Abstract
Interest in peroxisome proliferator–activated receptors (PPARs) has steadily increased over the past 15 years. The recognition that subclasses of this receptor played critical roles in regulation of metabolism led to the development of synthetic ligands and their widespread application in the treatment of type 2 diabetes. At the same time, emerging evidence demonstrated that the influence of PPARs extends well beyond metabolism and diabetes. A salient example of this can be seen in studies that explore the role of PPARs in lung cell biology. In fact, current literature suggests that PPAR receptors may well represent exciting new targets for treatment in a variety of lung disorders. In an attempt to keep the scientific and medical communities abreast of these developments, a symposium sponsored by the American Federation for Medical Research entitled “PPARγ: A Novel Molecular Target in Lung Disease” was convened on April 29, 2007, at the Experimental Biology Meeting in Washington, DC. During that symposium, 4 speakers reviewed the latest developments in basic and translational research as they relate to specific lung diseases. Jesse Roman, MD, professor and director of the Emory University Division of Pulmonary, Allergy, and Critical Care Medicine, reviewed the role of PPARγ in the pathogenesis of lung cancer and its implications for therapy. Raju Reddy, MD, assistant professor of Medicine at the University of Michigan, presented data regarding the immunomodula-tory role of PPARγ in alveolar macrophages. Patricia J. Sime, MD, associate professor of Medicine, Environmental Medicine, and Oncology at the University of Rochester School of Medicine, discussed the antifibrogenic potential of PPARγ ligands in pulmonary fibrosis. Finally, C. Michael Hart, MD, professor of Medicine at Emory University and chief of the Atlanta Veterans Affairs Medical Center Pulmonary Section, reviewed the role of PPARγ in pulmonary vascular disease. This brief introduction to the symposium will provide background information about PPARs to facilitate the general reader's appreciation of the more in-depth and disease-specific discussions that follow.
Keywords: PPARγ, TGF-β, fibroplast, thrazolidinedione, triterpenoid
Originally described in 1990, peroxisome proliferator–activated receptors (PPARs) are ligand-activated transcription factors belonging to the nuclear hormone receptor superfamily.1 Peroxisome proliferator–activated receptors regulate diverse physiological processes ranging from lipogenesis to inflammation and have been implicated in numerous disorders including cancer, diabetes, and atherosclerosis. Three distinct PPAR isotypes (PPARα, PPARβ/δ, and PPARγ) are encoded by separate genes and exhibit distinct tissue distribution and functions. Peroxisome proliferator–activated receptor α is predominantly expressed in liver, heart, kidney, and muscle where it regulates genes involved in lipid metabolism. Peroxisome proliferator–activated receptor β/δ is a more ubiquitously expressed isoform that stimulates fatty acid oxidation in heart and skeletal muscles2 and regulates metabolism.3 Finally, PPARγ, which is prominently expressed in adipose tissue, can also be found in a variety of other tissues where it regulates genes involved in cellular differentiation and growth, inflammation, apoptosis, and angiogenesis.4–6
The understanding of the biological roles of PPARγ in lung disease has been facilitated by an appreciation of normal PPAR function. Like other nuclear receptors,7 PPARs share a common structural organization with multiple distinct functional domains (Fig. 1). They also share a common mode of action to regulate target gene expression. Briefly, ligand binding to the PPAR receptor induces a conformational change in the receptor that permits heterodimerization with the retinoid X receptor, dissociation of corepressors, and concomitant association with coactivators.8 This activated complex binds to specific PPAR response elements in the promoter region of responsive genes to modulate transcriptional activity. The coactivator proteins either possess histone acetyltransferase activity or recruit other proteins with this activity to the transcription start site. Acetylation of histone proteins alters chromatin structure, facilitating the binding of RNA polymerase and the initiation of transcription.9 Peroxisome proliferator–activated receptors can also repress gene expression by interfering with other signaling pathways and by recruiting corepressors to unliganded PPARs.10 Peroxisome proliferator–activated receptors mediate these indirect repressive effects through transrepression mechanisms that inhibit the activity of key transcription factors via direct protein-protein interactions or by sequestrating cofactors necessary to their activity.11–14
FIGURE 1.
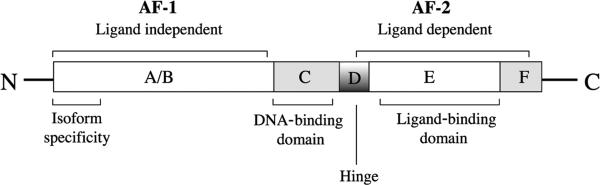
Schematic representation of the structural organization of nuclear receptors. All nuclear receptors share a common structural organization with multiple distinct functional domains. The N-terminal A/B domain contains at least 1 constitutively active transactivation region (activation function 1) and several autonomous transactivation domains. The C domain, the most conserved region, is responsible for DNA-binding specificity and for both homodimerization and heterodimerization of receptors. The D domain is a less conserved flexible hinge region between DNA-binding and the C-terminal ligand–binding domain E. The D domain contains the nuclear localization signal and also serves as docking site for cofactors. The E domain is a moderately conserved domain with a ligand-dependent transactivation function called activation function 2. Some members also have C-terminal F domain, whose sequence is extremely variable. AF-1 indicates activation function 1; AF-2, activation function 2.
Structurally diverse ligands activate PPARγ. For example, PPARγ ligands include the thiazolidinedione class of antidiabetic medications (eg, pioglitazone, rosiglitazone, and troglitazone),15 components of oxidized low-density lipoprotein,16 long-chain fatty acids and their metabolites, the PGD2 metabolite, 15-deoxy-Δ12,14-prostglandin J2,17,18 and nitroalkenes.19,20 However, despite this promiscuity for activating ligands and broad tissue distribution, specificity of PPAR-mediated tissue effects occurs, in part, through recruitment of ligand-specific populations of coactivator and corepressor molecules.21–23 As a result, PPARγ activation can lead to both the enhancement and reduction in the expression level of target genes in a broad variety of tissues. Emerging evidence indicates that PPARγ activation may be beneficial in a variety of disease processes. Synthetic and natural ligands have been used both in vitro and in vivo to elucidate the role of PPARγ in disease processes. However, several caveats should be considered when interpreting such studies (reviewed in the study by Han and Roman24). First, the natural ligands that regulate PPARs in vivo remain incompletely defined. Second, a number of PPARγ ligands exert effects through pathways independent of the receptor. Third, some PPARγ ligands exert both agonist and antagonist activity toward the PPARγ receptor.25
A rapidly emerging body of literature demonstrates the importance of PPARγ in the biology of the lung, where this receptor is expressed in parenchymal and immune cells. The expression of PPARγ in the lung, the availability of pharmacological ligands for this receptor,26 the evidence for depressed PPARγ expression in lung cells in several disorders,27–29 and the therapeutic efficacy of PPARγ ligands in selected nonpulmonary diseases26 suggest that the PPARγ receptor may not only play an important role in lung cell biology but may also serve as a novel therapeutic target in several forms of pulmonary disease. Therefore, this symposium highlights recent developments in lung cancer, lung immune cell function, pulmonary fibrosis, and pulmonary vascular disorders that indicate a role for PPARγ in disease pathogenesis and/or therapy. The studies reviewed below suggest that the intersection between PPARγ biology and pulmonary disease is a complex but promising avenue for future investigation that may provide novel insights into lung disease pathogenesis and treatment.
Footnotes
The proceedings of a symposium presented at the Experimental Biology Meeting in Washington, DC, on Sunday, April 29, 2007. This symposium was funded by educational grants from the National Institutes of Health and Takeda Pharmaceuticals of North America.
REFERENCES
- 1.Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347(6294):645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- 2.Gilde AJ, van der Lee KA, Willemsen PH, et al. Peroxisome proliferator–activated receptor (PPAR) alpha and PPARbeta/delta, but not PPARgamma, modulate the expression of genes involved in cardiac lipid metabolism. Circ Res. 2003;92(5):518–524. doi: 10.1161/01.RES.0000060700.55247.7C. [DOI] [PubMed] [Google Scholar]
- 3.Bedu E, Wahli W, Desvergne B. Peroxisome proliferator–activated receptor beta/delta as a therapeutic target for metabolic diseases. Expert Opin Ther Targets. 2005;9(4):861–873. doi: 10.1517/14728222.9.4.861. [DOI] [PubMed] [Google Scholar]
- 4.Chinetti G, Griglio S, Antonucci M, et al. Activation of proliferator-activated receptors alpha and gamma induces apoptosis of human monocyte-derived macrophages. J Biol Chem. 1998;273(40):25573–25580. doi: 10.1074/jbc.273.40.25573. [DOI] [PubMed] [Google Scholar]
- 5.Daynes RA, Jones DC. Emerging roles of PPARs in inflammation and immunity. Nat Rev Immunol. 2002;2:748–759. doi: 10.1038/nri912. [DOI] [PubMed] [Google Scholar]
- 6.Keshamouni VG, Reddy RC, Arenberg DA, et al. Peroxisome proliferator–activated receptor–gamma activation inhibits tumor progression in non-small-cell lung cancer. Oncogene. 2004;23(1):100–108. doi: 10.1038/sj.onc.1206885. [DOI] [PubMed] [Google Scholar]
- 7.Nuclear Receptors Nomenclature Committee A unified nomenclature system for the nuclear receptor superfamily. Cell. 1999;97(10):161–163. doi: 10.1016/s0092-8674(00)80726-6. [DOI] [PubMed] [Google Scholar]
- 8.Gearing KL, Gottlicher M, Teboul M, et al. Interaction of the peroxisome-proliferator–activated receptor and retinoid X receptor. Proc Natl Acad Sci U S A. 1993;90(4):1440–1444. doi: 10.1073/pnas.90.4.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosen ED, Spiegelman BM. PPARgamma: a nuclear regulator of metabolism, differentiation, and cell growth. J Biol Chem. 2001;276(41):37731–37734. doi: 10.1074/jbc.R100034200. [DOI] [PubMed] [Google Scholar]
- 10.Xu HE, Stanley TB, Montana VG, et al. Structural basis for antagonist-mediated recruitment of nuclear co-repressors by PPARalpha. Nature. 2002;415(6873):813–817. doi: 10.1038/415813a. [DOI] [PubMed] [Google Scholar]
- 11.Delerive P, De Bosscher K, Besnard S, et al. Peroxisome proliferator–activated receptor alpha negatively regulates the vascular inflammatory gene response by negative crosstalk with transcription factors NF-kappaB and AP-1. J Biol Chem. 1999;274(45):32048–32054. doi: 10.1074/jbc.274.45.32048. [DOI] [PubMed] [Google Scholar]
- 12.Wang N, Verna L, Chen NG, et al. Constitutive activation of peroxisome proliferator–activated receptor–gamma suppresses pro-inflammatory adhesion molecules in human vascular endothelial cells. J Biol Chem. 2002;277(37):34176–34181. doi: 10.1074/jbc.M203436200. [DOI] [PubMed] [Google Scholar]
- 13.Shi Y, Hon M, Evans RM. The peroxisome proliferator–activated receptor delta, an integrator of transcriptional repression and nuclear receptor signaling. Proc Natl Acad Sci U S A. 2002;99(5):2613–2618. doi: 10.1073/pnas.052707099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qi C, Zhu Y, Reddy JK. Peroxisome proliferator–activated receptors, coactivators, and downstream targets. Cell Biochem Biophys. 2000;32:187–204. doi: 10.1385/cbb:32:1-3:187. [DOI] [PubMed] [Google Scholar]
- 15.Lehmann JM, Moore LB, Smith-Oliver TA, et al. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator–activated receptor gamma (PPAR gamma). J Biol Chem. 1995;270(2):12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 16.Tontonoz P, Nagy L, Alvarez JG, et al. PPARgamma promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell. 1998;93(2):241–252. doi: 10.1016/s0092-8674(00)81575-5. [DOI] [PubMed] [Google Scholar]
- 17.Huang JT, Welch JS, Ricote M, et al. Interleukin-4–dependent production of PPAR-gamma ligands in macrophages by 12/15–lipoxygenase. Nature. 1999;400(6742):378–382. doi: 10.1038/22572. [DOI] [PubMed] [Google Scholar]
- 18.Nagy L, Tontonoz P, Alvarez JG, et al. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell. 1998;93(2):229–240. doi: 10.1016/s0092-8674(00)81574-3. [DOI] [PubMed] [Google Scholar]
- 19.Baker PR, Lin Y, Schopfer FJ, et al. Fatty acid transduction of nitric oxide signaling: multiple nitrated unsaturated fatty acid derivatives exist in human blood and urine and serve as endogenous peroxisome proliferator–activated receptor ligands. J Biol Chem. 2005;280(51):42464–42475. doi: 10.1074/jbc.M504212200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schopfer FJ, Lin Y, Baker PR, et al. Nitrolinoleic acid: an endogenous peroxisome proliferator–activated receptor gamma ligand. Proc Natl Acad Sci U S A. 2005;102(7):2340–2345. doi: 10.1073/pnas.0408384102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Camp HS, Li O, Wise SC, et al. Differential activation of peroxisome proliferator–activated receptor–gamma by troglitazone and rosiglitazone. Diabetes. 2000;49(4):539–547. doi: 10.2337/diabetes.49.4.539. [DOI] [PubMed] [Google Scholar]
- 22.Kodera Y, Takeyama K, Murayama A, et al. Ligand type–specific interactions of peroxisome proliferator–activated receptor gamma with transcriptional coactivators. J Biol Chem. 2000;275(43):33201–33204. doi: 10.1074/jbc.C000517200. [DOI] [PubMed] [Google Scholar]
- 23.Olefsky JM. Treatment of insulin resistance with peroxi-some proliferator–activated receptor gamma agonists. J Clin Invest. 2000;106(4):467–472. doi: 10.1172/JCI10843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han S, Roman J. Peroxisome proliferator–activated receptor gamma: a novel target for cancer therapeutics? Anticancer Drugs. 2007;18(3):237–244. doi: 10.1097/CAD.0b013e328011e67d. [DOI] [PubMed] [Google Scholar]
- 25.Reginato MJ, Bailey ST, Krakow SL, et al. A potent antidiabetic thiazolidinedione with unique peroxisome proliferator–activated receptor gamma–activating properties. J Biol Chem. 1998;273(49):32679–32684. doi: 10.1074/jbc.273.49.32679. [DOI] [PubMed] [Google Scholar]
- 26.Yki-Jarvinen H. Thiazolidinediones. N Engl J Med. 2004;351(11):1106–1118. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]
- 27.Ameshima S, Golpon H, Cool CD, et al. Peroxisome proliferator–activated receptor gamma (PPARgamma) expression is decreased in pulmonary hypertension and affects endothelial cell growth. Circ Res. 2003;92(10):1162–1169. doi: 10.1161/01.RES.0000073585.50092.14. [DOI] [PubMed] [Google Scholar]
- 28.Bonfield TL, Farver CF, Barna BP, et al. Peroxisome proliferator–activated receptor–gamma is deficient in alveolar macrophages from patients with alveolar proteinosis. Am J Respir Cell Mol Biol. 2003;29(6):677–682. doi: 10.1165/rcmb.2003-0148OC. [DOI] [PubMed] [Google Scholar]
- 29.Culver DA, Barna BP, Raychaudhuri B, et al. Peroxisome proliferator–activated receptor gamma activity is deficient in alveolar macrophages in pulmonary sarcoidosis. Am J Respir Cell Mol Biol. 2004;30(1):1–5. doi: 10.1165/rcmb.2003-0304RC. [DOI] [PubMed] [Google Scholar]


