Summary
Influenza vaccines must be updated regularly because influenza viruses continuously acquire mutations in antibody binding sites of hemagglutinin (HA). The majority of H3N2 strains circulating in the Northern Hemisphere during the 2014–2015 season are antigenically mismatched to the A/Texas/50/2012 H3N2 vaccine strain. Recent H3N2 strains possess several new HA mutations and it is unknown which of these mutations contribute to the 2014–2015 vaccine mismatch. Here, we use reverse-genetics to demonstrate that mutations in HA antigenic site B are primarily responsible for the current mismatch. Sera isolated from vaccinated humans and infected ferrets and sheep had reduced hemagglutination-inhibition and in vitro neutralization titers against reverse-genetics derived viruses possessing mutations in the HA antigenic site B. These data provide an antigenic explanation for the low influenza vaccine efficacy observed during the 2014–2015 influenza season. Further, our data support the World Health Organization’s decision to update the H3N2 component of future vaccine formulations.
Graphical Abstract
Introduction
Most neutralizing influenza antibodies (Abs) target the hemagglutinin (HA) glycoprotein. Seasonal influenza vaccines are designed to elicit HA Abs, however these vaccines are ineffective when viruses acquire mutations in HA Ab binding sites (Yewdell, 2011). Mid-season influenza vaccine efficiency rates during the 2014–2015 Northern Hemisphere season are extremely low (Flannery et al., 2015; Pebody et al., 2015) and recent H3N2 strains are antigenically distinct compared to the 2014–2015 A/Texas/50/2012 H3N2 vaccine strain (D’Mello et al., 2015). The 2014–2015 H3N2 strains can be grouped into at least three genetically distinct clades (Broberg et al., 2015). Viruses within each genetic clade possess several shared and unique HA mutations and it is currently unclear which of these mutations are antigenically relevant.
H3 HAs have at least 5 distinct antigenic sites (sites A–E) (Wiley et al., 1981). Seasonal influenza vaccine strains are routinely chosen based on antigenic analyses that utilize antisera prepared in ferrets (Stohr et al., 2012). Koel and colleagues recently demonstrated that most primary ferret Ab responses to H3N2 viruses are heavily focused on H3 antigenic sites A and B (Koel et al., 2013). Our studies and others have demonstrated that prior H1N1 influenza exposures can influence the specificity of Ab responses raised against new H1N1 influenza strains (Hensley, 2014; Li et al., 2013b; Linderman et al., 2014). We found that ferret antisera do not always recapitulate the different types of H1N1 Ab specificities that are found in individual humans with vastly different pre-exposure histories. Human Ab responses appear to be focused on antigenic site A of some H3 strains (Abe et al., 2004) and on antigenic site B of other H3 strains (Popova et al., 2012).
It is important to determine which HA residues are responsible for the observed antigenic drift of 2014–2015 H3N2 strains. This information can be useful for guiding the selection of viral strains for future vaccine formulations. Here, we completed serological assays using A/Texas/50/2012 H3N2 viruses engineered to have specific HA mutations that are present in currently circulating H3N2 strains. We find that mutations in H3 antigenic site B significantly decrease the binding of ferret, sheep, and human Abs elicited by the A/Texas/50/2012 H3N2 vaccine strain. The World Health Organization recently recommended that the H3 component of seasonal influenza vaccines should be updated to include A/Switzerland/9715293/2013-like strains (Anonymous, 2015). Our data support this recommendation, although we note that the majority of currently circulating H3N2 strains have a distinct antigenic site B compared to the A/Switzerland/9715293/2013 strain.
Results
2014-2015 H3N2 viruses possess several HA mutations
The H3N2 component of the 2014–2015 influenza vaccine is A/Texas/50/2012, which belongs to the 3C.1 HA genetic clade (Broberg et al., 2015). During the 2014–2015 season, H3N2 strains belonging to the phylogenetic 3C.2a, 3C.3 and 3C.3a HA clades predominated (Broberg et al., 2015). Compared to the A/Texas/50/2012 strain, 3C.2a viruses possess HA differences at L3I, N144S, N145S, F159Y, K160T, N225D and Q311H, 3C.3 viruses possess HA differences at T128A, R142G, and N145S, and 3C.3a viruses possess HA differences at T128A, A138S, R142G, N145S, F159S, and N225D (Table 1). HA clade 3C.2a and 3C.3a viruses are antigenically distinct compared to the A/Texas/50/2012 strain and the World Health Organization has recommended that the H3N2 component should be updated with an A/Switzerland/9715293/2013-like (HA 3C.3a) virus for the Northern Hemisphere 2015–2016 vaccine (Anonymous, 2015).
Table 1. HA mutations in 2014–2015 H3N2 viruses.
Shown are HA residues (H3 numbering) that differ between the A/Texas/50/2012 H3N2 vaccine strain and most clade 3C.2a, 3C.3, and 3C.3a viruses isolated during the 2014–2015 Northern Hemisphere influenza season.
| clades | ||
|---|---|---|
| 3C.2a | 3C.3 | 3C.3a |
| L3I | ||
| T128A | T128A | |
| A138S | ||
| R142G | R142G | |
| N144S | ||
| N145S | N145S | N145S |
| F159Y | F159S | |
| K160T | ||
| N225D | N225D | |
| Q311H | ||
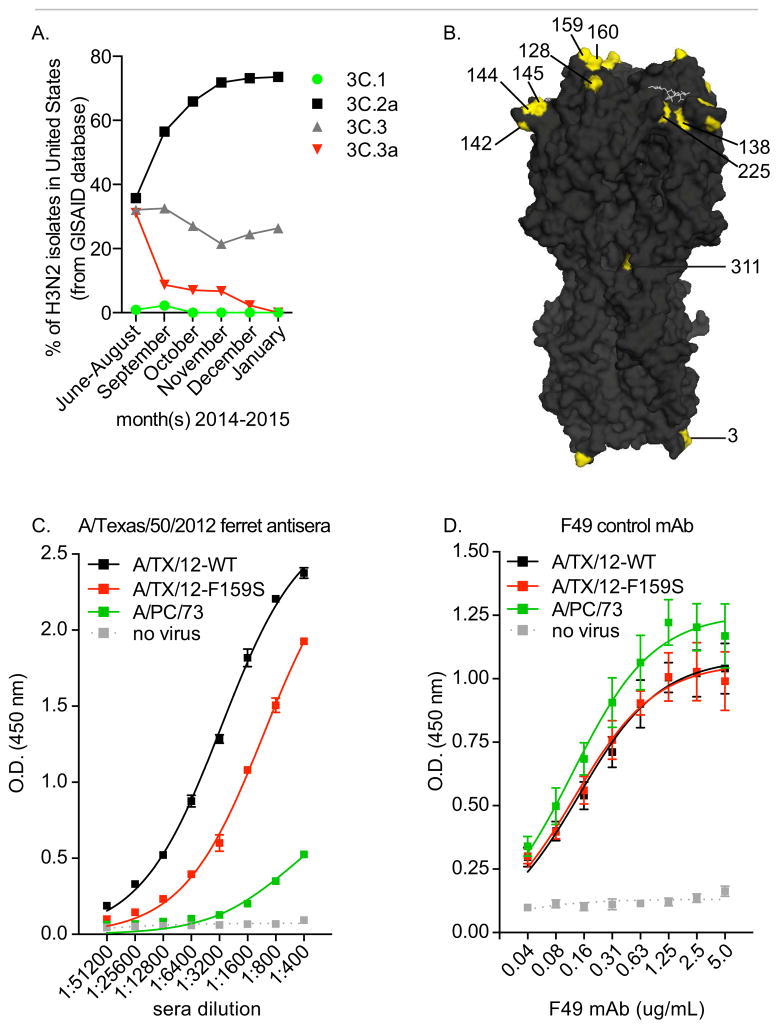
Analyses of HA sequences deposited in the GISAID database revealed that the majority of H3N2 viruses circulating in the United States in the fall and winter of 2014 belong to HA clade 3C.2a (Figure 1A). To understand the antigenic basis for the extremely poor H3N2 vaccine match during the 2014–2015 influenza season, we created a panel of A/Texas/50/2012 viruses that possessed HA mutations found in clade 3C.2a, 3C.3, and 3C.3a viruses (Table S1), and we completed antigenic analyses using sera isolated from ferret, sheep, and humans exposed to the A/Texas/50/2012 H3N2 vaccine strain (Tables 2–3). Our mutant viral panel includes every mutation from Table 1 that is located in the HA globular head (Figure 1B), with the exception of the F159Y and K160T mutations. We were able to successfully rescue A/Texas/50/2012 viruses that possessed the F159Y and K160T mutations, but these viruses failed to agglutinate red blood cells and quickly mutated when propagated for antigenic analyses. However, we were still able to assess the antigenic relevance of mutations in this area of HA since our mutant panel included a virus with the F159S HA mutation.
Figure 1. Genetically distinct H3N2 viruses circulated during 2014–2015 influenza season in the United States.
(A) HA sequences deposited on the GISAID database were analyzed. All sequences deposited by United States laboratories were included in the analysis. Shown are % of viruses that belong to each HA clade (as defined in the text). (B) The H3 structure (Protein Data Bank entry 1HGG) is shown with residues from Table 1 shown in yellow. (C-D) ELISAs were completed with plates coated with VLPs containing A/Texas/50/2012-WT HA (A/TX/12-WT), A/Texas/50/2012-F159S HA (A/TX/12-F159S), or A/Port Chalmers/1/1973 HA (A/PC/73). ELISAs were completed with A/Texas/50/2012 ferret anti-sera (C) or the F49 mAb (D) that binds to a conserved region of the HA stalk. Shown are mean and SEM of triplicate samples. Data are representative of 2 independent experiments.
Table 2. Analyses of ferret and sheep anti-sera raised against the A/Texas/50/2012 and the A/Switzerland/9715293/13 strains.
HAI assays were completed using antisera isolated from ferrets 19 days post-infection or sheep 28 days post-infection. Data are representative of 3 independent assays.
| sera | |||
|---|---|---|---|
| viruses | ferret α-A/Texas/50/12 | sheep α-A/Texas/50/12 | sheep α-A/Switzerland/9715293/13 |
| A/Texas/50/12-WT | 960 | 10240 | 2560 |
| A/Texas/50/12-N128A | 1280 | 10240 | 2560 |
| A/Texas/50/12-A138S | 480 | 5120 | 1280 |
| A/Texas/50/12-R142G | 640 | 7680 | 1920 |
| A/Texas/50/12-N144S+N145S | 1280 | 10240 | 3840 |
| A/Texas/50/12-N145S | 480 | 5120 | 1920 |
| A/Texas/50/12-F159S | 240 | 3840 | 3840 |
| A/Texas/50/12-N225D | 640 | 7680 | 2560 |
| A/Switzerland/9715293/13 | 60 | 1280 | 2560 |
Table 3. Analyses of sera isolated from humans pre- and post-vaccination with the 2014–2015 seasonal influenza vaccine that includes the A/Texas/50/2012 vaccine strain.
Sera were collected from humans pre- and post-vaccination with the 2014–2015 seasonal influenza vaccine. HAI assays were completed using A/Texas/50/2012 and A/Texas/50/2012 with the F159S HA mutation. Data are representative of 3 independent assays.
| pre-vaccination | post-vaccination | ||||
|---|---|---|---|---|---|
| sample ID | age (years) | A/Texas/50/2012 | A/Texas/50/2012-F159S | A/Texas/50/2012 | A/Texas/50/2012-F159S |
| 01 | 21 | 240 | 40 | 160 | 40 |
| 02 | 23 | 640 | 240 | 320 | 160 |
| 03 | 24 | 240 | 80 | 240 | 80 |
| 04 | 25 | 240 | 80 | 240 | 80 |
| 05 | 26 | 80 | <40 | 640 | 320 |
| 06 | 27 | 480 | <40 | 320 | <40 |
| 07 | 29 | 60 | <40 | 120 | 40 |
| 08 | 30 | 60 | 40 | 80 | 40 |
| 09 | 30 | <40 | <40 | 640 | 320 |
| 10 | 30 | 320 | 240 | 640 | 480 |
| 11 | 31 | 320 | 80 | 320 | 120 |
| 12 | 31 | 40 | <40 | 60 | <40 |
| 13 | 31 | <40 | <40 | 40 | <40 |
| 14 | 31 | <40 | <40 | 160 | <40 |
| 15 | 31 | 40 | <40 | 40 | <40 |
| 16 | 34 | 480 | 60 | 640 | 80 |
| 17 | 35 | 480 | 80 | 320 | 120 |
| 18 | 35 | 160 | 80 | 160 | 160 |
| 19 | 35 | 320 | 240 | 640 | 320 |
| 20 | 35 | 160 | <40 | 240 | <40 |
| 21 | 36 | 60 | <40 | 120 | <40 |
| 22 | 38 | <40 | <40 | 120 | <40 |
| 23 | 38 | <40 | <40 | 240 | <40 |
| 24 | 39 | 240 | 80 | 320 | 80 |
| 25 | 41 | 320 | 240 | 320 | 240 |
| 26 | 44 | <40 | <40 | 80 | 40 |
| 27 | 44 | 40 | <40 | 80 | 80 |
| 28 | 46 | <40 | <40 | 80 | 40 |
| 29 | 48 | 80 | <40 | 160 | 40 |
| 30 | 48 | <40 | <40 | 60 | <40 |
| 31 | 48 | <40 | <40 | 80 | <40 |
| 32 | 50 | 80 | 40 | 160 | 80 |
Ferrets and sheep infected with A/Texas/50/2012 mount Abs against HA antigenic site B
We first completed hemagglutination-inhibition (HAI) assays with our mutant A/Texas/50/2012 virus panel and sera collected from ferrets and sheep recovering from A/Texas/50/2012 infections (Table 2). As expected, anti-A/Texas/50/2012 Ab titers were drastically decreased using the antigenically distinct A/Switzerland/9715293/2013 clade 3C.3a virus. Strikingly, anti-A/Texas/50/2012 Ab titers were decreased 4-fold in HAI assays with viruses engineered to possess a single F159S HA mutation (Table 2). Residue 159 is located in a highly exposed region of antigenic site B of H3 HAs (Figure 1B). Other HA mutations had more subtle effects on anti-A/Texas/50/2012 HAI titers. For example, the A138S and N145S HA mutations each lead to 2-fold decreases in HAI titers. We previously showed that viruses that bind to red blood cells with a high avidity can escape Abs in HAI assays independently of antigenic change (Li et al., 2013a). We found that A138S and N145S mutant viruses bound to red blood cells with a higher avidity (Table S1), and the apparent decreased HAI titers using these viruses likely result from this increase in receptor binding avidity. We found that viruses possessing the F159S mutation bound to red blood cells with a decreased avidity (Table S1), and for this reason, we concluded that reduced HAI titers using this virus were the result of a genuine antigenic change.
To verify the antigenic relevance of the F159S HA mutation, we completed additional direct Ab ELISA binding assays with plates coated with viral like particles (VLPs) possessing A/Texas/50/2012-WT HA or A/Texas/50/2012-F159S HA (Figure 1C). As a control, we coated plates with VLPs possessing the antigenically distinct A/Port Chalmers/1/1973 HA (from a 1973 H3 virus). Anti-sera isolated from ferrets infected with A/Texas/50/2012 had reduced binding to VLPs possessing A/Texas/50/2012-F159S HA compared to VLPs possessing A/Texas/50/2012-WT HA (Figure 1C). We verified that equal amounts of VLPs were used in these assays by completing additional ELISA experiments with the F49 monoclonal Ab (mAb) that recognizes the conserved stalk region of H3 (Figure 1D). Collectively, these studies indicate that ferrets and sheep mount Ab responses against an HA epitope involving residue F159 following infection with A/Texas/50/2012, and for this reason we focused the rest of antigenic analyses on this region of HA.
Ferrets and sheep infected with A/Switzerland/9715293/2013 mount Ab responses that are not focused against HA epitope involving residue 159
We also completed HAI assays using our mutant viral panel and anti-sera isolated from sheep infected with A/Switzerland/9715293/2013 virus. Anti-sera isolated from A/Switzerland/9715293/2013-infected animals reacted to all mutant A/Texas/50/2012 viruses, including viruses that possessed the site B F159S HA mutation (Table 2). Therefore, the F159S HA mutation results in an asymmetrical antigenic change. These data are important since A/Switzerland/9715293/2013 (an HA clade 3C.3a virus) has been chosen as the H3 component of 2015–2016 seasonal vaccines, even though HA clade 3C.2a viruses predominated towards the end of the 2014–2015 influenza season (Figure 1). Antigenic site B of HA clades 3C.3a and 3C.2a viruses differ (Table 1), and future studies will need to be completed to rigorously define the specificity of Abs elicited by the A/Switzerland/9715293/2013 vaccine strain.
Anti-sera isolated from most humans vaccinated with A/Texas/50/2012 possess Abs against HA antigenic site B
We next completed HAI assays using human sera collected pre- and post-vaccination with the 2014–2015 seasonal influenza vaccine, which contains the A/Texas/50/2012-like H3N2 strain. We completed HAI assays using the A/Texas/50/2012 strain and the A/Texas/50/2012 strain engineered to have the F159S HA mutation. The A/Texas/50/2012 strain has been in seasonal influenza vaccines since the 2013–2014 season and many individuals in our study had high A/Texas/50/2012 Ab titers that were focused against antigenic site B prior to immunization (Table 3).
Following vaccination, most individuals either mounted or maintained Abs that had reduced reactivity to viruses possessing the F159S HA mutation (Table 3). Importantly, we were able to detect antigenic site B-specific Ab responses in humans that represent a fairly large age range (21–50 years old). It is interesting that some humans did not mount Ab responses that were specific for antigenic site B following vaccination. Our previous studies suggest that influenza pre-exposures can alter the immunodominance of H1N1 Ab responses (Li et al., 2013b; Linderman et al., 2014) and it is possible that individuals who do not mount site B-specific Ab responses have unique H3N2 pre-exposure histories.
Anti-A/Texas/50/2012 antigenic site B Abs are neutralizing
We next tested whether anti-A/Texas/50/2012 antigenic site B Abs identified in ferrets and humans are neutralizing. We completed in vitro neutralization assays with A/Texas/50/2012 and A/Texas/50/2012-F159S using sera samples isolated from humans 21 days post-vaccination. We focused on samples that had the largest HAI differences using A/Texas/50/2012 and A/Texas/50/2012-F159S viruses (Table 3). We also completed in vitro neutralization assays with antisera isolated from ferrets recovering from infection with the A/Texas/50/2012 strain. Our in vitro neutralization results mirrored our HAI results (Table S2). All 7 human sera samples and the ferret sera sample tested had dramatically decreased in vitro neutralization titers using the A/Texas/50/2012-F159S virus compared to the A/Texas/50/2012-WT virus (Table S2).
Discussion
There was a clear H3N2 vaccine mismatch during the 2014–2015 influenza season (Broberg et al., 2015; D’Mello et al., 2015; Flannery et al., 2015; Pebody et al., 2015) and it is important to identify specific HA mutations that have lead to this mismatch. This information is crucial for properly selecting viral strains to be used in future vaccine formulations. In this report, we demonstrate that sheep, ferrets, and humans exposed to the 2014–2015 A/Texas/50/2012 H3N2 vaccine strain mount Ab responses that are targeted against HA antigenic site B. The majority of H3N2 viruses circulating during the 2014–2015 season possessed mutations in antigenic site B (Figure 1A).
Our previous studies indicate that the specificity of human H1N1 Ab responses are shaped by prior H1N1 exposures (Li et al., 2013b; Linderman et al., 2014). We found that most human H1N1 Ab responses are narrowly focused on epitopes that were present in viral strains that circulated during each individual’s childhood. It is unclear if prior H3N2 exposures influence the development of Ab responses to drifted H3N2 strains in a similar manner. Our data indicate that most 21–50 year olds mount anti-A/Texas/50/2012 Abs against antigenic site B. It is important to note that some individuals in our study (4 of 32) mounted anti-A/Texas/50/2012 Ab responses that were not directed against antigenic site B (sera from these 4 individuals had < 2 fold change in HAI titer using the A/Texas/50/2012-WT and A/Texas/50/2012-F159S strains). Current studies are underway to investigate if these 4 individuals have evidence of unique H3N2 exposures.
The World Health Organization recently recommended that the H3N2 component of the 2015–2016 seasonal vaccine should be updated to include the A/Switzerland/9715293/2013 strain (Anonymous, 2015). We note that this clade 3C.3a virus differs in HA antigenic site B compared to clade 3C.2a viruses, which predominated towards the end of the 2014–2015 Northern Hemisphere influenza season (Figure 1). Clade 3C.2a viruses possess a new predicted glycosylation site in antigenic site B due to S159Y and K160T differences compared to A/Switzerland/9715293/2013. The addition of a new glycosylation site on top of the HA antigenic site B could potentially alter antigenicity and Ab access to this region of HA. In our studies, sera isolated from A/Switzerland/9715293/2013-infected animals reacted equally to the A/Switzerland/9715293/2013 strain and our A/Texas/50/2012 mutant panel, however additional studies need to be completed to precisely define the specificity of Abs elicited by A/Switzerland/9715293/2013 exposure.
Taken together, our data suggest that mutations in antigenic site B of 2014–2015 H3N2 strains have led to a major antigenic change. This antigenic change is likely responsible for the low vaccine efficacy during the 2014–2015 season. Our studies support the World Health Organization’s decision to update the H3N2 component of the 2015–2016 influenza vaccine.
Experimental Procedures
HA sequences
We obtained HA sequence data from the GISAID website, www.gisaid.org. Information related to the viral isolates used for these studies is reported in Table S3.
Viruses
We obtained the A/Switzerland/9715293/2013 strain from the National Institute for Biological Standards and Control (NIBSC) in Hertfordshire, UK. We created reverse genetics-derived viruses that possessed the A/Texas/50/2012 HA. Since we did not have the A/Texas/50/2012 strain when we initiated these experiments, we extracted RNA from the A/Victoria/361/2011 strain and cloned the HA of this virus into the pHW2000 reverse genetics plasmid. We then used Quickchange site-directed mutagenesis kits (Stratagene) to convert the A/Victoria/361/2011HA sequence to the A/Texas/50/2012 HA sequence (by adding T128N, G186V, S198P, S219F, N278K mutations). We then introduced additional mutations from Supplemental Table 1 into this A/Texas/50/2012 HA sequence. We rescued viruses that possessed the different mutated A/Texas/50/2012 HAs after transfecting 293T/MDCK cell co-cultures with the different HA plasmids and plasmids derived from A/Puerto Rico/8/1934 that encoded for the rest of the influenza genome. All viruses used for antigenic analyses were propagated in 10 day old fertilized chicken eggs. We used Sanger sequencing to verify that additional mutations did not arise during viral propagation.
Antisera
All sheep anti-sera used in this study were obtained from the National Institute for Biological Standards and Control (NIBSC) in Hertfordshire, UK. Antisera were collected from sheep 28 days post-infection. Ferret antisera used in this study were obtained from the Influenza Reagent Resource, Influenza Division, WHO Collaborating Center for Surveillance, Epidemiology and Control of Influenza, CDC, Atlanta, GA, USA. Antisera were collected from ferrets 19 days post-infection. Sera were also collected from humans prior to vaccination and 21 days following vaccination with the 2014–2015 seasonal influenza vaccine. All studies involving the collection and analysis of human sera were approved by the Institutional Review Boards of the Wistar Institute and Vaccine and Gene Therapy Institute of Florida. All sera were treated with receptor-destroying enzyme (RDE) for 3 hours prior to antigenic testing.
HAI assays
HAI titrations were performed in 96-well round bottom plates. Sera were serially diluted 2-fold and added to 4 agglutinating doses of virus in a total volume of 100 ul. Next, 12.5 ul of a 2% (vol/vol) turkey red blood cell solution was added. Agglutination was read out after incubating for 60 min at room temperature. HAI titers were recorded as the inverse of the highest dilution that inhibited hemagglutination of turkey red blood cells. Similar results were obtained using guinea pig red blood cells.
ELISA assays
VLPs expressing A/Texas/50/2012-WT HA, A/Texas/50/2012-F159S HA, or A/Port Chalmers/1/1973 HA were created. Codon-optimized sequences were cloned into the pCMV-Sport6 plasmid. VLPs were rescued by transfecting 293T cells with plasmids expressing HIV gag, A/Puerto Rico/8/1934 NA, HAT (human airway trypsin-like protease), and each HA. VLPs isolated from culture supernatants were concentrated using a 20% sucrose cushion and resuspended in PBS. VLP amounts were normalized in ELISAs using the F49 mAb (Clontech) that binds to a conserved region of the H3 stalk. Goat anti-ferret IgG conjugated to horseradish peroxidase (Abcam) was used to detect binding of A/Texas/50/2012 ferret antisera and goat anti-mouse IgG conjugated to horseradish peroxidase (MP Biomedicals) was used to detect the murine F49 mAb.
Neutralization assays
In vitro neutralization assays were performed in 96-well flat bottom plates. Sera were serially diluted and then added to 100 TCID50 units of A/Texas/50/2012-WT or A/Texas/50/2012-F159S virus and incubated at room temperature for 30 mins. The virus-sera mixtures were then incubated with MDCK cells for 1 hr at 37C. Next, cells were washed and then serum-free media with TPCK-treated trypsin was added. We visually determined cytopathic endpoints 3 days later. Data are expressed as the inverse of the highest dilution that caused neutralization.
Receptor binding assays
As previously described (Li et al., 2013a), turkey red blood cells were pretreated with different amounts of RDE (a neuraminidase) for 1 hr at 37 C. The red blood cells were washed with PBS and added (as 2% vol/vol solutions) to 4 agglutinating doses of each virus (as determined using non-treated red blood cells). After a 1 hr incubation, agglutination was measured. Viruses with higher receptor binding avidities are able to bind to red blood cells that are treated with high amounts of RDE (Li et al., 2013a).
Supplementary Material
Highlights.
Recent H3N2 strains are antigenically distinct compared to the 2014–2015 vaccine strain
Most humans produce antigenic site B HA antibodies
New mutations in antigenic site B of HA likely led to 2014–2015 vaccine mismatch
Acknowledgments
Research reported in this publication was supported by the NIAID of the National Institutes of Health under award numbers 1R01AI113047 (SEH) and 1R01AI108686 (SEH). We thank James Allen for collecting and processing blood from influenza vaccinated volunteers. We acknowledge the originating and submitting laboratories of the sequences from GISAID’s EpiFlu Database on which Figure 1A is based (Table S3). All submitters of sequence data may be contacted directly via the GISAID website www.gisaid.org. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Author Contributions
BSC created viruses by reverse-genetics, sequenced viruses, completed HAI and ELISA assays, designed experiments, and helped write the manuscript. KP completed in vitro neutralization assays. TMR collected and provided human sera samples. KA helped design experiments and edited the manuscript. SEH designed experiments, supervised all experiments, and wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe Y, Takashita E, Sugawara K, Matsuzaki Y, Muraki Y, Hongo S. Effect of the addition of oligosaccharides on the biological activities and antigenicity of influenza A/H3N2 virus hemagglutinin. Journal of virology. 2004;78:9605–9611. doi: 10.1128/JVI.78.18.9605-9611.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anonymous. Recommended composition of influenza virus vaccines for use in the 2015–2016 northern hemisphere influenza season. Weekly epidemiological record. 2015;90:97–108. [PubMed] [Google Scholar]
- Broberg E, Snacken R, Adlhoch C, Beaute J, Galinska M, Pereyaslov D, Brown C, Penttinen P on behalf of the WHO European Region and the European Influenza Surveillance Network. Start of the 2014/15 influenza season in Europe: drifted influenza A(H3N2) viruses circulate as dominant subtype. Eurosurveillance. 2015;20:21023. doi: 10.2807/1560-7917.es2015.20.4.21023. [DOI] [PubMed] [Google Scholar]
- D’Mello T, Brammer L, Blanton L, Kniss K, Smith S, Mustaquim D, Steffens C, Dhara R, Cohen J, Chaves SS, et al. Update: influenza activity - United States, september 28, 2014-february 21, 2015. MMWR Morbidity and mortality weekly report. 2015;64:206–212. [PMC free article] [PubMed] [Google Scholar]
- Flannery B, Clippard J, Zimmerman RK, Nowalk MP, Jackson ML, Jackson LA, Monto AS, Petrie JG, McLean HQ, Belongia EA, et al. Early estimates of seasonal influenza vaccine effectiveness - United States, January 2015. MMWR Morbidity and mortality weekly report. 2015;64:10–15. [PMC free article] [PubMed] [Google Scholar]
- Hensley SE. Challenges of selecting seasonal influenza vaccine strains for humans with diverse pre-exposure histories. Current opinion in virology. 2014;8C:85–89. doi: 10.1016/j.coviro.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koel BF, Burke DF, Bestebroer TM, van der Vliet S, Zondag GC, Vervaet G, Skepner E, Lewis NS, Spronken MI, Russell CA, et al. Substitutions near the receptor binding site determine major antigenic change during influenza virus evolution. Science. 2013;342:976–979. doi: 10.1126/science.1244730. [DOI] [PubMed] [Google Scholar]
- Li Y, Bostick DL, Sullivan CB, Myers JL, Griesemer SB, Stgeorge K, Plotkin JB, Hensley SE. Single Hemagglutinin Mutations That Alter both Antigenicity and Receptor Binding Avidity Influence Influenza Virus Antigenic Clustering. Journal of virology. 2013a;87:9904–9910. doi: 10.1128/JVI.01023-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Myers JL, Bostick DL, Sullivan CB, Madara J, Linderman SL, Liu Q, Carter DM, Wrammert J, Esposito S, et al. Immune history shapes specificity of pandemic H1N1 influenza antibody responses. The Journal of experimental medicine. 2013b;210:1493–1500. doi: 10.1084/jem.20130212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linderman SL, Chambers BS, Zost SJ, Parkhouse K, Li Y, Herrmann C, Ellebedy AH, Carter DM, Andrews SF, Zheng NY, et al. Potential antigenic explanation for atypical H1N1 infections among middle-aged adults during the 2013–2014 influenza season. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:15798–15803. doi: 10.1073/pnas.1409171111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pebody R, Warburton F, Ellis J, Andrews N, Thompson C, von Wissmann B, Green H, Cottrell S, Johnston J, de Lusignan S, et al. Low effectiveness of seasonal influenza vaccine in preventing laboratory-confirmed influenza in primary care in the United Kingdom: 2014/15 mid-season results. Eurosurveillance. 2015;20:21025. [PubMed] [Google Scholar]
- Popova L, Smith K, West AH, Wilson PC, James JA, Thompson LF, Air GM. Immunodominance of antigenic site B over site A of hemagglutinin of recent H3N2 influenza viruses. PloS one. 2012;7:e41895. doi: 10.1371/journal.pone.0041895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohr K, Bucher D, Colgate T, Wood J. Influenza virus surveillance, vaccine strain selection, and manufacture. Methods Mol Biol. 2012;865:147–162. doi: 10.1007/978-1-61779-621-0_9. [DOI] [PubMed] [Google Scholar]
- Wiley DC, Wilson IA, Skehel JJ. Structural identification of the antibody-binding sites of Hong Kong influenza haemagglutinin and their involvement in antigenic variation. Nature. 1981;289:373–378. doi: 10.1038/289373a0. [DOI] [PubMed] [Google Scholar]
- Yewdell JW. Viva la revolucion: rethinking influenza a virus antigenic drift. Current opinion in virology. 2011;1:177–183. doi: 10.1016/j.coviro.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.