Abstract
Ubiquinone, commonly called coenzyme Q10 (CoQ), is a lipophilic electron carrier and endogenous antioxidant found in all cellular membranes. In the mitochondrial inner membrane it transfers electrons to complex III of the electron transport chain. The short chain CoQ analogue idebenone is in clinical trials for a number of diseases that exhibit a mitochondrial etiology. Nevertheless, evidence that idebenone ameliorates neurological symptoms in human disease is inconsistent. Although championed as an antioxidant, idebenone can also act as a pro-oxidant by forming an unstable semiquinone at complex I. The antioxidant function of idebenone is critically dependent on two-electron reduction to idebenol without the creation of unstable intermediates. Recently, cytoplasmic NAD(P)H:quinone oxidoreductase 1 (NQO1) was identified as a major enzyme catalyzing idebenone reduction. While reduction allows idebenone to act as an antioxidant, evidence also suggests that NQO1 enables idebenone to shuttle reducing equivalents from cytoplasmic NAD(P)H to mitochondrial complex III, bypassing any upstream damage to the electron transport chain. In this mini-review we discuss how idebenone can influence mitochondrial function within the context of cytoprotection. Importantly, in the brain NQO1 is expressed primarily by glia rather than neurons. As NQO1 is an inducible enzyme regulated by oxidative stress and the nuclear factor erythroid 2-related factor 2 (Nrf2)/antioxidant response element (ARE) pathway, optimizing NQO1 expression in appropriate cell types within a specific disease context may be key to delivering on idebenone's therapeutic potential.
Keywords: Coenzyme Q10, quinone, mitochondria, complex I, NQO1, Friedreich's ataxia
Introduction
The mitochondrial electron transport chain (ETC) consists of complexes I-IV, the lipophilic inner membrane electron carrier ubiquinone and the soluble intermembrane space electron carrier cytochrome c [1]. The ATP synthase, which couples the electrochemical proton gradient established by the respiratory chain to phosphorylation of ADP to ATP, is often called complex V.
Ubiquinone, commonly known as coenzyme Q10 (CoQ), is present in excess relative to other components of the ETC [1,2]. Found in other cellular membranes in addition to mitochondria [3], the reduced ubiquinol form of CoQ is a powerful antioxidant which can accept electrons from free radicals and inhibit lipid peroxidation [2,4]. Largely for this radical scavenging property, CoQ supplements and skin cream are touted as “anti-aging” elixirs, though there is no hard scientific evidence that CoQ supplementation extends lifespan. Within the ETC, CoQ is a point of convergence for electrons originating from Complex I or II [1]. In nonneuronal cells, auxiliary respiratory chain components such as the electron-transferring flavoprotein–ETF ubiquinone oxidoreductase and glycerol-3-phosphate dehydrogenase also feed electrons to complex III via CoQ [1]. Due to its essential role in electron transport, CoQ is sometimes loosely advertised as an “energy” supplement useful for treating a wide variety of pathological conditions. Nevertheless, aside from rare disorders caused by CoQ deficiency, there is little evidence that CoQ therapy acts by mitigating electron transport chain deficiency [2].
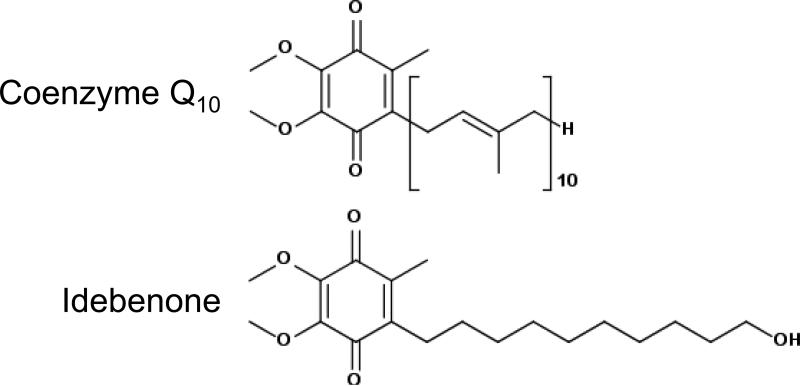
CoQ is poorly soluble in aqueous solutions. A series of short chain analogues of CoQ were synthesized with the aim of improving pharmacokinetics for patient treatment [2,5,6]. Idebenone (2-(10-hydroxydecyl)-5,6-dimethoxy-3-methyl-cyclohexa-2,5-diene-1,4-dione) is one such quinone developed in Japan in the 1980s for the treatment of neurodegenerative conditions (Fig. 1) [6]. Consistent with predicted antioxidant activity, idebenone inhibits lipid peroxidation in isolated brain mitochondria, synaptosomes, and cells [5–10]. In one study, idebenone was the most potent of 70 related quinones tested [5]. Cell-free experiments demonstrated that idebenone per se lacks antioxidant activity, with activity conferred solely by the reduced hydroquinone form idebenol [11]. In addition to impairing lipid peroxidation, idebenol is capable of detoxifying a wide variety of free radicals, including peroxyl and tyrosyl radicals and peroxynitrite [11].
Fig 1.
Chemical structures of coenzyme Q10 and idebenone
In vitro, idebenone attenuates oxidative injury to primary cortical neurons [12,13] and immortalized neural cells [14,15] caused by depletion of the endogenous intracellular antioxidant glutathione. 14C radiolabelling experiments established that idebenone crosses the blood-brain barrier following oral administration [16,17]. Idebenone protects against neuronal death caused by α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) glutamate receptor stimulation in vitro [18] and in vivo [19]. However, protection against N-methyl-D-aspartate (NMDA) receptor-induced death is observed only in vitro [18,19]. Nevertheless, neurological improvement in response to idebenone treatment was reported in rodent models of stroke [20] and Alzheimer's disease [9]. Idebenone is well-tolerated in humans even at daily doses of 1000-2000 mg and shows linear pharmacokinetics following single or repeated oral dosing [21]. Millions of humans have taken idebenone as part of clinical trials or as approved treatment in various countries [22]. Its excellent bioavailability and safety profile make idebenone an attractive neurotherapeutic drug candidate. But is it effective?
Idebenone in clinical trials: a short synopsis
Idebenone has been investigated most extensively for the treatment of Friedreich's ataxia, a rare autosomal recessive disease that typically affects children and young adults [22,23]. Friedreich's ataxia is a progressive disorder characterized by degeneration of spinal cord nerve tissue [23]. Adverse neurological symptoms include impaired movement and speech. Hypertrophic cardiomyopathy is a serious and prominent non-neurological feature [22,23].
The majority of Friedreich's ataxia patients are deficient in the mitochondrial protein frataxin, a protein involved in iron metabolism and redox homeostasis [23]. Frataxin deficiency results in loss of essential iron-sulfur containing proteins found in respiratory chain complexes I, II, and III, leading to impaired ETC function and reduced ATP production [24,25]. Dysfunctional mitochondria are further burdened by iron build-up, causing unmanaged oxidative stress [26]. Treatment of Friedreich's ataxia patients with idebenone generally led to a reduction in oxidative stress markers, with many patients also showing improvement of non-neurological symptoms [22,23]. However, although a six-month, randomized, double-blind, placebo-controlled trial for idebenone in Friedreich's ataxia patients found improvement in neurological function and activities associated with daily living [27], a subsequent trial with a separate cohort of patients failed to recapitulate the neurological benefits [28]. Nevertheless, a twelve-month open label extension of the second study provided evidence for neurological improvement at the highest idebenone dose [29]. Initially “authorized with conditions” for the treatment of Friedreich's ataxia in Canada under the trade name CATENA®, idebenone was voluntarily withdrawn from the Canadian market in 2013 by Santhera Pharmaceuticals without safety concerns, citing lack of efficacy.
Trials with idebenone for the most prevalent neurodegenerative disorder, Alzheimer's disease, also generated mixed results. An early double-blind trial with only a moderate patient number (102) found statistically significant improvement in memory, attention and behavior [30]. Several additional trials yielded favorable results [31–34]. However, idebenone failed to slow Alzheimer's-associated cognitive decline in a larger multicenter, double-blind, placebo-controlled trial [35]. Approval for the use of idebenone to treat Alzheimer's disease or related cognitive dementias in the U.S. has not been obtained. Thus, as in the case of Friedreich's ataxia, the initial high hopes for idebenone in the treatment of Alzheimer's disease remain unfulfilled.
A one-year, double-blind Huntington's disease trial for idebenone disappointingly found no significant improvement in primary outcome measures related to disease progression [36]. Idebenone has also seen clinical trials for the mitochondrial disorders Mitochondrial Encephalopathy Lactic Acidosis and Stroke-like Episodes (MELAS) and Leber's Hereditary Optic Neuropathy (LHON), as well as for Duchenne muscular dystrophy and multiple sclerosis (www.clinicaltrials.gov). While some trials are still ongoing, idebenone has yet to be approved by the U.S. Food and Drug Administration for the treatment of any human disease.
Not just an antioxidant: mitochondrial mechanisms of action
A greater understanding of how idebenone interacts with mitochondria in cells is needed to further clinical efforts with this drug. Because idebenone is described as a short chain CoQ analogue, a common misconception is that idebenone can substitute for the function of endogenous CoQ in the ETC. Although early work demonstrated that idebenone can restore complete succinate oxidation in CoQ-depleted brain mitochondria, NADH oxidation in the presence of idebenone was independent of downstream components of the ETC [37]. These findings suggest that idebenone is effective at transferring electrons from complex II (succinate dehydrogenase) but not from complex I (NADH dehydrogenase) to complex III. Experiments using intact cells, which oxidize primarily NADH-linked substrates, confirmed that idebenone cannot substitute for the electron transfer function of CoQ in CoQ-deficient fibroblasts [38]. However, several studies support the ability of idebenone to act as an effective electron carrier from complex II or glycerol-3-phosphate dehydrogenase to complex III [37,39,40].
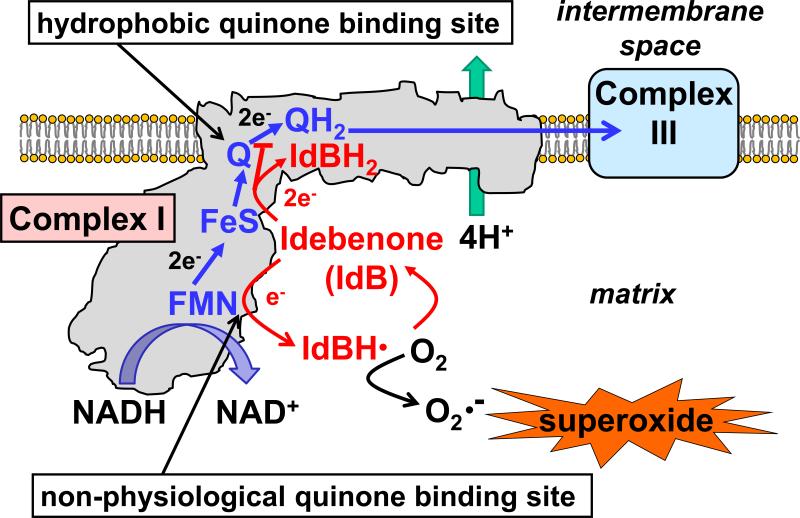
At apparent odds with its presumptive neuroprotective potential, multiple groups reported that idebenone can inhibit complex I and promote superoxide production [40–48]. Most of these studies were done with isolated complex I or submitochondrial particles—mitochondrial membrane fractions containing functional ETC complexes. However, it was also demonstrated that idebenone inhibits ADP-stimulated and uncoupled respiration by intact rat brain mitochondria in the presence of complex I-linked substrates [37]. Detailed biochemical studies by King et al. elucidated likely mechanisms of complex I inhibition and superoxide production by idebenone [46]. In the model of King et al., idebenone is reduced at the hydrophobic quinone-binding site within complex I like CoQ but exhibits very slow dissociation. Consequently it acts as a competitive substrate that impairs endogenous CoQ function without substituting for its electron transfer function to complex III (Fig. 2) [46]. Idebenone binds a second quinone-binding site within complex I that overlaps with the NADH binding site and flavin mononucleotide (FMN) moiety (Fig. 2) [46]. Due to its high lipophilicity, endogenous CoQ is incapable of binding this “non-physiological” hydrophilic quinone-binding site. However, idebenone, which is less lipophilic than CoQ, is reduced by the complex I flavin in the hydrophilic site to form an unstable semiquinone that generates superoxide (Fig. 2) [46].
Fig 2.
Model of how idebenone interacts with electron transport chain complex I. The oxidation of NADH by complex I results in the transfer of two electrons to complex III via ubiquinone/coenzyme Q10 (Q), accompanied by the pumping of four protons (H+) from the mitochondrial matrix to the intermembrane space. Idebenone (IdB) competes with endogenous Q at the physiological hydrophobic quinone binding site for electrons from upstream iron-sulfur (FeS) clusters. The two-electron reduction of idebenone results in a product, idebenol (IdBH2), with very slow dissociation kinetics from the quinone binding site. This slow dissociation not only makes idebenol ineffective at electron transfer to complex III but also allows it to competitively inhibit the electron transfer to complex III that normally occurs via endogenous ubiquinol (QH2). A second interaction of idebenone with complex I occurs at a non-physiological hydrophilic quinone binding site that overlaps with the flavin mononucleotide (FMN) moiety. Here, the one electron reduction of idebenone yields an unstable semiquinone intermediate (IdBH·). This semiquinone causes the one-electron reduction of oxygen (O2) to superoxide (O2·-), regenerating idebenone. The net results of idebenone-complex I interactions are impaired complex I functionality and superoxide generation
Mitochondrial complex I inhibition combined with superoxide generation sounds like a nice recipe for neurotoxicity. Both events are thought to contribute to dopaminergic neurodegeneration in Parkinson's disease [49]. So why isn't idebenone toxic? Interestingly, idebenone at concentrations ≥25 μM is in fact toxic to the human dopaminergic neuroblastoma cell line SH-SY5Y [50]. In addition, Giorgio et al. found that idebenone causes mitochondrial depolarization and NADH depletion within a fibroblast/osteosarcoma cybrid cell line [51]. These events are inhibited by cyclosporin A, an inhibitor of the cyclophilin D-regulated permeability transition pore implicated in neurodegeneration [52]. Since the permeability transition pore is a large conductance ion channel in the mitochondrial inner membrane that opens in response to oxidative stress and calcium overload [53,54], a likely explanation is that idebenone acts as a pro-oxidant in these cells by forming semiquinone radicals at complex I. Consistent with the possibility that idebenone promotes permeability transition pore opening via oxidative stress, N-ethylmaleimide, an agent that blocks permeability transition pore-regulating sulfhydryl groups [54], prevents idebenone from initiating pore-induced swelling of calcium-loaded mitochondria [51]. Remarkably, supplementation of idebenone with the reducing agent dithiothreitol not only attenuates permeability transition pore opening but enables idebenone to restore oxygen consumption in cells with deficient or inhibited complex I [51]. Respiration nevertheless remains sensitive to the complex III inhibitor antimycin A, indicating that distal components of the ETC are required for idebenone to rescue oxygen consumption. Idebenol but not idebenone maintains ATP synthesis in permeabilized cells incubated with complex I and II inhibitors rotenone and malonate, respectively [51], suggesting that idebenone in its reduced form idebenol can transfer electrons to complex III. Although complex I proton pumping remains impaired, proton pumping at complex III and complex IV is recovered, allowing restoration of proton-motive force for ATP synthesis.
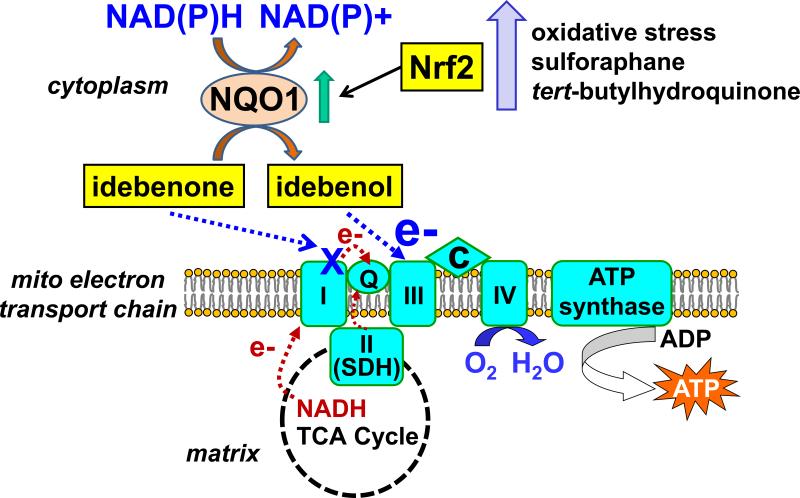
As outlined above, the human safety of idebenone is well-established. Therefore it follows that most cells must have an efficient means of reducing idebenone to idebenol to avert the potentially disastrous consequences of oxidative stress-induced permeability transition pore opening. Idebenone is reduced by succinate dehydrogenase, and succinate greatly potentiates the ability of idebenone to impede lipid peroxidation in brain mitochondria [7]. However, once idebenone reaches mitochondria, reduction at complex I is also likely to occur, leading to respiratory inhibition and superoxide production. Recent studies by Haefeli et al. identified NAD(P)H:quinone acceptor oxidoreductase (NQO) enzymes, also known as DT-diaphorases, as major enzymes catalyzing extramitochondrial idebenone reduction at the expense of cytoplasmic NAD(P)H [55]. Idebenone is able to rescue ATP levels in a variety of cell types exposed to the complex I inhibitor rotenone and the extent of rescue correlates with NQO1 mRNA expression level [55]. Idebenone also protects against the loss of retinal ganglion cells in vivo caused by intravitreal rotenone injection [56]. In vitro evidence supports an electron bypass model of cytoprotection whereby idebenone shuttles electrons from cytoplasmic NAD(P)H to complex III of the ETC in NQO1-expressing cells (Fig. 3), restoring mitochondrial ATP synthesis following complex I inhibition [5,55,56]. A similar NQO1-dependent complex I bypass mechanism was previously proposed for the related quinones CoQ1 and menadione [57,58].
Fig. 3.
Model of NQO1-dependent shuttling of reducing equivalents from cytoplasmic NAD(P)H to mitochondrial electron transport chain complex III via idebenone. The two-electron reduction of idebenone to idebenol is catalyzed by NAD(P)H:quinone oxidoreductase 1 (NQO1) and occurs primarily in the cytoplasm. Idebenol is hydrophilic enough to traverse the cytoplasm but lipophilic enough to mediate electron transfer to complex III in the mitochondrial inner membrane. NQO1-catalyzed idebenone reduction may elude the deleterious consequences of idebenone-complex I interaction while idebenol may exert cytoprotection by bypassing disease-associated inhibition of complex I or upstream tricarboxylic acid cycle (TCA) enzymes. This bypass would restore oxygen (O2) consumption at complex IV and ATP production by the ATP synthase. Stimuli which activate the nuclear factor erythroid 2-related factor 2 (Nrf2) transcription factor, including mild oxidative stress and the drugs sulforaphane and tert-butylhydroquinone, are predicted to synergize with idebenone by upregulating NQO1 expression
Notably, virtually no NQO1 mRNA expression is observed in SH-SY5Y cells [55], perhaps explaining the reported toxicity of idebenone to this cell type [50]. Regarding idebenone's neuroprotective potential, a critical question now emerges: where in the central nervous system is NQO1 expressed and can its expression be manipulated?
NQO1: an inducible enzyme expressed predominantly by glial cells
NQO1 is an antioxidant flavoprotein regulated by the nuclear factor erythroid 2-related factor 2 (Nrf2)/antioxidant response element (ARE) pathway [59,60]. While NQO1 is found mainly in the cytoplasm, small amounts localize to the mitochondria, endoplasmic reticulum and nucleus [61]. NQO1 catalyzes the two-electron reduction of quinones to hydroquinones [60,62]. This reaction counteracts the one-electron reduction of quinones to free radical semiquinones catalyzed by NADPH:cytochrome P450 reductase and other enzymes [62–64]. Thus, the antioxidant activity of NQO1 limits superoxide formation by minimizing redox cycling of quinones between their semiquinone and quinone forms [62,63]. Because NQO1 catalyzes the two-electron reduction of idebenone, NQO1 activity is predicted to limit the formation of semiquinones from idebenone at complex I or other one-electron reduction sites (see Fig. 2) while also supplying idebenol for electron transfer to complex III (Fig. 3).
NQO1 is expressed in many tissues throughout the body including the brain [65–67]. In the healthy brain, NQO1 expression is predominately restricted to astrocytes and a subset of oligodendrocytes [66], although expression in narrow neuronal subpopulations is also reported [67]. Because the transcription factor Nrf2 is implicated in basal as well as inducible NQO1 expression [68], the relative lack of neuronal NQO1 expression may be due to the low basal level of Nrf2 in these cells [69]. Given that NQO1 promotes the ability of idebenone to act as an effective antioxidant and electron carrier to complex III [55], the low expression of NQO1 in neurons may mean that idebenone has limited potential to protect these cells by cell autonomous mechanisms.
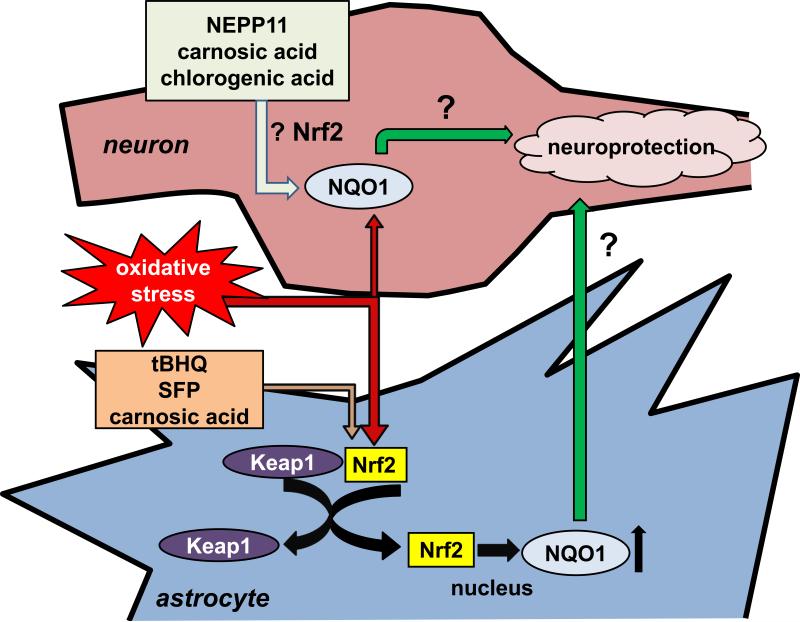
Fortuitously, NQO1 is an inducible enzyme [60]. Relevant to neurodisease, a primary inducer is oxidative stress [70]. Oxidation of cysteine residues on Kelch-like ECH-associated protein 1 (KEAP1) releases the transcription factor Nrf2 from an inhibitory cytoplasmic interaction, enabling Nrf2 relocalization to the nucleus where it initiates transcription of NQO1 and other ARE-controlled genes (Fig. 4) [71]. Interestingly, while NQO1 is expressed sparingly by neurons in the healthy brain, neuronal NQO1 is elevated in Alzheimer's disease [72–74] and Parkinson's disease [75], two neurodegenerative conditions with a major oxidative component [76]. In Alzheimer's disease, NQO1 expression and activity are observed in hippocampal neurons in close proximity to pathology, including in neurofibrillary tangles [72–74]. Therefore it is possible that oxidative stress associated with the disease process itself will enhance the antioxidant and electron transport functions of idebenone by upregulating NQO1. However, it is also possible that NQO1 is expressed primarily by dying neurons that are beyond rescue.
Fig. 4.
Nrf2 is inactive when sequestered by Keap1. Oxidative stress leads to Keap1 dissociation and Nrf2 activation. Nrf2-dependent gene expression can be activated by small molecule electrophiles, including sulforaphane (SFP) or tert-butylhydroquinone (tBHQ) in astrocytes, by carnosic acid in both astrocytes and neurons, and by NEPP11 in neurons. Chlorogenic acid may induce neuronal NQO1 expression by Nrf2-independent mechanisms [82], although this has yet to be firmly established
Idebenone and combination therapy: wave of the future?
To this point idebenone has been investigated in human disease as a single-agent therapy. What if we could upregulate NQO1 in the degenerating brain in a cell-specific and controlled fashion? Given the underwhelming success of idebenone in clinical trials, targeted induction of NQO1 in concert with idebenone treatment would seem to be the wave of the future. In addition to evidence that the Nrf2 antioxidant response pathway per se is neuroprotective, NQO1 is predicted to greatly increase the neuroprotective potential of idebenone by lessening unstable semiquinone formation while at the same time enhancing antioxidant and electron transfer activity. Therefore, idebenone and an Nrf2-inducing agent may be a strongly synergistic drug combination that is far more effective than either drug alone. Several small molecule electrophiles, including sulforaphane and tert-butylhydroquinone, initiate Nrf2-dependent gene expression (Fig. 3 and 4) [71,77]. However, in most cases induction of Nrf2-responsive genes by these agents is selective for astrocytes over neurons both in vitro and in vivo [59,69,77–79]. Neuroprotective changes in neuronal gene expression seem to depend on altered neuron-glial interactions initiated by Nrf2 activity in astrocytes (Fig. 4) [77,78]. While enhancing idebenone metabolism in astrocytes is indeed desirable as this may increase the neuroprotective potential of astrocytes, improving NQO1-catalyzed idebenone reduction in both neurons and astrocytes may ultimately yield the greatest therapeutic benefit.
Evidence that the Nrf2 pathway can also be recruited by small molecules in neurons as well as astrocytes is gradually accumulating. Uptake of a class of electrophilic neurite outgrowth-promoting prostaglandin compounds known as NEPPs is reportedly selective for neurons compared to astrocytes [80]. NEPP11 crosses the blood-brain barrier, activates the Nrf2 pathway in neurons in vitro and in vivo, and exhibits neuroprotection in an animal stroke model [80]. Carnosic acid was described by the same group to activate the Nrf2 pathway in both neurons and astrocytes and exhibit protection against focal ischemia/reperfusion brain injury [81]. Interestingly, chlorogenic acid, a compound found in both caffeinated and decaffeinated coffee, increases NQO1 expression in primary embryonic neurons without induction of other prototypical Nrf2-responsive genes such as heme oxygenase-1 [82].
The idea of combination therapy is not without caveats. One important caveat is that NQO1-dependent shuttling of electrons to complex III by idebenone has not been demonstrated to occur in neurons, astrocytes, or other glia; cell-specific metabolic differences may preclude this mechanism of action. Another caveat is that QS-10, a primary metabolite of idebenone [17], is not capable of transferring electrons to complex III though it is reduced by NQO1 [55]. Thus, idebenone metabolism upstream of NQO1 may limit the efficacy of the proposed electron bypass mechanism. A third caveat is that while the human safety of idebenone is well established, combination therapy may cause unexpected, dose-limiting toxicity that precludes usefulness. For example, a high level of cytoplasmic NAD(P)H oxidation by NQO1 may alter the redox state of cells if NAD(P)H-regenerating capacity is low or impaired [83,84].
Finally, cells may have enzymes in addition to NQO1 that can reduce idebenone effectively without semiquinone radical formation. As we learn more about the cellular metabolism of this intriguing molecule, new avenues for its therapeutic use may emerge. Efforts are underway to synthesize idebenone analogues with preserved electron transfer function but reduced ability to impair complex I activity [48]. It remains to be seen whether the ability of idebenone to inhibit complex I activity and stimulate mitochondrial superoxide production, phenomena so far observed only in vitro, impacted the success of idebenone in clinical trials.
Acknowledgments
The authors acknowledge support from National Institutes of Health R01 NS085165 to B.M.P. and from Sigma Tau Pharmaceuticals and the M. Jane Matjasko Endowment.
Abbreviations
- ARE
antioxidant response element
- CoQ
coenzyme Q10
- ETC
electron transport chain
- FMN
flavin mononucleotide
- IdBH2
idebenol
- Keap1
Kelch-like ECH-associated protein 1
- NQO
NAD(P)H:quinone acceptor oxidoreductase
- Nrf2
nuclear factor erythroid 2-related factor 2
References
- 1.Nicholls DG, Ferguson SJ. Bioenergetics 4. Academic Press; London: 2013. [Google Scholar]
- 2.Geromel V, Darin N, Chretien D, Benit P, DeLonlay P, Rotig A, Munnich A, Rustin P. Coenzyme Q(10) and idebenone in the therapy of respiratory chain diseases: rationale and comparative benefits. Mol Genet Metab. 2002;77:21–30. doi: 10.1016/s1096-7192(02)00145-2. [DOI] [PubMed] [Google Scholar]
- 3.Kalen A, Norling B, Appelkvist EL, Dallner G. Ubiquinone biosynthesis by the microsomal fraction from rat liver. Biochim Biophys Acta. 1987;926:70–78. doi: 10.1016/0304-4165(87)90183-8. [DOI] [PubMed] [Google Scholar]
- 4.Beyer RE, Segura-Aguilar J, di BS, Cavazzoni M, Fato R, Fiorentini D, Galli MC, Setti M, Landi L, Lenaz G. The role of DT-diaphorase in the maintenance of the reduced antioxidant form of coenzyme Q in membrane systems. Proc Natl Acad Sci U S A. 1996;93:2528–2532. doi: 10.1073/pnas.93.6.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erb M, Hoffmann-Enger B, Deppe H, Soeberdt M, Haefeli RH, Rummey C, Feurer A, Gueven N. Features of idebenone and related short-chain quinones that rescue ATP levels under conditions of impaired mitochondrial complex I. PLoS ONE. 2012;7:e36153. doi: 10.1371/journal.pone.0036153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suno M, Nagaoka A. Inhibition of lipid peroxidation by a novel compound, idebenone (CV-2619). Jpn J Pharmacol. 1984;35:196–198. doi: 10.1254/jjp.35.196. [DOI] [PubMed] [Google Scholar]
- 7.Suno M, Nagaoka A. Inhibition of lipid peroxidation by idebenone in brain mitochondria in the presence of succinate. Arch Gerontol Geriatr. 1989;8:291–297. doi: 10.1016/0167-4943(89)90010-1. [DOI] [PubMed] [Google Scholar]
- 8.Suno M, Nagaoka A. Inhibition of lipid peroxidation by a novel compound (CV-2619) in brain mitochondria and mode of action of the inhibition. Biochem Biophys Res Commun. 1984;125:1046–1052. doi: 10.1016/0006-291x(84)91389-5. [DOI] [PubMed] [Google Scholar]
- 9.Yamada K, Tanaka T, Han D, Senzaki K, Kameyama T, Nabeshima T. Protective effects of idebenone and alpha-tocopherol on beta-amyloid-(1-42)-induced learning and memory deficits in rats: implication of oxidative stress in beta-amyloid-induced neurotoxicity in vivo. Eur J Neurosci. 1999;11:83–90. doi: 10.1046/j.1460-9568.1999.00408.x. [DOI] [PubMed] [Google Scholar]
- 10.Cardoso SM, Pereira C, Oliveira CR. The protective effect of vitamin E, idebenone and reduced glutathione on free radical mediated injury in rat brain synaptosomes. Biochem Biophys Res Commun. 1998;246:703–710. doi: 10.1006/bbrc.1998.8563. [DOI] [PubMed] [Google Scholar]
- 11.Mordente A, Martorana GE, Minotti G, Giardina B. Antioxidant properties of 2,3-dimethoxy-5-methyl-6-(10-hydroxydecyl)-1,4-benzoquinone (idebenone). Chem Res Toxicol. 1998;11:54–63. doi: 10.1021/tx970136j. [DOI] [PubMed] [Google Scholar]
- 12.Murphy TH, Schnaar RL, Coyle JT. Immature cortical neurons are uniquely sensitive to glutamate toxicity by inhibition of cystine uptake. FASEB J. 1990;4:1624–1633. [PubMed] [Google Scholar]
- 13.Ratan RR, Murphy TH, Baraban JM. Oxidative stress induces apoptosis in embryonic cortical neurons. J Neurochem. 1994;62:376–379. doi: 10.1046/j.1471-4159.1994.62010376.x. [DOI] [PubMed] [Google Scholar]
- 14.Miyamoto M, Murphy TH, Schnaar RL, Coyle JT. Antioxidants protect against glutamate-induced cytotoxicity in a neuronal cell line. J Pharmacol Exp Ther. 1989;250:1132–1140. [PubMed] [Google Scholar]
- 15.Pereira CF, Oliveira CR. Oxidative glutamate toxicity involves mitochondrial dysfunction and perturbation of intracellular Ca2+ homeostasis. Neurosci Res. 2000;37:227–236. doi: 10.1016/s0168-0102(00)00124-3. [DOI] [PubMed] [Google Scholar]
- 16.Nagai Y, Yoshida K, Narumi S, Tanayama S, Nagaoka A. Brain distribution of idebenone and its effect on local cerebral glucose utilization in rats. Arch Gerontol Geriatr. 1989;8:257–272. doi: 10.1016/0167-4943(89)90008-3. [DOI] [PubMed] [Google Scholar]
- 17.Torii H, Yoshida K, Kobayashi T, Tsukamoto T, Tanayama S. Disposition of idebenone (CV-2619), a new cerebral metabolism improving agent, in rats and dogs. J Pharmacobiodyn. 1985;8:457–467. doi: 10.1248/bpb1978.8.457. [DOI] [PubMed] [Google Scholar]
- 18.Bruno V, Battaglia G, Copani A, Sortino MA, Canonico PL, Nicoletti F. Protective action of idebenone against excitotoxic degeneration in cultured cortical neurons. Neurosci Lett. 1994;178:193–196. doi: 10.1016/0304-3940(94)90757-9. [DOI] [PubMed] [Google Scholar]
- 19.Miyamoto M, Coyle JT. Idebenone attenuates neuronal degeneration induced by intrastriatal injection of excitotoxins. Exp Neurol. 1990;108:38–45. doi: 10.1016/0014-4886(90)90005-d. [DOI] [PubMed] [Google Scholar]
- 20.Nagaoka A, Suno M, Shibota M, Kakihana M. Effects of idebenone on neurological deficits, local cerebral blood flow, and energy metabolism in rats with experimental cerebral ischemia. Arch Gerontol Geriatr. 1989;8:193–202. doi: 10.1016/0167-4943(89)90002-2. [DOI] [PubMed] [Google Scholar]
- 21.Kutz K, Drewe J, Vankan P. Pharmacokinetic properties and metabolism of idebenone. J Neurol. 2009;256(Suppl 1):31–35. doi: 10.1007/s00415-009-1006-z. [DOI] [PubMed] [Google Scholar]
- 22.Meier T, Buyse G. Idebenone: an emerging therapy for Friedreich ataxia. J Neurol. 2009;256(Suppl 1):25–30. doi: 10.1007/s00415-009-1005-0. [DOI] [PubMed] [Google Scholar]
- 23.Parkinson MH, Schulz JB, Giunti P. Co-enzyme Q10 and idebenone use in Friedreich's ataxia. J Neurochem. 2013;126(Suppl 1):125–141. doi: 10.1111/jnc.12322. [DOI] [PubMed] [Google Scholar]
- 24.Rotig A, de LP, Chretien D, Foury F, Koenig M, Sidi D, Munnich A, Rustin P. Aconitase and mitochondrial iron-sulphur protein deficiency in Friedreich ataxia. Nat Genet. 1997;17:215–217. doi: 10.1038/ng1097-215. [DOI] [PubMed] [Google Scholar]
- 25.Cooper JM, Schapira AH. Friedreich's Ataxia: disease mechanisms, antioxidant and Coenzyme Q10 therapy. Biofactors. 2003;18:163–171. doi: 10.1002/biof.5520180219. [DOI] [PubMed] [Google Scholar]
- 26.Wong A, Yang J, Cavadini P, Gellera C, Lonnerdal B, Taroni F, Cortopassi G. The Friedreich's ataxia mutation confers cellular sensitivity to oxidant stress which is rescued by chelators of iron and calcium and inhibitors of apoptosis. Hum Mol Genet. 1999;8:425–430. doi: 10.1093/hmg/8.3.425. [DOI] [PubMed] [Google Scholar]
- 27.Di Prospero NA, Baker A, Jeffries N, Fischbeck KH. Neurological effects of high-dose idebenone in patients with Friedreich's ataxia: a randomised, placebo-controlled trial. Lancet Neurol. 2007;6:878–886. doi: 10.1016/S1474-4422(07)70220-X. [DOI] [PubMed] [Google Scholar]
- 28.Lynch DR, Perlman SL, Meier T. A phase 3, double-blind, placebo-controlled trial of idebenone in friedreich ataxia. Arch Neurol. 2010;67:941–947. doi: 10.1001/archneurol.2010.168. [DOI] [PubMed] [Google Scholar]
- 29.Meier T, Perlman SL, Rummey C, Coppard NJ, Lynch DR. Assessment of neurological efficacy of idebenone in pediatric patients with Friedreich's ataxia: data from a 6-month controlled study followed by a 12-month open-label extension study. J Neurol. 2012;259:284–291. doi: 10.1007/s00415-011-6174-y. [DOI] [PubMed] [Google Scholar]
- 30.Senin U, Parnetti L, Barbagallo-Sangiorgi G, Bartorelli L, Bocola V, Capurso A, Cuzzupoli M, Denaro M, Marigliano V, Tammaro AE, Fioravanti M. Idebenone in senile dementia of Alzheimer type: a multicentre study. Arch Gerontol Geriatr. 1992;15:249–260. doi: 10.1016/0167-4943(92)90060-h. [DOI] [PubMed] [Google Scholar]
- 31.Bergamasco B, Scarzella L, La CP. Idebenone, a new drug in the treatment of cognitive impairment in patients with dementia of the Alzheimer type. Funct Neurol. 1994;9:161–168. [PubMed] [Google Scholar]
- 32.Weyer G, Babej-Dolle RM, Hadler D, Hofmann S, Herrmann WM. A controlled study of 2 doses of idebenone in the treatment of Alzheimer's disease. Neuropsychobiology. 1997;36:73–82. doi: 10.1159/000119366. [DOI] [PubMed] [Google Scholar]
- 33.Gutzmann H, Kuhl KP, Hadler D, Rapp MA. Safety and efficacy of idebenone versus tacrine in patients with Alzheimer's disease: results of a randomized, double-blind, parallel-group multicenter study. Pharmacopsychiatry. 2002;35:12–18. doi: 10.1055/s-2002-19833. [DOI] [PubMed] [Google Scholar]
- 34.Gutzmann H, Hadler D. Sustained efficacy and safety of idebenone in the treatment of Alzheimer's disease: update on a 2-year double-blind multicentre study. J Neural Transm Suppl. 1998;54:301–310. doi: 10.1007/978-3-7091-7508-8_30. [DOI] [PubMed] [Google Scholar]
- 35.Thal LJ, Grundman M, Berg J, Ernstrom K, Margolin R, Pfeiffer E, Weiner MF, Zamrini E, Thomas RG. Idebenone treatment fails to slow cognitive decline in Alzheimer's disease. Neurology. 2003;61:1498–1502. doi: 10.1212/01.wnl.0000096376.03678.c1. [DOI] [PubMed] [Google Scholar]
- 36.Ranen NG, Peyser CE, Coyle JT, Bylsma FW, Sherr M, Day L, Folstein MF, Brandt J, Ross CA, Folstein SE. A controlled trial of idebenone in Huntington's disease. Mov Disord. 1996;11:549–554. doi: 10.1002/mds.870110510. [DOI] [PubMed] [Google Scholar]
- 37.Imada I, Fujita T, Sugiyama Y, Okamoto K, Kobayashi Y. Effects of idebenone and related compounds on respiratory activities of brain mitochondria, and on lipid peroxidation of their membranes. Arch Gerontol Geriatr. 1989;8:323–341. doi: 10.1016/0167-4943(89)90014-9. [DOI] [PubMed] [Google Scholar]
- 38.Lopez LC, Quinzii CM, Area E, Naini A, Rahman S, Schuelke M, Salviati L, Dimauro S, Hirano M. Treatment of CoQ(10) deficient fibroblasts with ubiquinone, CoQ analogs, and vitamin C: time- and compound-dependent effects. PLoS ONE. 2010;5:e11897. doi: 10.1371/journal.pone.0011897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rauchova H, Drahota Z, Bergamini C, Fato R, Lenaz G. Modification of respiratory-chain enzyme activities in brown adipose tissue mitochondria by idebenone (hydroxydecyl-ubiquinone). J Bioenerg Biomembr. 2008;40:85–93. doi: 10.1007/s10863-008-9134-1. [DOI] [PubMed] [Google Scholar]
- 40.Briere JJ, Schlemmer D, Chretien D, Rustin P. Quinone analogues regulate mitochondrial substrate competitive oxidation. Biochem Biophys Res Commun. 2004;316:1138–1142. doi: 10.1016/j.bbrc.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 41.Genova ML, Ventura B, Giuliano G, Bovina C, Formiggini G, Parenti CG, Lenaz G. The site of production of superoxide radical in mitochondrial Complex I is not a bound ubisemiquinone but presumably iron-sulfur cluster N2. FEBS Lett. 2001;505:364–368. doi: 10.1016/s0014-5793(01)02850-2. [DOI] [PubMed] [Google Scholar]
- 42.Genova ML, Pich MM, Biondi A, Bernacchia A, Falasca A, Bovina C, Formiggini G, Parenti CG, Lenaz G. Mitochondrial production of oxygen radical species and the role of Coenzyme Q as an antioxidant. Exp Biol Med (Maywood) 2003;228:506–513. doi: 10.1177/15353702-0322805-14. [DOI] [PubMed] [Google Scholar]
- 43.Esposti MD, Ngo A, Ghelli A, Benelli B, Carelli V, McLennan H, Linnane AW. The interaction of Q analogs, particularly hydroxydecyl benzoquinone (idebenone), with the respiratory complexes of heart mitochondria. Arch Biochem Biophys. 1996;330:395–400. doi: 10.1006/abbi.1996.0267. [DOI] [PubMed] [Google Scholar]
- 44.Imada I, Sato EF, Kira Y, Inoue M. Effect of CoQ homologues on reactive oxygen generation by mitochondria. Biofactors. 2008;32:41–48. doi: 10.1002/biof.5520320106. [DOI] [PubMed] [Google Scholar]
- 45.Ohnishi ST, Ohnishi T, Muranaka S, Fujita H, Kimura H, Uemura K, Yoshida K, Utsumi K. A possible site of superoxide generation in the complex I segment of rat heart mitochondria. J Bioenerg Biomembr. 2005;37:1–15. doi: 10.1007/s10863-005-4117-y. [DOI] [PubMed] [Google Scholar]
- 46.King MS, Sharpley MS, Hirst J. Reduction of hydrophilic ubiquinones by the flavin in mitochondrial NADH:ubiquinone oxidoreductase (Complex I) and production of reactive oxygen species. Biochemistry. 2009;48:2053–2062. doi: 10.1021/bi802282h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fato R, Bergamini C, Leoni S, Lenaz G. Mitochondrial production of reactive oxygen species: role of complex I and quinone analogues. Biofactors. 2008;32:31–39. doi: 10.1002/biof.5520320105. [DOI] [PubMed] [Google Scholar]
- 48.Fash DM, Khdour OM, Sahdeo SJ, Goldschmidt R, Jaruvangsanti J, Dey S, Arce PM, Collin VC, Cortopassi GA, Hecht SM. Effects of alkyl side chain modification of coenzyme Q10 on mitochondrial respiratory chain function and cytoprotection. Bioorg Med Chem. 2013;21:2346–2354. doi: 10.1016/j.bmc.2013.01.075. S0968-0896(13)00125-9. [DOI] [PubMed] [Google Scholar]
- 49.Sherer TB, Richardson JR, Testa CM, Seo BB, Panov AV, Yagi T, Matsuno-Yagi A, Miller GW, Greenamyre JT. Mechanism of toxicity of pesticides acting at complex I: relevance to environmental etiologies of Parkinson's disease. J Neurochem. 2007;100:1469–1479. doi: 10.1111/j.1471-4159.2006.04333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tai KK, Pham L, Truong DD. Idebenone induces apoptotic cell death in the human dopaminergic neuroblastoma SHSY-5Y cells. Neurotox Res. 2011;20:321–328. doi: 10.1007/s12640-011-9245-z. [DOI] [PubMed] [Google Scholar]
- 51.Giorgio V, Petronilli V, Ghelli A, Carelli V, Rugolo M, Lenaz G, Bernardi P. The effects of idebenone on mitochondrial bioenergetics. Biochim Biophys Acta. 2012;1817:363–369. doi: 10.1016/j.bbabio.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schinzel AC, Takeuchi O, Huang Z, Fisher JK, Zhou Z, Rubens J, Hetz C, Danial NN, Moskowitz MA, Korsmeyer SJ. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc Natl Acad Sci U S A. 2005;102:12005–12010. doi: 10.1073/pnas.0505294102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kowaltowski AJ, Castilho RF, Vercesi AE. Mitochondrial permeability transition and oxidative stress. FEBS Lett. 2001;495:12–15. doi: 10.1016/s0014-5793(01)02316-x. [DOI] [PubMed] [Google Scholar]
- 54.Petronilli V, Costantini P, Scorrano L, Colonna R, Passamonti S, Bernardi P. The voltage sensor of the mitochondrial permeability transition pore is tuned by the oxidation-reduction state of vicinal thiols. Increase of the gating potential by oxidants and its reversal by reducing agents. J Biol Chem. 1994;269:16638–16642. [PubMed] [Google Scholar]
- 55.Haefeli RH, Erb M, Gemperli AC, Robay D, Courdier F,I, Anklin C, Dallmann R, Gueven N. NQO1-dependent redox cycling of idebenone: effects on cellular redox potential and energy levels. PLoS ONE. 2011;6:e17963. doi: 10.1371/journal.pone.0017963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heitz FD, Erb M, Anklin C, Robay D, Pernet V, Gueven N. Idebenone protects against retinal damage and loss of vision in a mouse model of Leber's hereditary optic neuropathy. PLoS ONE. 2012;7:e45182. doi: 10.1371/journal.pone.0045182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.CONOVER TE, Ernster L. DT diaphorase. II. Relation to respiratory chain of intact mitochondira. Biochim Biophys Acta. 1962;58:189–200. doi: 10.1016/0006-3002(62)90998-8. [DOI] [PubMed] [Google Scholar]
- 58.Chan TS, Teng S, Wilson JX, Galati G, Khan S, O'brien PJ. Coenzyme Q cytoprotective mechanisms for mitochondrial complex I cytopathies involves NAD(P)H: quinone oxidoreductase 1(NQO1). Free Radic Res. 2002;36:421–427. doi: 10.1080/10715760290021270. [DOI] [PubMed] [Google Scholar]
- 59.Ahlgren-Beckendorf JA, Reising AM, Schander MA, Herdler JW, Johnson JA. Coordinate regulation of NAD(P)H:quinone oxidoreductase and glutathione-S-transferases in primary cultures of rat neurons and glia: role of the antioxidant/electrophile responsive element. Glia. 1999;25:131–142. [PubMed] [Google Scholar]
- 60.Dinkova-Kostova AT, Talalay P. NAD(P)H:quinone acceptor oxidoreductase 1 (NQO1), a multifunctional antioxidant enzyme and exceptionally versatile cytoprotector. Arch Biochem Biophys. 2010;501:116–123. doi: 10.1016/j.abb.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dong H, Shertzer HG, Genter MB, Gonzalez FJ, Vasiliou V, Jefcoate C, Nebert DW. Mitochondrial targeting of mouse NQO1 and CYP1B1 proteins. Biochem Biophys Res Commun. 2013;435:727–732. doi: 10.1016/j.bbrc.2013.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lind C, Hochstein P, Ernster L. DT-diaphorase as a quinone reductase: a cellular control device against semiquinone and superoxide radical formation. Arch Biochem Biophys. 1982;216:178–185. doi: 10.1016/0003-9861(82)90202-8. [DOI] [PubMed] [Google Scholar]
- 63.O'brien PJ. Molecular mechanisms of quinone cytotoxicity. Chem Biol Interact. 1991;80:1–41. doi: 10.1016/0009-2797(91)90029-7. [DOI] [PubMed] [Google Scholar]
- 64.Joseph P, Jaiswal AK. NAD(P)H:quinone oxidoreductase1 (DT diaphorase) specifically prevents the formation of benzo[a]pyrene quinone-DNA adducts generated by cytochrome P4501A1 and P450 reductase. Proc Natl Acad Sci U S A. 1994;91:8413–8417. doi: 10.1073/pnas.91.18.8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Siegel D, Ross D. Immunodetection of NAD(P)H:quinone oxidoreductase 1 (NQO1) in human tissues. Free Radic Biol Med. 2000;29:246–253. doi: 10.1016/s0891-5849(00)00310-5. [DOI] [PubMed] [Google Scholar]
- 66.Stringer JL, Gaikwad A, Gonzales BN, Long DJ, Jr., Marks LM, Jaiswal AK. Presence and induction of the enzyme NAD(P)H: quinone oxidoreductase 1 in the central nervous system. J Comp Neurol. 2004;471:289–297. doi: 10.1002/cne.20048. [DOI] [PubMed] [Google Scholar]
- 67.Schultzberg M, Segura-Aguilar J, Lind C. Distribution of DT diaphorase in the rat brain: biochemical and immunohistochemical studies. Neurosci. 1988;27:763–776. doi: 10.1016/0306-4522(88)90181-9. [DOI] [PubMed] [Google Scholar]
- 68.Bell KF, Al-Mubarak B, Fowler JH, Baxter PS, Gupta K, Tsujita T, Chowdhry S, Patani R, Chandran S, Horsburgh K, Hayes JD, Hardingham GE. Mild oxidative stress activates Nrf2 in astrocytes, which contributes to neuroprotective ischemic preconditioning. Proc Natl Acad Sci U S A. 2011;108:E1–E2. doi: 10.1073/pnas.1015229108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shih AY, Johnson DA, Wong G, Kraft AD, Jiang L, Erb H, Johnson JA, Murphy TH. Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress. J Neurosci. 2003;23:3394–3406. doi: 10.1523/JNEUROSCI.23-08-03394.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Greco T, Fiskum G. Neuroprotection through stimulation of mitochondrial antioxidant protein expression. J Alzheimers Dis. 2010;20(Suppl 2):S427–S437. doi: 10.3233/JAD-2010-100519. [DOI] [PubMed] [Google Scholar]
- 71.Li W, Kong AN. Molecular mechanisms of Nrf2-mediated antioxidant response. Mol Carcinog. 2009;48:91–104. doi: 10.1002/mc.20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Raina AK, Templeton DJ, Deak JC, Perry G, Smith MA. Quinone reductase (NQO1), a sensitive redox indicator, is increased in Alzheimer's disease. Redox Rep. 1999;4:23–27. doi: 10.1179/135100099101534701. [DOI] [PubMed] [Google Scholar]
- 73.SantaCruz KS, Yazlovitskaya E, Collins J, Johnson J, DeCarli C. Regional NAD(P)H:quinone oxidoreductase activity in Alzheimer's disease. Neurobiol Aging. 2004;25:63–69. doi: 10.1016/s0197-4580(03)00117-9. [DOI] [PubMed] [Google Scholar]
- 74.Wang Y, Santa-Cruz K, DeCarli C, Johnson JA. NAD(P)H:quinone oxidoreductase activity is increased in hippocampal pyramidal neurons of patients with Alzheimer's disease. Neurobiol Aging. 2000;21:525–531. doi: 10.1016/s0197-4580(00)00114-7. [DOI] [PubMed] [Google Scholar]
- 75.van Muiswinkel FL, de Vos RA, Bol JG, Andringa G, Jansen Steur EN, Ross D, Siegel D, Drukarch B. Expression of NAD(P)H:quinone oxidoreductase in the normal and Parkinsonian substantia nigra. Neurobiol Aging. 2004;25:1253–1262. doi: 10.1016/j.neurobiolaging.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 76.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 77.Kraft AD, Johnson DA, Johnson JA. Nuclear factor E2-related factor 2-dependent antioxidant response element activation by tert-butylhydroquinone and sulforaphane occurring preferentially in astrocytes conditions neurons against oxidative insult. J Neurosci. 2004;24:1101–1112. doi: 10.1523/JNEUROSCI.3817-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Habas A, Hahn J, Wang X, Margeta M. Neuronal activity regulates astrocytic Nrf2 signaling. Proc Natl Acad Sci U S A. 2013;110:18291–18296. doi: 10.1073/pnas.1208764110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Murphy TH, Yu J, Ng R, Johnson DA, Shen H, Honey CR, Johnson JA. Preferential expression of antioxidant response element mediated gene expression in astrocytes. J Neurochem. 2001;76:1670–1678. doi: 10.1046/j.1471-4159.2001.00157.x. [DOI] [PubMed] [Google Scholar]
- 80.Satoh T, Okamoto SI, Cui J, Watanabe Y, Furuta K, Suzuki M, Tohyama K, Lipton SA. Activation of the Keap1/Nrf2 pathway for neuroprotection by electrophilic phase II inducers. Proc Natl Acad Sci U S A. 2006;103:768–773. doi: 10.1073/pnas.0505723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Satoh T, Kosaka K, Itoh K, Kobayashi A, Yamamoto M, Shimojo Y, Kitajima C, Cui J, Kamins J, Okamoto S, Izumi M, Shirasawa T, Lipton SA. Carnosic acid, a catechol-type electrophilic compound, protects neurons both in vitro and in vivo through activation of the Keap1/Nrf2 pathway via S-alkylation of targeted cysteines on Keap1. J Neurochem. 2008;104:1116–1131. doi: 10.1111/j.1471-4159.2007.05039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim J, Lee S, Shim J, Kim HW, Kim J, Jang YJ, Yang H, Park J, Choi SH, Yoon JH, Lee KW, Lee HJ. Caffeinated coffee, decaffeinated coffee, and the phenolic phytochemical chlorogenic acid up-regulate NQO1 expression and prevent H(2)O(2)-induced apoptosis in primary cortical neurons. Neurochem Int. 2012;60:466–474. doi: 10.1016/j.neuint.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 83.Kim J, Kim SK, Kim HK, Mattson MP, Hyun DH. Mitochondrial Function in Human Neuroblastoma Cells Is Up-Regulated and Protected by NQO1, a Plasma Membrane Redox Enzyme. PLoS ONE. 2013;8:e69030. doi: 10.1371/journal.pone.0069030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dragan M, Dixon SJ, Jaworski E, Chan TS, O'brien PJ, Wilson JX. Coenzyme Q(1) depletes NAD(P)H and impairs recycling of ascorbate in astrocytes. Brain Res. 2006;1078:9–18. doi: 10.1016/j.brainres.2006.01.068. [DOI] [PubMed] [Google Scholar]