Abstract
Background
This study aims to assess the relationship of body mass index (BMI) status with respiratory conditions, asthma, and chronic obstructive pulmonary disease (COPD) in a state population.
Methods
Self-reported data from 11,868 adults aged ≥18 years in the 2012 South Carolina Behavioral Risk Factor Surveillance System telephone survey were analyzed using multivariable logistic regression that accounted for the complex sampling design and adjusted for sex, age, race/ethnicity, education, smoking status, physical inactivity, and cancer history.
Results
The distribution of BMI (kg/m2) was 1.5% for underweight (<18.5), 32.3% for normal weight (18.5-24.9), 34.6% for overweight (25.0-29.9), 26.5% for obese (30.0-39.9), and 5.1% for morbidly obese (≥40.0). Among respondents, 10.0% had frequent productive cough, 4.3% had frequent shortness of breath (SOB), 7.3% strongly agreed that SOB affected physical activity, 8.4% had current asthma, and 7.4% had COPD. Adults at extremes of body weight were more likely to report having asthma or COPD, and to report respiratory conditions. Age-adjusted U-shaped relationships of BMI categories with current asthma and strongly agreeing that SOB affected physical activity, but not U-shaped relationship with COPD, persisted after controlling for the covariates (p<0.001). Morbidly obese but not underweight or obese respondents were significantly more likely to have frequent productive cough and frequent SOB than normal weight adults after adjustment.
Conclusion
Our data confirm that both underweight and obesity are associated with current asthma and obesity with COPD. Increased emphasis on exercise and nutrition may improve respiratory conditions.
Keywords: Body Mass Index, chronic obstructive pulmonary disease, asthma, respiratory conditions, population-based study
Introduction
A large number of studies support that obesity is a major risk factor for respiratory symptoms and chronic conditions such as cardiovascular disease, asthma, and chronic obstructive pulmonary disease (COPD) [1-6]. In addition, a few studies reported that underweight was also associated with reduced respiratory function and asthma [7-9]. Breathlessness and exercise intolerance are common in clinical studies of patients with obesity and/or respiratory conditions [7,10-14]. A U-shaped relationship of body mass index (BMI) was reported with dyspnea in men and with symptomatic airway hyper-responsiveness, which are asthma-related symptoms that include wheezing and dyspnea in men [7,8,15,16]. Other studies have not observed a significant relationship between BMI and airway hyper-responsiveness [17,18]. However, the existing research on the relationships between extremes of BMI with respiratory conditions and respiratory symptoms is limited—often because of small sample sizes in clinical studies or exclusion of underweight participants or not distinguishing between obesity and morbid obesity [7-9,15,16,18-20].
This study aims to assess the relationship of BMI levels with self-reported respiratory symptoms and respiratory conditions using the 2012 population-based South Carolina Behavioral Risk Factor Surveillance System (BRFSS) survey. While the BRFSS relies on self-reported characteristics rather than clinical measurements, it provides the opportunity to assess potential relationships in a large general population, which is better representative of the heterogeneous types of patients that would enter a physician's office than subjects commonly selected for clinical studies.
Material and Methods
The BRFSS is an annual random-digital-dialed telephone survey that is conducted by state health departments in collaboration with the Centers for Disease Control and Prevention (CDC) in all 50 states, the District of Columbia, and US territories. The respondents in households with either landline or cellular telephones were selected using a complex stratified sampling method and new weighting procedures have been adopted since 2011 in order to better reflect the nation's health status (http://www.cdc.gov/surveillancepractice/reports/brfss/brfss.html). The South Carolina (SC) BRFSS in 2012 included questions about socio-demographic characteristics, risk behaviors, chronic diseases, respiratory symptoms, self-reported height, and self-reported weight. The combined response rate (the number of respondents who completed the survey as a proportion of all eligible and likely-eligible persons using the American Association of Public Opinion Research Response Rate Formula #4) was 48.6% for SC respondents aged ≥18 years in the survey (http://www.cdc.gov/brfss/annual_data/2012/pdf/SummaryDataQualityReport2012_20130712.pdf). The survey data do not contain personal identification information and this study is a secondary data analysis, which is exempt from Institutional Review Board review.
Of 12,795 respondents aged ≥18 years in the 2012 SC BRFSS, 11,868 (92.8%) adults who provided complete information were included in this study after exclusion of those missing data on sex, age, race/ethnicity, education, body weight and/or body height, smoking status, physical inactivity, cancer history, asthma, or COPD.
Respiratory Conditions
A history of COPD was defined as an affirmative response to the question “Has a doctor, nurse, or other health professional ever told you that you have chronic obstructive pulmonary disease or COPD, emphysema or chronic bronchitis?” Over 76.4% of respondents with self-reported COPD also reported having had a breathing test such as spirometry. Current asthma was defined for persons who answered in the affirmative to the following two questions: “Has a doctor, nurse, or other health professional ever told you that you have asthma?” and “Do you still have asthma?” Persons who reported ‘don’t know/not sure' as a response to either COPD or asthma were defined as not having been diagnosed with that condition. Three respiratory symptom questions are described as follows: 1)“How often do you cough up mucus or phlegm?” A frequent productive cough was defined when respondents chose either ‘everyday’ or ‘most days a week’ to compare with respondents with responses of ‘a few days a month’, ‘only with occasional colds or chest infections’, or ‘never’; 2)“During the past 30 days, how often did you feel short of breath (SOB)?” Frequent SOB was defined when respondents chose either ‘all the time’ or ‘most of the time’ to compare to respondents with other responses; and 3)“Thinking about your physical activity during the last 12 months, do you agree slightly or strongly, or disagree slightly or strongly with the following statement: I do less now than I used to because of my breathing problems”, we compared ‘strongly agree’ to respondents with the other responses.
Body Mass Index (BMI)
All respondents were asked “about how much (pounds) do you weigh without shoes” and “About how tall (feet and inches) are you without shoes?” BMI was then calculated as kilograms/meter2 and grouped into five categories: underweight (<18.5 kg/m2), normal weight (18.5-24.9 kg/m2), overweight (25.0-25.9 kg/m2), obese (30.0-39.9 kg/m2), and morbidly obese (≥40.0 kg/m2).
Covariates
Selected socio-demographic characteristics included age (18-24, 25-34, 35-44, 45-64, or ≥65 years), sex, and race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, or non-Hispanic other). Respondents were asked the highest grade or year of school completed and responses were grouped into less than a high school diploma (≤grade 11), high school graduate or equivalent (grade 12 or general education development certificate), and at least some college (1 to 3 years college or college ≥4 years).
A history of cancer was defined as an affirmative response to the question “Has a doctor, nurse, or other health professional ever told you that you had cancer (excluding skin cancer)?” Cancer is often highly associated with underweight so we included a history of cancer as a confounder in the model to ascertain whether underweight status was explained by comorbid cancer. For these analyses, never smokers were defined by a negative response to the first tobacco question “Have you smoked at least 100 cigarettes in your entire life?” Former smokers were defined by an affirmative response to the first tobacco question and a response of “not at all” to a second tobacco use question, “Do you now smoke cigarettes every day, some days, or not at all?” Current smokers were defined by an affirmative response to the first question and a response of “every day” or “some days” to the second. Physical inactivity was defined by a negative response to “During the past month, other than your regular job, did you participate in any physical activities or exercises such as running, calisthenics, golf, gardening, or walking for exercise?”
Statistical Analyses
First, we examined the distributions of selected characteristics among study respondents overall and then by levels of BMI. We also compared the distribution of BMI levels for respondents defined by COPD status and current asthma status. The age-adjusted prevalence and 95% confidence interval (CI) of respiratory symptoms, COPD, and asthma by BMI levels were obtained from separate logistic regression models that included age as the covariate. Finally, we assessed the adjusted prevalence ratios (PR) and 95% CI for the likelihood of having respiratory symptoms or respiratory conditions associated with BMI levels using a multivariable logistic regression model that included sex, age, race/ethnicity, education, current smoking status, and physical inactivity as covariates. We repeated the multivariable logistic analyses with the addition of cancer history as a covariate to determine whether cancer may confound the relationship, particularly among underweight persons. The complex sampling design in this study requires a statistical software that can take into account stratification, clustering, and sample weights to obtain more representative estimates of population prevalence and the associated CIs. Therefore, SAS-callable SUDAAN (Release 10.0.1, Research Triangle Institute, NC) was used to analyze the data.
Results
Table 1 shows the distribution of selected characteristics among 11,868 adults aged ≥18 years. Among SC adults, 1.5% were underweight, 32.3% were normal weight, 34.6% were overweight, 26.5% were obese, 5.1% were morbidly obese, 23.0% were current smokers, 24.4% reported physical inactivity in the past month, 10.0% had a frequent productive cough, 4.3% had SOB, 7.3% strongly agreed that SOB affected physical activity, 8.4% had current asthma, 7.4% had COPD, and 6.6% had a history of cancer. Only 2.6% of the study population had both COPD and asthma.
Table 1. The distribution of selected characteristics among 11,868 adults aged ≥18 years: South Carolina, 2012.
| Characteristic | Na | % (95% CI)b |
|---|---|---|
| Sex | ||
| Men | 4,777 | 49.5 (48.2-50.8) |
| Women | 7,091 | 50.5 (49.2-51.8) |
| Age (years) | ||
| 18-24 | 590 | 13.2 (12.1-14.4) |
| 25-34 | 1,115 | 16.6 (15.6-17.8) |
| 35-44 | 1,402 | 16.4 (15.4-17.5) |
| 45-64 | 4,654 | 34.5 (33.3-35.7) |
| 65+ | 4,107 | 19.2 (18.4-20.1) |
| Race | ||
| Non-Hispanic White | 7,983 | 66.8 (65.5-68.1) |
| Non-Hispanic Black | 3,291 | 25.6 (24.4-26.7) |
| Other/multiracial | 594 | 7.6 ( 6.8- 8.6) |
| Education | ||
| Less than high school diploma | 1,391 | 16.3 (15.1-17.5) |
| High school graduate or equivalent | 3,714 | 30.8 (29.5-32.0) |
| At least some college | 6,763 | 53.0 (51.6-54.3) |
| Smoking status | ||
| Current smokers | 2,054 | 23.0 (21.8-24.2) |
| Former smokers | 3,501 | 25.9 (24.8-27.0) |
| Never smokers | 6,110 | 51.1 (49.8-52.5) |
| Physical inactivity in past month | 3,184 | 24.4 (23.3-25.6) |
| Body Mass Index (BMI, kg/m2) | ||
| Underweight (<18.5) | 184 | 1.5 ( 1.2- 1.9) |
| Normal weight (18.5-24.9) | 3,639 | 32.3 (31.0-33.6) |
| Overweight (25.0-29.9) | 4,255 | 34.6 (33.3-35.9) |
| Obese (30.0-39.9) | 3,146 | 26.5 (25.4-27.7) |
| Morbidly obese (≥40.0) | 644 | 5.1 ( 4.6- 5.7) |
| Chronic Conditions | ||
| Any cancer excluding skin cancer | 1,184 | 6.6 ( 6.1- 7.2) |
| Current asthma | 942 | 8.4 ( 7.7- 9.2) |
| Chronic obstructive pulmonary disease | 1,033 | 7.4 ( 6.7- 8.1) |
| Frequent productive cough | 1,173 | 10.0 (9.2-10.8) |
| Frequent shortness of breath (SOB) | 518 | 4.3 ( 3.8- 4.9) |
| Strongly agree that shortness of breath affects physical activity | 890 | 7.3 ( 6.6- 8.0) |
Unweighted sample size.
Weighted percentage and 95% confidence interval (CI).
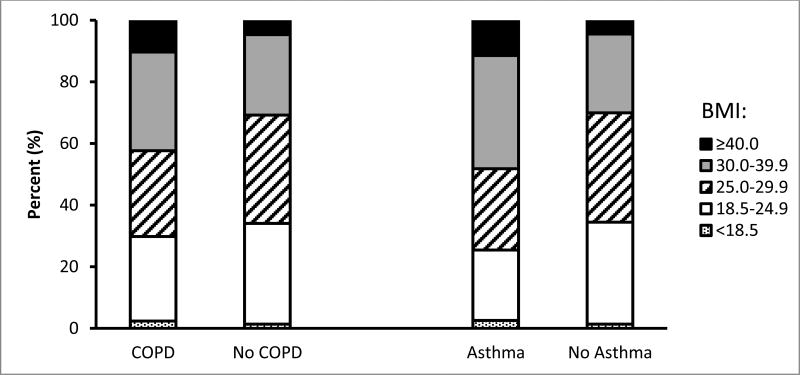
Figure 1 demonstrates that respondents who reported having COPD had a significantly higher percentage of obesity (32.0% vs. 26.1%, p=0.01) and morbid obesity (10.3% vs. 4.7%, p<0.001) but not underweight status (2.4% vs. 1.4%, p=0.10) than those without COPD. Similarly, a significantly higher percentage of obesity (36.8% vs. 25.6%, p<0.001) and morbid obesity (11.5% vs. 4.5%, p<0.001) but not underweight (2.6% vs. 1.4%, p=0.12) was also reported among respondents who had current asthma than those without current asthma.
Figure 1. Distribution of levels of body mass index among adults aged ≥18 years by chronic obstructive pulmonary disease and current asthma status: South Carolina, 2012.
The percentage of selected characteristics by BMI levels are presented in Table 2. Compared to persons with normal weight, underweight adults were significantly (p<0.05) more likely to have current asthma, COPD, and to strongly agree that SOB affects physical activity. Obese or morbidly obese adults were significantly (p<0.05) more likely than adults with normal weight to have asthma, COPD, physical inactivity, frequent SOB, and to strongly agree that SOB affects physical activity. Overweight, obese and morbidly obese adults were significantly (p<0.05) less likely than adults with normal weight to be current smokers. In contrast, overweight and obese adults were significantly (p<0.05) more likely than adults with normal weight to be former smokers. A history of cancer did not differ by BMI level (Table 2). In an age-adjusted logistic regression model, only those who were underweight were more likely to have cancer compared to normal weight respondents (PR=1.66; 95% CI:1.01-2.72); however, that relationship was no longer significant after adjustment for other covariates (data not shown).
Table 2. Percentage of selected characteristics among adults aged ≥18 years, by level of body mass index: South Carolina, 2012.
| Characteristic | |||||
|---|---|---|---|---|---|
| Underweight(<18.5 kg/m2)[N=184]% (95% CI) | Normal Weight(18.5-24.9 kg/m2)[N=3,639]% (95% CI) | Overweight(25.0-29.9 kg/m2)[N=4,255]% (95% CI) | Obese(30.0-39.9 kg/m2)[N=3,146]% (95% CI) | Morbidly Obese(≥40.0 kg/m2)[N=644]% (95% CI) | |
| Women | 63.2 (49.7-74.8) | 55.6 (53.1-58.1) | 42.9 (40.8-45.1) | 50.5 (48.0-53.1) | 65.4 (59.7-70.7) |
| Age, years | |||||
| 18-24 | 28.3 (17.0-43.1) | 19.8 (17.5-22.3) | 11.3 ( 9.5-13.3) | 7.4 ( 5.8- 9.3) | 10.1 ( 6.4-15.5) |
| 25-34 | 21.7 (13.8-32.6) | 18.4 (16.3-20.6) | 15.4 (13.7-17.3) | 15.8 (13.8-18.0) | 16.9 (12.7-22.1) |
| 35-44 | 2.7 ( 1.1- 6.1)* | 14.2 (12.6-16.0) | 16.2 (14.5-18.1) | 19.1 (17.0-21.2) | 22.2 (18.1-26.9) |
| 45-64 | 27.0 (18.5-37.6) | 28.8 (26.8-30.9) | 35.0 (33.0-37.0) | 39.8 (37.4-42.3) | 42.0 (36.7-47.5) |
| 65+ | 20.3 (14.2-28.0) | 18.8 (17.4-20.3) | 22.1 (20.6-23.6) | 18.0 (16.4-19.6) | 8.8 ( 6.7-11.6) |
| Race/ethnicity | |||||
| Non-Hispanic, white | 69.6 (56.8-79.9) | 71.6 (69.2-73.9) | 69.3 (67.1-71.4) | 61.1 (58.6-63.6) | 48.4 (42.9-54.1) |
| Non-Hispanic, black | 20.1 (12.2-31.1) | 20.2 (18.2-22.3) | 22.8 (21.0-24.7) | 32.0 (29.7-34.4) | 46.1 (40.6-51.7) |
| Other | 10.4 ( 4.1-24.1)* | 8.2 ( 6.7-10.1) | 7.8 ( 6.4- 9.6) | 6.9 ( 5.4- 8.8) | 5.4 ( 3.0- 9.7)* |
| Education | |||||
| Less than high school education | 20.0 (10.6-34.5)* | 16.0 (13.9-18.3) | 13.1 (11.4-15.0) | 19.5 (17.3-21.8) | 21.7 (17.0-27.3) |
| High school graduate or equivalent | 33.9 (24.2-45.2) | 27.7 (25.5-29.9) | 32.4 (30.4-34.5) | 32.7 (30.4-35.1) | 28.1 (23.6-33.1) |
| At least some college | 46.1 (34.8-57.8) | 56.4 (53.9-58.9) | 54.5 (52.2-56.7) | 47.8 (45.3-50.4) | 50.2 (44.6-55.8) |
| Physically inactive in past month | 32.2 (23.0-43.1) | 21.6 (19.6-23.7) | 20.8 (19.1-22.6) | 28.5 (26.3-30.8) | 43.2 (37.7-48.8) |
| Smoking status | |||||
| Current smoker | 34.3 (24.5-45.6) | 27.4 (25.1-29.8) | 21.5 (19.7-23.5) | 19.6 (17.5-21.9) | 19.2 (14.6-24.9) |
| Former smoker | 14.5 ( 9.2-22.1) | 20.8 (19.1-22.7) | 28.5 (26.5-30.5) | 29.8 (27.5-32.2) | 23.3 (19.2-28.1) |
| Never smoker | 51.2 (39.5-62.8) | 51.8 (49.3-54.3) | 50.0 (47.8-52.2) | 50.6 (48.0-53.2) | 57.5 (51.7-63.0) |
| Chronic Conditions | |||||
| Any cancer, excluding skin cancer | 9.7 ( 5.7-16.1) | 5.9 ( 5.0- 6.8) | 6.9 ( 6.1- 7.9) | 7.0 ( 6.0- 8.1) | 7.1 ( 5.0-10.0) |
| Current asthma | 14.5 ( 8.3-24.1) | 6.0 ( 4.9- 7.2) | 6.4 ( 5.4- 7.5) | 11.6 ( 9.9-13.6) | 19.0 (14.8-23.9) |
| Chronic obstructive pulmonary disease | 11.8 ( 7.4-18.5) | 6.3 ( 5.3- 7.4) | 5.9 ( 5.0- 7.1) | 8.9 ( 7.6-10.5) | 14.9 (11.3-19.3) |
| Frequent productive cough | 15.1 ( 9.7-22.9) | 9.7 ( 8.3-11.2) | 9.7 ( 8.4-11.2) | 9.7 ( 8.3-11.4) | 13.7 (10.3-18.1) |
| Frequent shortness of breath | 7.0 ( 3.4-13.8)* | 3.8 ( 2.9- 4.9) | 2.9 ( 2.3- 3.8) | 5.3 ( 4.3- 6.5) | 10.4 ( 7.5-14.2) |
| Strongly agree that shortness of breath affects physical activity | 17.9 (10.3-29.1) | 5.7 ( 4.6- 7.0) | 5.2 ( 4.4- 6.2) | 9.9 ( 8.5-11.5) | 14.3 (11.0-18.5) |
Unreliable estimate due to small sample size.
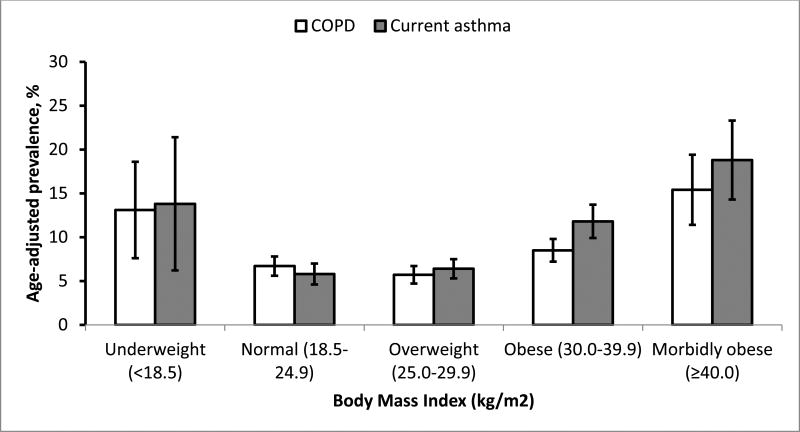
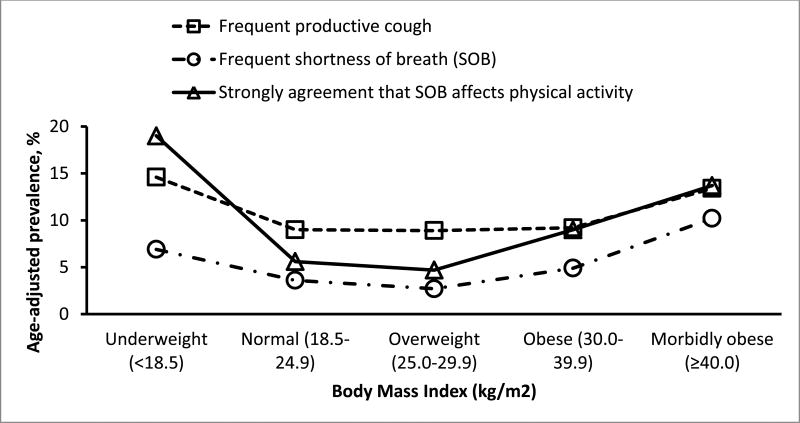
U-shaped relationships of BMI levels with age-adjusted prevalence of COPD and current asthma history were demonstrated in Figure 2 and with the three respiratory symptoms in Figure 3.
Figure 2. Age-adjusted prevalence of chronic obstructive pulmonary disease and current asthma among adults aged ≥18 years, by level of body mass index: South Carolina, 2012.
Figure 3. Age-adjusted percentage of frequent productive cough, frequent shortness of breath (SOB), and strong agreement that SOB affects physical activity among adults aged ≥18 years, by level of body mass index: South Carolina, 2012.
The modest U-shaped relationships of BMI levels with current asthma and SOB affected physical activity persisted (p<0.001) after controlling for the covariates (Table 3). Obese or morbidly obese but not underweight respondents were still significantly more likely to have COPD than normal weight adults after adjustment for covariates. In addition, morbidly obese adults but not obese adults were still significantly more likely to have a frequent productive cough and frequent SOB than normal weight adults after the adjustment (Table 3).
Table 3. Age-adjusted prevalence and adjusted likelihood of having respiratory symptoms and respiratory conditions associated with level of body mass index among adults aged ≥18 years: South Carolina, 2012.
| Body Mass Index (kg/m2) | Age-adjusted prevalence | Model 1 | Model 2 |
|---|---|---|---|
| % (95% CI) | Adjusted prevalence ratio (95% CI) | Adjusted prevalence ratio (95% CI) | |
| Chronic Obstructive Pulmonary Disease (COPD) | |||
| Underweight (<18.5) | 13.1 ( 7.6-18.6) | 1.55 (1.00-2.41) | 1.49 (0.95-2.32) |
| Normal weight (18.5-24.9) | 6.7 ( 5.6- 7.8) | 1.00 (referent) | 1.00 (referent) |
| Overweight (25.0-29.9) | 5.7 ( 4.7- 6.7) | 0.93 (0.73-1.18) | 0.92 (0.73-1.17) |
| Obese (30.0-39.9) | 8.5 ( 7.2- 9.9) | 1.30 (1.03-1.64) | 1.29 (1.02-1.64) |
| Morbidly obese (≥40.0) | 15.4 (11.4-19.4) | 2.25 (1.65-3.08) | 2.22 (1.62-3.04) |
| Current Asthma | |||
| Underweight (<18.5) | 13.8 ( 6.2-21.3) | 2.15 (1.22-3.81) | 2.13 (1.21-3.76) |
| Normal weight (18.5-24.9) | 5.8 ( 4.6- 6.9) | 1.00 (referent) | 1.00 (referent) |
| Overweight (25.0-29.9) | 6.4 ( 5.3- 7.5) | 1.18 (0.91-1.53) | 1.17 (0.90-1.52) |
| Obese (30.0-39.9) | 11.8 ( 9.9-13.7) | 1.99 (1.53-2.57) | 1.98 (1.53-2.57) |
| Morbidly obese (≥40.0) | 18.8 (14.3-23.4) | 2.86 (2.08-3.93) | 2.84 (2.06-3.91) |
| Frequent productive cough | |||
| Underweight (<18.5) | 14.6 ( 8.5-20.7) | 1.40 (0.91-2.13) | 1.36 (0.88-2.08) |
| Normal weight (18.5-24.9) | 9.0 ( 7.7-10.4) | 1.00 (referent) | 1.00 (referent) |
| Overweight (25.0-29.9) | 8.9 ( 7.6-10.2) | 1.04 (0.84-1.27) | 1.03 (0.84-1.27) |
| Obese (30.0-39.9) | 9.2 ( 7.7-10.6) | 1.05 (0.84-1.31) | 1.04 (0.83-1.31) |
| Morbidly obese (≥40.0) | 13.4 ( 9.6-17.2) | 1.54 (1.11-2.13) | 1.52 (1.10-2.11) |
| Frequent shortness of breath (SOB) | |||
| Underweight (<18.5) | 6.9 ( 2.1-11.7) | 1.48 (0.73-2.99) | 1.39 (0.70-2.77) |
| Normal weight (18.5-24.9) | 3.6 ( 2.7- 4.5) | 1.00 (referent) | 1.00 (referent) |
| Overweight (25.0-29.9) | 2.7 ( 2.0- 3.4) | 0.79 (0.55-1.14) | 0.79 (0.55-1.13) |
| Obese (30.0-39.9) | 4.9 ( 3.9- 6.0) | 1.20 (0.87-1.67) | 1.20 (0.87-1.65) |
| Morbidly obese (≥40.0) | 10.2 ( 7.0-13.5) | 2.20 (1.43-3.37) | 2.13 (1.39-3.29) |
| Strongly agree that SOB affects physical activity | |||
| Underweight (<18.5) | 19.0 ( 9.6-28.5) | 2.78 (1.65-4.67) | 2.68 (1.57-4.57) |
| Normal weight (18.5-24.9) | 5.6 ( 4.4- 6.8) | 1.00 (referent) | 1.00 (referent) |
| Overweight (25.0-29.9) | 4.7 ( 3.9- 5.5) | 0.85 (0.66-1.11) | 0.85 (0.65-1.10) |
| Obese (30.0-39.9) | 9.0 ( 7.6-10.3) | 1.38 (1.07-1.79) | 1.38 (1.07-1.78) |
| Morbidly obese (≥40.0) | 13.7 (10.1-17.2) | 1.91 (1.33-2.74) | 1.87 (1.30-2.69) |
Model 1: a multivariable logistic regression model that includes sex, age, race/ethnicity, education, smoking status, and any exercise as covariates in addition to body mass index;
Model 2: a multivariable logistic regression model that includes history of cancer (excluding skin cancer) in addition to covariates in model 1;
Discussion
We examined the relationship of BMI with respiratory symptoms, current asthma, and COPD in a state-based health survey of nearly 12,000 adults who resided in SC in 2012. The data showed that persons who were at extremes of BMI (underweight, obese, or morbidly obese) had a higher prevalence of self-reported, provider-diagnosed current asthma and/or COPD than persons who were of normal weight. The frequency of respiratory symptoms also appeared to differ with BMI level. SOB affecting physical activities was reported most often among persons who were underweight (BMI ≤ 18.5 kg/m2) or morbidly obese (BMI ≥ 40 kg/m2). Morbidly obese persons were also more likely to report frequent SOB than respondents with normal weight. The association of obese or morbidly obese with COPD is consistent to the results in previous studies [10-13], however, the relationship of underweight with asthma is a new finding. These associations persisted after adjustment for potential confounders, including age, sex, race/ethnicity, education, smoking status, physical activity, and a history of cancer.
The relationship of BMI and obstructive lung diseases is complex and has been extensively researched. One particularly important phenotype that has emerged from this research is the underweight COPD patient. It has been reported in some clinical studies that 12-25% of COPD patients are underweight or cachectic [21,22]. This occurs more frequently in advanced disease and is associated with a marked increase in mortality rates [23]. Several pathways that can result in COPD patients having low body weight include: 1) having baseline low body weight prior to developing COPD [24]; 2) developing low body weight due to COPD complications [25,26]; and 3) as observed in our study, a tendency for higher rates of current smoking among persons who are underweight and/or have COPD, which is consistent with observations from cross-sectional US surveys. One longitudinal study over a decade showed that men who have a low BMI have 2.7 fold higher risk of developing COPD compared to others [24]. In some COPD patients, advanced disease may lead to developing a low BMI from the substantial dyspnea, hypoxia, and inflammation, which further raises energy expenditure, decreases energy intake, and leads to nutritional depletion and weight loss [27]. In our study, underweight respondents reported SOB affecting physical activities more frequently, greater physical inactivity, asthma, and COPD than normal weight persons. We also found that persons with low BMI reported the highest rates of current smoking. Guerra et al have suggested that a low BMI is related to emphysema while obesity is related to chronic bronchitis [28]. However, our study could not address these relationships because of survey question limitations in the BRFSS. A recent analysis of COPDGene study subjects found that among the 4 different phenotypic clusters of COPD patients, the group with relatively low BMI had more extensive evidence of emphysema, more severe disease, and frequent exacerbations, and more extensive tobacco use intensity [29]. The group that had relatively high BMIs were more likely to be female, had predominantly airways disease, lower tobacco use intensity than the low BMI group, and relatively less emphysema [29]. Our underweight and morbidly obese subjects share some of these same characteristics. Little is published regarding interventions that are able to maintain higher body mass in these types of patients. Smoking cessation may be especially important in this type of patient in order to slow progression of COPD and promote weight gain.
Another well-studied weight-related phenotype of obstructive lung disease is the obese asthmatic. Obesity is an important risk factor for both prevalent and incident asthma [8,30]. Our data also show higher asthma prevalence in obese persons; especially in the morbidly obese group where there was a three-fold higher rate compared to normal or overweight persons. Etiologies for higher rates of asthma in obesity include altered respiratory mechanics leading to an increased work of breathing, physical inactivity, increased inflammation, and altered responses to drug therapies [16]. Nearly one-half of the morbidly obese persons in our survey reported physical inactivity in the last month and frequent SOB was two times more likely to occur than in normal weight persons. Previous studies have suggested that obesity may increase the resistance of airway and decrease respiratory muscle endurance, and further cause dyspnea, wheezing, hypoxia [10,31,32], and even asthma [8,16]. In addition, obesity is also highly related to chronic and low-grade inflammation, which may contribute to the development of COPD [33]. Although greater dyspnea may be associated with obesity and over-diagnosis of asthma may occur, two studies have reported that over-diagnosis of asthma in the obese population is not observed to any greater extent than in non-obese persons [34,35]. Most studies have defined obese asthma patients as those with BMI ≥ 30 kg/m2 and few have compared obese and morbidly obese [18,30,36,37]. One study that included both obese and morbidly obese subjects showed that lung volumes were incrementally decreased as the BMI increased [38]. Obese and especially morbidly obese persons breath at low lung volumes and therefore are more likely to have more severe respiratory symptoms compared to non-obese persons. Subsequently, respiratory symptoms are often more likely to be reported in the obese asthmatic who has less severe airflow obstruction as measured by spirometry. Some studies report that obese or morbidly obese females are more likely to be diagnosed with asthma than males [28]. However, this is not true in another study [39]. Considering the higher prevalence of obesity in females in the general population, there may be a greater number of obese females with asthma. In our study population, nearly two-thirds of the morbidly obese group were female, nearly one-half were African American, and frequent SOB was 2 times more likely among morbidly obese than normal weight persons. Although severe disease occurs in less than 10% of all asthmatics, one study reported a third of severe asthmatics required chronic oral corticosteroid use [40]. Chronic systemic steroid use and associated weight gain could contribute to obesity in asthma. Bariatric surgery in morbidly obese persons has led to decreased airway hyperreactivity, improved lung volumes, and lessened asthma severity [41]. The role of pulmonary rehabilitation in obese COPD patients has been studied and compared to lower weight COPD such that obese subjects present with less severe impairment in pulmonary function tests and poorer exercise performance, but outcomes associated with pulmonary rehabilitation are similar in obese and non-obese persons [42]. Another study examining the effects of pulmonary rehabilitation on obese COPD subjects showed improvement in some measures such as quality of life, but not 6-minute walk test [43].
Unlike the COPD population, relatively few studies have reported that low BMI affects the prevalence or severity of asthma [44]. We found that underweight persons were two times as likely to report asthma as were normal or over-weight persons. Schatcher also reported a U-shaped relationship for asthma where low and very high BMI led to worse asthma symptoms as well as increased airway hyper-responsiveness in low BMI, but not morbidly obese BMI [18]. One study in China reported more severe asthma in low BMI persons [8] and another study in children reported relatively severe asthma in those who were underweight [45]. Therefore, additional studies are needed to explore the relationship between low BMI and asthma prevalence and severity.
The link between obesity and COPD has become more widely recognized. We found that the morbidly obese group reported the highest prevalence of COPD compared to all groups except the underweight group. Steuten et al and Eisner reported a higher prevalence of obesity in GOLD stage I and II than in the general population [46,47]. Both of these studies reported that obesity was more likely to be present in mild to moderate than severe COPD. The classic clinical COPD phenotype including “blue bloater” has typically been associated with chronic bronchitis in overweight persons [48]. The Tucson prospective cohort study of obstructive lung diseases showed a higher prevalence of chronic bronchitis in persons with a BMI ≥ 28 kg/m2 [28]. Although frequent productive cough was reported more frequently in the morbidly obese persons in our study, this was not statistically different.
The strength of this study is the relatively large sample of adult, which allowed analysis of relationship of BMI particularly among underweight or morbidly obese respondents with respiratory symptoms and respiratory conditions. A few prior population studies reported similar findings only among obese adults [32,49]. However, this study has some limitations. First, the BRFSS survey is a cross-sectional study; therefore causal relationships of BMI with respiratory symptoms and respiratory conditions cannot be established. Second, the analyses relied upon self-reported information from telephone interviews and could not be validated with medical record data. Finally, our results, which were obtained from households, were only representative of the non-institutionalized population in SC. Persons with more severe COPD and respiratory symptoms may be more likely to be residing in nursing homes; therefore the observed relationship may be underestimated. However, because the BRFSS appears to be a useful tool to describe the relationship between BMI and obstructive lung diseases, implementation and analysis of these questions in other states would help confirm our findings.
Conclusion
We observed that both underweight and obesity are associated with asthma and obesity with COPD from one state population data. In addition, underweight respondents reported much higher respiratory symptoms and respiratory conditions. Providing nutritional and smoking cessation counseling may help to improve respiratory symptoms and respiratory conditions.
Abbreviations
- COPD
chronic obstructive pulmonary disease
- BRFSS
Behavioral Risk Factor Surveillance System
- SOB
short of breath
- BMI
Body Mass Index
Footnotes
Publisher's Disclaimer: Disclaimer: The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
All authors have provided no conflict of interest statements.
References
- 1.Carey IM, Cook DG, Strachan DP. The effects of adiposity and weight change on forced expiratory volume decline in a longitudinal study of adults. Int J Obes Relat Metab Disord. 1999;23:979–985. doi: 10.1038/sj.ijo.0801029. [DOI] [PubMed] [Google Scholar]
- 2.Chen Y, Horne SL, Dosman JA. Body weight and weight gain related to pulmonary function decline in adults: a six year follow up study. Thorax. 1993;48:375–380. doi: 10.1136/thx.48.4.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chinn DJ, Cotes JE, Reed JW. Longitudinal effects of change in body mass on measurements of ventilatory capacity. Thorax. 1996;51:699–704. doi: 10.1136/thx.51.7.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kannel WB, Cupples LA, Ramaswami R, Stokes J, III, Kreger BE, Higgins M. Regional obesity and risk of cardiovascular disease: The Framingham Study. J Clin Epidemiol. 1991;44:183–190. doi: 10.1016/0895-4356(91)90265-b. [DOI] [PubMed] [Google Scholar]
- 5.Ray CS, Sue DY, Bray G, Hansen JE, Wasserman K. Effects of obesity on respiratory function. Am Rev Respir Dis. 1983;128:501–506. doi: 10.1164/arrd.1983.128.3.501. [DOI] [PubMed] [Google Scholar]
- 6.Thomas PS, Cowen ER, Hulands G, Milledge JS. Respiratory function in the morbidly obese before and after weight loss. Thorax. 1989;44:382–386. doi: 10.1136/thx.44.5.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Subhan MM, Ali SA, Bokhari SS, Khan MN, Ahmad HR. Underweight and overweight men have greater exercise-induced dyspnea than normal weight men. Upsala J Med Sci. 2012;117:383–389. doi: 10.3109/03009734.2012.714416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Celedon JC, Palmer LJ, Litonjua AA, et al. Body mass index and asthma in adults in families of subjects with asthma in Anqing, China. Am J Respir Crit Care Med. 2001;164(Pt 1):1835–1840. doi: 10.1164/ajrccm.164.10.2105033. [DOI] [PubMed] [Google Scholar]
- 9.Yamaguchi Y, Hibi S, Ishii M, et al. Pulmonary features associated with being underweight in older men. J Am Geriatr Soc. 2011;59:1558–1560. doi: 10.1111/j.1532-5415.2011.03536.x. [DOI] [PubMed] [Google Scholar]
- 10.Gibson GJ. Obesity, respiratory function and breathlessness. Thorax. 2000;55(Suppl 1):S41–S44. doi: 10.1136/thorax.55.suppl_1.s41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franssen FME, O'Donnell DE, Goossens GH, Blaak EE, Schols AMWJ. Obesity and the lung: 5 obesity and COPD. Thorax. 2008;63:1110–1117. doi: 10.1136/thx.2007.086827. [DOI] [PubMed] [Google Scholar]
- 12.Ora J, Laveneziana P, Ofir D, Deesomchok A, Webb KA, O'Donnell DE. Combined effects of obesity and chronic obstructive pulmonary disease on dyspnea and exercise tolerance. Am J Respir Crit Care Med. 2009;180:964–971. doi: 10.1164/rccm.200904-0530OC. [DOI] [PubMed] [Google Scholar]
- 13.O'Donnell DE, O'Donnell CDJ, Webb KA, Guenette JA. Respiratory consequences of mild-to-moderate obesity: impact on exercise performance in health and in chronic obstructive pulmonary disease. Pulmonary Med. 2012;2012 doi: 10.1155/2012/818925. 818925 ( http://dx.doi.org/10.1155/2012/818925) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zutler M, Singer JP, Omachi TA, et al. Relationship of obesity with respiratory symptoms and decreased functional capacity in adults without established COPD. Prim Care Respir J. 2012;21:194–201. doi: 10.4104/pcrj.2012.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chinn S, Jarvis D, Burney P. Relation of bronchial responsiveness to body mass index in the ECRHS. European Community Respiratory Health Survey. Thorax. 2002;57:1028–1033. doi: 10.1136/thorax.57.12.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dixon AE, Holguin F, Sood A, et al. An official American Thoracic Society Workshop report: obesity and asthma. Proc Am Thorac Soc. 2010;7:325–335. doi: 10.1513/pats.200903-013ST. [DOI] [PubMed] [Google Scholar]
- 17.Bustos P, Amigo H, Oyarzún M, Rona RJ. Is there a causal relation between obesity and asthma? Evidence from Chile. Int J Obes (Lond) 2005;29:804–809. doi: 10.1038/sj.ijo.0802958. [DOI] [PubMed] [Google Scholar]
- 18.Schachter LM, Salome CM, Peat JK, Woolcock AJ. Obesity is a risk for asthma and wheeze but not airway hyperresponsiveness. Thorax. 2001;56:4–8. doi: 10.1136/thorax.56.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sin DD, Jones RL, Man SFP. Obesity is a risk factor for dyspnea but not for airflow obstruction. Arch Intern Med. 2002;62:1477–1481. doi: 10.1001/archinte.162.13.1477. [DOI] [PubMed] [Google Scholar]
- 20.O'Donnell DE, Deesomchok A, Lam Y-M, et al. Effects of BMI on static lung volumes in patients with airway obstruction. Chest. 2011;40:461–468. doi: 10.1378/chest.10-2582. [DOI] [PubMed] [Google Scholar]
- 21.Congleton J. The pulmonary cachexia syndrome: aspects of energy balance. Proc Nutr Soc. 1999;8:321–328. doi: 10.1017/s0029665199000439. [DOI] [PubMed] [Google Scholar]
- 22.Van den Bemt L, Smeele IJ, Kolkman M, Grol M, van Weel C, Schermer TR. Low body mass index, airflow obstruction, and dyspnea in a primary care COPD patient population. Prim Care Respir J. 2010;19:118–123. doi: 10.4104/pcrj.2009.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson DO, Rogers RM, Wright EC, Anthonisen NR. Body weight in Chronic Obstructive Pulmonary Disease: The National Institutes of Health Intermittent Positive-Pressure Breathing Trial. Am Rev Respir Dis. 1989;139:1435–1438. doi: 10.1164/ajrccm/139.6.1435. [DOI] [PubMed] [Google Scholar]
- 24.Harik-Khan RI, Fleg JL, Wise RA. Body mass index and risk of COPD. Chest. 2002;21:370–376. doi: 10.1378/chest.121.2.370. [DOI] [PubMed] [Google Scholar]
- 25.Agusti AG, Noguera A, Sauleda J, Sala E, Pons J, Busquets X. Systemic effects of chronic obstructive pulmonary disease. Eur Respir J. 2003;1:347–360. doi: 10.1183/09031936.03.00405703. [DOI] [PubMed] [Google Scholar]
- 26.Schols AM, Slangen J, Volovics L, Wouters EF. Weight loss is a reversible factor in the prognosis of chronic obstructive pulmonary disease. Am J Respir Care Med. 1998;157(Pt 1):1791–1797. doi: 10.1164/ajrccm.157.6.9705017. [DOI] [PubMed] [Google Scholar]
- 27.Decramer M, De Benedetto F, Del Ponte A, Marinari S. Systemic effects of COPD. Respir Med. 2005;99(Suppl B):S3–S10. doi: 10.1016/j.rmed.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 28.Guerra S, Sherrill DL, Bobadilla A, Martinez FD, Barbee RA. The relation of body mass index to asthma, chronic bronchitis, and emphysema. Chest. 2002;122:1256–1263. doi: 10.1378/chest.122.4.1256. [DOI] [PubMed] [Google Scholar]
- 29.Castaldi PJ, Dy J, Ross R, et al. Cluster analysis in the COPDGene study identifies subtypes of smokers with distinct patterns of airways disease and emphysema. Thorax. 2014;69:415–422. doi: 10.1136/thoraxjnl-2013-203601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ford ES. The epidemiology of obesity and asthma. J Allergy Clin Immunol. 2005;115(5):897–909. doi: 10.1016/j.jaci.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 31.Babb TG, Ranasinghe KG, Comeau LA, Semon TL, Schwartz B. Dyspnea on exertion in obese women: association with an increased oxygen cost of breathing. Am J Respir Crit Care Med. 2008;178:116–123. doi: 10.1164/rccm.200706-875OC. [DOI] [PubMed] [Google Scholar]
- 32.Wannamethee SG, Shaper AG, Whincup PH. Body fat distribution, body composition, and respiratory function in elderly men. Am J Clin Nutr. 2005;82:996–1003. doi: 10.1093/ajcn/82.5.996. [DOI] [PubMed] [Google Scholar]
- 33.Zammit C, Liddicoat H, Moonsie I, Makker H. Obesity and respiratory diseases. Int J Gen Med. 2010;3:335–343. doi: 10.2147/IJGM.S11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pakhale S, Doucette S, Vandemmeem K, et al. Comparison of obese and non-obese people with asthma: exploring an asthma/obesity interaction. Chest. 2010;137:1316–1323. doi: 10.1378/chest.09-2491. [DOI] [PubMed] [Google Scholar]
- 35.van Huisstede A, Cabezas MC, van de Geijn GM, et al. Underdiagnosis and overdiagnosis of asthma in the morbidly obese. Respir Med. 2013;107:1356–1364. doi: 10.1016/j.rmed.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 36.Al-Alwan A, Bates JH, Chapman DG, et al. The nonallergic asthma of obesity. A matter of distal lung compliance. Am J Respir Crit Care Med. 2014;189:1494–1502. doi: 10.1164/rccm.201401-0178OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mahadev S, Salome CM, Berend N, King GG. The effect of low lung volume on airway function in obesity. Respir Physiol Neurobiol. 2013;188:192–199. doi: 10.1016/j.resp.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 38.Jones RL, Nzekwu MM. The effects of body mass index on lung volumes. Chest. 2006;130:827–833. doi: 10.1378/chest.130.3.827. [DOI] [PubMed] [Google Scholar]
- 39.Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic data. Am J Respir Crit Care Med 1. 2007;75:661–666. doi: 10.1164/rccm.200611-1717OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moore WC, Bleecker ER, Curran-Everett D, et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute's Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119:405–413. doi: 10.1016/j.jaci.2006.11.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boulet LP, Turcotte H, Martin J, Poirier P. Effect of bariatric surgery on airway response and lung function in obese subjects with asthma. Respir Med. 2012;106:651–660. doi: 10.1016/j.rmed.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 42.Ramachandran K, McCusker C, Connors M, Zuwallack R, Lahiri B. The influence of obesity on pulmonary rehabilitation outcomes in patients with COPD. Chronic Respir Dis. 2008;5:205–209. doi: 10.1177/1479972308096711. [DOI] [PubMed] [Google Scholar]
- 43.Sava F, Laviolette L, Bernard S, Breton M, Bourbeau J, Maltais F. The impact of obesity on walking and cycling performance and response to pulmonary rehabilitation in COPD. BMC Pulmonol Med. 2010;10:55. doi: 10.1186/1471-2466-10-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Akerman MJ, Calacanis CM, Madsen MK. Relationship between asthma severity and obesity. J Asthma. 2004;41:521–526. doi: 10.1081/jas-120037651. [DOI] [PubMed] [Google Scholar]
- 45.Chu YT, Chen WY, Wang TN, Tseng HI, Wu JR, Ko YC. Extreme BMI predicts higher asthma prevalence and is associated with lung function impairment in school-aged children. Pediatric Pulmonol. 2009;44:472–479. doi: 10.1002/ppul.21023. [DOI] [PubMed] [Google Scholar]
- 46.Eisner MD, Blanc PD, Sidney S, et al. Body composition and functional limitation in COPD. Respir Res. 2007;8:7. doi: 10.1186/1465-9921-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steuten LM, Creutzberg EC, Vrijhoef HJ, Wouters EF. COPD as a multicomponent disease: inventory of dyspnoea, underweight, obesity and fat free mass depletion in primary care. Prim Care Respir J. 2006;15:84–91. doi: 10.1016/j.pcrj.2005.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Filley GF, Buckwitt HJ, Reeves JT. Chronic obstructive bronchopulmonary disease. II Oxygen transport in two clinical types. Am J Med. 1968;44:26–38. doi: 10.1016/0002-9343(68)90234-9. [DOI] [PubMed] [Google Scholar]
- 49.Canoy D, Luben R, Welch A, et al. Abdominal obesity and respiratory function in men and women in the EPIC-Norfolk Study, United Kingdom. Am J Epidemiol. 2004;159:1140–1149. doi: 10.1093/aje/kwh155. [DOI] [PubMed] [Google Scholar]