Abstract
Combination chemotherapy with gemcitabine and cisplatin in patients with metastatic urothelial cancer of the bladder frequently results in the development of acquired drug resistance. Availability of cell culture models with acquired resistance could help to identify candidate treatments for an efficient second-line therapy. Six cisplatin- and six gemcitabine-resistant cell lines were established. Cell viability assays were performed to evaluate the sensitivity to 16 different chemotherapeutic substances. The activity of the drug transporter ATP-binding cassette transporter, subfamily B, member 1 (ABCB1, a critical mediator of multidrug resistance in cancer) was evaluated using fluorescent ABCB1 substrates. For functional assessment, cells overexpressing ABCB1 were generated by transduction with a lentiviral vector encoding for ABCB1, while zosuquidar was used for selective inhibition. In this study, 8 of 12 gemcitabine- or cisplatin-resistant cell lines were cross-resistant to carboplatin, 5 to pemetrexed, 4 to methotrexate, 3 to oxaliplatin, 5-fluorouracil, and paclitaxel, and 2 to cabazitaxel, larotaxel, docetaxel, topotecan, doxorubicin, and mitomycin c, and 1 of 12 cell lines was cross-resistant to vinflunine and vinblastine. In one cell line with acquired resistance to gemcitabine (TCC-SUPrGEMCI20), cross-resistance seemed to be mediated by ABCB1 expression. Our model identified the vinca alkaloids vinblastine and vinflunine, in Europe an already approved second-line therapeutic for metastatic bladder cancer, as the most effective compounds in urothelial cancer cells with acquired resistance to gemcitabine or cisplatin. These results demonstrate that this in vitro model can reproduce clinically relevant results and may be suitable to identify novel substances for the treatment of metastatic bladder cancer.
Introduction
Patients with metastatic urothelial cancer of the bladder are treated with cisplatin containing systemic chemotherapies (e.g., gemcitabine/cisplatin, GC) as a standard of care [1,2]. Unfortunately, the treatment success is limited resulting in a median survival of 12 to 14 months. Treatment failure is commonly caused by development of resistance to chemotherapy [1,2].
ATP-binding cassette transporter, subfamily B, member 1 (ABCB1) is a cell membrane efflux pump with broad substrate specificity. Overexpression of ABCB1 in tumor cells develops mostly as a specific response to ABCB1 substrates (e.g., vinca alkaloids, taxanes, or anthracyclines) and confers resistance to these substances. However, ABCB1 may also be upregulated as part of a generalized stress response to different toxic drugs (such as gemcitabine and cisplatin), which are not ABCB1 substrates [3,4]. Expression of ABCB1 was detected in both pre-chemotherapy and post-chemotherapy tumor tissue samples from patients with bladder cancer with higher expression in post-chemotherapy patients [5–11]. Therefore, efficient second-line chemotherapies or targeted therapies for the treatment of bladder cancer need to be tested especially in a context of specific resistance mechanisms such as ABCB1 overexpression.
Development of acquired cancer cell drug resistance is difficult to study in a clinical setting. Since acquisition of tumor biopsies represents an invasive procedure, possibilities to obtain serial tumor biopsies from patients under chemotherapy are limited by technical as well as ethical barriers [12]. Moreover, significance of biopsies may be affected by intratumor heterogeneity [13]. “Liquid biopsies” including circulating tumor cells and tumor DNA may be valuable sources for detection of molecular changes associated with resistance in the future [14] but may be unsuitable for functional studies. Therefore, experimental in vitro models are needed to identify potential markers of resistance and novel drug targets.
Drug-adapted cancer cell lines have been successfully used to study cancer cell mechanisms of resistance [15,16]; however, comprehensive cell line panels are missing. A panel of 18 urothelial cancer cell lines consisting of six parental chemosensitive cell lines and their gemcitabine- or cisplatin-resistant sublines was used to study the activity of 16 anticancer drugs. The cell lines are part of the Resistant Cancer Cell Line collection. This collection consists of cell lines of 15 different cancer entities including the six gemcitabine- and six cisplatin-resistant urothelial cancer cell lines that were used here.
Materials and Methods
Drugs
Cisplatin (solvent: 0.9% aqueous NaCl solution) was purchased from Gry-Pharma (Kirchzarten, Germany), gemcitabine (solvent: 0.9% aqueous NaCl solution) from Lilly (Bad Homburg, Germany), vinflunine [solvent: phosphate-buffered saline (PBS)] from Pierre Fabre (Freiburg, Germany), pemetrexed (solvent: DMSO) from Lilly, methotrexate (solvent: PBS) from Hexal (Holzkirchen, Germany), carboplatin (solvent: 5% aqueous glucose solution) from Hexal, oxaliplatin (solvent: PBS) from Teva (Basel, Switzerland), paclitaxel (solvent: DMSO) from Bristol-Myers Squibb (New York, NY), topotecan (solvent: dH2O) from GlaxoSmithKline (London, United Kingdom), docetaxel (solvent: DMSO) from Sanofi (Paris, France), cabazitaxel (solvent: DMSO) from Sanofi, larotaxel (solvent: DMSO) from Shanghai Fuhe Chemistry Technology (Shanghai, China), vinblastine (solvent: PBS) from Teva, doxorubicin (solvent: 0.9% aqueous NaCl solution) from Sigma-Aldrich (St Louis, MO), mitomycin c (solvent: dH2O) from Medac (Wedel, Germany), and 5-fluorouracil (solvent: 0.9% aqueous NaCl solution) from Medac.
Cell Lines and Lentiviral Transduction
The cell lines RT112, RT4, 5637, T24, HT1376, and TCC-SUP were obtained from the American Type Culture Collection (Manassas, VA). Drug-resistant sublines were established by continuous exposure to increasing drug concentrations and are part of the Resistant Cancer Cell Line (RCCL) collection (http://www.kent.ac.uk/stms/cmp/RCCL/RCCLabout): RT112rCDDP1000 (cisplatin-resistant, 1000 ng/ml cisplatin), RT112rGEMCI20 (gemcitabine-resistant, 20 ng/ml gemcitabine), RT4rCDDP1000, RT4rGEMCI10, 5637rCDDP1000, 5637rGEMCI20, T24rCDDP1000, T24rGEMCI20, T24rVBL20 (vinblastine-resistant, 20 ng/ml vinblastine), HT1376rCDDP1000, HT1376rGEMCI20, TCC-SUPrCDDP1000, TCC-SUPrGEMCI20, and TCC-SUPrVBL20.
Cell line adaptation was started with drug concentrations that were two-fold higher than the respective IC50. The doses were stepwise increased during subculturing until resistance to clinically achievable plasma concentration was reached. The establishment of readily growing resistant cell lines required 1 to 2 years in dependence on the used cell line and the drug.
The ABCB1-expressing cell lines TCC-SUPABCB1 and T24ABCB1 and the corresponding control cell lines TCC-SUPCER2 and T24CER2 were established by lentiviral transduction using the Lentiviral Gene Ontology vector technology as described previously [17,18].
All cell lines were grown in Iscove’s modified Dulbecco’s medium (IMDM) supplemented with 10% fetal calf serum (FCS; Gibco, Karlsruhe, Germany). Cell line authentication was performed by short tandem repeats (STR) profiling.
Growth Curves
To determine cell growth kinetics, 4000 cells per cm2 were seeded in cell culture flasks containing IMDM supplemented with 10% FCS. Cell counts were determined using a Neubauer chamber in the presence of trypan blue. Doubling time (DT) was calculated using the formula DT = culture time/cell doubling. Cell doubling = ln(Nf/Ni)/ln2, where Ni represents seeded cell number and Nf represents the harvested cell number [19].
Cell Viability Assay
Cell viability was determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) dye reduction assay after 120-hour incubation as described previously [20]. Drug resistance was defined by resistance factors defined as IC50 drug-resistant cells/IC50 parental cells. Cell lines were regarded to be resistant to a drug if the resistance factor was > 2 [21].
Flow Cytometry
Antibodies directed against ABCB1 (20 μl per sample with an antibody concentration of 25 ng/μl; Alexis Biochemicals through AXXORA Deutschland, Lörrach, Germany) followed by secondary antibodies labeled with phycoerythrin were used to detect protein expression by flow cytometry (FACSCalibur; BD Biosciences, Heidelberg, Germany). Mouse IgG2a antibodies were used as isotype control.
For washout experiments, cells were incubated for 1 hour with 1 μM rhodamine 123 (R123, ABCB1 substrate). Zosuquidar (Sigma-Aldrich), an inhibitor of ABCB1, was added immediately. Cells were resuspended in supplemented growth medium, and cellular fluorescence was measured at FL1 channel by flow cytometry.
Statistical Analysis
Results are expressed as mean ± SD of at least three independent experiments. For statistical analysis, Student's t test, analysis of variance, and Student-Newman-Keuls test were performed whenever applicable. Significance was defined at values of P ≤ .05.
Results
Cell Growth Kinetics
Four of six gemcitabine-resistant sublines showed decreased growth rates compared to their parental cell lines [5637 (DT: 0.96 day) vs 5637rGEMCI20 (DT: 1.74 day); HT1376 (DT: 1.29 day) vs HT1376rGEMCI20 (DT: 1.74 day); TCC-SUP (DT: 2.05 day) vs TCC-SUPrGEMCI20 (DT: 4.91 day); T24 (DT: 0.97 day) vs T24rGEMCI20 (DT: 1.08 day)], while no significant differences were found for RT112 cells [RT112 (DT: 1.07 day) vs RT112rGEMCI20 (DT: 1.11 day)] and RT4 cells [RT4 (DT: 2.70 day) vs RT4rGEMCI10 (DT: 2.34 day)]. Three of six cisplatin-resistant sublines [RT112rCDDP1000 (DT: 0.96 day), RT4rCDDP1000 (DT: 1.65 day), and TCC-SUPrCDDP1000 (DT: 1.30 day)] displayed enhanced growth rates compared to their parental cell lines, while growth rate of 5637rCDDP1000 (DT: 1.23 day) cells was decreased relative to 5637 cells. For HT1376 vs HT1376rCDDP1000 (DT: 1.30 day) and T24 vs T24rCDDP1000 (DT: 1.03 day), no significant difference in growth rate was found (Figure 1).
Figure 1.

Growth curves of urothelial carcinoma cell lines; 4000 cells per cm2 were seeded in cell culture flasks at day 0 containing IMDM supplemented with 10% FCS. Cell counts were determined using a Neubauer chamber in the presence of trypan blue. Values are displayed as mean ± SD. *P ≤ .05 relative to parental cell line.
Cross-Resistance Profiles
The effects of a panel of 16 anticancer drugs were determined on the viability of all 18 urothelial cancer cell lines by MTT assay. Cisplatin-resistant cell lines showed resistance factors to cisplatin (IC50 resistant cell line/IC50 parental cell line) ranging from 2.78 (HT1376rCDDP1000) to 28.86 (TCC-SUPrCDDP1000). Gemcitabine-resistant cell lines displayed resistance factors to gemcitabine ranging from 7.11 (RT4rGEMCI10) to 73.28 (RT112rGEMCI20) relative to parental cell lines (Suppl. Table 1).
The effects of a panel of 16 anticancer drugs were determined on the viability of all 18 urothelial cancer cell lines by MTT assay. Cisplatin-resistant cell lines showed resistance factors to cisplatin (IC50 resistant cell line/IC50 parental cell line) ranging from 2.78 (HT1376rCDDP1000) to 28.86 (TCC-SUPrCDDP1000). Gemcitabine-resistant cell lines displayed resistance factors to gemcitabine ranging from 7.11 (RT4rGEMCI10) to 73.28 (RT112rGEMCI20) relative to parental cell lines (Suppl. Table 1).
The parental cell line panel included cell lines derived from low-risk carcinomas (RT4 and 5637) rarely requiring systemic chemotherapy in vivo [22,23] and those from high-risk carcinomas (HT1376, RT112, T24, and TCC-SUP) that are commonly treated by chemotherapy when metastasized. Gemcitabine-resistant sublines of the low-risk carcinoma cell lines RT4 and 5637 showed resistance to three of the investigated 16 anticancer drugs (RT4rGEMCI10: gemcitabine, 5-fluorouracil, and carboplatin; 5637rGEMCI20: gemcitabine, methotrexate, and pemetrexed). Cisplatin-resistant sublines were resistant to three (RT4rCDDP1000: cisplatin, carboplatin, and 5-fluorouracil) and four (5637rCDDP1000: cisplatin, carboplatin, methotrexate, and topotecan) of the 16 drugs (Suppl. Table 1).
The parental cell line panel included cell lines derived from low-risk carcinomas (RT4 and 5637) rarely requiring systemic chemotherapy in vivo [22,23] and those from high-risk carcinomas (HT1376, RT112, T24, and TCC-SUP) that are commonly treated by chemotherapy when metastasized. Gemcitabine-resistant sublines of the low-risk carcinoma cell lines RT4 and 5637 showed resistance to three of the investigated 16 anticancer drugs (RT4rGEMCI10: gemcitabine, 5-fluorouracil, and carboplatin; 5637rGEMCI20: gemcitabine, methotrexate, and pemetrexed). Cisplatin-resistant sublines were resistant to three (RT4rCDDP1000: cisplatin, carboplatin, and 5-fluorouracil) and four (5637rCDDP1000: cisplatin, carboplatin, methotrexate, and topotecan) of the 16 drugs (Suppl. Table 1).
Gemcitabine-resistant sublines derived from high-risk urothelial carcinoma RT112, T24, HT1376, and TCC-SUP showed resistance to up to 11 of the tested substances of the 16 anticancer agents. Cisplatin-resistant sublines of RT112, T24, HT1376, and TCC-SUP were cross-resistant to up to five agents. In summary, most pronounced cross-resistance to other chemotherapeutic agents was observed in gemcitabine-resistant sublines of high-risk urothelial carcinoma cells (Suppl. Table 1).
Gemcitabine-resistant sublines derived from high-risk urothelial carcinoma RT112, T24, HT1376, and TCC-SUP showed resistance to up to 11 of the tested substances of the 16 anticancer agents. Cisplatin-resistant sublines of RT112, T24, HT1376, and TCC-SUP were cross-resistant to up to five agents. In summary, most pronounced cross-resistance to other chemotherapeutic agents was observed in gemcitabine-resistant sublines of high-risk urothelial carcinoma cells (Suppl. Table 1).
Moreover, resistance profiles differed among the investigated drugs. Eight of 12 investigated resistant cell lines were cross-resistant to carboplatin, 5 of 12 to pemetrexed, 4 of 12 to methotrexate, 3 of 12 to oxaliplatin, 5-fluorouracil, and paclitaxel, and 2 of 12 to cabazitaxel, larotaxel, docetaxel, topotecan, doxorubicin, and mitomycin c. Only one resistant cell line (TCC-SUPrGEMCI20) was cross-resistant to vinflunine and vinblastine (Figure 2 and Suppl. Table 1).
Figure 2.
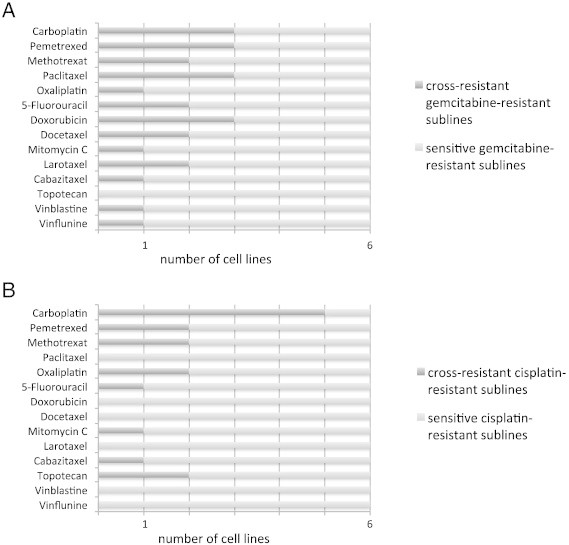
(A) The number of gemcitabine-resistant sublines that displayed cross-resistance to additional anticancer drugs is presented. Cross-resistance was defined as IC50 (as determined by MTT assay) resistant subline/IC50 respective parental cell line > 2. (B) Cisplatin-resistant sublines that displayed cross-resistance to additional anticancer drugs.
Moreover, resistance profiles differed among the investigated drugs. Eight of 12 investigated resistant cell lines were cross-resistant to carboplatin, 5 of 12 to pemetrexed, 4 of 12 to methotrexate, 3 of 12 to oxaliplatin, 5-fluorouracil, and paclitaxel, and 2 of 12 to cabazitaxel, larotaxel, docetaxel, topotecan, doxorubicin, and mitomycin c. Only one resistant cell line (TCC-SUPrGEMCI20) was cross-resistant to vinflunine and vinblastine (Figure 2 and Suppl. Table 1).
ABCB1 Expression in Drug-Resistant Urothelial Carcinoma Cell Lines
Since ABCB1 overexpression is a major mechanism of resistance to chemotherapy, we evaluated its role in this model of urothelial bladder cancer cell lines with acquired drug resistance. TCC-SUPrGEMCI20 and T24rGEMCI20 cells showed cross-resistance to ABCB1 substrates docetaxel, paclitaxel, and doxorubicin (Suppl. Table 1). The ABCB1 inhibitor zosuquidar sensitized TCC-SUPrGEMCI20 cells to vinflunine and vinblastine but not to gemcitabine (Suppl. Table 2). Vinflunine was described to be a weaker ABCB1 substrate than other vinca alkaloids [24]. In accordance, the relative resistance IC50 TCC-SUPrGEMCI20/IC50 TCC-SUP and IC50 T24rGEMCI20/IC50 T24 was lower for vinflunine than for vinblastine (Suppl. Tables 1–3).
Since ABCB1 overexpression is a major mechanism of resistance to chemotherapy, we evaluated its role in this model of urothelial bladder cancer cell lines with acquired drug resistance. TCC-SUPrGEMCI20 and T24rGEMCI20 cells showed cross-resistance to ABCB1 substrates docetaxel, paclitaxel, and doxorubicin (Suppl. Table 1). The ABCB1 inhibitor zosuquidar sensitized TCC-SUPrGEMCI20 cells to vinflunine and vinblastine but not to gemcitabine (Suppl. Table 2). Vinflunine was described to be a weaker ABCB1 substrate than other vinca alkaloids [24]. In accordance, the relative resistance IC50 TCC-SUPrGEMCI20/IC50 TCC-SUP and IC50 T24rGEMCI20/IC50 T24 was lower for vinflunine than for vinblastine (Suppl. Tables 1–3).
R123 is a fluorescent dye that is transported by ABCB1. A flow cytometric assay with R123 was used to determine functional activity of ABCB1 [25]. Flow cytometry indicated a strong increase of R123 fluorescence after treatment with zosuquidar in TCC-SUPrGEMCI20, TCC-SUPrVBL20, TCC-SUPABCB1, T24rGEMCI20, T24rVBL20, and T24ABCB1 cells compared to cell lines that served as a control (Figures 3 and 4).
Figure 3.
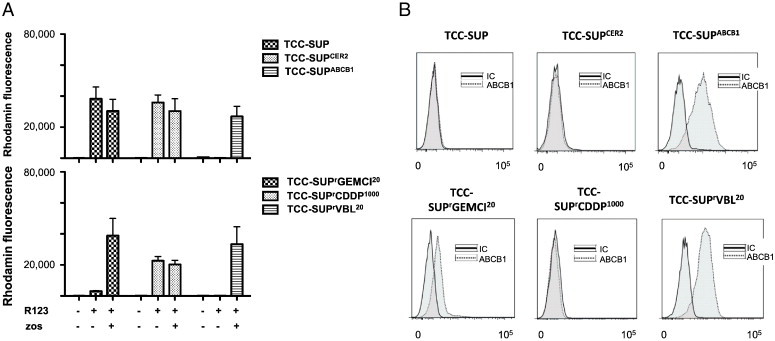
(A) R123 fluorescence of TCC-SUP, TCC-SUPCER2, TCC-SUPABCB1, TCC-SUPrGEMCI20, TCC-SUPrCDDP1000, and TCC-SUPrVBL20 cells after staining with R123 alone and in combination with 1.25 μM zosuquidar. Values are means ± SD. (B) ABCB1 expression in TCC-SUP, TCC-SUPCER2, TCC-SUPABCB1, TCC-SUPrGEMCI20, TCC-SUPrCDDP1000, and TCC-SUPrVBL20 cells. IC, isotype control. *P ≤ .05 relative to parental cell line.
Figure 4.

(A) R123 fluorescence of T24, T24CER2, T24ABCB1, T24rGEMCI20, T24rCDDP1000, and T24rVBL20 cells after staining of R123 alone and in combination with 1.25 μM zosuquidar. Values are means ± SD. (B) ABCB1 expression in T24, T24CER2, T24ABCB1, T24rGEMCI20, T24rCDDP1000, and T24rVBL20 cells. *P ≤ .05 relative to parental cell line.
Next, we compared ABCB1 expression and drug sensitivity profiles in TCC-SUP and T24, TCC-SUPrGEMCI20 and T24rGEMCI20, TCC-SUPABCB1 and T24ABCB1 (TCC-SUP and T24 cells transduced with a lentiviral vector encoding for ABCB1), TCC-SUPCER2 and T24CER2 (TCC-SUP and T24 cells transduced with a control vector), and TCC-SUPrVBL20 and T24rVBL20 (TCC-SUP and T24 cells with acquired resistance to vinblastine, ABCB1 substrate). Successful transduction of the ABCB1 encoding plasmid in TCC-SUPABCB1 and T24ABCB1 cells was verified by a significant increase of ABCB1 expression compared to cells transduced with a control vector (Figures 3 and 4). Compared to TCC-SUPABCB1, T24ABCB1, TCC-SUPrVBL20, and T24rVBL20, gemcitabine-resistant sublines of TCC-SUP and T24 cells showed lower ABCB1 expression (Figures 3 and 4). Zosuquidar sensitized ABCB1-expressing cell lines to ABCB1 substrates with exemption of T24rGEMCI20 cells (Suppl. Tables 2 and 3).
Next, we compared ABCB1 expression and drug sensitivity profiles in TCC-SUP and T24, TCC-SUPrGEMCI20 and T24rGEMCI20, TCC-SUPABCB1 and T24ABCB1 (TCC-SUP and T24 cells transduced with a lentiviral vector encoding for ABCB1), TCC-SUPCER2 and T24CER2 (TCC-SUP and T24 cells transduced with a control vector), and TCC-SUPrVBL20 and T24rVBL20 (TCC-SUP and T24 cells with acquired resistance to vinblastine, ABCB1 substrate). Successful transduction of the ABCB1 encoding plasmid in TCC-SUPABCB1 and T24ABCB1 cells was verified by a significant increase of ABCB1 expression compared to cells transduced with a control vector (Figures 3 and 4). Compared to TCC-SUPABCB1, T24ABCB1, TCC-SUPrVBL20, and T24rVBL20, gemcitabine-resistant sublines of TCC-SUP and T24 cells showed lower ABCB1 expression (Figures 3 and 4). Zosuquidar sensitized ABCB1-expressing cell lines to ABCB1 substrates with exemption of T24rGEMCI20 cells (Suppl. Tables 2 and 3).
Discussion
In this study, we established a panel of urothelial cancer cell lines with acquired resistance to gemcitabine or cisplatin, the standard therapeutics for patients with metastasized urothelial cancer of the bladder [1].
First, we compared tumor cell growth differences, since rapidly dividing tumor cells might be more vulnerable to chemotherapy. There was no consistent correlation between cell growth kinetics and drug sensitivity in the investigated cell lines. This suggests that the cell growth kinetics are not critical for the drug response in our models.
The most effective compounds among our anticancer drug panel were vinflunine and vinblastine, since most gemcitabine- or cisplatin-resistant cell lines were still sensitive to these drugs. Notably, vinflunine was approved by the European Medicines Agency for second-line treatment of urothelial bladder cancer on the basis of a survival advantage of 2.4 months over best supportive care [26]. Vinblastine is a constituent of methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC), an alternative therapy protocol for metastasized urothelial cancer [27]. This suggests that testing of drug candidates in drug-resistant cell lines holds potential for identification of next-line therapies.
Several studies demonstrated ABCB1 overexpression in tumor cells established from different human carcinomas (e.g., ovarian, stomach, colon) after adaptation to cisplatin [28–30]. Since cisplatin is not an ABCB1 substrate, this is probably the consequence of a long-term non-specific stress response [31,32]. Interestingly, only gemcitabine- but not cisplatin-resistant cells displayed ABCB1 up-regulation in our study, being to our knowledge the first report describing gemcitabine-induced ABCB1 up-regulation in urothelial tumor cells. Thus, ABCB1 expression may affect the efficacy of candidate drugs for second-line therapies of urothelial cancer after GC failure. In addition, ABCB1 may also modulate the malignant properties of cancer cells (e.g., cell survival, cell proliferation, and cell invasion) independently of the transporter-mediated drug efflux [4,33]. Indeed, ABCB1 expression correlated with an advanced tumor grade or with an increasing risk of recurrence in urothelial carcinoma patients [7,9]. Therefore, it will be important to show to which extent GC treatment is associated to an increased ABCB1 expression.
Cabazitaxel is a taxane that was recently approved for treatment of castration-resistant prostate cancer [34]. Currently, two clinical trials that investigate cabazitaxel in advanced bladder cancer are ongoing (NCT01616875 and NCT01668459). In contrast to taxanes including paclitaxel and docetaxel that have been used for decades, cabazitaxel is a weaker ABCB1 substrate [34]. In accordance, ABCB1-expressing TCC-SUPrGEMCI20 cells remained sensitive to cabazitaxel. Interestingly, cabazitaxel's resistance profile differed from those of the other three taxanes in our study. TCC-SUPrGEMCI20 and T24rGEMCI20 cells displayed cross-resistance to docetaxel, paclitaxel, and larotaxel, another taxane under clinical evaluation [35]. Moreover, HT1376rGEMCI20 cells were cross-resistant to larotaxel and paclitaxel. In contrast, RT112rGEMCI20 and RT112rCDDP1000 cells showed cross-resistance to cabazitaxel. Our results show how complex the effects of apparently closely related compounds can be and that cancer cell line panels are suitable to identify such differences.
Among platinum derivates, cisplatin and carboplatin are thought to share a very similar mode of anticancer action [36,37]. In urothelial carcinoma, cisplatin was found to be superior to carboplatin in a randomized phase 2 study [38]. The European Association of Urology recommends carboplatin as a less toxic alternative in patients that are unfit for cisplatin because of bad performance status or elevated creatinine levels [39]. In concert with the anticipated closely related mechanisms of action of cisplatin and carboplatin [36,37], five of six cisplatin-resistant cell lines were also resistant to carboplatin. Interestingly, also three of six gemcitabine-resistant sublines displayed decreased sensitivity to cisplatin (RT112rGEMCI20, TCC-SUPrGEMCI20, and T24rGEMCI20), and additionally, three of six gemcitabine-resistant sublines showed reduced sensitivity to carboplatin (RT112rGEMCI20, TCC-SUPrGEMCI20, and RT4rGEMCI10). Therefore, cross-resistance against cisplatin and carboplatin seems to be common after gemcitabine resistance.
Oxaliplatin supposedly differs in its anticancer mechanism of action from those exerted by cisplatin and carboplatin [40]. In concordance with this, oxaliplatin differed clearly in its activity profile from the cisplatin and carboplatin efficacy patterns. Only one gemcitabine- (TCC-SUPrGEMCI20) and two cisplatin-resistant cell lines (RT112rCDDP1000 and TCC-SUPrCDDP1000) showed cross-resistance to oxaliplatin. In this context, gemcitabine/oxaliplatin combination therapy was suggested as an alternative for urothelial cancer patients unfit for cisplatin [41,42].
Conclusions
Here, we established a novel panel of gemcitabine- and cisplatin-resistant urothelial cancer cell lines. Cross-resistance profiles identified vinflunine, the European Medicines Agency–approved second-line therapeutic for urothelial cancer, together with vinblastine as the most effective drugs. This emphasizes the potential of panels of cancer cell lines with acquired drug resistance as preclinical models for the identification of potential next-line therapies after treatment failure. Notably, ABCB1 expression was detected in two gemcitabine-resistant cell lines although gemcitabine is not an ABCB1 substrate. The clinical relevance of these findings needs to be further investigated. Larger cell line panels may better reflect the complex processes of resistance formation in urothelial cancer cells [15]. Thus, the panel of drug-resistant urothelial carcinoma cell lines will be further expanded and characterized during ongoing research.
The following are the supplementary data related to this article.
Concentrations of 16 Different Cytotoxic Substances that Reduce Urothelial Cancer Cell Viability by 50% (IC50)
Concentrations of Docetaxel, Paclitaxel, Vinblastine, Vinflunine, Cabazitaxel, Larotaxel, Doxorubicin, Methotrexate, and Gemcitabine that Reduce TCC-SUP, TCC-SUPrGEMCI20, TCC-SUPrVBL20, TCC-SUPCER2, and TCC-SUPABCB1 Cell Viability by 50% (IC50)
Concentrations of Docetaxel, Paclitaxel, Vinblastine, Vinflunine, Cabazitaxel, Larotaxel, Doxorubicin, Methotrexate, and Gemcitabine that Reduce T24, T24rGEMCI20, T24rVBL20, T24CER2, and T24ABCB1 Cell Viability by 50% (IC50)
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.tranon.2015.04.002.
Footnotes
This work was supported by the charity Hilfe für krebskranke Kinder Frankfurt e.V., its trust Frankfurter Stiftung für krebskranke Kinder, the Patenschaftsmodell programme of the University Hospital Frankfurt, and the Kent Cancer Trust. Conflict of interest statement: None of the authors has any financial or other interest with regard to the submitted manuscript that might be constructed as a conflict of interest.
This article refers to supplementary materials, which are designated by Suppl. Tables 1 to 3 and are available online at www.transonc.com.
References
- 1.von der Maase H, Sengelov L, Roberts JT, Ricci S, Dogliotti L, Oliver T, Moore MJ, Zimmermann A, Arning M. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23:4602–4608. doi: 10.1200/JCO.2005.07.757. [DOI] [PubMed] [Google Scholar]
- 2.Pectasides D, Pectasides M, Economopoulos T. Systemic chemotherapy in locally advanced and/or metastatic bladder cancer. Cancer Treat Rev. 2006;32:456–470. doi: 10.1016/j.ctrv.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Stordal B, Hamon M, McEneaney V, Roche S, Gillet JP, O'Leary JJ, Gottesman M, Clynes M. Resistance to paclitaxel in a cisplatin-resistant ovarian cancer cell line is mediated by P-glycoprotein. PLoS One. 2012;7:e40717. doi: 10.1371/journal.pone.0040717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breier A, Gibalova L, Seres M, Barancik M, Sulova Z. New insight into p-glycoprotein as a drug target. Anticancer Agents Med Chem. 2013;13:159–170. [PubMed] [Google Scholar]
- 5.Petrylak DP, Scher HI, Reuter V, O'Brien JP, Cordon-Cardo C. P-glycoprotein expression in primary and metastatic transitional cell carcinoma of the bladder. Ann Oncol. 1994;5:835–840. doi: 10.1093/oxfordjournals.annonc.a059013. [DOI] [PubMed] [Google Scholar]
- 6.Kakehi Y, Wu WJ, Kim WJ, Arao S, Fukumoto M, Yoshida O. Comparison of multidrug resistance gene expression levels with malignant potentials and influence of chemotherapy in urothelial cancers. Int J Urol. 1995;2:309–315. [PubMed] [Google Scholar]
- 7.Chen Z, Zhang Y, Zhang X, Du G, Yang W, Hu Z, Li J, Zhang Y. Expression of multidrug-associated protein, P-glycoprotein, P53 and Bcl-2 proteins in bladder cancer and clinical implication. J Tongji Med Univ. 2001;21:56–58. doi: 10.1007/BF02888038. [DOI] [PubMed] [Google Scholar]
- 8.Hour TC, Chen J, Huang CY, Guan JY, Lu SH, Hsieh CY, Pu YS. Characterization of chemoresistance mechanisms in a series of cisplatin-resistant transitional carcinoma cell lines. Anticancer Res. 2000;20:3221–3225. [PubMed] [Google Scholar]
- 9.Serretta V, Pavone C, Allegro R, Vella M, Sanguedolce R, Porcasi R, Morello V, Tomasino RM, Pavone-Macaluso M. Correlation between GP-170 expression, prognosis, and chemoresistance of superficial bladder carcinoma. J Cancer Res Clin Oncol. 2003;129:472–476. doi: 10.1007/s00432-003-0455-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffmann AC, Wild P, Leicht C, Bertz S, Danenberg KD, Danenberg PV, Stöhr R, Stöckle M, Lehmann J, Schuler M. MDR1 and ERCC1 expression predict outcome of patients with locally advanced bladder cancer receiving adjuvant chemotherapy. Neoplasia. 2010;12:628–636. doi: 10.1593/neo.10402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rioja J, Bandrés E, Rosell Costa D, Rincón A, López I, Zudaire Bergera JJ, García Foncillas J, Gil MJ, Panizo A, Plaza L. Association of steroid and xenobiotic receptor (SXR) and multidrug resistance 1 (MDR1) gene expression with survival among patients with invasive bladder carcinoma. BJU Int. 2010;107:1833–1838. doi: 10.1111/j.1464-410X.2010.09653.x. [DOI] [PubMed] [Google Scholar]
- 12.Aparicio S, Caldas C. The implications of clonal genome evolution for cancer medicine. N Engl J Med. 2013;368:842–851. doi: 10.1056/NEJMra1204892. [DOI] [PubMed] [Google Scholar]
- 13.Crockford A, Jamal-Hanjani M, Hicks J, Swanton C. Implications of intratumour heterogeneity for treatment stratification. J Pathol. 2014;232:264–273. doi: 10.1002/path.4270. [DOI] [PubMed] [Google Scholar]
- 14.Pantel K, Alix-Panabières C. Real-time liquid biopsy in cancer patients: fact or fiction? Cancer Res. 2013;73:6384–6388. doi: 10.1158/0008-5472.CAN-13-2030. [DOI] [PubMed] [Google Scholar]
- 15.Sharma SV, Haber DA, Settleman J. Cell line-based platforms to evaluate the therapeutic efficacy of candidate anticancer agents. Nat Rev Cancer. 2010;10:241–253. doi: 10.1038/nrc2820. [DOI] [PubMed] [Google Scholar]
- 16.Domingo-Domenech J, Vidal SJ, Rodriguez-Bravo V, Castillo-Martin M, Quinn SA, Rodriguez-Barrueco R, Bonal DM, Charytonowicz E, Gladoun N, de la Iglesia-Vicente J. Suppression of acquired docetaxel resistance in prostate cancer through depletion of notch- and hedgehog-dependent tumor-initiating cells. Cancer Cell. 2012;22:373–388. doi: 10.1016/j.ccr.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rothweiler F, Michaelis M, Brauer P, Otte J, Weber K, Fehse B, Doerr HW, Wiese M, Kreuter J, Al-Abed Y. Anticancer effects of the nitric oxide-modified saquinavir derivative saquinavir-NO against multidrug-resistant cancer cells. Neoplasia. 2010;12:1023–1030. doi: 10.1593/neo.10856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weber K, Thomaschewski M, Benten D, Fehse B. RGB marking with lentiviral vectors for multicolor clonal cell tracking. Nat Protoc. 2012;5:839–849. doi: 10.1038/nprot.2012.026. [DOI] [PubMed] [Google Scholar]
- 19.Rutigliano L, Corradetti B, Valentini L, Bizzaro D, Meucci A, Cremonesi F, Lange-Consiglio A. Molecular characterization and in vitro differentiation of feline progenitor-like amniotic epithelial cells. Stem Cell Res Ther. 2013;30:133. doi: 10.1186/scrt344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michaelis M, Rothweiler F, Barth S, Cinatl J, van Rikxoort M, Löschmann N, Voges Y, Breitling R, von Deimling A, Rödel F. Adaptation of cancer cells from different entities to the MDM2 inhibitor nutlin-3 results in the emergence of p53-mutated multi-drug-resistant cancer cells. Cell Death Dis. 2011;2:e243. doi: 10.1038/cddis.2011.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rohde D, Brehmer B, Kapp T, Valdor M, Jakse G. Induction of drug-resistant bladder carcinoma cells in vitro: impact on polychemotherapy with cisplatin, methotrexate and vinblastine (CMV) Urol Res. 1998;26:249–257. doi: 10.1007/s002400050053. [DOI] [PubMed] [Google Scholar]
- 22.Rigby CC, Franks LM. A human tissue culture cell line from a transitional cell tumour of the urinary bladder: growth, chromosone pattern and ultrastructure. Br J Cancer. 1970;24:746–754. doi: 10.1038/bjc.1970.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee YG, Macoska JA, Korenchuk S, Pienta KJ. MIM, a potential metastasis suppressor gene in bladder cancer. Neoplasia. 2002;4:291–294. doi: 10.1038/sj.neo.7900231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Etievant C, Barret JM, Kruczynski A, Perrin D, Hill BT. Vinflunine (20′,20′-difluoro-3′,4′-dihydrovinorelbine), a novel vinca alkaloid, which participates in P-glycoprotein (Pgp)-mediated multidrug resistance in vivo and in vitro. Invest New Drugs. 1998;16:3–17. doi: 10.1023/a:1006022811895. [DOI] [PubMed] [Google Scholar]
- 25.van der Kolk DM, de Vries EG, van Putten WJ, Verdonck LF, Ossenkoppele GJ, Verhoef GE, Vellenga E. P-glycoprotein and multidrug resistance protein activities in relation to treatment outcome in acute myeloid leukemia. Clin Cancer Res. 2000;6:3205–3214. [PubMed] [Google Scholar]
- 26.Gerullis H. Vinflunine: a fluorinated vinca alkaloid for bladder cancer therapy. Drugs Today (Barc) 2011;47:17–25. doi: 10.1358/dot.2011.47.1.1576693. [DOI] [PubMed] [Google Scholar]
- 27.Bamias A, Dafni U, Karadimou A, Timotheadou E, Aravantinos G, Psyrri A, Xanthakis I, Tsiatas M, Koutoulidis V, Constantinidis C. Prospective, open-label, randomized, phase III study of two dose-dense regimens MVAC versus gemcitabine/cisplatin in patients with inoperable, metastatic or relapsed urothelial cancer: a Hellenic Cooperative Oncology Group study (HE 16/03) Ann Oncol. 2013;24:1011–1017. doi: 10.1093/annonc/mds583. [DOI] [PubMed] [Google Scholar]
- 28.Yang LY, Trujillo JM, Siciliano MJ, Kido Y, Siddik ZH, Su YZ. Distinct P-glycoprotein expression in two subclones simultaneously selected from a human colon carcinoma cell line by cis-diamminedichloroplatinum (II) Int J Cancer. 1993;53:478–485. doi: 10.1002/ijc.2910530321. [DOI] [PubMed] [Google Scholar]
- 29.Yang X, Pagé M. P-glycoprotein expression in ovarian cancer cell line following treatment with cisplatin. Oncol Res. 1995;7:619–624. [PubMed] [Google Scholar]
- 30.Xu H, Choi SM, An CS, Min YD, Kim KC, Kim KJ, Choi CH. Concentration-dependent collateral sensitivity of cisplatin-resistant gastric cancer cell sublines. Biochem Biophys Res Commun. 2005;11:618–622. doi: 10.1016/j.bbrc.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 31.Hamaguchi K, Godwin AK, Yakushiji M, O'Dwyer PJ, Ozols RF, Hamilton TC. Cross-resistance to diverse drugs is associated with primary cisplatin resistance in ovarian cancer cell lines. Cancer Res. 1993;53:5225–5232. [PubMed] [Google Scholar]
- 32.Stordal B, Hamon M, McEneaney V, Roche S, Gillet JP, O'Leary JJ, Gottesman M, Clynes M. Resistance to paclitaxel in a cisplatin-resistant ovarian cancer cell line is mediated by P-glycoprotein. PLoS One. 2012;7:e40717. doi: 10.1371/journal.pone.0040717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fletcher JI, Haber M, Henderson MJ, Norris MD. ABC transporters in cancer: more than just drug efflux pumps. Nat Rev Cancer. 2013;10:147–156. doi: 10.1038/nrc2789. [DOI] [PubMed] [Google Scholar]
- 34.Bouchet BP, Galmarini CM. Cabazitaxel, a new taxane with favorable properties. Drugs Today (Barc) 2010;46:735–742. doi: 10.1358/dot.2010.46.10.1519019. [DOI] [PubMed] [Google Scholar]
- 35.Sternberg CN, Skoneczna IA, Castellano D, Theodore C, Blais N, Voog E, Bellmunt J, Peters F, Le-Guennec S, Cerbone L. Larotaxel with Cisplatin in the first-line treatment of locally advanced/metastatic urothelial tract or bladder cancer: a randomized, active-controlled, phase III trial (CILAB) Oncology. 2013;85:208–215. doi: 10.1159/000354085. [DOI] [PubMed] [Google Scholar]
- 36.Rixe O, Ortuzar W, Alvarez M, Parker R, Reed E, Paull K, Fojo T. Oxaliplatin, tetraplatin, cisplatin, and carboplatin: spectrum of activity in drug-resistant cell lines and in the cell lines of the National Cancer Institute's Anticancer Drug Screen panel. Biochem Pharmacol. 1996;52:1855–1865. doi: 10.1016/s0006-2952(97)81490-6. [DOI] [PubMed] [Google Scholar]
- 37.Heffeter P, Jungwirth U, Jakupec M, Hartinger C, Galanski M, Elbling L, Micksche M, Keppler B, Berger W. Resistance against novel anticancer metal compounds: differences and similarities. Drug Resist Updat. 2008;11:1–16. doi: 10.1016/j.drup.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 38.Dogliotti L, Carteni G, Siena S, Bertetto O, Martoni A, Bono A, Amadori D, Onat H, Marini L. Gemcitabine plus cisplatin versus gemcitabine plus carboplatin as first-line chemotherapy in advanced transitional cell carcinoma of the urothelium: results of a randomized phase II trial. Eur Urol. 2007;52:134–141. doi: 10.1016/j.eururo.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 39.Stenzl A, Cowan NC, De Santis M, Kuczyk MA, Merseburger AS, Ribal MJ, Sherif A, Witjes JA. Treatment of muscle-invasive and metastatic bladder cancer: update of the EAU guidelines. Eur Urol. 2011;59:1009–1018. doi: 10.1016/j.eururo.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 40.Burger H, Loos WJ, Eechoute K, Verweij J, Mathijssen RH, Wiemer EA. Drug transporters of platinum-based anticancer agents and their clinical significance. Drug Resist Updat. 2011;14:22–34. doi: 10.1016/j.drup.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 41.Eroglu Z, Fruehauf JP. A phase II study of gemcitabine and oxaliplatin in advanced transitional cell carcinoma of the bladder. Cancer Chemother Pharmacol. 2013;72:263–267. doi: 10.1007/s00280-013-2178-x. [DOI] [PubMed] [Google Scholar]
- 42.Carles J, Esteban E, Climent M, Font A, Gonzalez-Larriba JL, Berrocal A, Garcia-Ribas I, Marfa X, Fabregat X, Albanell J. Gemcitabine and oxaliplatin combination: a multicenter phase II trial in unfit patients with locally advanced or metastatic urothelial cancer. Ann Oncol. 2007;18:1359–1362. doi: 10.1093/annonc/mdm160. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Concentrations of 16 Different Cytotoxic Substances that Reduce Urothelial Cancer Cell Viability by 50% (IC50)
Concentrations of Docetaxel, Paclitaxel, Vinblastine, Vinflunine, Cabazitaxel, Larotaxel, Doxorubicin, Methotrexate, and Gemcitabine that Reduce TCC-SUP, TCC-SUPrGEMCI20, TCC-SUPrVBL20, TCC-SUPCER2, and TCC-SUPABCB1 Cell Viability by 50% (IC50)
Concentrations of Docetaxel, Paclitaxel, Vinblastine, Vinflunine, Cabazitaxel, Larotaxel, Doxorubicin, Methotrexate, and Gemcitabine that Reduce T24, T24rGEMCI20, T24rVBL20, T24CER2, and T24ABCB1 Cell Viability by 50% (IC50)


