Abstract
OBJECTIVE
To estimate whether alcohol use at the initiation of an in vitro fertilization (IVF) cycle is associated with IVF outcomes.
METHODS
In this prospective cohort study, men and women completed a self-administered questionnaire before their first IVF cycle. Participants reported alcohol type, amount, and frequency consumed. Discrete survival analysis was applied to calculate the odds ratio (OR) and 95% confidence interval (CI) for live birth—the primary outcome. Secondary outcomes were cycle characteristics and points of failure in the IVF process (cycle cancellation, failed fertilization, implantation failure, and spontaneous abortion). We conducted multicycle analyses with final models adjusted for potential confounders that included cycle number, cigarette use, body mass index, and age.
RESULTS
A total of 2,545 couples contributed 4,729 cycles. Forty-one percent of women and 58% of men drank one to six drinks per week. Women drinking at least four drinks per week had 16% less odds of a live birth rate compared with those who drank fewer than four drinks per week (OR 0.84, CI 0.71–0.99). For couples in which both partners drank at least four drinks per week, the odds of live birth were 21% lower compared with couples in which both drank fewer than four drinks per week (OR 0.79; CI 0.66–0.96).
CONCLUSION
Consumption of as few as four alcoholic drinks per week is associated with a decrease in IVF live birth rate.
It is well known that alcohol use during pregnancy is linked to birth defects.1 However, the effects of alcohol on fertility are not clearly defined. Epidemiologic studies of spontaneous pregnancies suggest that women with high consumption of alcohol were more likely to present with infertility and that fecundability was decreased among women drinking moderate or heavy amounts of alcohol.2,3 Conversely, women with moderate intake did not have an increased time to pregnancy.4,5 With regard to alcohol type, time to pregnancy was shorter with wine drinkers, unchanged with beer use, and variable with liquor.6
The association between men’s alcohol use and male infertility is also contradictory. Whereas some studies have demonstrated decreased fecundability with heavy use, other studies have not demonstrated an association with decreased fecundability with any amount or type of alcohol.3,4,7
Only one study exists on the effects of alcohol use on in vitro fertilization (IVF) cycles.8 In women, an increase of one drink per day was associated with 13% fewer oocytes retrieved. Also, those who drank alcohol had a nonsignificant decreased chance of pregnancy. The strongest association of men’s intake, pregnancy, and spontaneous abortion was seen when the consumption occurred closest to the time of semen sample collection.
Owing to the high costs of IVF—in terms of time, money, and emotional stress—it is critical that each cycle be optimized. Any environmental or lifestyle factor that may have a detrimental effect on IVF cycle success should be identified and avoided. The limited amount of research on alcohol use in the IVF population prompted us to conduct the current study. Our objective was to estimate whether alcohol affects IVF cycle outcomes.
MATERIALS AND METHODS
Couples newly enrolled for IVF treatment between 1994 and 2003 at three Boston-area clinics were eligible. We excluded couples using donor gametes or gestational carriers. The study was introduced to potential couples by the health practitioner, and those indicating initial interest were given self-administered baseline questionnaires and consent forms for the woman and her male partner to complete. Questionnaires assessed demographic history, menstrual and fertility history, past contraceptive and hormone use, genital hygiene and infections, physical activity, smoking history, caffeine, alcohol, vitamin use, weight, and height. We calculated body mass index (BMI, calculated as weight (kg)/[height (m)]2). Couples were given $25 for their participation. This study was approved by the Brigham and Women’s Hospital Institutional Review Board.
Alcohol use was assessed only at the time of IVF cycle start, not during the IVF cycle or pregnancy. Participants answered the following question: “How much beer do you currently consume?” They specified the amount per day or per week or both. The question was then repeated for white wine, red wine, and hard liquor. We calculated alcohol content per drink: white wine, 4 oz=12.1 g; red wine, 4 oz=12.5 g; beer, 12 oz=13.9 g; hard liquor, 1.5 oz=14 g.9
Information on the couple’s infertility history and cycle-specific data were abstracted from medical records. Specifically, the data included the planned procedure (IVF or gamete intrafallopian transfer), estradiol (E2) levels, number of oocytes retrieved, semen characteristics, whether or not a clinical pregnancy occurred, and the outcome of a clinical pregnancy. The cycle reference date was defined as the day that gonadotropins began. Abstracting was done on site using a portable computer with customized data entry software. Ten percent of records were re-abstracted and less than 1% of data were observed to have an entry error, and this very low proportion did not vary across time.
The original study population included 2,687 couples undergoing a variety of assisted reproductive technologies procedures. For the purposes of this methodological paper, only IVF cycles that included the transfer of at least one embryo were considered in the analysis. In addition, any cycles subsequent to a non-IVF cycle were omitted, as were 69 cycles missing covariates and three implausible records.
The primary outcome was live birth rate among women who completed cycle medications. We defined two types of secondary outcomes, points of failure in the IVF cycle and cycle characteristics. Points of failure were rate of cycle cancellation, fertilization failure, implantation failure, and spontaneous abortion. Cycle cancellation occurred if there was not sufficient gonadotropin stimulation and the oocytes retrieval did not occur or if the retrieval did occur, but no oocytes were retrieved. Failed fertilization was defined as the absence of embryos available for transfer. The woman was diagnosed with implantation failure if the embryo transfer occurred, but she did not have a biochemical or clinical pregnancy. Spontaneous abortion was defined as a clinical pregnancy without a delivery. In addition, cycle characteristics were sperm concentration (postprocessing), sperm motility, sperm morphology, E2 level, number of oocytes retrieved, and fertilization rate.
Women who underwent oocyte retrieval served as the comparison group for women with a cancelled cycle; women who had an embryo transfer were the comparison group for failed fertilization; women who had at least a chemical pregnancy were the comparison group for failed implantation; and women who had a delivery served as the comparison group for pregnancies with a spontaneous abortion.
|
| ||
| Failed Implantation: Positive β-hCG‡ | ||
|
| ||
| n (%) | Crude OR (95% CI) | MV OR (95% CI) |
|
| ||
| 1,832 (54.8) | 1.00 (referent) | 1.00 (referent) |
| 553 (58.4) | 1.14 (0.98–1.32) | 1.06 (0.92–1.24) |
| 1,958 (55.2) | 1.00 (referent) | 1.00 (referent) |
| 411 (57.2) | 1.08 (0.91–1.27) | 1.07 (0.91–1.26) |
| 1,840 (54.9) | 1.00 (referent) | 1.00 (referent) |
| 518 (58.1) | 1.14 (0.98–1.33) | 1.09 (0.94–1.27) |
| 1,794 (54.0) | 1.00 (referent) | 1.00 (referent) |
| 552 (60.9) | 1.30 (1.12–1.51) | 1.22 (1.05–1.43) |
| 2,249 (55.5) | 1.00 (referent) | 1.00 (referent) |
| 138 (57.0) | 1.06 (0.81–1.37) | 0.99 (0.76–1.30) |
|
| ||
Alcohol use was analyzed by categorical and continuous variables (use compared with no use, or amount). Dichotomous outcomes were used for the following semen analyses parameters: motility (less than 50% motile compared with 50% motile or higher), morphology (strict Kruger criteria: less than 5% compared with 5% or more normal forms), postprocessing concentration (less than 20 million/mL or at least 20 million/mL). We considered all variables as potential confounders of the association between alcohol and IVF outcomes. If addition, if that variable to the model changed the odds ratio by 10% or more, it was identified as a confounder and was kept in all models. Age, BMI, cycle number, and cigarette use were observed to be confounders and were adjusted for in each analysis.
|
| ||
| Failed Implantation: Positive β-hCG‡ | ||
|
| ||
| n (%) | Crude OR (95% CI) | MV OR (95% CI) |
|
| ||
| 1,215 (54.4) | 1.00 (referent) | 1.00 (referent) |
| 1,164 (57.0) | 1.09 (0.97–1.23) | 1.05 (0.93–1.19) |
| 1,021 (55.2) | 1.00 (referent) | 1.00 (referent) |
| 1,183 (54.9) | 0.98 (0.86–1.11) | 0.99 (0.87–1.12) |
| 1,803 (55.6) | 1.00 (referent) | 1.00 (referent) |
| 538 (56.3) | 1.04 (0.90–1.20) | 0.98 (0.84–1.13) |
| 1,997 (55.0) | 1.00 (referent) | 1.00 (referent) |
| 371 (59.6) | 1.20 (1.01–1.42) | 1.10 (0.92–1.32) |
| 2,038 (55.2) | 1.00 (referent) | 1.00 (referent) |
| 318 (57.7) | 1.10 (0.92–1.32) | 1.03 (0.85–1.24) |
|
| ||
We conducted discrete survival analyses, which accounts for the number of cycles until success as the event time, for the dichotomous outcomes; mixed-effect models with unstructured covariance for peak E2 level; Poisson regression for the number of follicles and the number of oocytes retrieved; and generalized estimating equations for fertilization rate (the proportion of normally fertilized embryos). Ninety-five percent confidence intervals were calculated, and P values were based on 2-sided tests, with P<.05 indicating statistical significance.
RESULTS
A total of 2,545 couples underwent 4,729 cycles and most participants underwent two IVF cycles (Table 1). The mean ages of the women and men were 35 years plus or minus 4 years and 37 years plus or minus 6 years, respectively. Approximately 11% of the women and 17% of the men had BMIs of 30 or higher. Nine percent of the women and 10% of the men were current cigarette smokers. Most of the women (87%) had not had a previous IVF cycle, whereas 51% of the women and 26% of the men’s partners had experienced a previous pregnancy.
Table 1.
Demographic Characteristics Among 2,545 Couples Undergoing In Vitro Fertilization
| Characteristic | Women | Men |
|---|---|---|
| Body mass index (kg/m2) | ||
| Less than 18.5 | 76 (3.0) | 15 (0.6) |
| 18.5–24.9 | 1685 (67.0) | 805 (31.6) |
| 25.0–29.9 | 486 (19.3) | 1288 (50.6) |
| 30.0–34.9 | 151 (6.0) | 328 (12.9) |
| 35.0–39.9 | 79 (3.1) | 91 (3.6) |
| 40.0 or higher | 39 (1.6) | 18 (0.7) |
| Age (y) | ||
| 34 or younger | 921 (36.2) | 734 (28.8) |
| 35–37 | 611 (24.0) | 571 (22.5) |
| 38–39 | 368 (14.4) | 367 (14.4) |
| 40–43 | 549 (21.6) | 501 (19.7) |
| 44 or older | 96 (3.8) | 372 (14.6) |
| Primary diagnosis | ||
| Unexplained | 781 (30.9) | — |
| Male | — | 857 (33.9) |
| Female | 890 (35.2) | — |
| Race or ethnicity | ||
| White | 2282 (89.7) | 2291 (90.6) |
| African American | 85 (3.3) | 90 (3.6) |
| Hispanic | 41 (1.6) | 36 (1.4) |
| Asian | 110 (4.3) | 89 (3.5) |
| Other | 27 (1.1) | 22 (0.9) |
| Current smoker | ||
| No | 1680 (90.6) | 2273 (90.0) |
| Yes | 175 (9.4) | 253 (10.0) |
Data are n (%).
Fifty-five percent of the women consumed less than one drink per week, 41% drank one to six drinks per week, and 4% drank at least daily. Thirty-three percent of men consumed less than one drink per week, 58% drank one to six drinks per week, and 9% drank at least daily. Of those participants who consumed more than one drink per week, most of the women drank wine (white wine, 23%; red wine, 22%), whereas 57% of the men drank beer.
We dichotomized at 50 g of alcohol per week, as this was the median amount consumed in the population of women who consumed more than 0 g of alcohol. Fifty grams is approximately four drinks. Women participants who drank at least four drinks per week had a 16% lower likelihood of live birth compared with women who drank fewer than four drinks per week (OR 0.84; CI 0.71–0.99; Table 2). Specifically, women drinking white wine weekly were observed to have a decreased likelihood of live birth (OR 0.83; CI 0.70–0.98).
Table 2.
The Association of Women’s Alcohol Use and Live Birth, Failed Fertilization, and Failed Implantation in a Cohort of 2,545 Couples Undergoing In Vitro Fertilization
| Per Week | Live Birth: No Live Birth*
|
Failed Fertilization: Successful Fertilization†
|
||||
|---|---|---|---|---|---|---|
| n (%) | Crude OR (95% CI) |
MV OR (95% CI) |
n (%) | Crude OR (95% CI) |
MV OR (95% CI) |
|
| Total alcohol | ||||||
| 0–49 g | 994 (27.2) | 1.00 (referent) | 1.00 (referent) | 194 (5.4) | 1.00 (referent) | 1.00 (referent) |
| 50 g or more | 237 (22.4) | 0.78 (0.67–0.92) | 0.84 (0.71–0.99) | 84 (8.0) | 1.55 (1.19–2.02) | 1.48 (1.13–1.95) |
| Beer | ||||||
| Less than 1 drink | 1028(26.5) | 1.00 (referent) | 1.00 (referent) | 217 (5.6) | 1.00 (referent) | 1.00 (referent) |
| 1–7 drinks | 201 (25.2) | 0.94 (0.79–1.121 | 0.92 (0.77–1.10) | 59 (7.4) | 1.36 (1.01–1.83) | 1.37 (1.02–1.86) |
| Red wine | ||||||
| Less than 1 drink | 972 (26.5) | 1.00 (referent) | 1.00 (referent) | 196 (5.4) | 1.00 (referent) | 1.00 (referent) |
| 1–7 drinks | 246 (24.7) | 0.90 (0.77–1.06) | 0.97 (0.82–1.14) | 79 (8.0) | 1.52 (1.16–1.99) | 1.51 (1.14–1.99) |
| White wine | ||||||
| Less than 1 drink | 997 (27.4) | 1.00 (referent) | 1.00 (referent) | 206 (5.7) | 1.00 (referent) | 1.00 (referent) |
| 1–7 drinks | 222 (22.3) | 0.77 (0.65–0.91) | 0.83 (0.70–0.98) | 63 (6.3) | 1.13 (0.85–1.52) | 1.10 (0.82–1.48) |
| Hard liquor | ||||||
| Less than 1 drink | 1174 (26.3) | 1.00 (referent) | 1.00 (referent) | 266 (6.0) | 1.00 (referent) | 1.00 (referent) |
| 1–7 drinks | 59 (22.9) | 0.83 (0.62–1.13) | 0.92 (0.67–1.25) | 13 (5.0) | 0.83 (0.47–1.48) | 0.80 (0.45–1.43) |
n, number of cycles; OR, odds ratio; CI, confidence interval; MV, multivariable.
Multivariable OR adjusted for cycle number and women’s age, body mass index, and cigarette use in the women’s drinking exposure models; and for cycle number, women’s age, and men’s age, body mass index, and cigarette use in the men’s drinking exposure models.
Comparison participants are women who completed cycle medications but did not achieve a live birth.
Comparison participants are those who had oocyte retrieval and had fertilized eggs.
Comparison participants are those who had an embryo transfer and had positive β-hCG.
Although the effect of male drinking overall was not statistically significantly associated with live birth (OR 0.90; CI 0.79–1.03), the odds of a live birth were 35% lower for men drinking beer daily (OR 0.65; CI 0.48–0.89) (Table 3).
Table 3.
The Association of Men’s Alcohol Use and Live Birth, Failed Fertilization, and Failed Implantation in a Cohort of 2,545 Couples Undergoing In Vitro Fertilization
| Per Week | Live Birth: No Live Birth*
|
Failed Fertilization: Successful Fertilization†
|
||||
|---|---|---|---|---|---|---|
| n (%) | Crude OR (95% CI) |
MV OR (95% CI) |
n (%) | Crude OR (95% CI) |
MV OR (95% CI) |
|
| Total alcohol | ||||||
| 0–49 g | 678 (27.9) | 1.00 (referent) | 1.00 (referent) | 123 (5.1) | 1.00 (referent) | 1.00 (referent) |
| 50 g or more | 550 (24.4) | 0.85 (0.75–0.97) | 0.90 (0.79–1.03) | 151 (6.7) | 1.38 (1.08–1.76) | 1.36 (1.06–1.74) |
| Beer | ||||||
| Less than 1 drink | 546 (26.9) | 1.00 (referent) | 1.00 (referent) | 104 (5.2) | 1.00 (referent) | 1.00 (referent) |
| 1–7 drinks | 627 (26.6) | 0.99 (0.87–1.13) | 0.98 (0.86–1.13) | 151 (6.4) | 1.26 (0.98–1.63) | 1.32 (1.01–1.71) |
| Red wine | ||||||
| Less than 1 drink | 939 (26.5) | 1.00 (referent) | 1.00 (referent) | 204 (5.8) | 1.00 (referent) | 1.00 (referent) |
| 1–7 drinks | 272 (25.8) | 0.96 (0.82–1.12) | 1.04 (0.88–1.22) | 61 (5.8) | 1.00 (0.75–1.35) | 1.00 (0.74–1.35) |
| White wine | ||||||
| Less than 1 drink | 1074 (27.0) | 1.00 (referent) | 1.00 (referent) | 224 (5.7) | 1.00 (referent) | 1.00 (referent) |
| 1–7 drinks | 150 (21.8) | 0.76 (0.62–0.92) | 0.85 (0.70–1.04) | 46 (6.7) | 1.20 (0.87–1.67) | 1.18 (0.84–1.65) |
| Hard liquor | ||||||
| Less than 1 drink | 1,084 (26.8) | 1.00 (referent) | 1.00 (referent) | 231 (5.7) | 1.00 (referent) | 1.00 (referent) |
| 1–7 drinks | 140 (23.1) | 0.83 (0.68–1.01) | 0.91 (0.74–1.12) | 38 (6.3) | 1.11 (0.78–1.59) | 1.04 (0.73–1.49) |
n, number of cycles; OR, odds ratio; CI, confidence interval; MV, multivariable.
Multivariable OR adjusted for cycle number and women’s age, body mass index, and cigarette use in the women’s drinking exposure models; and for cycle number, women’s age, and men’s age, body mass index, and cigarette use in the men’s drinking exposure model.
Comparison participants are women who completed cycle medications but did not achieve a live birth.
Comparison participants are those who had oocyte retrieval and had fertilized eggs.
Comparison participants are those who had an embryo transfer and had positive β-hCG.
In addition, the odds of having a live birth were 21% lower among couples in which both members of the couple drank at least four drinks per week, compared with couples in which both members drank fewer than four drinks per week (OR 0.79; CI 0.66–0.96) (Fig. 1).
Fig. 1.
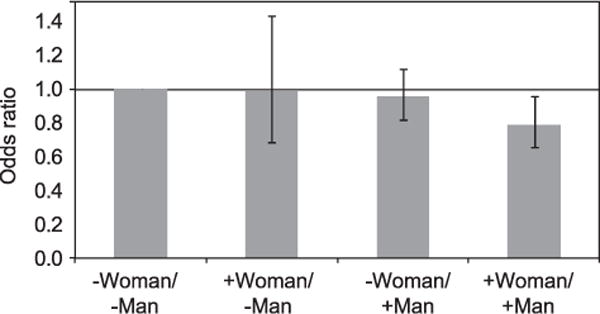
Likelihood of live birth by couple’s alcohol intake in a cohort of 2,545 couples undergoing in vitro fertilization (IVF). −, intake of less than 50 g (four drinks) of alcohol per week; +, intake of at least 50 g (four drinks) of alcohol per week. Bars represent 95% confidence intervals.
Rossi. Alcohol and IVF Outcomes. Obstet Gynecol 2011.
Subsetting on women who had an embryo transfer, the effect of alcohol on live birth was attenuated slightly and was not statistically significant (women: OR 0.87; CI 0.73–1.03; men: OR 0.92; CI 0.80–1.05; couple OR 0.83; CI 0.68–1.00) (data not shown). These results suggest that alcohol may have the strongest effect on points of failure in the IVF cycle before embryo transfer.
Indeed, associations were observed between alcohol use and an increased likelihood of failed fertilization (Tables 2 and 3). Women and men drinking more than four drinks per week had 48% greater odds of failed fertilization (women: OR 1.48; CI 1.13–1.95; men: OR 1.36; CI 1.06–1.74). There were also 18% greater odds of failed implantation among women drinking one to seven drinks per week (OR 1.18; CI 1.03–1.35) (Table 2). Women drinking white wine weekly had 22% greater odds of failed implantation (OR 1.22; CI 1.05–1.43). Men drinking beer daily also were observed to have a greater likelihood of failed implantation (OR 1.36; CI 1.04–1.78).
In general, women drinkers had lower peak E2 levels than nondrinkers (Table 4). A linear relation was observed in that nondrinkers had the highest E2 levels and women who drank daily had the lowest E2 levels, on average. Women drinking white wine weekly had significantly fewer oocytes retrieved compared with nondrinkers of white wine (10 compared with 11 oocytes, P=.04) (data not shown). There were no other significant associations observed between alcohol use and number of oocytes retrieved (data not shown).
Table 4.
The Association Between Alcohol Use and Peak Estradiol Among Women in a Cohort of 2,545 Couples Undergoing In Vitro Fertilization
| n | Adjusted Mean Estradiol | P | |
|---|---|---|---|
| Women’s Use (per week) | |||
| Total alcohol | |||
| 0–49 g | 3,572 | 1,732 | |
| 50 g or more | 1,034 | 1,602 | .002 |
| Beer | |||
| Less than 1 drink | 3,810 | 1,727 | |
| Weekly | 771 | 1,624 | .03 |
| Red wine | |||
| Less than 1 drink | 3,588 | 1,727 | |
| Weekly | 968 | 1,644 | .05 |
| White wine | |||
| Less than 1 drink | 3,558 | 1,737 | |
| Weekly | 977 | 1,604 | .002 |
| Hard liquor | |||
| Less than 1 drink | 4,363 | 1,737 | |
| Weekly | 249 | 1,637 | .29 |
n, number of in vitro fertilization cycles; weekly, 1–7 drinks/wk. Estradiol means adjusted for cycle number and women’s age, body mass index, and pack-years.
Men who consumed beer daily had 27% lower odds of having poor sperm motility (OR 0.73; CI 0.55–0.95) (data not shown). Wine, on the other hand, was observed to be inversely associated with sperm morphology and concentration. Weekly use of white wine increased the odds of poor sperm morphology by 43% (CI 1.07–1.91), and weekly use of red wine increased the odds of poor sperm concentration by 23% (CI 1.04–1.47) (data not shown).
DISCUSSION
In this prospective study of couples undergoing IVF, we observed an association between alcohol use and IVF outcomes. Our primary outcome was live birth rate, as we feel that this is ultimately the most important information when counseling patients. We considered secondary outcomes to better understand the mechanism for the association between alcohol and decreased live birth. For couples drinking as few as four drinks per week, their odds of live birth were decreased by 21% and they had 48% greater odds of failed fertilization.
Rates of any alcohol use in our population with infertility presenting for IVF were approximately 45–66%. These rates are lower than reported rates in the general U.S. population, as 74% of men and 62% of women age 25–44 years in the United States classify themselves as current drinkers.10 Drinking behavior may be similar in infertile women or different in women presenting for IVF. As fertile women are trying to become pregnant, they may drink less in anticipation of pregnancy. Conversely, they may assume that they cannot achieve pregnancy without IVF and thus have similar drinking patterns to women who are not attempting pregnancy.
We calculated alcohol use from participants’ self-administered questionnaires. The questionnaire presented the alcohol questions among a larger series of questions regarding food and drink consumption. Alcohol can be underreported as a result of the social perception that alcohol is not acceptable in pregnancy. Studies that used retrospective data collection or included women with heavy use demonstrated a greater discrepancy between reported.11,12 However, the use of a questionnaire, asking about current amount and type of alcohol, has been shown to give a more accurate account.11,12 If underreported, estimates in the current study are likely to be uncorrelated with outcome owing to the prospective design and, therefore, would attenuate observed associations.
We observed differences in several outcomes based on alcohol type, namely decreased likelihood of live birth, fertilization, and implantation in men who drank beer daily and women drinking wine weekly. Various types of alcohol may have different biologic influences on reproduction. Juhl et al described differences in time to pregnancy depending on type of alcohol; however, there was no distinction between white and red wine.6 With regard to cardiovascular disease, the antioxidant or antiinflammatory effects of red wine, and to a lesser effect white wine, may be protective.13 We can speculate that different effects on reproduction also exist, but the exact consequences are unknown.
The mechanism for the detrimental effect of alcohol on IVF outcome is not known. Studies in mice demonstrate that alcohol may affect follicular growth, induce nondisjunction in fertilized eggs, interfere with germ cell spindle and chromosomal segregation, facilitate embryo degeneration, and impair implantation or embryo hatching.14–18 These animal studies describe possible mechanism; however, they do not fully explain what was seen in our study. If aneuploidy was more common in the oocytes or embryos, we would expect to have observed a higher rate of spontaneous abortion in the drinkers, which was not expressed in our data, although sample sizes were small. In addition, we considered alcohol use at the start of the IVF cycle only. Knowledge of alcohol use after implantation may elucidate a mechanism. More research, specifically with human germ cells, must occur to clearly define our clinical findings.
Women drinkers had lower peak E2 levels than the nondrinkers. Acute alcohol ingestion has been associated with significantly higher E2 levels compared with placebo.19,20 Follicle-stimulating hormone is cleared by the kidney and liver.21 Theoretically, if alcohol affected the hepatic metabolism or clearance of the gonadotropins, follicular growth and subsequent E2 levels may be altered. However, the only significant difference in number of oocytes retrieved was seen in one to seven drinks of white wine per week.
In conclusion, alcohol use in men or women at the time of IVF cycle start can have a negative effect on cycle outcomes, specifically failure of fertilization and live birth. It is essential to note that an increase in negative outcomes was observed at even modest amounts of alcohol consumption (four drinks per week). There are many factors that contribute to IVF cycle outcomes, such as age, over which patients have no control. However, alcohol use is a modifiable risk factor and counseling patients to decrease or stop alcohol use, before IVF cycle start, may contribute to cycle success.
Acknowledgments
Supported by grant HD32153 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Footnotes
Financial Disclosure
Dr. Hornstein serves on the medical advisory board of WIN Fertility and has received honoraria from Up-To-Date. The otiter authors did not report any potential conflicts of interest.
Presented in part at the 66th Annual Meeting of the American Society for Reproductive Medicine, October 17–21, 2009, Atlanta, Georgia.
LEVEL OF EVIDENCE: II
References
- 1.Surgeon General’s advisory on alcohol use in pregnancy. Office of the Surgeon General, U.S. Department of Health and Human Services; Available at: http://www.surgeongeneral.gov/pressreleases/sg02222005.html. [Google Scholar]
- 2.Eggert J, Theobald H, Engfeldt P. Effects of alcohol consumption on female fertility during an 18-year period. Fertil Steril. 2004;81:379–83. doi: 10.1016/j.fertnstert.2003.06.018. [DOI] [PubMed] [Google Scholar]
- 3.Jensen TK, Hjollund NH, Henriksen TB, Scheike T, Kolstad H, Giwercman A, et al. Does moderate alcohol consumption affect fertility? Follow up study among couples planning first pregnancy. BMJ. 1998;317:505–10. doi: 10.1136/bmj.317.7157.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curtis KM, Savitz DA, Arbuckle TE. Effects of cigarette smoking, caffeine consumption, and alcohol intake on fecundability. Am J Epidemiol. 1997;146:32–41. doi: 10.1093/oxfordjournals.aje.a009189. [DOI] [PubMed] [Google Scholar]
- 5.Juhl M, Nyboe Andersen AM, Grønbaek M, Olsen J. Moderate alcohol consumption and waiting time to pregnancy. Hum Reprod. 2001;16:2705–9. doi: 10.1093/humrep/16.12.2705. [DOI] [PubMed] [Google Scholar]
- 6.Juhl M, Olsen J, Andersen AM, Grønbaek M. Intake of wine, beer and spirits and waiting time to pregnancy. Hum Reprod. 2003;18:1967–71. doi: 10.1093/humrep/deg376. [DOI] [PubMed] [Google Scholar]
- 7.Hassan MA, Killick SR. Negative lifestyle is associated with a significant reduction in fecundity. Fertil Steril. 2004;81:384–92. doi: 10.1016/j.fertnstert.2003.06.027. [DOI] [PubMed] [Google Scholar]
- 8.Klonoff-Cohen H, Lam-Kruglick P, Gonzalez C. Effects of maternal and paternal alcohol consumption on the success rates of in vitro fertilization and gamete intrafallopian transfer. Fertil Steril. 2003;79:330–9. doi: 10.1016/s0015-0282(02)04582-x. [DOI] [PubMed] [Google Scholar]
- 9.USDA National Nutrient Database for Standard Reference, Release 18. Nutrient Data Laboratory Home Page; http://www.nal.usda.gov/fhic/foodcomp/search/ [Google Scholar]
- 10.Center for Disease Control and Prevention, National Center for Health Statistics. Lifetime alcohol drinking status among adults 18 years of age and over, by selected characteristics: United States, selected years 1997–2006. Table 68. Available at http://www.cdc.gov/nchs/data/hus/hus08.pdf#068. Retrieved 12 2009.
- 11.Feunekes GL, van’t Veer P, van Staveren WA, Kok FJ. Alcohol intake assessment: the sober facts. Am J Epidemiol. 1999;150:105–12. doi: 10.1093/oxfordjournals.aje.a009909. [DOI] [PubMed] [Google Scholar]
- 12.Midanik L. The validity of self-reported alcohol consumption and alcohol problems: a literature review. Br J Addict. 1982;77:357–82. doi: 10.1111/j.1360-0443.1982.tb02469.x. [DOI] [PubMed] [Google Scholar]
- 13.Opie LH, Lecour S. The red wine hypothesis: from concepts to protective signalling molecules. Eur Heart J. 2007;28:1683–93. doi: 10.1093/eurheartj/ehm149. [DOI] [PubMed] [Google Scholar]
- 14.Sandor S, Mureşan C. The influence of beer, cognac and ethanol upon the follicular state of mouse ovaries on day 4 of pregnancy. Rom J Morphol Embryol. 1995;41:3–6. [PubMed] [Google Scholar]
- 15.Kaufman MH, Bain IM. Influence of ethanol on chromosome segregation during the first and second meiotic divisions in the mouse egg. J Exp Zool. 1984;230:315–20. doi: 10.1002/jez.1402300217. [DOI] [PubMed] [Google Scholar]
- 16.Kaufman MH. Ethanol-induced chromosomal abnormalities at conception. Nature. 1983;302:258–60. doi: 10.1038/302258a0. [DOI] [PubMed] [Google Scholar]
- 17.Checiu M. The effect of ethanol upon early development in mice and rats: XXI. the effect of acute maternal ethanol intoxication upon in vitro hatching of mouse embryos. Rom J Morphol Embryol. 1995;41:13–8. [PubMed] [Google Scholar]
- 18.Fazakas-Todea I. The effect of ethanol upon early development in mice and rats: XX. the effect of chronic biparental beer intake in mice upon early implantation events. Rom J Morphol Embryol. 1995;41:7–12. [PubMed] [Google Scholar]
- 19.Mendelson JH, Lukas SE, Mello NK, Amass L, Ellingboe J, Skupny A. Acute alcohol effects on plasma estradiol levels in women. Psychopharmacology (Berl) 1988;94:464–7. doi: 10.1007/BF00212838. [DOI] [PubMed] [Google Scholar]
- 20.Ginsburg ES, Mello NIC, Mendelson JH, Barbieri RL, Teoh SK, Rothman M, et al. Effects of alcohol ingestion on estrogens in postmenopausal women. JAMA. 1996;276:1747–51. doi: 10.1001/jama.1996.03540210055034. [DOI] [PubMed] [Google Scholar]
- 21.Ben-Rafael Z, Levy T, Schoemaker J. Pharmacokinetics of follicle-stimulating hormone: clinical significance. Fertil Steril. 1995;63:689–700. doi: 10.1016/s0015-0282(16)57467-6. [DOI] [PubMed] [Google Scholar]


