Abstract
Dengue viruses (DENV1-4) cause 390 million clinical infections every year, several hundred thousand of which progress to severe hemorrhagic and shock syndromes. Preexisting immunity resulting from a previous DENV infection is the major risk factor for severe dengue during secondary heterologous infections. During primary infections in infants, maternal antibodies pose an analogous risk. At the same time, maternal antibodies are likely to prevent induction of endogenous anti-DENV antibodies in response to current live, attenuated virus (LAV) vaccine candidates. Any effective early life dengue vaccine has to overcome maternal antibody interference (leading to ineffective vaccination) and poor induction of antibody responses (increasing the risk of severe dengue disease upon primary infection). In a previous study, we demonstrated that a non-propagating Venezuelan equine encephalitis virus replicon expression vector (VRP), expressing the ectodomain of DENV E protein (E85), overcomes maternal interference in a BALB/c mouse model. We report here that a single immunization with a tetravalent VRP vaccine induced NAb and T-cell responses to each serotype at a level equivalent to the monovalent vaccine components, suggesting that this vaccine modality can overcome serotype interference. Furthermore, neonatal immunization was durable and could be boosted later in life to further increase NAb and T-cell responses. Although the neonatal immune response was lower in magnitude than responses in adult BALB/c mice, we demonstrate that VRP vaccines generated protective immunity from a lethal challenge after a single neonatal immunization. In summary, VRP vaccines expressing DENV antigens were immunogenic and protective in neonates, and hence are promising candidates for safe and effective vaccination in early life.
Introduction
The four serotypes of dengue virus (DENV) are the leading cause of the most important mosquito-borne viral disease worldwide, with annual estimates of approximately 390 million infections (1). The World Health Organization also estimates that up to half a million people are hospitalized with severe dengue disease (Dengue Hemorrhagic Fever/Dengue Shock Syndrome; DHF/DSS), and among them a large proportion are children (2). Children and adults are at increased risk of severe dengue upon a secondary infection with a different serotype. In addition, infants born to dengue immune mothers are at an increased risk of DHF/DSS during a primary infection, and account for more than 5% of all DHF cases (3, 4). This increased risk in infants seems to correlate with maternal antibody titers dropping to sub-neutralizing levels, and becoming potentially enhancing (3, 4). At present, there are no licensed dengue vaccines available, and the ones in development may not be effective in infants.
In addition to the challenges inherent to immunizing early in life, when the immune system is suboptimal, additional unique challenges are encountered in the development of dengue vaccines. (A) A dengue vaccine must be tetravalent (TV) and induce equivalent and durable neutralizing antibodies (NAbs) against all 4 serotypes simultaneously, due to the theoretical enhanced risk of severe disease if incomplete immunity is induced. (B) Serotype interference has been described among the components of some TV LAV vaccines in development. The dominant serotype prevents other serotype(s) from inducing adequate responses, resulting in incomplete immunity and the need for additional vaccinations over a one year period to achieve a tetravalent response (5). (C) In dengue endemic areas, most children are born with maternal antibodies (Abs) to DENV. These Abs protect in the first months, but also have the potential to interfere with and reduce the efficacy of LAV. Therefore, there is a need for early life vaccines that can induce balanced NAb responses after a single immunization given early in life, and that are not subject to maternal antibody interference.
Venezuelan equine encephalitis virus replicon particles (VRP) are non-propagating viral vectors that can express high levels of an antigen protein after a single round of replication. VRP-based vaccines expressing various antigens induced protective immunity in rodent models (6-13), and in non-human primates (NHP) (14, 15). A VRP-based dengue vaccine candidate is immunogenic and protective in adult mice and NHP (16, 17). Furthermore, VRP expressing DENV2 prME was immunogenic in weanling mice even in the presence of maternal antibodies that prevented immunization with live virus (16).
Here we hypothesize that the VRP vectors are well suited as an effective early life vaccine platform for dengue. First, VRP are propagation incompetent, and therefore safe. Second, VRP immunization is not dependent on vector propagation and amplification. Therefore, serotype interference is minimized as indicated by balanced responses to the 4 dengue serotypes in adult mice and non-human primates. Third, VRP induce strong Th1 immune responses, potentially overcoming one of the deficiencies in the neonatal immune response. And finally, since VRP contain no DENV antigens, maternal antibodies are less likely to interfere with the vaccine, as was demonstrated previously (16).
Materials & Methods
Cells and Viruses
Vero81, BHK-21, and C6/36 cells were obtained from the American Type Culture Collection (ATCC) and appropriately maintained as described previously (17).
WHO reference DENV strains were used in the neutralization assays: DENV1 WP, DENV2 S-16803, DENV3 CH53489, and DENV4 TVP-360 (provided by R. Putnak from WRAIR). The viruses were propagated no more than three times in C6/36 cells, titrated on Vero cells, and stored at -80°C. The mouse-adapted, neurovirulent New Guinea C (NGC) strain of DENV2 used for challenge studies (provided by the late Robert Shope, UTMB, Galveston, TX) was amplified no more than twice in C6/36 cells and stored at -80°C at a concentration of 1×107PFU/ml.
DV1-4 VRP Vaccines
The construction and production of VRP vectors expressing a C-terminal truncated protein representing 85% of the E protein (E85) from DENV1-4 as well as their source have been described in detail elsewhere (17).
Mice and immunizations
Pregnant and adult BALB/c mice were purchased from Charles River and were housed at the Global Vaccines Inc. or at the University of North Carolina. The animals were housed and cared for in accordance with the protocols approved by each facility's Institutional Animal Care and Use Committees. Pregnant mice were closely observed for date and approximate time of delivery. Adult mice (6-8 weeks old) or neonatal mice (7 days old) were anesthetized (only adults) and injected in both rear footpads with vaccine or diluent (5μl in each rear footpad.
Neutralization assay
Neutralizing antibody titers specific for DENV1-4 were quantified using a flow cytometry-based neutralization assay on Vero cells, as previously described (17). MAb 2H2 (binds prM protein) was used for intracellular staining of dengue-infected cells.
Surface and intracellular staining (ICS)
Mouse spleens were harvested, homogenized, and strained through 40μm cell strainers to get single cell suspensions. The CD8/CD4 T cell responses were elicited after splenocytes were cultured in RPMI-10 media containing brefeldin A (GolgiPlug, BD Biosciences) with the appropriate peptide pool (2 μg/ml) (see figure legends for detail) for 18 h at 37°C. Cells were stained for CD8 and CD4 (eBioscience) in PBS at 4°C, fixed and permeabilized in Cytofix/Cytoperm (BD). For ICS, fixed cells were stained with antibody specific for IFN-γ (eBioscience). Cells were analyzed on an Accuri™ C6 Flow Cytometer (BD). The final results were obtained after normalizing the value in each sample by subtracting the value obtained in mock-stimulated cells. Spleens harvested from GFP-VRP immunized mice were also included as control.
IFN-γ enzyme-linked immunospot (ELISPOT) assay
An IFN-γ ELISPOT assay was performed to measure DENV3 E-specific IFN-γ-secreting cells in the popliteal draining lymph nodes of neonatally immunized mice. Mouse IFN-γ capture antibody (R&D Systems) was incubated on nitrocellulose membrane plates (96 well; Millipore) overnight at 4°C. Single cell suspensions in RPMI-10 (2.5×105 cells per well) from popliteal DLN were added after washing the plates. Peptides (see figure legends for detail) were added at final concentration of 2 μg/ml for 36 h. IFN-γ spots were developed according to manufacturer's protocol (R&D Systems) and the plates scanned by Cellular Technologies Ltd. [Shaker Heights, OH].
Challenge
Survival after DENV challenge was studied in mice three weeks after MV and TV neonatal immunization. Immunized and PBS control mice were anesthetized and challenged by intracranial injection with 5×104 PFU of mouse-adapted strain DENV2 NGC virus. Mice wereobserved for 25 days for weight change, morbidity, and mortality. Mice were euthanized if their weight dropped below 80% of their initial weight.
Statistical analysis
Neutralization titers and immune cell values were evaluated for statistically significant differences by either the ANOVA or Mann–Whitney tests (GraphPad Prism). Statistical significance is reported as follows (*, p < 0.05; **, p < 0.01; ***, p < 0.001).
Results
VRP expressing monovalent dengue E85 protein induces a sustained neutralizing antibody response after a single immunization
The kinetics of the Nab response after one immunization with DENV3 E85-VRP was compared in adult and neonatal mice. Adult and neonatal mice were given a single immunization with 1×106 IU of DV3-VRP. Mice were bled at 3, 6, and 13 (15 in neonates) weeks post immunization (wpi) (Figures 1A & 1B). A single immunization in both adult and neonatal mice induced a NAb response in all animals. In neonates, the NAb titers against DENV3 remained below the limit of detection at 3 wpi, increased and peaked at around 6 wpi and remained relatively stable for up to 15 wpi (last time-point tested). While induction of NAbs in adult mice showed similar kinetics, titers were significantly higher than those in neonates. A VRP vaccine expressing E85 from a serotype 2 (DV2-VRP) yielded serotype-specific NAbs in neonates with similar kinetics to DV3-VRP (data not shown).
Figure 1.
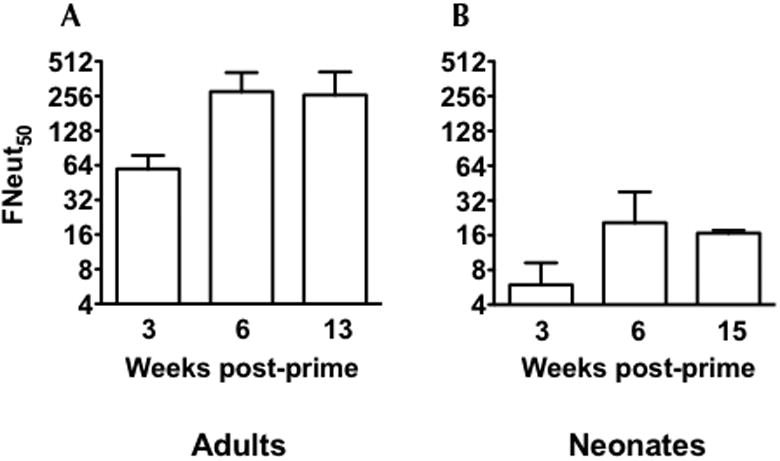
DENV3-VRP vaccine induces neutralizing antibody response after a single immunization in neonates and adult mice. Adult mice were anesthetized before immunization by intraperitoneal (i.p.) injection with a 4/1 mixture (vol/vol) of ketamine (50 μg/g body weight) and xylazine (15 μg/g body weight). (A) Six adult BALB/c mice (6 weeks old) were immunized with 1×106 IU of monovalent DV3 -VRP and neutralizing antibody titers were measured in the sera at 3, 6, and 13 weeks post-immunization. (B) Six neonatal mice were immunized on day 7 after birth with 1×106 IU of monovalent DV3 -VRP. Three, 6, and 15 weeks after the immunization, neutralization antibody titers were measured in the sera of the immunized mice. Note: There were only five samples for 6 weeks time-point. Data are presented as 50% neutralization titers (Neut50) (GMT +/- 95% CI). The limit of detection for this assay was 10.
Neutralizing antibody titers induced by monovalent (MV) and tetravalent (TV) vaccination
To determine whether each serotype VRP vaccine was immunogenic, and whether there was serotype interference among the 4 components in the TV formulation, adult and neonatal mice were immunized with each MV component separately or in the TV mix, and NAbs were measured. In adult mice, two immunizations with TV-VRP induced comparable NAb titers to each dengue serotype (Figure 2A-D). In addition, the NAb titers induced by each serotype when administered as MV vs. TV vaccine were similar (p>0.2). In neonates, a single immunization with TV vaccine induced NAbs to all 4 serotypes in every animal. Like in adult mice, NAb titers for serotypes 1, 2, and 3 (serotype 4 was lost to analysis) were not significantly different between the TV and MV formulations, (Figure 2E-H), suggesting that serotype interference was minimized when using the VRP vaccine approach.
Figure 2.
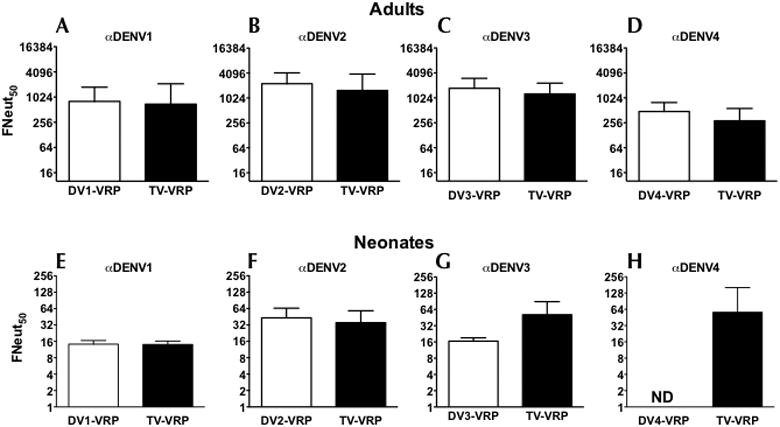
Serotype-specific Nab titers induced by each MV and TV vaccine in adult and neonatal mice. (A-D) Eight adult BALB/c mice per group were immunized and boosted (under anesthesia) 4 weeks later with 2×106 IU of each monovalent vaccine, DV1-VRP, DV2-VRP, DV3-VRP, or DV4-VRP or with a tetravalent cocktail of the above four VRP vaccines at 106 IU each. NAbs were determined at 3 weeks post boost. Graphs A-D represent Neut50 titers against serotypes 1-4 respectively. Each graph includes NAbs induced by the monovalent (white bars) or the tetravalent (black bars) vaccine. Data are presented as GMT +/- 95% CI. The limit of detection for this assay was 10. (p values: DENV1 = 0.59; DENV2 = 0.24; DENV3 = 0.24; DENV4 = 0.26). (E-H) Neonatal mice were immunized on day 7 after birth with 1×106 IU of either monovalent DV1-VRP (N=7), DV2-VRP (N=9) or DV3-VRP (N=6). A tetravalent immunization with 1×106 IU of each DV1-, DV2, DV3, and DV4-VRP expression vectors were administered to a group of 6 neonatal mice. Neut50 titers were determined at 15 weeks post immunization. (p values: DENV1 = 0.95; DENV2 = 0.52; DENV3 = 0.01). Monovalent DV4-VRP immunization data was lost to analysis (Figure 2H).
VRP induces T cell responses to DENV3 E protein in mice immunized with monovalent or tetravalent vaccine
To determine whether DENV envelope-specific T-cells are induced upon VRP immunization, adult mice were immunized with either 106 IU of DV3-VRP or a TV formulation with 106 IU each of the four serotypes. All mice were boosted with the homologous vaccine before harvesting spleens 8 days post-boost for ICS. In cells stimulated with a pool of DV3 E peptides, we observed IFNγ+ CD8+ T-cell responses against DENV3 envelope for both MV and TV immunizations, and these did not differ significantly, p=0.49 (Figure 3A). Furthermore, DENV3 E-specific IFNγ+ CD4+ T cells were induced and were not significantly different between MV and TV vaccines (p=0.07). In a separate experiment, a similar result was observed (data not shown).
Figure 3.
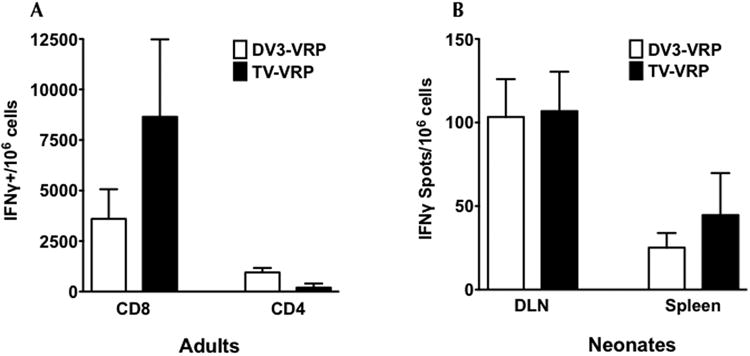
Induction of DENV E-specific cell-mediated immunity (CMI) after monovalent and tetravalent VRP immunization in adult and neonatal mice. (A) CD4+ and CD8+ T cell responses in adult mice. Adult BALB/c mice (4 per group) were immunized and boosted (under anesthesia) 4 weeks later with 106 IU of either monovalent DV3-VRP or a tetravalent cocktail of 106 IU each of DV1-VRP, DV2-VRP, DV3-VRP, and DV4-VRP (TV-VRP). Splenocytes were obtained 8 days after booster immunization and stimulated with peptide in vitro. Overlapping peptides derived from DENV3 E protein were used (68 DENV3 E peptides, BEI NR-9228), which were resuspended in DMSO and pooled. Cells were stained by ICS for IFNγ after surface staining of CD4 and CD8. Data were normalized to mock-stimulated splenocytes of each individual mouse. (B) CMI after a single immunization in neonatal mice. Six neonatal mice per group (five in tetravalent DLN group) were immunized with 106 IU of either monovalent DV3-VRP or a tetravalent cocktail of 106 IU each of DV1-VRP, DV2-VRP, DV3-VRP, and DV4-VRP (TV-VRP). At 8 days post immunization spleens and DLN were harvested. The two popliteal DLNs from each mouse were combined. For immune cell stimulation, 68 DENV3 E peptides obtained from BEI (NR-9228) were individually resuspended in DMSO, and pooled in three panels: A (peptides 1 to 23), B (peptides 24 to 46), and C (peptides 47 to 68). Unpublished observations in adult mice showed the highest immune response to E was in peptide pool B (includes amino acid sequence from 174 to 343), DLN immune cells were stimulated with E peptide pool B. IFNγ-producing cells were quantified by ELISPOT assay. Data were normalized to mock-stimulated DLN cells of each individual mouse. All results were graphed as mean +/- SEM.
Neonatal mice received a single immunization with 106 IU DV3-VRP (Figure 3B). DLN and spleens were harvested on 8 dpi. Due to the limited number of immune cells present in neonatal DLN, an IFNγ-ELISPOT assay was performed instead of ICS. We observed that both MV and TV immunizations induced similar levels of IFNγ-secreting cells in the neonatal DLN (p=0.93). Antigen-specific IFNγ-secreting cell numbers in the spleen were reduced; however, they were not significantly different for MV and TV immunized mice (p=0.94). Since the harvested immune cells were stimulated with a panel of pooled DENV3 envelope peptides, we infer that most, if not all, of the IFNγ secreting cells were antigen-specific T-cells.
Neonatal priming with VRP improves immune responses upon a later adult immunization
Three groups of mice were immunized on d7 post-birth, on d7 & d35, or only on d35. All mice received 106 IU DV3-VRP (Figure 4A). On d56, serum was collected for neutralization assays. Significantly higher Neut50 titers were observed in mice that had been primed as neonates and boosted as adults (GMT = 601) compared to immunization on d7 only (GMT = 95; p <0.05) The increase after the booster immunization was mostly due to the prime, mice immunized on d35 only had reduced NAb titers (GMT = 121; p=0.0519). A parallel experiment with DV2-VRP was set up to investigate the CD8 T-cell responses, in which the spleens were harvested on day 43. Using ICS, single cell suspensions of splenocytes were evaluated for the presence of IFNγ-secreting CD8 T-cells following stimulation with a panel of pooled DENV2 E-protein peptides. As seen for the NAb response, there was a significantly higher T-cell response in mice receiving two immunizations compared to those that only received immunization on d35 only or day 7 only (Figure 4B).
Figure 4.
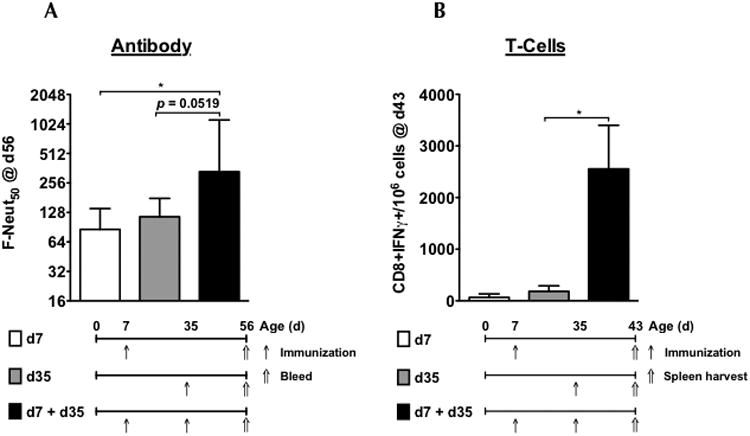
Neonatal immunization with VRP vaccine primes robust neutralizing antibody and T-cell responses. (A) Neutralizing antibody responses – Three groups of mice were immunized with 106 IU of DV3-VRP as follows: on day 7 after birth, groups 1 (n=6, white bars) and group 2 (n=6, black bars) received VRP vaccine, and group 3 (n=5, gray bars) received diluent (PBS + 0.1%HSA). On day 35, groups 2 and 3 received VRP vaccine, and group 1 received diluent (all under anesthesia). On day 56, all groups were bled and Neut50 titers vs. DENV2 were determined. Bars represent GMT +/- 95% CI. (B) T-cell responses – Three groups of mice were immunized with 106 IU of DV2-VRP as described in figure 4A. Groups 1 and 2 had 3 mice each and group 3 had 4 mice. On day 43 (8 days post boost) splenocytes from all mice were harvested followed by stimulation with DENV2 E-protein peptide cocktail (67 peptides, BEI NR-507), surface staining for CD8, and intracellular cytokine staining for IFNγ. Data were normalized to mock-stimulated splenocytes of each individual mouse, and expressed as number of CD8+ T cells per 106 cells. Results were graphed as mean +/- SEM. * indicates p<0.05.
A single neonatal immunization with the VRP-based vaccine confers protection from dengue challenge
Protection was determined using an intracranial DENV2 challenge model. Neonatal mice immunized with TV-VRP, DV2-VRP or PBS were challenged 3 weeks later with a mouse brain-adapted DENV2 strain. While all the PBS immunized mice succumbed to challenge by day 17, mice receiving either the MV DV2-VRP or the TV vaccine had significantly better protection with 5/6 (p=0.0024) and 4/6 (p=0.0018) mice surviving the challenge, respectively (Figure 5). There was no significant difference between the MV and TV immunized groups. In a separate experiment, weanling BALB/c mice (21 days old) vaccinated with 106 IU DV2-VRP were protected (8 out of 9 animals) from a lethal challenge at three weeks post-immunization. The control mice immunized with 106 IU HA-VRP (expressing an irrelevant influenza HA antigen) did not confer any protection (0/8) (supplementary figure 1).
Figure 5.
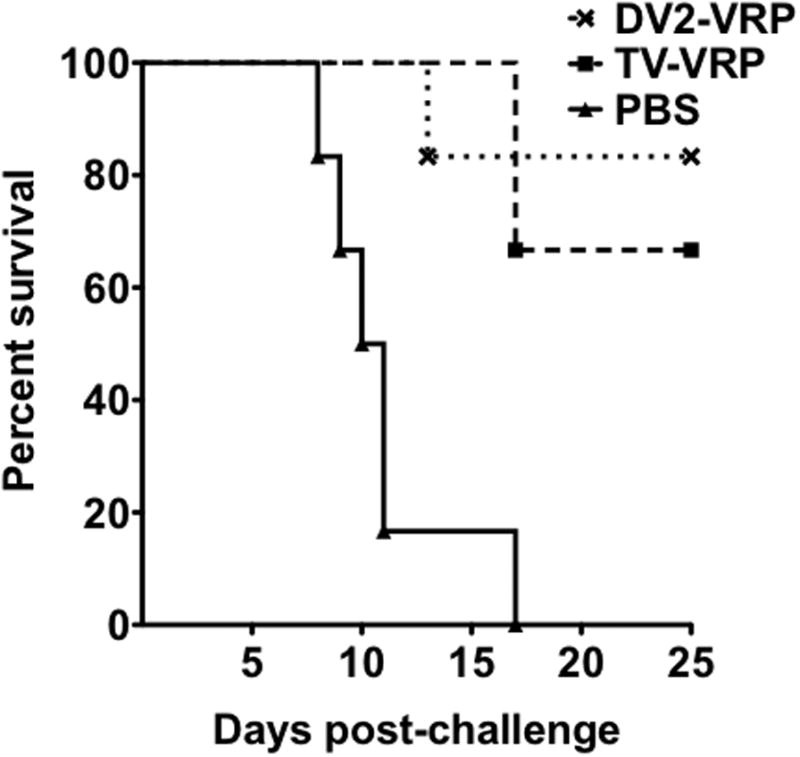
VRP expression vector immunization is protective in mice after a single immunization on day 7. Six 7-day-old neonatal BALB/c mice were immunized with 106 IU of either monovalent DV2-VRP or a tetravalent cocktail of 106 IU each of DV1-VRP, DV2-VRP, DV3-VRP, and DV4-VRP (TV-VRP). Six control neonatal mice were immunized with diluent. Three weeks after immunization, all mice were challenged intracranially (under anesthesia) with 5×104 PFU of a mouse-adapted, neurovirulent New Guinea C strain of dengue virus serotype 2. The mice were observed daily for 25 days after challenge. Results are presented as percent survival.
Discussion
There is currently no licensed vaccine for dengue, and the vaccine candidates in advanced clinical trials do not target children <12-15 months of age. Developing a dengue vaccine that can be administered during the first year of life is important for 2 reasons. First, in dengue endemic areas, most children are born with dengue maternal antibodies, which are protective during the first few months of life. However, these maternal antibodies become a risk factor for severe disease via ADE when they wane to sub-neutralizing titers (3, 4). An early life vaccine would protect these infants at risk of DHF/DSS. Second, vaccinating early in life in endemic regions would have the advantage of vaccinating a population more likely to be uniformly DENV-naïve, avoiding having to vaccinate older subjects with differences in pre-existing immunity, which may affect vaccine-induced immunity in unknown ways.
Our study is the first to address the challenges of immunizing against dengue early in life, and to demonstrate that the VRP vaccine platform is an effective early life dengue vaccine candidate. The present study demonstrates that VRP vaccines can induce NAbs in neonatal mice after a single immunization and that the titers remain stable until at least 15wpi. Although these titers were reduced at all time-points post-immunization compared to those in adult mice, this finding was not surprising, as the neonatal immune response is known to be weaker (18-21), resulting in lower serum antibody titers (22, 23) of shorter duration (24, 25).
Dengue vaccines in development seek to induce robust NAbs to all 4 serotypes (26). However, results from the first dengue phase IIb efficacy trial suggest that cell-mediated immunity may be needed in a dengue vaccine (27), and a recent study suggests that the DENV-specific CD8+ T-cell response in humans correlates with protection in an HLA-specific manner (28). VRP vaccines induce robust CD8+ CTL responses in adult mice to other antigens (29). Even though neonates induce significantly reduced CTL responses (21), our studies showed dengue-specific IFNγ-secreting cells in the DLN of neonatal mice, which we infer to be predominantly CD8+ (Figure 3B). This is based on observations in adult mice, which showed an approximately 10-fold higher quantity of dengue-reactive CD8+ cells compared to CD4+ cells (Figure 3A). Direct comparison between neonates and adults in the current study is difficult, because there was only a single neonatal immunization, while adult mice were immunized twice. However, this is the first study to our knowledge that shows induction of a DENV-specific T-cell response in neonatal mice after a single immunization. We predict that addition to the immunization cocktail of VRP expressing DENV NS3 and/or NS5, major targets of T-cell responses, will result in a stronger antigen-specific CD8+ T-cell response.
The induction of equivalent NAb responses has been a major challenge for vaccines based on replicating antigens, where different rates of replication or immunodominance result in unbalanced responses (5, 30). In a TV, non-propagating VRP vaccine, the 4 components presumably have similar replication rates, reducing the chances of serotype interference. Indeed, we show here a VRP-based TV vaccine induced equivalent titers to each serotype after a single dose in neonates, and the NAbs induced were comparable to those induced by each individual component when tested as a MV vaccine.
It is possible that the serotype-specific NAbs measured 3 weeks after TV vaccination could be attributed to cross-reactive NAbs induced by the dominant serotypes. This is unlikely for two reasons. First, studies in adult mice indicate that MV E85-VRP vaccines predominantly induce homologous NAbs with very low amounts of cross-reactive NAbs (data not shown). Second, since cross-reactive NAbs induced by one serotype are short-lived (31, 32), measuring NAbs to all 4 serotypes long after immunization reflects a true TV response. This was indeed demonstrated 73-weeks after immunization of adult mice with two doses of TV VRP vaccine expressing DENV1-4 prME (supplementary figure 2).
A previous study demonstrated that a DENV-VRP vaccine was not subject to otherwise interfering maternal antibodies when it was used to immunize weanling mice born to DENV-immune dams (16). Maternal antibody interference is one of the most important obstacles to developing an early life dengue vaccine, especially because LAV are likely to be ineffective at this age due to maternal antibodies interfering with the replication of the vaccine strains.
Reducing the risk of DHF in early life after a single immunization will allow opportunities for a second immunization to further improve the immune response. Anti-vector immunity is undetectable-to-low after a single VRP immunization in NHP, and does not prevent a second VRP immunization from having a booster effect on dengue-specific NAbs (17). This suggested that infants primed with a VRP vaccine could be safely and effectively boosted with a second dose of a VRP vaccine. Neonatal mice primed on d7 appear to produce more NAb after a boost on d35 than animals that received a single inoculation on d35 (p=0.0519). Similarly, the CD8+ T-cell response is significantly higher when the mice were first primed as neonates (p=0.0498). Although these p-values hover around the limit of significance, we feel that immunizing neonatal animals not only will have a salutary effect on protection of neonates as maternal NAb wanes, but also will prime an improved boost response going forward. The second immunization as an adult could utilize a homologous or heterologous vaccine platform to broaden and strengthen the immune response. Although anti-vector immunity is a concern that remains to be addressed in humans, studies in adult mice (unpublished data) and macaques (17), have shown anti-VEE NAbs do not have any significant effect on the induction of NAbs to vaccine antigens. Another potential concern is the chance of in vivo recombination if vaccination occurs at the same time as infection with wild type virus. This event is highly unlikely to result in recombinant virus of more virulence than the wild-type infecting virus itself.
Supplementary Material
Acknowledgments
This work was supported by Public Health Service grant 5UO1-AI078060 awarded to L.J.W by the National Institute of Allergy and Infectious Diseases (NIAID), titled “A tetravalent dengue vaccine based on Alphavirus replicons.”
We thank Martha Collier for VRP production, Alan Whitmore for excellent technical help, Jan Woraratnadharm for coordination of animal facility, and members of Global Vaccines Inc. for helpful discussions. The following reagents were obtained through the NIH Biodefense and Emerging Infections Research Resources Repository, NIAID, NIH: Peptide Array, Dengue Virus Type 2, New Guinea C (NGC), E Protein, NR-507 and Peptide Array, Dengue Virus Type 3, Sleman/1978, E Protein, NR-511.
R.E.J was a co-inventor of the VRP vectors, and will share in any remuneration received by the University of North Carolina from the commercialization of this technology. He also holds an equity interest in AlphaVax, a privately held company that holds commercial rights to the VRP vectors. L.J.W and R.E.J are currently employed at Global Vaccines, Inc. (GVI). GVI is a not-for-profit vaccine company. Therefore, its employees have no equity interest in the company.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013 doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. Dengue guidelines for diagnosis, treatment, prevention and control. World Health Organization; Geneva, Switzerland: 2009. http://wwwwhoint/tdr/publications/documents/dengue-diagnosispdf. [PubMed] [Google Scholar]
- 3.Halstead SB, Lan NT, Myint TT, Shwe TN, Nisalak A, Kalyanarooj S, et al. Dengue hemorrhagic fever in infants: research opportunities ignored. Emerging infectious diseases. 2002;8(12):1474–9. doi: 10.3201/eid0812.020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kliks SC, Nimmanitya S, Nisalak A, Burke DS. Evidence that maternal dengue antibodies are important in the development of dengue hemorrhagic fever in infants. Am J Trop Med Hyg. 1988;38(2):411–9. doi: 10.4269/ajtmh.1988.38.411. [DOI] [PubMed] [Google Scholar]
- 5.Guy B, Barban V, Mantel N, Aguirre M, Gulia S, Pontvianne J, et al. Evaluation of interferences between dengue vaccine serotypes in a monkey model. Am J Trop Med Hyg. 2009;80(2):302–11. [PubMed] [Google Scholar]
- 6.Lee JS, Hadjipanayis AG, Welkos SL. Venezuelan equine encephalitis virus-vectored vaccines protect mice against anthrax spore challenge. Infection and immunity. 2003;71(3):1491–6. doi: 10.1128/IAI.71.3.1491-1496.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee JS, Pushko P, Parker MD, Dertzbaugh MT, Smith LA, Smith JF. Candidate vaccine against botulinum neurotoxin serotype A derived from a Venezuelan equine encephalitis virus vector system. Infection and immunity. 2001;69(9):5709–15. doi: 10.1128/IAI.69.9.5709-5715.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marth T, Kleen N, Stallmach A, Ring S, Aziz S, Schmidt C, et al. Dysregulated peripheral and mucosal Th1/Th2 response in Whipple's disease. Gastroenterology. 2002;123(5):1468–77. doi: 10.1053/gast.2002.36583. [DOI] [PubMed] [Google Scholar]
- 9.Pushko P, Bray M, Ludwig GV, Parker M, Schmaljohn A, Sanchez A, et al. Recombinant RNA replicons derived from attenuated Venezuelan equine encephalitis virus protect guinea pigs and mice from Ebola hemorrhagic fever virus. Vaccine. 2000;19(1):142–53. doi: 10.1016/s0264-410x(00)00113-4. [DOI] [PubMed] [Google Scholar]
- 10.Pushko P, Geisbert J, Parker M, Jahrling P, Smith J. Individual and bivalent vaccines based on alphavirus replicons protect guinea pigs against infection with Lassa and Ebola viruses. J Virol. 2001;75(23):11677–85. doi: 10.1128/JVI.75.23.11677-11685.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson JA, Bray M, Bakken R, Hart MK. Vaccine potential of Ebola virus VP24, VP30, VP35, and VP40 proteins. Virology. 2001;286(2):384–90. doi: 10.1006/viro.2001.1012. [DOI] [PubMed] [Google Scholar]
- 12.Schultz-Cherry S, Dybing JK, Davis NL, Williamson C, Suarez DL, Johnston R, et al. Influenza virus (A/HK/156/97) hemagglutinin expressed by an alphavirus replicon system protects chickens against lethal infection with Hong Kong-origin H5N1 viruses. Virology. 2000;278(1):55–9. doi: 10.1006/viro.2000.0635. [DOI] [PubMed] [Google Scholar]
- 13.Velders MP, McElhiney S, Cassetti MC, Eiben GL, Higgins T, Kovacs GR, et al. Eradication of established tumors by vaccination with Venezuelan equine encephalitis virus replicon particles delivering human papillomavirus 16 E7 RNA. Cancer research. 2001;61(21):7861–7. [PubMed] [Google Scholar]
- 14.Hevey M, Negley D, Pushko P, Smith J, Schmaljohn A. Marburg virus vaccines based upon alphavirus replicons protect guinea pigs and nonhuman primates. Virology. 1998;251(1):28–37. doi: 10.1006/viro.1998.9367. [DOI] [PubMed] [Google Scholar]
- 15.Johnston RE, Johnson PR, Connell MJ, Montefiori DC, West A, Collier ML, et al. Vaccination of macaques with SIV immunogens delivered by Venezuelan equine encephalitis virus replicon particle vectors followed by a mucosal challenge with SIVsmE660. Vaccine. 2005;23(42):4969–79. doi: 10.1016/j.vaccine.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 16.White LJ, Parsons MM, Whitmore AC, Williams BM, de Silva A, Johnston RE. An immunogenic and protective alphavirus replicon particle-based dengue vaccine overcomes maternal antibody interference in weanling mice. J Virol. 2007;81(19):10329–39. doi: 10.1128/JVI.00512-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.White LJ, Sariol CA, Mattocks MD, Wahala MPBW, Yingsiwaphat V, Collier ML, et al. An alphavirus vector-based tetravalent dengue vaccine induces a rapid and protective immune response in macaques that differs qualitatively from immunity induced by live virus infection. J Virol. 2013;87(6):3409–24. doi: 10.1128/JVI.02298-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adkins B, Bu Y, Guevara P. Murine neonatal CD4+ lymph node cells are highly deficient in the development of antigen-specific Th1 function in adoptive adult hosts. J Immunol. 2002;169(9):4998–5004. doi: 10.4049/jimmunol.169.9.4998. [DOI] [PubMed] [Google Scholar]
- 19.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4(7):553–64. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 20.Li L, Lee HH, Bell JJ, Gregg RK, Ellis JS, Gessner A, et al. IL-4 utilizes an alternative receptor to drive apoptosis of Th1 cells and skews neonatal immunity toward Th2. Immunity. 2004;20(4):429–40. doi: 10.1016/s1074-7613(04)00072-x. [DOI] [PubMed] [Google Scholar]
- 21.Siegrist CA. Neonatal and early life vaccinology. Vaccine. 2001;19(25-26):3331–46. doi: 10.1016/s0264-410x(01)00028-7. [DOI] [PubMed] [Google Scholar]
- 22.Giuliano M, Mastrantonio P, Giammanco A, Piscitelli A, Salmaso S, Wassilak SG. Antibody responses and persistence in the two years after immunization with two acellular vaccines and one whole-cell vaccine against pertussis. The Journal of pediatrics. 1998;132(6):983–8. doi: 10.1016/s0022-3476(98)70395-6. [DOI] [PubMed] [Google Scholar]
- 23.Tiru M, Hallander HO, Gustafsson L, Storsaeter J, Olin P. Diphtheria antitoxin response to DTP vaccines used in Swedish pertussis vaccine trials, persistence and projection for timing of booster. Vaccine. 2000;18(21):2295–306. doi: 10.1016/s0264-410x(99)00539-3. [DOI] [PubMed] [Google Scholar]
- 24.Pihlgren M, Friedli M, Tougne C, Rochat AF, Lambert PH, Siegrist CA. Reduced ability of neonatal and early-life bone marrow stromal cells to support plasmablast survival. J Immunol. 2006;176(1):165–72. doi: 10.4049/jimmunol.176.1.165. [DOI] [PubMed] [Google Scholar]
- 25.Pihlgren M, Schallert N, Tougne C, Bozzotti P, Kovarik J, Fulurija A, et al. Delayed and deficient establishment of the long-term bone marrow plasma cell pool during early life. Eur J Immunol. 2001;31(3):939–46. doi: 10.1002/1521-4141(200103)31:3<939::aid-immu939>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 26.Whitehead SS, Blaney JE, Durbin AP, Murphy BR. Prospects for a dengue virus vaccine. Nature reviews Microbiology. 2007;5(7):518–28. doi: 10.1038/nrmicro1690. [DOI] [PubMed] [Google Scholar]
- 27.Sabchareon A, Wallace D, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S, et al. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomised, controlled phase 2b trial. Lancet. 2012;380(9853):1559–67. doi: 10.1016/S0140-6736(12)61428-7. [DOI] [PubMed] [Google Scholar]
- 28.Weiskopf D, Angelo MA, de Azeredo EL, Sidney J, Greenbaum JA, Fernando AN, et al. Comprehensive analysis of dengue virus-specific responses supports an HLA-linked protective role for CD8+ T cells. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1305227110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moran TP, Collier M, McKinnon KP, Davis NL, Johnston RE, Serody JS. A novel viral system for generating antigen-specific T cells. J Immunol. 2005;175(5):3431–8. doi: 10.4049/jimmunol.175.5.3431. [DOI] [PubMed] [Google Scholar]
- 30.Osorio JE, Brewoo JN, Silengo SJ, Arguello J, Moldovan IR, Tary-Lehmann M, et al. Efficacy of a tetravalent chimeric dengue vaccine (DENVax) in Cynomolgus macaques. Am J Trop Med Hyg. 2011;84(6):978–87. doi: 10.4269/ajtmh.2011.10-0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy BR, Whitehead SS. Immune response to dengue virus and prospects for a vaccine. Annual review of immunology. 2011;29:587–619. doi: 10.1146/annurev-immunol-031210-101315. [DOI] [PubMed] [Google Scholar]
- 32.Rothman AL. Immunity to dengue virus: a tale of original antigenic sin and tropical cytokine storms. Nat Rev Immunol. 2011;11(8):532–43. doi: 10.1038/nri3014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


