IVC, PV and SMV and had APACHE II scores on admission of 15, 18 and 18. Root-cause analysis showed that 2 patients died of multi-organ failure and disseminated intravascular coagulopathy within 48 h of the resection after receiving a median of 27 units of blood during the damage control operation. The third patient died after 24 days of multi-organ failure, acute respiratory distress syndrome, and resistant acinetobacter and pseudomonas-related intra-abdominal sepsis.
Discussion
This prospective single-centre observational cohort analysis is unique in several respects. To the authors knowledge this is the largest series documenting an emergency pancreatoduodenectomy in injured patients with severe trauma of the proximal pancreas and duodenum. There are no existing data on the results of a proximal pancreatic resection and reconstruction in severely injured patients performed by or under the supervision of experienced HPB surgeons using established pancreatobiliary operative techniques adapted for trauma. In this cohort of patients reconstruction is frequently technically difficult as the ducts are non-dilated and the surrounding organs damaged or oedematous which necessitates modification of conventional biliary and pancreatic anastomoses.1,2,4,11 Unlike previous publications, a novel feature in this study was the ability to do a PPPD in a substantial proportion of injured patients. Importantly, in those patients who, in addition to maximal injuries to the pancreas, also had severe injuries to adjacent vascular, biliary, enteric, colonic or solid organs and had persistent shock, an initial damage control operation was followed by a delayed pancreatoduodenectomy and reconstruction when the patient was stable.
Most authors agree that a pancreaticoduodenectomy for trauma is seldom necessary and should be reserved for the select small group of patients with severe injuries of the head of pancreas and duodenum in whom lesser procedures with preservation of the pancreas and duodenum is not possible.12,13 However, the mortality rate for a Whipple resection in severely injured and unstable patients is prohibitive, and in this and other series, those who survive also have a high post-operative complication rate.1,14 When faced with a devitalized head of the pancreas and duodenum, an avulsed ampulla or a near-complete traumatic resection, a surgeon may have no recourse but to proceed and complete the resection provided the patient is haemodynamically stable and the necessary surgical expertise is available.2,4 McKone has proposed specific indications for a pancreatoduodenectomy for trauma: (i) extensive devitalization of the head of the pancreas and duodenum in whom there is no prospect of a repair; (ii) ductal disruption in the pancreatic head with AAST grade 5 injuries of the duodenum and distal common bile duct; (iii) injury to the ampulla of Vater, with disruption of the main pancreatic duct from the duodenum.22 It should be emphasized that only patients who had devitalised non-reconstructable injury were considered for a pancreatoduodenectomy in the study. Other authors23 have recently suggested that lesser procedures may be applicable for grade V injuries but this is not an option in patients with a disrupted and devitalized duodenum and pancreas.
The reputation of an emergency pancreaticoduodenectomy is tarnished by high mortality rates reported in the literature. In an analysis of 61 publications which reported 220 pancreatoduodenectomies for trauma, Krige et al. found an overall mortality of 34%.1 Substantial experience is scant. Only seven series have previously treated ten or more patients with a pancreatoduodenectomy for trauma24–30 (Table 2).
Table 2.
Pancreatoduodenectomy for trauma
| Author | Year | Site | No of patients | Mortality |
|---|---|---|---|---|
| Yellin24 | 1975 | LAC + USC Medical Center, Los Angeles, USA | 10 | 6 |
| Balasegaram25 | 1979 | General Hospital, Kuala Lumpur, Malaysia | 12 | 5 |
| Jones26 | 1985 | Parkland Memorial Hospital, Dallas, USA | 12 | 7 |
| Oreskovich27 | 1984 | Harbourview Medical Center, Seattle, USA | 10 | 0 |
| Feliciano28 | 1987 | Ben Taub General Hospital, Houston, USA | 13 | 6 |
| Asensio29 | 2003 | LAC + USC Medical Center, Los Angeles, USA | 18 | 6 |
| Thompson30 | 2013 | Harbourview Medical Center, Seattle, USA | 15 | 2 |
| This study | 2014 | Groote Schuur Hospital, Cape Town, South Africa | 19 | 3 |
A pancreatoduodenectomy for trauma is perhaps the most demanding of all pancreatic resections because the procedure is performed under the most difficult circumstances with severe operative constraints. Management of the associated and collateral damage is crucial in ensuring survival in this group of desperately injured patients and, in particular, injuries to adjacent large visceral splanchnic veins are frequently immediately life-threatening and require priority intervention.10 Urgent vascular access to a lacerated retropancreatic portal or superior mesenteric vein in an exsanguinating patient is often problematic and accelerated exposure and rapid control is necessary.1 Assessment of the extent of the pancreatic injury and the need for resection requires mature judgement and skilled evaluation and in these situations intra-operative appraisal by an HPB surgeon provides invaluable assistance to the trauma surgeon. The decision to do a pancreatoduodenectomy may be obvious, especially if blunt injury has resulted in a near complete de facto resection.1 However, in gunshot injuries of the pancreatic head, assessment may be difficult and crucial strategic decisions benefit from the opinion of an experienced pancreatic surgeon. In some circumstances a lesser procedure is both appropriate and technically feasible without resorting to a pancreatoduodenectomy.
The conduct and execution of an emergency pancreaticoduodenectomy for trauma differs from the elective operation.1,2 There is general agreement that patients who have a major pancreatic injury with associated major visceral injuries and are haemodynamically unstable in spite of vigorous resuscitation and are coagulopathic, acidotic and hypothermic and have received a massive intra-operative blood transfusion should have an abbreviated laparotomy with a damage control procedure and subsequent re-exploration, resection and reconstruction when stable.10,12,30–32 While some authors recommend that a pancreaticoduodenectomy for trauma should always be performed as a two-stage procedure, this has not been the authors experience. In this series, five patients who were unstable in spite of optimal resuscitation had an initial damage control operation to achieve haemostasis with staple closure of hollow viscera and external drainage of pancreas and common bile duct. Resection and anastomoses were completed at either the second or third reoperation 48 or 72 h later when the patient was stable.
This study has several specific limitations. In spite of the fact that the data generated are from a high-volume tertiary academic centre, the patient numbers are small and may reflect an inherent referral and treatment bias. The analysis is based on a select high-risk surgical cohort treated in a centre with constant access to specialist multidisciplinary HPB care which may not be representative of or applicable to lesser resourced hospitals where such facilities are not freely available. A strength of this study is the prospective documentation of a robust dataset conducted in a single centre using uniform criteria in a defined and homogenous population of consecutive patients supervised by a single surgeon (J.E.K.) for the duration of the study period.
In conclusion, a pancreaticoduodenectomy for trauma is seldom necessary and is reserved for maximal injuries involving the head of the pancreas and duodenum in which repair is not feasible and where the decision to do a pancreaticoduodenectomy is unavoidable. This study has shown that a PPPD is technically feasible in the trauma situation. A pancreatogastrostomy is an option when a conventional pancreatojejunostomy is difficult as a result of an edematous jejunum. Initial damage control with delayed resection and reconstruction is applicable in a select group of patients. While an emergency pancreatoduodenectomy has significant morbidity and appreciable mortality owing to complicating factors, associated injuries and shock, a resection may be the only option in complex injuries with ampullary destruction or a devitalized duodenum. The pancreas is an unforgiving organ, especially if severely damaged and it is prudent to call an experienced HPB surgeon to assist with operative decisions as the procedure is technically demanding and crucial procedural decisions must be made during resection and reconstruction. For the patients benefit, this should not be the time for a trauma surgeon to be doing his or her first unsupervised Whipple’s resection. The current data show that these are patients with complex problems associated with significant post-operative morbidity and should be managed collaboratively by both trauma and HPB surgical teams.
Conflicts of interest
None declared.
Author contributions
Study conception and design: Krige, Nicol, Navsaria.
Acquisition of data: Krige.
Analysis and interpretation of data: Krige, Nicol, Navsaria.
Drafting of manuscript: Krige, Nicol, Navsaria.
Critical revision: Krige, Nicol, Navsaria.
References
- Krige JEJ, Bornman PC, Terblanche J. The role of pancreatoduodenectomy in the management of complex pancreatic trauma. In: Hanyu F, Takasaki K, editors. Pancreatoduodenectomy. Tokyo: Springer-Verlag; 1997. pp. 49–62. [Google Scholar]
- Krige JE, Beningfield SJ, Nicol AJ, Navsaria P. The management of complex pancreatic injuries. S Afr J Surg. 2005;43:92–102. [PubMed] [Google Scholar]
- Krige JE, Kotze UK, Hameed M, Nicol AJ, Navsaria PH. Pancreatic injuries after blunt abdominal trauma: an analysis of 110 patients treated at a level 1 trauma centre. S Afr J Surg. 2011;49:62–64. 58, 60. [PubMed] [Google Scholar]
- Krige JE, Thomson SR. Operative strategies in pancreatic trauma – keep it safe and simple. S Afr J Surg. 2011;49:106–109. [PubMed] [Google Scholar]
- Kao LS, Bulger EM, Parks DL, Byrd GF, Jurkovich GJ. Predictors of morbidity after traumatic pancreatic injury. J Trauma. 2003;55:898–905. doi: 10.1097/01.TA.0000090755.07769.4C. [DOI] [PubMed] [Google Scholar]
- Smego DR, Richardson JD, Flint LM. Determinants of outcome in pancreatic trauma. J Trauma. 1985;25:771–776. doi: 10.1097/00005373-198508000-00007. [DOI] [PubMed] [Google Scholar]
- Hwang SY, Choi YC. Prognostic determinants in patients with traumatic pancreatic injuries. J Korean Med Sci. 2008;23:126–130. doi: 10.3346/jkms.2008.23.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scollay JM, Yip VS, Garden OJ, Parks RW. A population-based study of pancreatic trauma in Scotland. World J Surg. 2006;30:2136–2141. doi: 10.1007/s00268-006-0039-z. [DOI] [PubMed] [Google Scholar]
- Chinnery GE, Krige JE, Kotze UK, Navsaria P, Nicol A. Surgical management and outcome of civilian gunshot injuries to the pancreas. Br J Surg. 2012;99(Suppl. 1):140–148. doi: 10.1002/bjs.7761. [DOI] [PubMed] [Google Scholar]
- Wang GF, Li YS, Li JS. Damage control surgery for severe pancreatic trauma. Hepatobiliary Pancreat Dis Int. 2007;6:569–571. [PubMed] [Google Scholar]
- Krige JEJ, Bornman PC, Beningfield SJ, Funnell IC. Pancreatic Trauma. In: Pitt HA, Carr-Locke DL, Ferrucci JT, editors. Hepatobiliary and Pancreatic Disease. 1st edn. Boston: Little, Brown and Company; 1995. pp. 421–435. [Google Scholar]
- Chrysos E, Athanasakis E, Xynos E. Pancreatic trauma in the adult: current knowledge in diagnosis and management. Pancreatology. 2002;2:365–378. doi: 10.1159/000065084. [DOI] [PubMed] [Google Scholar]
- Subramanian A, Dente CJ, Feliciano DV. The management of pancreatic trauma in the modern era. Surg Clin North Am. 2007;87:1515–1532. doi: 10.1016/j.suc.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Krige JEJ, Beningfield SJ, Bornman PC. Management strategies in pancreatic trauma. In: Johnson C, Taylor I, editors. Recent Advances in Surgery. 29th edn. London: Royal Society of Medicine; 2006. pp. 95–118. [Google Scholar]
- Farrell RJ, Krige JE, Bornman PC, Knottenbelt JD, Terblanche J. Operative strategies in pancreatic trauma. Br J Surg. 1996;83:934–937. doi: 10.1002/bjs.1800830715. [DOI] [PubMed] [Google Scholar]
- Moore EE, Cogbill TH, Malangoni MA, Jurkovich GJ, Champion HR, Gennarelli TA, et al. Organ injury scaling, II: pancreas, duodenum, small bowel, colon, and rectum. J Trauma. 1990;30:1427–1429. [PubMed] [Google Scholar]
- Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. International Study Group on Pancreatic Fistula Definition. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- Moore FA, Sauaia A, Moore EE, Haenel JB, Burch JM, Lezotte DC. Postinjury multiple organ failure: a bimodal phenomenon. J Trauma. 1996;40:501–510. doi: 10.1097/00005373-199604000-00001. [DOI] [PubMed] [Google Scholar]
- Imanaga H. A new method of pancreaticoduodenectomy designed to preserve liver and pancreatic function. Surgery. 1960;47:577–586. [PubMed] [Google Scholar]
- McKone TK, Bursch LR, Scholten DJ. Pancreaticoduodenectomy for trauma: a life-saving procedure. Am Surg. 1988;54:361–364. [PubMed] [Google Scholar]
- van der Wilden GM, Yeh DD, Hwabejire JO, Klein EN, Fagenholz PJ, King DR, et al. Trauma Whipple: do or don’t after severe pancreaticoduodenal injuries? An analysis of the National Trauma Data Bank (NTDB) World J Surg. 2014;38:335–340. doi: 10.1007/s00268-013-2257-5. [DOI] [PubMed] [Google Scholar]
- Yellin AE, Rosoff L. Pancreatoduodenectomy for combined pancreatoduodenal injuries. Arch Surg. 1975;110:1117–1183. doi: 10.1001/archsurg.1975.01360160015001. [DOI] [PubMed] [Google Scholar]
- Balasegaram M. Surgical management of pancreatic trauma. Curr Probl Surg. 1979;16:1–59. doi: 10.1016/s0011-3840(79)80013-1. [DOI] [PubMed] [Google Scholar]
- Jones RC. Management of pancreatic trauma. Am J Surg. 1985;150:698–704. doi: 10.1016/0002-9610(85)90412-x. [DOI] [PubMed] [Google Scholar]
- Oreskovich MR, Carrico CJ. Pancreaticoduodenectomy for trauma: a viable option? Am J Surg. 1984;147:618–623. doi: 10.1016/0002-9610(84)90126-0. [DOI] [PubMed] [Google Scholar]
- Feliciano DV, Martin TD, Cruse PA, Graham JM, Burch JM, Mattox KL, et al. Management of combined pancreatoduodenal injuries. Ann Surg. 1987;205:673–680. doi: 10.1097/00000658-198706000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asensio JA, Petrone P, Roldán G, Kuncir E, Demetriades D. Pancreaticoduodenectomy: a rare procedure for the management of complex pancreaticoduodenal injuries. J Am Coll Surg. 2003;197:937–942. doi: 10.1016/j.jamcollsurg.2003.07.019. [DOI] [PubMed] [Google Scholar]
- Thompson CM, Shalhub S, DeBoard ZM, Maier RV. Revisiting the pancreaticoduodenectomy for trauma: a single institution’s experience. J Trauma Acute Care Surg. 2013;75:225–228. doi: 10.1097/TA.0b013e31829a0aaf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glancy KE. Review of pancreatic trauma. West J Med. 1989;151:45–51. [PMC free article] [PubMed] [Google Scholar]
- Nicol AJ, Navsaria PH, Krige JE. Damage control surgery. S Afr J Surg. 2010;48:4–5. [PubMed] [Google Scholar]


